Abstract
Since the advent of immunotherapy revolutionized the treatment of metastatic renal cell carcinoma (mRCC), the attention of oncologists has been unavoidably shifted from tyrosine kinase inhibitors (TKIs) to immune checkpoint blockade, with the associated risk of listing cabozantinib as just one of many available TKIs. On the contrary, we think that cabozantinib represents a very good option for mRCC treatment, with outstanding outcomes in terms of response rate, progression-free survival, overall survival and quick time to treatment response. Its safety profile is acceptable and its discontinuation rate, due to toxicity, is similar to those of other TKIs. It is still not clear if the effectiveness of this drug is justified by its wide spectrum of multikinase activity, extended to the MET and AXL kinases, or by the simple maintenance of a ‘vascular endothelial growth factor receptor pressure’ after another previous TKI. Early-phase studies are currently ongoing to investigate the potential activity and safety of cabozantinib in association with immunotherapy, albeit with the risk of an overly toxic combination. Thus, future opportunities to improve the clinical use of this drug will probably be represented by a smart treatment sequence.
Keywords: cabozantinib, kidney cancer treatment, metastatic renal cell carcinoma, renal cell carcinoma, tyrosine kinase inhibitors
Introduction
After the advent of immunotherapy revolutionized the treatment of metastatic renal cell carcinoma (mRCC), with the approval of nivolumab for second-line therapy of this disease, the attention of oncologists has been unavoidably shifted from tyrosine kinase inhibition to immune checkpoint blockade, with the associated risk of listing cabozantinib as just one of many available tyrosine kinase inhibitors (TKIs).1
Indeed, the overall survival (OS) benefit obtained with cabozantinib, considering the similar results achieved with nivolumab in the same treatment setting, seems perhaps not so remarkable to justify the heavier toxicity profile of a TKI compared with those of the new immune checkpoint inhibitors (CKIs). Nevertheless, after careful analysis of the results obtained with cabozantinib in recent clinical trials, it is clear that this new TKI represents a further significant step towards the improvement of mRCC treatment, for which immune checkpoint blockade probably will not replace the targeting of the vascular endothelial growth factor (VEGF) pathway.
According to the most recent evidence, it seems to emerge that cabozantinib may be the most efficacious TKI for mRCC, not only for pretreated patients but also in the first line setting.2,3
In this light, it is even more important to discuss the role of this drug in the management of the optimal treatment sequence for patients with mRCC, undoubtedly tailoring the therapeutic strategy case by case, but also outlining a clear profile of this new valuable resource in such an amazing evolving landscape of treatment.
The drug’s profile
Cabozantinib is an oral small molecule inhibitor of multiple tyrosine kinase receptors with activity toward VEGF receptor 2 (VEGFR-2) and MET (hepatocyte growth factor receptor), but also targeting RET (rearranged during transfection), KIT (mast/stem cell growth factor receptor), AXL (anexelekto), TIE2 (angiopoietins receptor) and FLT3 (Fms-like tyrosine kinase), which are important mediators of tumor cell survival, metastasis and tumor angiogenesis.4
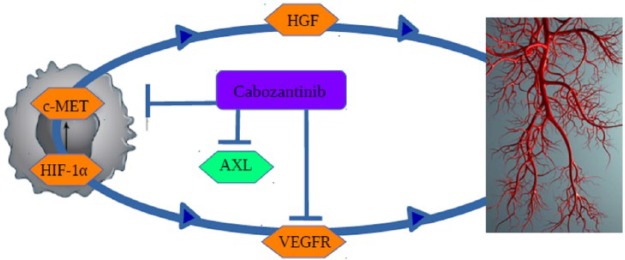
The mechanism of action is summarized in Figure 1 and the rationale for the use of this drug in mRCC is justified by the effect on the VEGF pathway together with the possibility to overcome secondary resistance to the prior TKI, by targeting MET and AXL.5 MET is a receptor tyrosine kinase (RTK) that transduces signals from the extracellular matrix into the cytoplasm, binding to the hepatocyte growth factor (HGF) ligand and so regulating many physiological processes including proliferation, scattering, morphogenesis and survival of cells; moreover, it can also regulate cortical bone osteogenesis.6 AXL is a RTK that transduces signals from the extracellular matrix into the cytoplasm, binding to the growth factor growth arrest specific 6 (GAS6); it is involved in several cellular functions including growth, migration, aggregation and differentiation in multiple cell types.7
Figure 1.
