Abstract
Sustained remission is an ultimate treatment goal in the management of patients with rheumatoid arthritis (RA). Historically the frequency of sustained remission was low but the frequency of achieved sustained remission is increasing over time. The last years’ clinical studies of tight control targeted treatment and intervention trials of early use of intensive strategy suggest that these treatment strategies are associated with higher rates of sustained remission. Achievement of sustained remission, in particular but not limited to early sustained remission, can provide tapering and stopping disease-modifying antirheumatic drugs (DMARDs). With new treatment strategies drug-free sustained remission is becoming an achievable goal. Sustained remission is associated with improved outcomes in regard to function, patient-reported outcomes and survival. Drug-free sustained remission is characterized by normalized function ability and survival. Sustained remission and, in particular, drug-free sustained remission offer hope that early identification of patients with arthritis, early improved novel treatments and treatment with target to achieve remission may potentially transform the progressive course of RA disease and disrupt RA chronicity.
In this review we summarize the recent evidence on sustained remission in patients with RA, treatment strategies to achieve sustained remission, management of patients in sustained remission and significance of sustained remission from the patient perspective.
Keywords: rheumatoid arthritis, sustained remission, drug-free remission, treatment strategy, outcome, disability
Introduction
During recent decades, the target of treatment of rheumatoid arthritis (RA) has changed from symptomatic relief to prevention of disability and radiologic damage, and further to early remission and persistent remission. This last target has become achievable because of a change in the paradigm of RA treatment: from ‘go low go slow’ to early use of disease-modifying antirheumatic drugs (DMARDs), from a pyramid approach of DMARD monotherapy to ‘step-up’ combination therapy, treatment-targeted tight strategy and early ‘step-down’ combination therapy (i.e. an inverse pyramid approach). With changes in the therapy approach, the course of the disease nowadays can be truly modified. These existing advantages have transformed the view on the ultimate course of RA as a chronic disabling disease to a potentially curable condition by possibility to switch off autoimmunity and induce immune tolerance. As such, novel therapy outcomes are then required to identify an individual benefit/risk profile at each therapy decision. These must facilitate a balanced therapy approach when inflammation is suppressed and remission is achieved.
The shift in the disease perspectives brings forth a shift in outcome measures such as remission. Remission outcomes as American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) and Disease Activity Score (DAS) remission measure treatment efficacy at one timepoint and are undoubtedly the first crucial step in informed, successful disease management. The next logical step is an achievement of durable remission and management during durable remission once it is achieved. This review aims to describe definitions of sustained remission, to review its frequency, to discuss whether it can be induced or whether it reflects the natural course of disease and to review what benefits sustained remission offers.
How is sustained remission defined?
RA is characterized by chronic, progressively destructive inflammation. Disease activity and flares over time are associated with disease progression, functional deterioration and development of radiographic damage.1 Hence, the primary target of treatment of RA is to achieve clinical remission.2,3 It has been proved that the target-steered approach in the management of RA conveys better outcomes than routine care.4 It is likely that stable remission could be a stronger predictor of a better RA prognosis than remission at a single timepoint. Conceptually, remission and absence of disease, chronic by its nature, should include a time perspective and should be defined at an individual patient level in clinical practice.
Although the concept of sustained remission has become accepted, there is currently no uniform definition of sustained remission. The ACR/EULAR Boolean-based definition of remission is developed for use in clinical trials.5 Remission criteria according to DAS28 using the erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) level and more stringent DAS-based criteria, as Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI), measure disease activity in RA both in daily clinical practice as well as in clinical trials on a group level as well as for an individual patient.6,7 All these are important outcome measures at any timepoint of the disease and predict later good radiographic and functional outcomes on a group level. However, index-based remission criteria allow residual swollen joints on conventional clinical examination. Thus, absence of disease and synovitis at a patient level could not be defined using current criteria.
According to the recommendations on RA management, regular monitoring of disease activity should guide decisions on choice and changes in therapy to reach a state of clinical remission.2 The treatment target is moved forward to the goal of maintenance of remission in the last 2016 update of the EULAR treatment recommendations.8 As a developing definition, a minimum of 6 months is mentioned as a time frame for the target-state of sustained remission. However, the strict approach of sustained remission could only be recommended after validation of application of this criterion in prospective RA clinical settings if the preselected remission duration could differentiate the long-term outcomes.
To date, different definitions of sustained remission have been applied in the studies with various remission criteria, frequency of assessments and a minimum duration of remission sustenance. The usage of different criteria corresponds to variance in study results. To test the hypothesis of sustained remission and in the absence of consensus on definition, in the meantime, DAS remission criteria along with ACR/EULAR remission criteria have been adopted in the studies.
What is sustained DMARD-free remission?
Once a good response on DMARDs and stable remission are achieved, the treatment could be tapered; and once remission is further sustained, the treatment could be stopped.9 Drug-free remission has been described in several patient groups which supports that drug-free remission could truly be achieved.
The approach of benefit/risk assessment of continuation or stopping DMARDs was examined as early as the 1990s in a double-blind placebo-controlled study.10 In this study, RA patients with a good long-term therapeutic response, after median duration of DMARD therapy of 5 years, were randomized to continue therapy, n = 142, or to receive placebo, n = 143. At 52 weeks of follow up, as many as 62% of the patients who were randomized to placebo and 78% of the patients who were randomized to continue therapy did not have a flare, defined as recurrence of synovitis. Side-effects that necessitated dose reduction or discontinuation occurred equally in each group.
A sustained DMARD-free remission is not uncommon in RA patients who are early and successfully treated with conventional DMARDs. In the large observational inception cohort from the Leiden Early Arthritis Clinic (EAC), The Netherlands (454 patients), and the British Early Rheumatoid Arthritis Study (ERAS; 895 patients), who fulfilled the ACR 1987 RA criteria and were initially treated with DMARDs, sustained DMARD-free remission was achieved in 9–15% of patients during follow up.11 The remission in the EAC cohort was defined as a sustained absence of clinical synovitis for at least 1 year after cessation of DMARDs.
From the Dutch Behandel-Strategieën (BeSt) randomized controlled trial (RCT) we have learned that early-targeted treatments, irrespective of initial treatment strategy, and subsequent strict monitoring of disease state during the whole follow up improve sustenance of remission after discontinuation of DMARDs.12 According to the protocol of the BeSt study, the patients were randomized to group 1: sequential monotherapy, group 2: step-up combination therapy, group 3: combination therapy with methotrexate (MTX), sulfasalazine and high-dose tapered prednisone; and group 4: combination therapy with MTX and infliximab. During all extended 10-year follow up, 3-monthly DAS calculations were performed to steer treatment and to adjust medication according to strategy protocols. If DAS was ⩽2.4 for at least 6 months, therapy was tapered; first, combination therapy to monotherapy, then monotherapy to maintenance dose. From year 3, patients who had been in DAS < 1.6 remission for at least 6 months on maintenance dose, had to taper and stop their last DMARD. Treatment was restarted if DAS increased ⩾1.6.
During the first 5 years of the study, 115/508 (23%) of patients at some time achieved drug-free remission. The 3-monthly DAS monitoring by protocol continued through the whole study. Overall, 53 patients (46%) lost remission and restarted treatment after a median duration of 5 months, 74% of these patients re-achieved remission within 3–6 months. Patients who could not maintain remission were more likely to be anti-citrullinated protein antibody (ACPA)-positive, had higher DAS and used sulfasalazine (but not MTX) as the last DMARD before they had achieved remission. Radiological damage progression was rare after drug discontinuation. A total of 59 patients (51%) remained in drug-free remission for a median duration of 23 months. At the end of the 7th year, as many as 13%, 16%, 16% and 14% of patients in treatment groups 1–4, respectively, were still in drug-free remission.12 Further analysis after 10 years showed that beneficial effects of DAS-targeted strategy persisted.1 Of all, 14% of patients were in drug-free remission without differences among the strategies; and radiographic damage was limited for all treat-to-target strategies.
The early strict DAS-steered therapy principle provides higher rates of achieved DMARD-free sustained remission also in the presence of autoantibodies. The retrospective comparison in 508 recent-onset RA patients treated with DAS-driven therapy (DAS ⩽ 2.4) in the BeSt cohort, and in 424 patients who received non-DAS-driven therapy in the prospective inception EAC cohort has shown that DAS-driven therapy may improve the chance of sustained drug-free remission in ACPA-positive patients. Among ACPA-positive patients, drug-free remission was more frequently achieved after DAS-driven than after non-DAS-driven therapy, 5.4% versus 2.1% of patients.13 This observation suggests that early-targeted treatment strategy may indeed modify the disease course.
Immuno-dysregulation has been described several years before onset of symptoms of arthritis. Breakdown of immune tolerance arises in the predisposed persons which manifests in clinical symptoms of the disease. As an autoimmune disease, RA has a tendency to become chronic with a time. Biological mechanisms distinguishing between preclinical, early and established disease have been proposed.14 The reports that DMARD-free sustained remission could be achieved more frequently in early disease and in patients fulfilling the 2010 criteria of RA compared with those fulfilling the 1987 criteria15 raise the hypothesis that early intensified antirheumatic therapy may have higher potential to restore self-tolerance and, thence, to modify the course and the phenotype of RA disease.
Is sustained remission uncommon?
In general, sustained remission in established RA might be achievable in fewer patients than in early RA, and it is likely not as common in clinical practice as in trials. Higher rates of remission can be achieved in both observational datasets and clinical trials when a treat-to-target strategy is adopted. Currently, there is no definite answer on how often sustained remission could be achieved and how long sustained remission could be maintained because of several different definitions of sustained remission used in the studies.
Firstly, the time frame to assess remission at consecutive visits ranges from two consecutive assessments with at least 1 month apart,16 to biannual assessments,17,18 annual assessments,19–21 or longer periods between assessments.22–25 The rate of persistent DAS-based remission in these studies ranges from 5–45%.
Hence, a definition of accurate timeframes to assess remission is required. This question has been investigated in the recent pooled analysis of patient-level clinical trial data.26 It has been shown that 3-month changes are critical timepoints for the target of remission (while 6-months changes for the target of low disease activity), and that type of treatment or early versus established RA (initial MTX or a biologic in early RA, combination of MTX plus a biologic in early RA, step-up from MTX to a combination of MTX and a biologic in insufficient responders) did not significantly influence the accuracy of the 3-month changes to predict response. In the observational setting, 3-month monitoring has also been shown as a minimum timeframe to assess remission, taking into account prediction of good functional and good overall outcome.27
Secondly, the definition of duration of remission is required. The longer the time in remission is required, the fewer patients could be defined as maintaining remission because a number of patients will experience a flare with time. The reported definition of remission duration ranges from several weeks (mostly in clinical trials) to over 12 months (in observational cohorts). The most often applied time to define sustained remission before tapering or withdrawing of treatment is 6 months.9
The greatest loss of remission status occurs after a single remission visit, and once remission is seen on two subsequent visits, the chance of remaining in remission becomes much higher. Thus, in 621 RA patients from a typical outpatient setting, frequency of remission dropped from approximately 40% at a single random visit to 17–20% if two subsequent visits in remission were required.16 Further, in the recent-onset RA patients treated with conventional DMARDs, sustained clinical remission at all 3-, 4- and 5-year follow ups was achieved only in 11% of patients, half as likely as remission at a single timepoint.19 Evaluation in 394 patients with established RA disease from the Brigham and Women’s Rheumatoid Arthritis Sequential Study (BRASS) not in remission at baseline has shown that <50% of RA patients in remission at a single time remained in remission 1 year later, and that the likelihood of patients experiencing active disease after remission decreases as the years in remission increase.21 The important observation in the latter study is that more patients with disease duration ⩽5 years who achieved DAS28-CRP < 2.6 remission at ⩾1 visit can further maintain remission, compared with patients who achieved remission >5 years after onset of disease. The maintenance of remission the first years after that remission had been achieved, were similar for seropositive and seronegative patients in this study. These results suggest that the prognosis of the disease course in both seropositive and seronegative RA may be defined by the efficacy of therapeutic interventions applied the first years after diagnosis.
Thirdly, the reported rates of sustained remission range depending on the choice of remission criteria, treatment regimen and RA patient population. Higher rates of sustained remission, not surprisingly, are observed with DAS-based criteria than with the more stringent ACR-EULAR index-based criteria. The highest rates of sustained remission could likely be achieved in early RA patients receiving early intensive therapy. In early RA patients assessed 3-monthly during the first year of the combination therapy with prednisolone tapering from 30 mg/day and escalating dose of MTX to 25 mg/week in the Combinatietherapie Bij Reumatoïde Artritis (COBRA) light trial, the highest rates of sustained remission over at least two consecutive visits have been reported for DAS28 in 40% of patients, followed by DAS in about 30% of patients, and CDAI, SDAI and Boolean criteria remission in 7–17% of patients.27 In the post hoc analyses of the Abatacept study to Gauge Remission in Early Erosive rheumatoid arthritis (AGREE study), a higher proportion of patients receiving abatacept plus MTX attained sustained SDAI and CDAI remission than patients receiving MTX alone.28 The median time to achieve first remission in this study was longer for SDAI and CDAI remission criteria than for DAS28 criteria, which also indicates greater stringency of the index-based criteria. High early sustained remission rates at both the 3- and 12-month visits were achieved in recent-onset arthritis patients treated according to treat-to-target principles in the Finnish rheumatology clinic; the rate of sustained DAS28 remission was 57% in RA patients and 56% in patients with undifferentiated arthritis; sustained Boolean remission was achieved in 16% and 12% of patients.29
Application of the stringent remission criteria likely differentiates a particular subgroup of patients with a particular favorable functional status. The highest chance for good functional outcome, as expected, has been demonstrated for ACR-EULAR index-based criteria, followed by DAS-based criteria.27,28
Lower rates of sustained remission have been reported from the registry of unselected patients with established RA treated in usual care; in these patients sustained DAS28 remission was achieved in 16% and SDAI remission in 8%.30 Choice of prolonged remission at all visits after 1, 2, 5 and 8-years after inclusion into the observational study of patients in usual care similarly resulted in low sustained remission rates: 14% by DAS28 remission, 5% by SDAI and 3% by Boolean criteria.24
The DAS remission criterion has been criticized for allowing classification of patients as being in remission in spite of having several swollen joints, not compatible with a state of remission. However, in the group of patients who achieved DAS28-sustained remission in the BARFOT study, only a minority had more than a few tender or swollen joints.24 By applying CDAI, SDAI and the Boolean remission criteria some remission may be missed due to non-RA-related conditions. The low frequency of sustained remission by these criteria suggests that they may be too stringent to suite for long-term observational studies. In clinical practice, it seems reasonable and feasible to use the DAS-based remission criteria for the definition of sustained remission.