Mechanism of action: cabozantinib inhibits VEGF receptor 2 (VEGFR-2) and MET (hepatocyte growth factor receptor), but also several other tyrosine kinase receptors involved in tumor progression and neoangiogenesis, such as RET (rearranged during transfection), KIT (mast/stem cell growth factor receptor), AXL (anexelekto), TIE2 (angiopoietins receptor) and FLT3 (Fms-like tyrosine kinase), HGF (hepatocyte growth factor) and HIF-1α (hypoxia inducible factor 1α).
Preclinical results suggest that chronic treatment with antiangiogenic TKIs can induce secondary resistance through the activation of AXL and MET signaling. In this light, the suppression of their activity in cancer cells could represent an important strategy to overcome resistance. AXL protein levels were found to be elevated in tumors of patients with mRCC treated with sunitinib. Furthermore, high MET levels correlated with poor OS and progression free survival (PFS) of patients with renal cancer, regardless of treatment. Finally, it has been demonstrated in vivo that that the blockade of AXL and MET activation by cabozantinib suppressed both epithelial–mesenchymal transition and VEGF secretion induced by chronic sunitinib treatment, providing the rationale for overcoming the acquired resistance.5
Cabozantinib was approved in April 2016 by the US Food and Drug Administration (FDA)8 for the treatment of patients with mRCC after prior antiangiogenic therapy. A few months later, it was also approved by the European Medicines Agency (EMA)9 for the treatment of mRCC following VEGF-targeted therapy, on the basis of the results of the METEOR phase III randomized trial.2
Cabozantinib is available in two formulations, tablets and capsules, which are not bioequivalent nor interchangeable. For mRCC, the drug is orally administered in the form of tablets at the daily dose of 60 mg (with the possibility of dose reductions to 40 or 20 mg). It is mainly eliminated by the hepatobiliary system, as well as its six inactive metabolites, while urine excretion occurs only for metabolites.4 Pharmacokinetics are characterized by a half life of 99 h, accumulation with daily dosing and moderately high variability in exposure. It can be affected by cytochrome P450 3A4 (CYP3A4) inducers and inhibitors, high-fat meals, hepatic impairment and minimally by renal failure.10 Maximum tolerated dose of 175 mg was reached with capsules of cabozantinib in the first phase I trial.11
A specific phase I trial was conducted with different doses of cabozantinib (from 140 mg to 20 mg daily) administered in 25 heavily pretreated patients with mRCC, reaching a response rate (RR) of 28%, a disease control rate (DCR) of 80%, a median PFS of 12.9 months and a median OS of 15 months.12 These early-phase results were already undoubtedly noteworthy in such a late setting of treatment, demonstrating the significant activity and safety of this drug in renal cancer.
Treatment settings: latest findings, clinical potential and ongoing developments
Evidence about cabozantinib in mRCC is provided by three major clinical trials: the phase I study cited above,12 from which first emerged a promising efficacy and a manageable toxicity profile; the METEOR phase III pivotal trial,2 from which cabozantinib was approved for clinical use in second- and third-line settings; and finally, the most recent CABOSUN phase II randomized trial,3 comparing the drug to sunitinib as first-line therapy in a subset of patients characterized by intermediate or poor risk features as per the International Metastatic Renal Cell Carcinoma Database Consortium criteria.13
Interesting further findings about cabozantinib subsequently emerged from the analysis of its biological properties, with useful clinical implications (namely its potential activity on bone remodeling), and finally from the subgroup analysis of the trials cited above, adding knowledge and providing new hopes to improve its clinical use.
Elective indication in pretreated patients with mRCC: does the treatment line or the ‘VEGF-pressure’ maintenance matter most?