The constant frequency of DMARD-free remission, 10–15%, over the last two decades is reported across the observational cohort studies in RA patients treated with conventional DMARDs.11,31,32 In RCTs in established RA, rates of successful drug withdrawal are also low and risk of flare after therapy withdrawal is high.33,34 These observations have given rise to questions on whether the patients who achieve DMARD-free sustained remission would also achieve remission (i.e. spontaneous remission) without any DMARDs.35 Spontaneous remission per definition would occur regardless of treatment and thus the rates of spontaneous remission would be similar through the years. On the contrary, treatment-induced remission would occur at different rates with explicitly different treatments. Therefore, with earlier and intensive treatment strategies, higher overall remission rates and higher rates of sustained remission as well as sustainability of remission for a longer time are expected.
Indeed, evidence supporting the concept of modifiable achievement of drug-free remission is expanding. With early treatment and improved therapeutic strategies the last decade, sustained remission is not uncommon anymore. Thus, the early treatment including combination DMARDs in the treat-to target studies Cyclosporine, Methotrexate, Steroid in RA (CIMESTRA) and the BeSt trial has benefited by higher rates of DMARD-free remission in 16–25% of patients.36,37 In early RA patients from the Leiden EAC observational cohort, the cumulative percent of patients achieving sustained DMARD-free remission over time was increasing from 13% in the inclusion period 1993–1995, 18% between 1996–1998, to 26% between 1999–2004 and 30% in the inclusion period after 2005, p < 0.001, between-group difference.38
Altogether, early rapid and sustained suppression of disease activity the first year guided by criteria of low disease activity may provide sustained remission and therapy de-escalation. Further, early aggressive treatment of RA guided by remission criteria may be considered as ‘induction’ therapy of, ideally, a prolonged remission after therapy discontinuation. Thus, in the BeSt study, 67/120 (56%) of the DMARD-naïve patients with active early RA, who were initially treated with infliximab in combination with MTX 25 mg/week, achieved sustained low disease activity DAS ⩽ 2.4 and could discontinue infliximab after a median of 9.9 months, with a median MTX dosage of 10 mg/week after 2 years.39 The concept of early intensive treatment targeted at remission has been first examined in the placebo-controlled double-blind pilot study, with or without infliximab, in addition to escalating MTX doses up to 25 mg/week in early poor-prognosis RA.40 After 1 year from the discontinuation of infliximab in this study, 7/10 patients (70%) of patients still had sustained clinical response with manifest advantages in quality of life and function. Rapid disease suppression in the active infliximab arm was paralleled by a rapid (<14 weeks), sustained (104 weeks), and significant improvement in function and quality of life scores.
The recent Dutch study from the Rotterdam Early Arthritis Cohort (tREACH) reassured that with early intensive treatment many patients with early RA can reach sustained remission and maintain it after tapering conventional and biological DMARDs.41 In this study 281 patients were randomized to initial induction strategies with triple DMARD therapy and glucocorticoid bridging or MTX 25 mg/week monotherapy with glucocorticoid bridging. Patients were evaluated every 3 months and treated-to-target of DAS ⩽ 2.4. In cases where DAS was >2.4, treatment was switched to a tumor necrosis factor (TNF)-blocker combined with MTX 25 mg/week. If remission was stable, a DAS < 1.6 at two consecutive timepoints was achieved, and as long as sustained remission was maintained, DMARDs were stepwise tapered according to the study protocol. During 2 years of follow up, as many as 57% of patients achieved sustained remission regardless of the initial treatment strategy. A higher frequency of early sustained remission within 6 months was among patients treated with triple DMARD therapy compared with the MTX monotherapy group, 52% versus 37%, but the rate of sustained remission within 1 year was comparable between the treatment groups, 84% versus 76%. Tapering involved a conventional DMARD in 84% of the patients and a biological DMARD in 16% of the patients in this study. Flares within 12 months occurred in 41% of patients after tapering conventional DMARDs and in 7% of patients after tapering TNF-blockers. After a flare, 65% of patients regained remission within 6 months after treatment intensification. The somewhat low rate of regained remission in this study could depend on inclusion of patients with a high risk of developing persistent arthritis and that therapy was steered by criteria of low disease activity. Yet, in this study on RA patients with a poor prognosis, tapering of all DMARDs was successful in 34 patients (12%) who achieved remission DAS < 1.6. Of these, 11 and 7 patients remained in drug-free remission for at least 3 and 6 months, respectively.
The benefits of early intensive treatment with tocilizumab (TCZ) compared with methotrexate monotherapy to achieve sustained remission have been demonstrated in the U-Act-Early randomized clinical trial.42 The treatment target in the study was sustained DAS28 < 2.6 remission during at least 24 weeks. Sustained remission was substantially more frequent achieved in patients initially treated with TCZ plus MTX or TCZ monotherapy compared with patients initially treated with MTX monotherapy: 86% of patients versus 84% versus 44% of patients, respectively, relative risk for sustained remission 2.00 [95% confidence interval (CI) 1.59–2.51] for TCZ + MTX versus MTX, and 1.86 (1.48–2.32) for TCZ versus MTX, p < 0.001 for both comparisons. The time to onset of sustained remission was shorter in the TCZ arms than in the MTX arm: median (interquartile range, IQR) of 8.0 (4.4–16.0), 9.4 (4.1–21.0) and 34.6 (18.7–45.3) weeks for the TCZ + MTX, TCZ and MTX arm, respectively, p < 0.001. If sustained remission was achieved, the dose of all drugs was stepwise reduced and then discontinued, provided that sustained remission persisted. The proportion of patients achieving sustained drug-free remission for ⩾12 weeks after tapering and discontinuing all DMARDs was significantly higher in both TCZ arms compared with the MTX arm, 35% and 27% of patients (initial TCZ + MTX and TCZ therapy, respectively) versus 11% of patients with initial MTX therapy, p < 0.001. This study therefore suggested that rates of early sustained remission are different in patients depending on the previous treatment strategies.
The very important concept that sustained drug-free remission is a modifiable event induced by treatment and not just the result of natural disease course has been also proved in trials of early biological DMARD usage in DMARD-naïve RA patients. Thus, in the Productivity and Remission in a randomIZed controlled trial of etanercept in Early rheumatoid arthritis (PRIZE) study, one of four patients who were in DAS28 remission induced by early initial treatment with etanercept and MTX were still in remission more than 1 year after the cessation of both drugs.43 Patients maintained remission significantly longer after remission induction with a biological DMARD than patients who were initially treated with MTX alone in this study. Similarly, in A Very Early Rehabilitation Trial (AVERT study), treatment withdrawal resulted in 15% sustained drug-free remission at 18 months in patients initially treated with abatacept plus MTX, while only 8% of patients initially treated with MTX alone was able to maintain drug-free remission after induction of remission.44 Longer duration in DAS-defined remission on treatment predicted sustenance of drug-free remission following withdrawal of abatacept. Although suggesting that drug-free remission could be modified by treatments, the follow up of both the U-Act-Early study and the RCT studies are too short to prove the hypothesis that drug-free remission can be induced by a certain drug or a certain treatment strategy.
Does sustained remission matter?
The patient perception of remission was examined in the inductive thematic analysis of the prespecified guided focus group discussions with RA patients in Austria, The Netherlands and UK.45 Patients identified duration of remission as an important aspect of the concept of remission and characterized remission by the feeling of a return to normality.
The relevance of achieving drug-free sustained remission has been recently evaluated in the Leiden EAC cohort in 155 RA patients who achieved DMARD-free sustained remission during follow up.38 At remission, the median Health Assessment Questionnaire (HAQ) score was 0.13, which means that functional ability in these patients was normalized, as in the normal population the mean HAQ of 0.25 is reported.46 The Visual Analogue Scale (VAS)-scores on pain and fatigue (a 100 mm scale) at remission was 6 and 10 respectively, which are lower compared with the corresponding reference values of 11.5 and 20.5.47–50
Achievement of DMARD-free remission at any timepoint in the disease course in the EAC cohort was related to better outcomes compared with outcomes among patients who did not achieve such remission. Importantly, better outcomes were demonstrated in all patients who achieved remission, irrespective whether remission was achieved early (within 3 years after inclusion), intermediate (3–5 years of disease) or late (5–13 years) after RA diagnosis. Thus, the longitudinal course of HAQ, VAS disease activity, VAS pain, VAS fatigue, VAS morning stiffness, likewise CRP, ESR and tender-joint count (TJC) was substantially similar in the patients who achieved remission in early, intermediate or late phases of RA disease.38 Characteristics of the patients that achieved DMARD-free sustained remission at different time points during RA disease did not differ either at baseline or at the time when DMARD-free sustained remission was achieved. This finding implies that resolution of arthritis is possible and not limited to early disease but indeed can occur even in longstanding disease. Whether standard disease measures are not sensitive to measure the differences of remission achieved in different stages of disease, and whether pathophysiological mechanisms of transition from chronic inflammatory state to DMARD-free sustained remission may differ in different stages of RA can only be speculated on.
RA is a fluctuating disease with fluctuating disease activity over time. Overall disease activity is a risk factor for radiographic progression, loss of function and early mortality. The state of sustained remission should ideally indicate absence of disease activity and thence a halt of further joint damage. Indeed, in patients with established RA treated in usual care, physical function measured by HAQ improved in patients reaching DAS28-sustained remission for at least 6 months at two consecutive visits compared with patients who only occasionally reached remission; and the HAQ continued to improve while in sustained remission.30 The relationship between time in remission and radiographic outcome has been also assessed in RA patients with a broad spectrum of disease activity and disease duration in the observational cohort of the BRASS.20 In this analysis, an increased number of visits in remission by any criteria (ACR/EULAR remission, DAS28, SDAI and CDAI criteria), assessed in 535 patients at baseline, 1 and 2 years, was associated with reduced radiographic damage after 2 years of follow up. Similarly, sustenance of remission resulted in less subsequent radiographic damage progression in the PREMIER trial.51 In this RCT of adalimumab plus MTX or adalimumab alone or MTX alone in early RA, sustained remission SDAI ⩽ 3.3 during the second year of trial was associated with arrest of joint damage, irrespective of the therapy given. The mean change in the modified Sharp van der Heijde score (SHS) was 1.19 in those with 3 additional months of remission versus 0.20 in those with 6 additional months of remission and −0.32 in those with 9 additional months of remission,p < 0.05.
The likelihood of experiencing active disease decreases as duration in remission increases, which is reassuring for systematical treatment tapering in patient with sustained remission. On the other hand, even after years in remission, a patient’s disease can become more active. However, even after the loss of remission at follow up, sustained remission in the course of the disease may be beneficial in respect to long-term clinical outcomes. Further, once sustained remission is achieved, a chance to regain remission after flare is high. Thus, in the BRASS observational cohort of established RA,21 patients who were in DAS28-CRP < 2.6 remission at two subsequent annual visits but lost remission at the subsequent annual visit, had a median DAS28-CRP score of 3.7, median TJC of 5, swollen joint (SJC) of 4, CRP of 8.3 mg/l, and general health on VAS of 18 mm; while patients who were in remission only at one visit and lost remission at the subsequent annual visit had a worse measurements at follow-up assessment: DAS28-CRP score of 3.8, TJC of 6, SJC of 4, CRP of 6.6 mg/l, and VAS general health of 25 mm. Further, after flare, 31% of patients who were in remission at two subsequent annual visits but lost remission at the subsequent visit could regain remission, compared with 23% of those who were in remission only at one visit. The percentage of patients who regained remission after a flare was low in this cohort, likely because of the long disease duration (a median of 8 years) and 1-year interval between visits.
Sustained absence of disease activity also suggests a halt of functional deterioration and normalized survival. This has been reported in patients with early inflammatory polyarthritis recruited to the Norfolk Arthritis Register, NOAR cohort.52,53 The most persistent remission under the most stringent definition of remission in this cohort was associated with the lowest probability of long-term disability. Thus, the number of times in remission, defined as absence of clinically detectable joint inflammation on a 51-joint count assessed at 1, 2, and 3 years after inclusion, correlated with the odds of disability, with a mean decrease in the probability of disability, defined as HAQ ⩾ 1, of ~64% for each additional timepoint in remission, odds ratio (OR) (95% CI) 0.38 (0.28–0.52). Remission according to less stringent criteria based on the 40- and 28-joint counts showed a weaker protection against future disability. Similarly, the number of assessments in remission was associated with a proportional decrease of the mortality risk, hazard ratio (HR) (95% CI) 0.86 (0.74–0.99). Patients who were in remission 1 year after the baseline assessments and had persistent remission over time had the greatest reduction in mortality risk compared with patients who never achieved remission within the first 3 years of follow up, HR 0.58 (0.37–0.91). Remission according to less stringent definitions was associated with progressively lower beneficial effect for overall survival.