Cabozantinib is currently the only drug that has improved PFS, objective RR and OS for patients with mRCC in a pivotal phase III trial after one or more prior VEGFR TKIs.2,14
Previously, only two drugs have been able to demonstrate an OS benefit compared with standard agents in other phase III trials, namely the INTORSECT trial, with an OS advantage of sorafenib over temsirolimus in the second-line setting,15 and the pivotal trial that demonstrated an OS advantage of first-line temsirolimus over interferon.16 To date, nivolumab has also reached this milestone, with the outstanding OS of 25 months in the second-line setting, but without benefit in terms of PFS.17
The first METEOR trial results demonstrated a median PFS of 7.4 months for cabozantinib versus 3.8 months with everolimus [hazard ratio (HR) 0.58; 95% confidence interval (CI) 0.45–0.75; p < 0.001] and a RR of 21% for cabozantinib versus 5% for the control arm (p < 0.001; only partial responses, no complete remissions).2 Then, the final analysis of survival eventually showed a median OS of 21.4 months (95% CI 18.7–not estimable) for cabozantinib compared with that of 16.5 months (95% CI 14.7–18.8) with everolimus (HR 0.66; 95% CI 0.53–0.83; p = 0.00026), overall achieving the best performance (in terms of RR, PFS and OS together) of a systemic treatment in this setting.14
The PFS and OS results are undoubtedly impressive. The maintenance of a long-lasting survival difference suggests that cabozantinib activity could overcome disease progression. The survival curves overlap until the first 4–5 months (approximately corresponding to the median PFS of everolimus) and then they become separated and constantly parallel after about 7 months (approximately corresponding to the median PFS of cabozantinib). Of note, a 3.5-month difference between the respective median PFS results in a wider difference (of 4.9 months) in terms of median OS. We hypothesize a certain effect of cabozantinib on OS also beyond disease progression, suggested by the curves despite the use of subsequent TKIs in the control arm. Indeed, 47% of patients (155/328) received a TKI after everolimus in the control arm (27% of patients, 90/328, received axitinib), while 24% of patients (79/330) received a TKI after cabozantinib in the experimental arm (17% of patients, 57/330, received axitinib).2,14
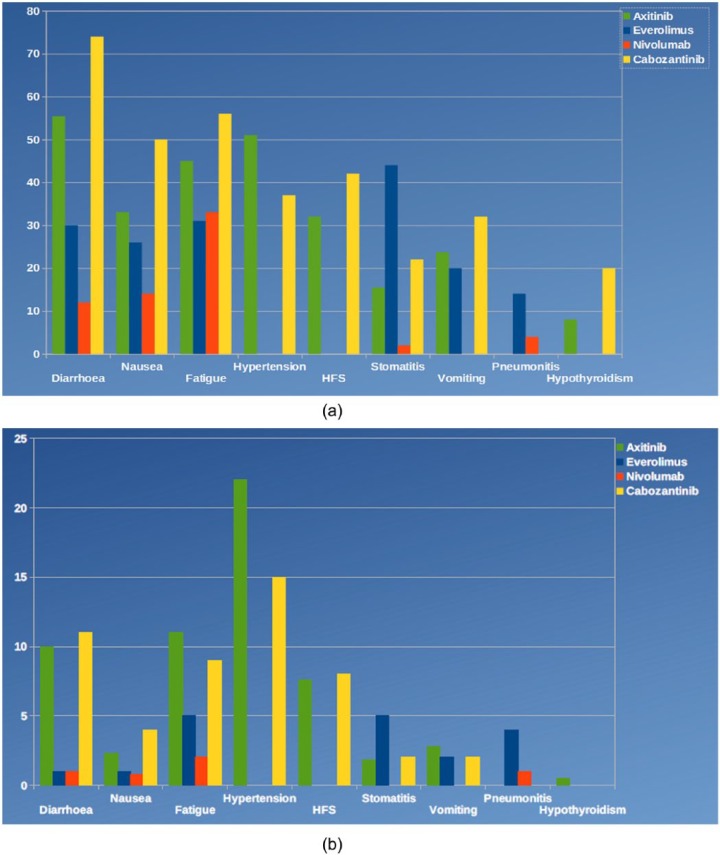
Regarding the safety profile (Figure 2), non-negligible rates of grade 3 or 4 hypertension (15% in the cabozantinib group versus 4% in the control arm), diarrhea (13% versus 2%), fatigue (11% versus 7%) and palmar-plantar erythrodysesthesia syndrome (8% versus 1%) were reported in the METEOR trial. Nevertheless, the reported toxicity included all adverse events, regardless of their real correlation to the study drug; this could have resulted in an overestimation of toxicity rates. Indeed, the toxicity reported for everolimus in the control arm of this study is much higher than those reported in the pivotal trial of nivolumab (Checkmate025) for the analogue control arm. In the present trial, serious adverse events of grade 3 or worse occurred in 39% of patients receiving cabozantinib and in 40% of cases in the everolimus group, with a similar overall rate.2,14,17
Figure 2.
Safety profile of cabozantinib in the phase III METEOR trial compared with those of other drugs currently approved for renal cancer treatment. (a) Represents the main toxicities of all grades and (b) shows grade 3–4 toxicities.
HFS, hand-foot syndrome.
It is uncertain whether the inhibition of MET, RET or AXL drives the major clinical activity of cabozantinib or whether the benefit is simply due to a VEGFR inhibitory effect, as stated by Quinn and Lara in their editorial.18 This doubt is also supported both by the evidence that cabozantinib efficacy is independent of tumor MET expression (as demonstrated in the METEOR trial subanalysis) and by its typical ‘VEGF inhibition related’ toxicity profile. Moreover, these findings suggest, once again, according to several prospective randomized phase II and phase III trials,15,19–21 that maintaining or recovering ‘VEGF pressure’ works well in the treatment sequence strategy for mRCC.22
Of note, METEOR is the first clinical phase III trial exploring a direct comparison between the outcome of a TKI–TKI sequence with those of a TKI–mTORi (mammalian target of rapamycin inhibitor) sequence. It could be hypothesized that similar results may have been reached using another TKI (like axitinib) instead of cabozantinib in the same study design. The results of the AXIS trial are not transposable because of different statistical design and the comparison with another TKI instead of everolimus in the control arm, namely sorafenib.20 The performance of cabozantinib is undoubtedly powerful, but the PFS is not so far from those of axitinib after sunitinib or cytokines (6.7 months). Possible similar performances of different TKI–TKI sequences with ‘old TKIs’ such as pazopanib and sunitinib could possibly be expected if explored, in line with the concept of a ‘good strategy’ being more powerful than a ‘good drug’. The results of the randomized phase II trial with lenvatinib are also consistent with this hypothesis.19
Elective indication in pretreated patients with mRCC: cabozantinib versus nivolumab
Beyond mechanistic speculations among different VEGFR TKIs, the main consideration in mRCC second-line treatment is currently represented by the comparison and the choice between cabozantinib and nivolumab. Despite the first predictions suggesting that, because of the high rate of dose reductions due to side effects (60%) and the lack of a significant benefit in terms of OS, cabozantinib would not have preceded nivolumab in the therapeutic sequence, with it instead being relegated as a third-line or later choice in competition with other VEGFR inhibitors,18 it is now clear that the clinician’s decision about the optimal second line for patients with mRCC is anything but obvious.
The National Comprehensive Cancer Network (NCCN) guidelines for kidney cancer (version 3.2016) provide the same category 1 recommendations based on ‘high-level evidence where there is uniform NCCN consensus that the intervention is appropriate’ for either cabozantinib or nivolumab, and similarly the European Society of Medical Oncology updated guidelines recommend both drugs for second-line treatment of patients with mRCC.23,24
From a purely clinical point of view, a careful patient-based evaluation of clinical conditions, comorbidity and disease features could represent the unique criteria for treatment selection. Nevertheless, the current knowledge about the molecular and immunological modulations generated by the exposure to each drug should be carefully taken into consideration to plan a smart, personalized, sequential strategy.