The relation between sustained remission and survival outcome has been studied in 556 patients with early RA or undifferentiated arthritis (UA) included in the Leiden EAC cohort between 1993 and 1998.54 The DMARD-free sustained remission (defined as persistent absence of synovitis for at least 1 year after cessation of DMARD therapy) was achieved in 22% patients within 3 years after inclusion and in 33% patients during the total follow up of median 15 years in this cohort. Patients who achieved DMARD-free sustained remission within 3 years had a reduced mortality compared with patients without remission, HR 0.60 (0.39–0.93). Compared with the mortality rates of matched controls from the general Dutch population, patients who achieved DMARD-free sustained remission within the first 3 years or during the total follow up did not have an increased standardized mortality rate (SMR) (95% CI) 0.70 (0.47–1.02) in the patients and SMR 0.66 (0.47–0.92) in the matched population. The concept of improved long-term outcomes achieved by targeted treatment strategies has been also demonstrated in the BeSt study in patients with early RA. Treat-to-target at DAS ⩽ 2.4 at every 3-month visit has been associated with drug-free remission with prevention of functional deterioration and normalized mortality rate after 10 years.55
Altogether these observations imply that sustained remission is a desirable outcome relevant from the patient perspective and associates with a halt of joint damage progression, improved function and survival prognosis. Sustained drug-free remission reflects normalized health state and survival expectations close to these in the general population.
Could we predict achievement of sustained remission?
Sustained remission is likely to be more prevalent in patients commencing DMARD therapy earlier and less prevalent in ACPA-positive patients.56,57 Thus, DMARD-free remission was more frequent in ACPA-negative patients in the Leiden EAC cohort through the study period from 1996 to 2011.38 The presence of auto-antibodies did not absolutely impede achievement of DMARD-free sustained remission in this observational cohort but the chance of RA persistence was higher and the chance of DMARD-free sustained remission was lower in patients with auto-antibodies. Patients who achieved DMARD-free sustained remission in the EAC cohort, compared with those who did not, were less often ACPA or rheumatoid factor-positive (18% versus 62%, and 31% versus 65%, both p < 0.001) and had a shorter symptom duration at inclusion (median (IQR) of 3 months (2–7) versus 5 months (2–9),p < 0.001). However, patients who achieved DMARD-free sustained remission in this cohort, compared with those who still had persistent RA during follow up, did not have milder disease characteristics at baseline in terms of SJC, TJC, ESR, CRP, HAQ, morning stiffness, fatigue and frequency of high titre of ACPA.
As anticipated, ACPA presence seems likely to predict flares after tapering or stopping conventional and biological DMARD treatment in RA patients with sustained remission as it has been shown in the Reduction of Therapy in patients with Rheumatoid arthritis in Ongoing remission (RETRO) study.58 In this study, ACPA-positive patients in sustained remission DAS28 < 2.6 for >6 months showed a gradual increase of relapse rates in respect to allocation to continuing DMARDs, tapering DMARDs by 50% or stopping DMARDs after 6 months tapering, while ACPA-negative patients showed rather low prevalence for relapse, independent from whether treatment was continued, tapered or stopped.
In the first clinical study with drug-free remission as a treatment goal, the IMPROVED study in early RA and UA patients treated according to DAS < 1.6, ACPA status was not associated with achievement of early drug-free remission but with sustenance of drug-free remission.59 Patients who lost remission were more often ACPA-positive than those who sustained remission, 72% versus 47%, p = 0.01. In ACPA-positive patients drug-free remission was less often sustained than in ACPA-negative patients, 58% versus 80%, p = 0.013.The importance to treat early and to treat-to-target in early RA is supported by results of several studies. Thus, in early RA in the Finnish Rheumatoid Arthritis Combination Therapy trial (FIN-RACo) cohort it has been shown that remission is more often sustained if treatment is started early and if combinations of DMARDs is used compared with treatment with a single DMARD.60
Further, in the Dutch study from the Nijmegen early RA cohort, the median time to sustained remission was 10 months in the subcohort included between 2001–2005 and 14 months in the subcohort included between 1985–1990.61 Overall, the median time-to-remission in patients with sustained remission was 9 months (IQR 4–13 months), while time-to-remission in patients with nonsustained remission was 13 months (IQR 7–24 months), p < 0.001. The chance on sustained remission decreased with every additional month of time-to-remission. The OR (95% CI) of the relation between time-to-remission and having sustained remission was 1.11 (1.10–1.12), p < 0.001, which means a 1.11 higher odds on sustained remission if achieving first remission 1 month earlier (compared with achieving first remission 1 month later), a 1.37 higher odds to remain in remission if achieving remission 3 months earlier, and in case of 1 year earlier remission the odds to remain in remission increased to 3.5. The relationship between time-to-remission and sustained remission was similar across all calendar years of inclusion in this study, 1985–1990, 1991–1995, 1996–2000, 2001–2005. This observation implies the importance of achieving remission with any treatment strategy as early as possible in the course of the disease.
Next, the data from the BeST trial of treat-to-target treatment strategies in patients with recent-onset RA have demonstrated that the longer and the better a good clinical state is maintained, the greater the likelihood of remaining in that state.62 Thus, the probability to maintain DAS ⩽ 2.4 3 months after a first DAS ⩽ 2.4 was 74% in this trial, this probability increased to 85% after two preceding DAS ⩽ 2.4 and to 88–97% after one to two preceding DAS< 1.6.
The meta-analyses of raw data of the studies reporting on achieving DMARD-free sustained remission has proved the importance of early treatment initiation in the ‘window of opportunity’.57 Longer symptom duration at initiation of DMARDs was independently associated with a decreased chance to achieve DMARD-free sustained remission, HR (95% CI) of 0.989 (0.982–0.995) per week increase in symptom duration. Assuming a linear correlation with time in the early disease stage, the HR for achieving DMARD-free sustained remission was 0.88 in cases of 12 weeks’ symptom duration at treatment initiation (the suggested period of the window of opportunity).
The importance of early and intensified antirheumatic therapy in achievement of drug-free sustained remission has been demonstrated in the Leiden EAC cohort. From this cohort we have learned that achievement of DMARD-free sustained remission is increased in the recent years of early improved antirheumatic strategies. The time to achieve DMARD-free remission was shown to be shorter and the prevalence of remission higher in the patients included the last years than in those included in the 1990s.38 The mean time-to-remission (SD) was 7.8 (3.4), 6.9 (4.0), 4.8 (2.7) and 3.1 (1.4) years for RA patients included between 1993–1995, 1996–1998, 1999–2004 and 2005–2011, respectively, p < 0.001. The role of treatment strategy for chance on DMARD-free remission was described in this study as HRs (95% CI) for achieving remission of 1.5 (0.6–3.4) for the patients included 1996–1998 (initially treated with mild DMARDs), HR of 2.9 (1.3–6.4) for the patients included 1999–2004 (initially treated with MTX) and HR of 5.3 (2.3–12.1) for the patients included 2005–2011 (initially treated with MTX and DAS-steered strategy), as reference the inclusion period 1993–1995 (initial treatment with NSAIDs) was used in the analysis. The concept of RA disease course and outcomes with the development of treatment strategies is presented in figure 1.
Figure 1.
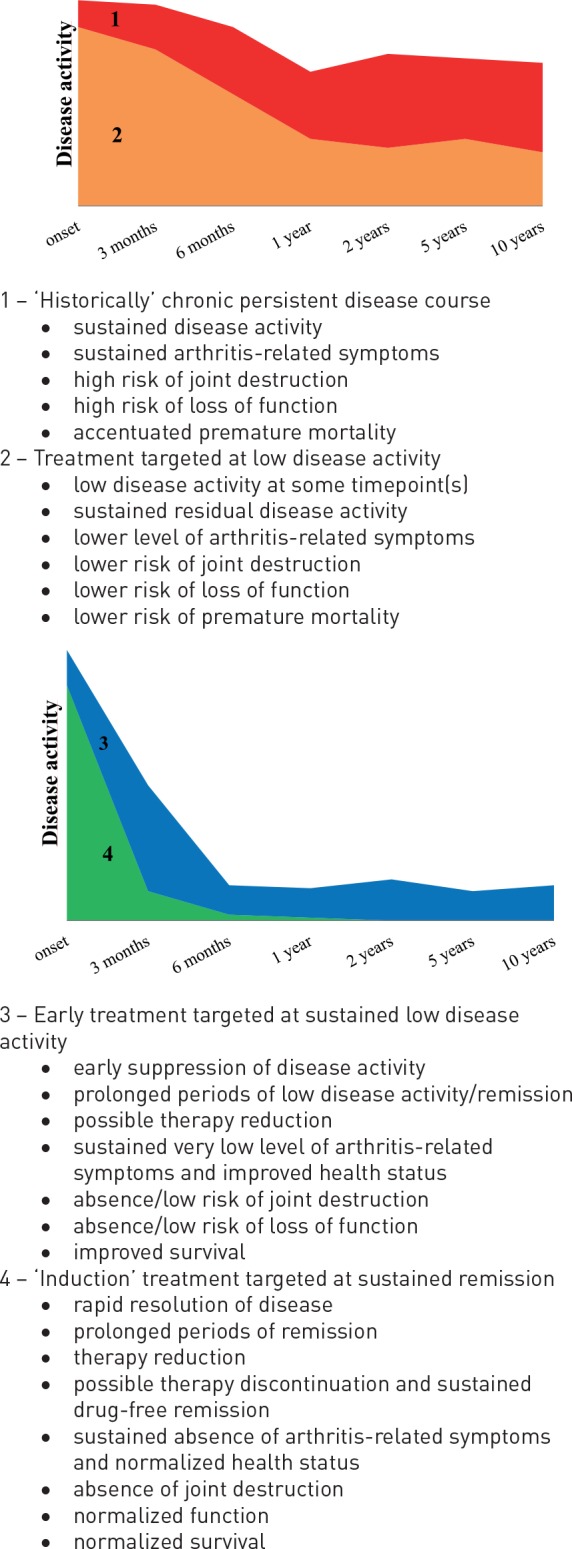
The concept of rheumatoid arthritis disease course and outcomes with the development of treatment strategies.
The treatment steered at the treatment target of remission is important in order to improve chance on achievement of early remission and drug-free remission. Thus, from the DAS-remission-steered strategies aiming at drug-free remission in early arthritis, the IMPROVED study, we have learned that DAS < 1.6 remission 4 months after DMARD initiation (early remission) is associated with a higher chance on drug-free remission. In this study, 610 patients with early RA or UA were treated with MTX and tapered high-dose prednisone. Patients in remission DAS < 1.6 after 4 months tapered and stopped medication. Patients who did not achieve early DAS remission were randomized to MTX plus hydroxychloroquine plus sulfasalazine plus low dose prednisone (arm 1), or to MTX plus adalimumab (arm 2). Monitored 4-monthly, medication was tapered and stopped if DAS was <1.6; medication was restarted, increased or switched if DAS was ⩾1.6. After 1 year of DAS-remission-steered treatment, 32% of the patients who had achieved early remission after 4 months were able to taper medication and achieved drug-free remission. A total of 65% of patients in drug-free remission at 1 year were still in drug-free remission after 16 months.59 After 2 years of remission-steered treatment, 29% patients in the early remission group were still in drug-free remission, while the overall drug-free remission rate was 21%. The frequency of drug-free remission did not differ between the treatment arms. More UA patients achieved drug-free remission compared with early RA patients, 34% versus 19%, p < 0.001. Radiographic outcome was excellent for all patients, median (IQR) SHS progression was 0 (0–0) across all patients in this study.63
Conclusion
In short, initial targeted intensive treatments and early remission seem to be essential to achieve sustained remission and hence essential to modify the course of RA disease. Sustained remission is associated with improved long-term outcomes as measured by function, patient-reported outcomes and survival. With therapeutic strategies targeted at a low disease activity sustained remission can be achieved in many patients. Sustained remission is achievable in patients with both early and established disease. Sustained remission allows withdrawal of biological DMARDs and tapering of conventional DMARDs in some patients. Sustained remission means suppressed disease activity in the patients who may flare during follow up. Disease activity should be monitored and therapy should be adjusted during the whole disease course.
In a proportion of patients, sustained remission means re-establishment of immune tolerance. In these patients, remission is sustained after DMARD discontinuation. Drug-free remission is characterized by normalization of function and general health status. Early therapy targeted at remission is likely to improve the chance to achieve drug-free remission. The efficacy of therapies alters along the disease course and potential reversibility of autoimmunity in RA seems to decrease over time. There is likely more potential to alter the ultimate course of the disease during the early period of ‘the window of opportunity’. In future, with understanding of pathways behind initiation and perturbation of immune regulation, (new) targeted interventions at an individual patient level would influence mechanisms driving occurrence of sustained drug-free remission, and hopefully, could offer a cure.
Footnotes
Funding: Sofia Ajeganova is supported by grants from King Gustav V 80 year’s Foundation. Tom Huizinga is supported by the Dutch Arthritis Foundation and grants from the IMI project BT-CURE.
Conflict of interest statement: Tom Huizinga and the Department of Rheumatology, Leiden University Medical Center, has received lecture fees/consultancy fees from Merck, UCB, Bristol Myers Squibb, Biotest AG, Janssen, Pfizer, GSK, Novartis, Roche, Sanofi-Aventis, Abbott, Crescendo Bioscience, Nycomed, Boeringher, Takeda, Zydus, Epirus and Eli Lilly.
Contributor Information
Sofia Ajeganova, Leids Universitair Medisch Centrum, Albinusdreef 2, Leiden, 2300 RC, The Netherlands; Leiden University Medical Center, Leiden, The Netherlands; Karolinska Institutet, Department of Medicine Huddinge, Stockholm, Sweden.
Tom Huizinga, Leiden University Medical Center, Leiden, The Netherlands.