Some reflections should be highlighted to help in choosing; for example, only 12% of patients experienced progressive disease as best response with cabozantinib in the METEOR trial compared with 35% of primary refractory patients treated with nivolumab. However, the criteria for response evaluation (RECIST 1.1) may have affected these results in favor of the TKI, considering the possibility of pseudo progression in the case of immunotherapy.25,26
Moreover, despite the scarce clinical significance of the subgroup analyses from the METEOR trial, subsequent evidence based on several aspects of the different therapeutic alternatives at our disposal for second-line treatment suggests that cabozantinib could be a good choice in case of high tumor growth rate, bone metastases and prolonged response to first-line TKI.27–29 The median time to the achievement of an objective response, of 1.9 months, especially compared with that of 3.5 months for nivolumab (range 1.4–24.8 months), surely suggests cabozantinib is to be preferred in the case of a rapidly progressive disease with multiple sites of metastases or visceral lesions.2,17
With the limitation of an indirect evaluation and of a different primary endpoint of the nivolumab trial (OS) compared with that of the cabozantinib trial (PFS), a comparison in terms of OS has also been attempted. HR for OS of nivolumab versus cabozantinib varies over time, favoring cabozantinib in the first months of treatment but nivolumab afterwards. This analysis from the two pivotal trials, obtained with the Butcher method, offers a possible indication that patients with poor prognosis could benefit more from cabozantinib in terms of survival, while nivolumab should favor patients with better prognosis.30
First-line treatment: intermediate–poor risk patients or only the beginning?
Most recently, cabozantinib has been tested in the first-line setting for a subgroup of patients with mRCC, providing apparently exciting results, which in our opinion should be carefully considered before being translated into clinical practice.31
Indeed, cabozantinib improved PFS and RR compared with sunitinib in the randomized phase II CABOSUN trial, enrolling treatment-naive patients with mRCC of International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate- and poor-risk groups.3
Its efficacy in this population was undeniable, with an observed median PFS of 8.2 months and a RR of 46%, but an unexpected poor performance emerged for the comparator sunitinib, with a PFS of 5.6 months (in spite of which we noticed a wide overlap of confidence intervals, 6.2–8.8 and 3.4–8.1, respectively, for the cabozantinib and sunitinib arms). The objective RR of 18% was unexpectedly low for sunitinib, even in a poor risk population, and the median number of 6-week cycles administered in the trial was only two (versus five for cabozantinib).
However, adverse events were similar in the two arms, confirming the manageable toxicity profile of cabozantinib in contrast to the ‘general impression’ of it as a poorly tolerated drug.
The bias of the investigators assessing PFS as the primary endpoint, without independent review and without blindness, in our opinion, further affects the strength of the trial.31
Supposing that cabozantinib could demonstrate better activity and efficacy than those of any other TKI, and in the light of its benefit in all subgroups of patients and diseases in the second-line METEOR trial, it would be more interesting to test it against the standard first-line treatment (currently pazopanib or sunitinib) in the overall mRCC population.
While we wait for definitive data from the CABOSUN trial about OS, which unfortunately will probably be invalidated by the subsequent treatment lines, the only certainty about first-line treatment of mRCC remains the gold standard with ‘a strong TKI’ in all risk groups, with the hope to better investigate which could be the best one with a proper phase III trial design.
Special issues
Some crucial issues considering the mechanism of action of cabozantinib have not been addressed in the previously cited pivotal trials.
First, the predictive factors, such as activating RET and RAS mutations, already known from the phase III EXAM trial in medullary thyroid cancer, as well as MET mutations/amplification, which do not seem to affect the response.32
Second, the papillary histology, which can represent an excellent target for a MET inhibitor.33
Finally, the bone metastasis, considering several elements: the unmet clinical need, with the clear evidence that bone sites of disease are not well controlled by the standard VEGF inhibitors34; the key role of the hepatocyte growth factor–MET pathway in bone metastasis development,35 and the clinical evidence of an important improvement of bone scans, pain, analgesic use, measurable soft tissue disease, circulating tumor cells and bone biomarkers through the modification of the bone microenvironment with this drug.36 This point is also supported by a wide preclinical background; cabozantinib demonstrated significant activity as an inhibitor of osteoclast differentiation and bone resorption; it was also shown to downmodulate the expression of osteoclast marker genes, TRAP, CATHEPSIN K and receptor activator of nuclear factor κB (RANK), to increase osteoprotegerin mRNA and protein levels and to downmodulate RANK ligand (RANKL) at both mRNA and protein levels. Finally, direct cell to cell contact between cabozantinib-pretreated osteoblasts and untreated osteoclasts confirmed the indirect antiresorptive effect of cabozantinib.37
All these elements undoubtedly deserve further investigations and some of them have been considered in the planning of clinical trials currently ongoing (see the last paragraph).