References
- 1. Markusse IM, Dirven L, Gerards AH, et al. Disease flares in rheumatoid arthritis are associated with joint damage progression and disability: 10-year results from the BeSt study. Arthritis Res Ther 2015; 17: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016; 75: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Combe B, Landewe R, Daien CI, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2016; 76: 948–959. [DOI] [PubMed] [Google Scholar]
- 4. Stoffer MA, Schoels MM, Smolen JS, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis 2016; 75: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011; 63: 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prevoo ML, van Gestel AM, van THMA, et al. Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatism Association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol 1996; 35: 1101–1105. [DOI] [PubMed] [Google Scholar]
- 7. Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005; 23: S100–S108. [PubMed] [Google Scholar]
- 8. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017; 76: 960–977. [DOI] [PubMed] [Google Scholar]
- 9. Schett G, Emery P, Tanaka Y, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis 2016; 75: 1428–1437. [DOI] [PubMed] [Google Scholar]
- 10. Ten Wolde S, Breedveld FC, Hermans J, et al. Randomised placebo-controlled study of stopping second-line drugs in rheumatoid arthritis. Lancet 1996; 347: 347–352. [DOI] [PubMed] [Google Scholar]
- 11. Van der Woude D, Young A, Jayakumar K, et al. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: results from two large early arthritis cohorts. Arthritis Rheum 2009; 60: 2262–2271. [DOI] [PubMed] [Google Scholar]
- 12. Van den Broek M, Lems WF, Allaart CF. BeSt practice: the success of early-targeted treatment in rheumatoid arthritis. Clin Exp Rheumatol 2012; 30: S35–S38. [PubMed] [Google Scholar]
- 13. Van der Woude D, Visser K, Klarenbeek NB, et al. Sustained drug-free remission in rheumatoid arthritis after DAS-driven or non-DAS-driven therapy: a comparison of two cohort studies. Rheumatology 2012; 51: 1120–1128. [DOI] [PubMed] [Google Scholar]
- 14. Yarwood A, Huizinga TW, Worthington J. The genetics of rheumatoid arthritis: risk and protection in different stages of the evolution of RA. Rheumatology 2016; 55: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burgers LE, van Nies JA, Ho LY, et al. Long-term outcome of rheumatoid arthritis defined according to the 2010-classification criteria. Ann Rheum Dis 2014; 73: 428–432. [DOI] [PubMed] [Google Scholar]
- 16. Mierau M, Schoels M, Gonda G, et al. Assessing remission in clinical practice. Rheumatology 2007; 46: 975–979. [DOI] [PubMed] [Google Scholar]
- 17. Listing J, Strangfeld A, Rau R, et al. Clinical and functional remission: even though biologics are superior to conventional DMARDs overall success rates remain low—results from RABBIT, the German biologics register. Arthritis Res Ther 2006; 8: R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Combe B, Logeart I, Belkacemi MC, et al. Comparison of the long-term outcome for patients with rheumatoid arthritis with persistent moderate disease activity or disease remission during the first year after diagnosis: data from the ESPOIR cohort. Ann Rheum Dis 2015; 74: 724–729. [DOI] [PubMed] [Google Scholar]
- 19. Jayakumar K, Norton S, Dixey J, et al. Sustained clinical remission in rheumatoid arthritis: prevalence and prognostic factors in an inception cohort of patients treated with conventional DMARDS. Rheumatology 2012; 51: 169–175. [DOI] [PubMed] [Google Scholar]
- 20. Lillegraven S, Prince FH, Shadick NA, et al. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Ann Rheum Dis 2012; 71: 681–686. [DOI] [PubMed] [Google Scholar]
- 21. Prince FH, Bykerk VP, Shadick NA, et al. Sustained rheumatoid arthritis remission is uncommon in clinical practice. Arthritis Res Ther 2012; 14: R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen G, Gossec L, Dougados M, et al. Radiological damage in patients with rheumatoid arthritis on sustained remission. Ann Rheum Dis 2007; 66: 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gandjbakhch F, Haavardsholm EA, Conaghan PG, et al. Determining a magnetic resonance imaging inflammatory activity acceptable state without subsequent radiographic progression in rheumatoid arthritis: results from a followup MRI study of 254 patients in clinical remission or low disease activity. J Rheumatol 2014; 41: 398–406. [DOI] [PubMed] [Google Scholar]
- 24. Svensson B, Andersson ML, Bala SV, et al. Long-term sustained remission in a cohort study of patients with rheumatoid arthritis: choice of remission criteria. BMJ Open 2013; 3: e003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hmamouchi I, Combe B, Fautrel B, et al. Prevalence and concordance of early and sustained remission assessed by various validated indices in the early arthritis “ESPOIR” cohort. Joint Bone Spine 2014; 81: 409–415. [DOI] [PubMed] [Google Scholar]
- 26. Aletaha D, Alasti F, Smolen JS. Optimisation of a treat-to-target approach in rheumatoid arthritis: strategies for the 3-month time point. Ann Rheum Dis 2016; 75: 1479–1485. [DOI] [PubMed] [Google Scholar]
- 27. Konijn NP, van Tuyl LH, Boers M, et al. Short and sustained periods of ACR/EULAR remission predict good functional outcome, but do not predict good radiographic outcome in early rheumatoid arthritis patients with low overall damage progression. Arthritis Care Res. Epub ahead of print 1 October 2016. DOI: 10.1002/acr.23112. [DOI] [PubMed] [Google Scholar]
- 28. Smolen JS, Wollenhaupt J, Gomez-Reino JJ, et al. Attainment and characteristics of clinical remission according to the new ACR-EULAR criteria in abatacept-treated patients with early rheumatoid arthritis: new analyses from the Abatacept study to Gauge Remission and joint damage progression in methotrexate (MTX)-naïve patients with Early Erosive rheumatoid arthritis (AGREE). Arthritis Res Ther 2015; 17: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rannio T, Asikainen J, Kokko A, et al. Early remission is a realistic target in a majority of patients with DMARD-naïve rheumatoid arthritis. J Rheumatol 2016; 43: 699–706. [DOI] [PubMed] [Google Scholar]
- 30. Einarsson JT, Geborek P, Saxne T, et al. Sustained remission improves physical function in patients with established rheumatoid arthritis, and should be a treatment goal: a prospective observational cohort study from southern Sweden. J Rheumatol 2016; 43: 1017–1123. [DOI] [PubMed] [Google Scholar]
- 31. Wolfe F, Hawley DJ. Remission in rheumatoid arthritis. J Rheumatol 1985; 12: 245–252. [PubMed] [Google Scholar]
- 32. Tiippana-Kinnunen T, Paimela L, Kautiainen H, et al. Can disease-modifying anti-rheumatic drugs be discontinued in long-standing rheumatoid arthritis? A 15-year follow-up. Scand J Rheumatol 2010; 39: 12–18. [DOI] [PubMed] [Google Scholar]
- 33. O’Mahony R, Richards A, Deighton C, et al. Withdrawal of disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2010; 69: 1823–1826. [DOI] [PubMed] [Google Scholar]
- 34. Huizinga TW, Conaghan PG, Martin-Mola E, et al. Clinical and radiographic outcomes at 2 years and the effect of tocilizumab discontinuation following sustained remission in the second and third year of the ACT-RAY study. Ann Rheum Dis 2015; 74: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harrison BJ, Symmons DP, Brennan P, et al. Natural remission in inflammatory polyarthritis: issues of definition and prediction. Br J Rheumatol 1996; 35: 1096–1100. [DOI] [PubMed] [Google Scholar]
- 36. Hetland ML, Stengaard-Pedersen K, Junker P, et al. Radiographic progression and remission rates in early rheumatoid arthritis - MRI bone oedema and anti-CCP predicted radiographic progression in the 5-year extension of the double-blind randomised CIMESTRA trial. Ann Rheum Dis 2010; 69: 1789–1795. [DOI] [PubMed] [Google Scholar]
- 37. Klarenbeek NB, van der Kooij SM, Guler-Yuksel M, et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann Rheum Dis 2011; 70: 315–319. [DOI] [PubMed] [Google Scholar]
- 38. Ajeganova S, van Steenbergen HW, van Nies JA, et al. Disease-modifying antirheumatic drug-free sustained remission in rheumatoid arthritis: an increasingly achievable outcome with subsidence of disease symptoms. Ann Rheum Dis 2016; 75: 867–873. [DOI] [PubMed] [Google Scholar]
- 39. van der Bijl AE, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Infliximab and methotrexate as induction therapy in patients with early rheumatoid arthritis. Arthritis Rheum 2007; 56: 2129–2134. [DOI] [PubMed] [Google Scholar]
- 40. Quinn MA, Conaghan PG, O’Connor PJ, et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2005; 52: 27–35. [DOI] [PubMed] [Google Scholar]
- 41. Kuijper TM, Luime JJ, de Jong PH, et al. Tapering conventional synthetic DMARDs in patients with early arthritis in sustained remission: 2-year follow-up of the tREACH trial. Ann Rheum Dis 2016; 75: 2119–2123. [DOI] [PubMed] [Google Scholar]
- 42. Bijlsma JW, Welsing PM, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016; 388: 343–355. [DOI] [PubMed] [Google Scholar]
- 43. Emery P, Hammoudeh M, FitzGerald O, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med 2014; 371: 1781–1792. [DOI] [PubMed] [Google Scholar]
- 44. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis 2015; 74: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Tuyl LH, Hewlett S, Sadlonova M, et al. The patient perspective on remission in rheumatoid arthritis: ‘You’ve got limits, but you’re back to being you again’. Ann Rheum Dis 2015; 74: 1004–1010. [DOI] [PubMed] [Google Scholar]
- 46. Krishnan E, Sokka T, Hakkinen A, et al. Normative values for the Health Assessment Questionnaire disability index: benchmarking disability in the general population. Arthritis Rheum 2004; 50: 953–960. [DOI] [PubMed] [Google Scholar]
- 47. Sokka T. Assessment of pain in rheumatic diseases. Clin Exp Rheumatol 2005; 23: S77–S84. [PubMed] [Google Scholar]
- 48. Slatkowsky-Christensen B, Mowinckel P, Loge JH, et al. Health-related quality of life in women with symptomatic hand osteoarthritis: a comparison with rheumatoid arthritis patients, healthy controls, and normative data. Arthritis Rheum 2007; 57: 1404–1409. [DOI] [PubMed] [Google Scholar]
- 49. Bellamy N, Wilson C, Hendrikz J. Population-based normative values for the Western Ontario and McMaster (WOMAC) Osteoarthritis Index: part I. Semin Arthritis Rheum 2011; 41: 139–148. [DOI] [PubMed] [Google Scholar]
- 50. Bellamy N, Wilson C, Hendrikz J. Population-based normative values for the Australian/Canadian (AUSCAN) Hand Osteoarthritis Index: part 2. Semin Arthritis Rheum 2011; 41: 149–156. [DOI] [PubMed] [Google Scholar]
- 51. Aletaha D, Funovits J, Breedveld FC, et al. Rheumatoid arthritis joint progression in sustained remission is determined by disease activity levels preceding the period of radiographic assessment. Arthritis Rheum 2009; 60: 1242–1249. [DOI] [PubMed] [Google Scholar]
- 52. Scire CA, Verstappen SM, Mirjafari H, et al. Reduction of long-term disability in inflammatory polyarthritis by early and persistent suppression of joint inflammation: results from the Norfolk Arthritis Register. Arthritis Care Res 2011; 63: 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scire CA, Lunt M, Marshall T, et al. Early remission is associated with improved survival in patients with inflammatory polyarthritis: results from the Norfolk Arthritis Register. Ann Rheum Dis 2014; 73: 1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van Nies JA, van der Helm-van Mil AH. Is early remission associated with improved survival or is arthritis persistency associated with increased mortality in early arthritis? Comparisons with the general population. Ann Rheum Dis 2013; 72: e25. [DOI] [PubMed] [Google Scholar]
- 55. Markusse IM, Akdemir G, Dirven L, et al. Long-term outcomes of patients with recent-onset rheumatoid arthritis after 10 years of tight controlled treatment: a randomized trial. Ann Intern Med 2016; 164: 523–531. [DOI] [PubMed] [Google Scholar]
- 56. van den Broek M, Dirven L, Klarenbeek NB, et al. The association of treatment response and joint damage with ACPA status in recent-onset RA: a subanalysis of the 8-year follow-up of the BeSt study. Ann Rheum Dis 2012; 71: 245–248. [DOI] [PubMed] [Google Scholar]
- 57. van Nies JA, Krabben A, Schoones JW, et al. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis 2014; 73: 861–870. [DOI] [PubMed] [Google Scholar]
- 58. Haschka J, Englbrecht M, Hueber AJ, et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic therapy: interim results from the prospective randomised controlled RETRO study. Ann Rheum Dis 2016; 75: 45–51. [DOI] [PubMed] [Google Scholar]
- 59. Wevers-de Boer KV, Heimans L, Visser K, et al. Determinants of reaching drug-free remission in patients with early rheumatoid or undifferentiated arthritis after one year of remission-steered treatment. Rheumatology 2015; 54: 1380–1384. [DOI] [PubMed] [Google Scholar]
- 60. Makinen H, Kautiainen H, Hannonen P, et al. Sustained remission and reduced radiographic progression with combination disease-modifying antirheumatic drugs in early rheumatoid arthritis. J Rheumatol 2007; 34: 316–321. [PubMed] [Google Scholar]
- 61. Schipper LG, Fransen J, den Broeder AA, et al. Time to achieve remission determines time to be in remission. Arthritis Res Ther 2010; 12: R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van der Kooij SM, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Probability of continued low disease activity in patients with recent onset rheumatoid arthritis treated according to the disease activity score. Ann Rheum Dis 2008; 67: 266–269. [DOI] [PubMed] [Google Scholar]
- 63. Heimans L, Akdemir G, Boer KV, et al. Two-year results of disease activity score (DAS)-remission-steered treatment strategies aiming at drug-free remission in early arthritis patients (the IMPROVED study). Arthritis Res Ther 2016; 18: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]