Immunomodulating effects: rationale for sequences?
In the light of investigating the optimal treatment sequence between cabozantinib and immune checkpoint inhibitors, it would be desirable to know the possible immunomodulating effects of this new TKI and if any preparatory effect could be carried out with cabozantinib to improve the subsequent immunotherapy approach. Cabo-zantinib demonstrated an effect on programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-antigen 4 (CTLA-4) expression on regulatory T cells (T-regs) in a study of metastatic urothelial cancer.38 T-reg levels may represent a predictive and prognostic marker: their dosing prior to cabozantinib treatment has been shown to predict therapeutic responsiveness and OS. When assessing myeloid-derived suppressor cells (MDSCs) and T-regs in patients undergoing treatment with cabozantinib, patients with low T-reg levels measured in peripheral blood samples at baseline had an improved outcome (RR, PFS and OS). T-reg levels significantly decreased and PD-1 expression in regulatory T cells significantly increased after cabozantinib treatment. Smaller changes in PD-1 levels tended to correlate with an improved PFS; a decrease in MDSC expression during treatment was in turn associated with improved PFS. These results suggest that changes in T-reg checkpoint molecule expression and MDSC expression may potentially acquire prognostic and predictive value in patients treated with cabozantinib, and this effect, linked to the function of the immune system rather than to the tumor itself, may be observed regardless of the primary cancer type.38
Future directions for cabozantinib in mRCC
Despite the great potential demonstrated by cabozantinib, it seems that no further ideas have been formulated about its definitive setting in the current landscape of mRCC therapy. The few trials ongoing with cabozantinib in patients with renal cancer are reported in Table 1. Three major issues remain to be addressed: the competition with nivolumab as the best choice for second-line treatment, the confirmation of its possible use in the first-line setting, and its possible role in combination with CKIs.
Table 1.
Trials ongoing with cabozantinib in patients with renal cancer.
| ClinicalTrials.gov identifier and type | Treatment line | Arms of therapy | Eligibility | No. of planned patients | Primary endpoint | Estimated completion date |
|---|---|---|---|---|---|---|
| PAPMET NCT02761057 phase II |
First to second line | Cabozantinib Sunitinib Crizotinib Volitinib |
Advanced papillary renal cell carcinoma (type 1 or type 2) | 180 | PFS | 2019 |
|
NCT02496208 phase Ib |
From second line | Cabozantinib + nivolumab Cabozantinib + nivolumab + ipilimumab |
Advanced genitourinary tumors (renal cancer and all cancers of the urinary tract) | 113 | Dose-limiting toxicity | 2017 |
|
NCT02293980 phase I |
From second to fourth line | PT2385 PT2385 + nivolumab PT2385 + cabozantinib |
Advanced clear cell renal carcinoma | 151 | Maximum tolerated dose | 2018 |
PFS, progression-free survival.
Addressing the issue of special populations, the randomized PAPMET phase II study is currently enrolling patients with advanced papillary renal cancer to receive multiple MET kinase inhibitors, including cabozantinib, crizotinib, volitinib or sunitinib, with PFS as primary endpoint.39
Otherwise, two early-phase studies are currently ongoing to investigate the potential activity and safety of cabozantinib in association with immunotherapy.
In a phase Ib trial, the combination of cabozantinib and nivolumab with or without ipilimumab is being assessed in patients with metastatic genitourinary tumors, including mRCC. The coprimary endpoints are represented by safety, identification of dose-limiting toxicity and determination of the recommended phase II doses for the combinations. Outcomes in terms of objective RR, PFS and OS are secondary endpoints.40
Another phase I study is investigating cabozantinib in association with a hypoxia inducible factor 2α inhibitor (PT2385) in patients with advanced mRCC.41
Conclusions
In conclusion, cabozantinib represents a very good option for mRCC treatment, with outstanding outcome in terms of RR, PFS, OS and quick time to treatment response. Its safety profile is acceptable, as well as its discontinuation rate due to toxicity, which is not so different from those of other TKIs. It is still not clear if the effectiveness of this drug is justified by its wide spectrum of multikinase activity, extended to the MET and AXL kinases, or by the simple maintenance of a ‘VEGFR pressure’ after another previous TKI.
Possibly, a further role of cabozantinib in the neoadjuvant setting should be discussed and investigated, considering its good cytoreductive activity.
We finally think that the illusion of being able to identify the ideal candidate for cabozantinib treatment, especially considering the inconclusive subgroup analyses from pivotal trials,27 must be debunked by the knowledge that the strategy matters more than the single choice.
In light of the previously described rationale about the immunomodulating effects of cabozantinib, rather than its possibly overly toxic combination with CKIs and beyond its use in small special subgroups of patients with mRCC (such as papillary RCC or poor-risk patients), we are more likely to believe that the future opportunity to improve the clinical use of this drug will probably be represented by a smart treatment sequence.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Melissa Bersanelli received grants/reimbursements from IPSEN, BMS, NOVARTIS and PFIZER for participation in scientific events, received honoraria from MUNDIPHARMA for an advisory role and from PFIZER as a speaker at scientific events. Dr Sebastiano Buti received honoraria for an advisory role and grants/reimbursements for participation in scientific events from IPSEN, ASTRA ZENECA, JANSSEN and NOVARTIS, and received honoraria from PFIZER and NOVARTIS as a speaker at scientific events.
Contributor Information
Melissa Bersanelli, University Hospital of Parma, Medical Oncology Unit, Via Gramsci 14, 43126, Parma (PR), Italy.
Sebastiano Buti, University Hospital of Parma, Medical Oncology Unit, Parma (PR), Italy.
References
- 1. Buti S, Bersanelli M. The ‘Nivolution’ in renal cell carcinoma: behind the scenes of clinical trials. Future Oncology 2016; 12(18): 2061–2063. [DOI] [PubMed] [Google Scholar]
- 2. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373(19): 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol 2017; 35(6): 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lacy S, Miles DR, Nguyen LT. Clinical pharmacokinetics and pharmacodynamics of cabozantinib. Clin Pharmacokinet 2017; 56(5): 477–491. [DOI] [PubMed] [Google Scholar]
- 5. Zhou L, Liu XD, Sun M. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 2016; 35(21): 2687–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Genetics Home Reference. MET gene, https://ghr.nlm.nih.gov/gene/MET#normalfunction (accessed 28 March 2017).
- 7. Genetics Home Reference. AXL gene, https://ghr.nlm.nih.gov/gene/AXL#normalfunction (accessed 28 March 2017).
- 8. FDA, https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm497483.htm (accessed 28 March 2017).
- 9. European Medicines Agency, http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004163/human_med_002018.jsp&mid=WC0b01ac058001d124 (accessed 28 March 2017).
- 10. Cabozantinib. Summary of product characteristics, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004163/WC500214071.pdf (accessed 28 March 2017).
- 11. Kurzrock R, Sherman S, Ball D, et al. Activity of Xl184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 2011; 29: 2660–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choueiri TK, Pal SK, McDermott DF, et al. A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann Oncol 2014; 25(8): 1603–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 2013; 14(2): 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016; 17(7): 917–927. [DOI] [PubMed] [Google Scholar]
- 15. Hutson TE, Escudier B, Esteban E, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 2014; 32(8): 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hudes C, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007; 356(22): 2271–2281. [DOI] [PubMed] [Google Scholar]
- 17. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373(19): 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quinn DI, Lara PN., Jr. Renal-cell cancer – targeting an immune checkpoint or multiple kinases. N Engl J Med 2015; 373(19): 1872–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015; 16(15): 1473–1482. [DOI] [PubMed] [Google Scholar]
- 20. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011; 378(9807): 1931–1939. [DOI] [PubMed] [Google Scholar]
- 21. Motzer RJ, Porta C, Vogelzang NJ, et al. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15(3): 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buti S, Bersanelli M. Combination therapy in kidney cancer: the next revolution? Lancet Oncol 2015; 16(15): 1441–1442. [DOI] [PubMed] [Google Scholar]
- 23. Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2016, https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
- 24. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27(Suppl 5): v58–v68. [DOI] [PubMed] [Google Scholar]
- 25. de Velasco G, Krajewski KM, Albiges L, et al. Radiologic heterogeneity in responses to anti-PD-1/PD-L1 therapy in metastatic renal cell carcinoma. Cancer Immunol Res 2016; 4(1): 12–17. [DOI] [PubMed] [Google Scholar]
- 26. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18: e143–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Subgroup analyses of METEOR, a randomized phase 3 trial of cabozantinib versus everolimus in patients with advanced renal carcinoma. 2016 Genitourinary Cancers Symposium. J Clin Oncol 2016; 34(Suppl 2S): abstract 499. [Google Scholar]
- 28. Outcomes based on prior VEGFR TKI and PD-1 checkpoint inhibitor therapy in METEOR, a randomized phase 3 trial of cabozantinib vs everolimus in advanced renal cell carcinoma. J Clin Oncol 2016; 34(Suppl 2S): abstract 4557. [Google Scholar]
- 29. Grande E, Martínez-Sáez O, Gajate-Borau P, et al. Translating new data to the daily practice in second line treatment of renal cell carcinoma: the role of tumor growth rate. World J Clin Oncol 2017; 8(2): 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wiecek W, Karcher H. Nivolumab versus cabozantinib: comparing overall survival in metastatic renal cell carcinoma. PLoS One 2016; 11(6): e0155389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buti S, Bersanelli M. Is cabozantinib really better than sunitinib as first-line treatment of metastatic renal cell carcinoma? J Clin Oncol 2017; 35(16): 1858–1859. [DOI] [PubMed] [Google Scholar]
- 32. Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. Clin Cancer Res 2014; 20(13): 3411–3421.24658158 [Google Scholar]
- 33. Albiges L, Guegan J, Le Formal A, et al. MET is a potential target across all papillary renal cell carcinomas: result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res 2014; 20(13): 3411–3421. [DOI] [PubMed] [Google Scholar]
- 34. Plimack E, Tannir N, Lin E, et al. Patterns of disease progression in metastatic renal cell carcinoma patients treated with antivascular agents and interferon: impact of therapy on recurrence patterns and outcome measures. Cancer 2009; 115: 1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ono K, Kamiya S, Akatsu T, et al. Involvement of hepatocyte growth factor in the development of bone metastasis of a mouse mammary cancer cell line. BALB/c-MC Bone 2006; 39: 27–34. [DOI] [PubMed] [Google Scholar]
- 36. Smith DC, Smith MR, Sweeney CJ, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol 2013; 31(4): 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fioramonti M, Santini D, Iuliani M, et al. Cabozantinib targets bone microenvironment modulating human osteoclast and osteoblast functions. Oncotarget 2017; 8(12): 20113–20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Apolo AB, Tomita Y, Lee M-J, et al. Effect of cabozantinib on immunosuppressive subsets in metastatic urothelial carcinoma. ASCO Annual Meeting. J Clin Oncol 2014; 32(Suppl 5S): abstract 4501. [Google Scholar]
- 39. Cabozantinib-S-Malate, Crizotinib, Volitinib, or Sunitinib Malate in Treating Patients with Locally Advanced or Metastatic Kidney Cancer, https://clinicaltrials.gov/ct2/show/NCT02761057 (accessed 28 March 2017).
- 40. Cabozantinib-s-malate and nivolumab with or without ipilimumab in treating patients with metastatic genitourinary tumors, https://clinicaltrials.gov/ct2/show/NCT02496208 (accessed 28 March 2017).
- 41. A phase 1, Dose-escalation trial of PT2385 Tablets in patients with advanced clear cell renal cell carcinoma, https://clinicaltrials.gov/ct2/show/NCT02293980 (accessed 28 March 2017).