Abstract
Cleft palate is a common birth defect caused by disruption of palatogenesis during embryonic development. Although mutations disrupting components of the Wnt signaling pathway have been associated with cleft lip and palate in humans and mice, the mechanisms involving canonical Wnt signaling and its regulation in secondary palate development are not well understood. Here, we report that canonical Wnt signaling plays an important role in Pax9-mediated regulation of secondary palate development. We found that cleft palate pathogenesis in Pax9-deficient embryos is accompanied by significantly reduced expression of Axin2, an endogenous target of canonical Wnt signaling, in the developing palatal mesenchyme, particularly in the posterior regions of the palatal shelves. We found that expression of Dkk2, encoding a secreted Wnt antagonist, is significantly increased whereas the levels of active β-catenin protein, the essential transcriptional coactivator of canonical Wnt signaling, is significantly decreased in the posterior regions of the palatal shelves in embryonic day 13.5 Pax9-deficent embryos in comparison with control littermates. We show that small molecule–mediated inhibition of Dickkopf (DKK) activity in utero during palatal shelf morphogenesis partly rescued secondary palate development in Pax9-deficient embryos. Moreover, we found that genetic inactivation of Wise, which is expressed in the developing palatal shelves and encodes another secreted antagonist of canonical Wnt signaling, also rescued palate morphogenesis in Pax9-deficient mice. Furthermore, whereas Pax9del/del embryos exhibit defects in palatal shelf elevation/reorientation and significant reduction in accumulation of hyaluronic acid—a high molecular extracellular matrix glycosaminoglycan implicated in playing an important role in palatal shelf elevation—80% of Pax9del/del;Wise-/- double-mutant mouse embryos exhibit rescued palatal shelf elevation/reorientation, accompanied by restored hyaluronic acid accumulation in the palatal mesenchyme. Together, these data identify a crucial role for canonical Wnt signaling in acting downstream of Pax9 to regulate palate morphogenesis.
Keywords: cell signaling, cleft palate, craniofacial biology, transcription factors, Sostdc1, Wnt antagonist
Introduction
The mammalian secondary palate arises from the oral side of embryonic maxillary prominences and initially grows vertically to form a pair of palatal shelves flanking the developing tongue. As development proceeds, the palatal shelves undergo remodeling and reorient to the horizontal position above the tongue, subsequently fusing to form the roof of oral cavity (Lan et al. 2015; Li et al. 2017). These developmental processes, including palatal shelf growth, elevation/reorientation, and fusion, are often disrupted by genetic or environmental perturbations, resulting in a high frequency of cleft palate defects in humans (Dixon et al. 2011; Bush and Jiang 2012). Extensive genetic and developmental studies with multiple animal model systems have identified crucial roles of multiple signaling pathways and transcription factors in palate development (Bush and Jiang 2012; Lan et al. 2015). However, critical gaps remain in current knowledge regarding the molecular networks integrating various signaling pathways and transcription factors in regulation of palate development, and no effective strategy has been developed for prevention of cleft palate.
Several studies have demonstrated that Shh, Bmp4, and Fgf10 signaling pathways act in a molecular network containing the Msx1, Osr2, and Pax9 transcription factors in regulating palatal shelf growth (reviewed by Bush and Jiang 2012; Lan et al. 2015). Shh and Fgf10 are expressed in palatal shelf epithelium and mesenchyme, respectively, and function in a positive feedback loop during palatal shelf outgrowth (Rice et al. 2004; Lan and Jiang 2009). Msx1 regulates anterior palatal mesenchyme proliferation through activation of Bmp4 expression in the palatal mesenchyme (Zhang et al. 2002). Osr2 and Pax9 are 2 key regulators of palatogenesis expressed throughout the developing palatal mesenchyme (Peters et al. 1998; Lan et al. 2004; Zhou et al. 2013). Mouse embryos lacking Pax9 function exhibit significantly reduced expression of Bmp4, Fgf10, Msx1, and Osr2 messenger RNAs (mRNAs) in the palatal mesenchyme as well as significantly reduced expression of Shh mRNAs in the palatal epithelium. Remarkably, restoration of Osr2 expression in the palatal mesenchyme was able to partly rescue palatal morphogenesis in the absence of Pax9 function (Zhou et al. 2013), suggesting that modulation of signaling pathways downstream of Pax9 and Osr2 could prevent cleft palate.
In addition to roles in palatogenesis, Msx1, Osr2, and Pax9 are important regulators of tooth development (reviewed by Lan et al. 2014). Mice lacking either Msx1 or Pax9 exhibit bud-stage developmental arrest of all tooth germs (Satokata and Maas 1994; Peters et al. 1998; Zhou et al. 2011). Mice lacking Osr2 exhibit Msx1-dependent induction of supernumerary tooth formation (Zhang et al. 2009). Msx1 and Osr2 were recently shown to act antagonistically to regulate expression of several secreted Wnt antagonists, including Dkk2 and Sfrp2, in developing tooth mesenchyme (Jia et al. 2016). Remarkably, genetic deletion of Sfrp2/Sfrp3 with inhibition of Dickkopf (DKK) function partly rescued molar tooth morphogenesis in Msx1-/- mice (Jia et al. 2016). Although many components of the Wnt signaling pathway are required for palatogenesis in humans and mice (reviewed by He et al. 2008; Chiquet et al. 2008; Menezes et al. 2010; Liu et al. 2015), the mechanisms involving Wnt signaling and the relationship between the Msx1, Osr2, Pax9 transcription factors and Wnt signaling in palate development are not well understood.
Here we report that Pax9-deficent mouse embryos exhibit decreased canonical Wnt signaling in the developing palatal shelves and that pharmacologic inhibition of DKK function or genetic inactivation of Wise (also known as ectodin or Sostdc1), which encodes a distinct Wnt antagonist (Itasaki et al. 2003; Laurikkala et al. 2003; Lintern et al. 2009; reviewed by Cruciat and Niehrs 2013), partly rescued secondary palate development in Pax9-deficient mice. Our data identify a novel role for Pax9 in integrating the Wnt signaling pathway in the regulation of palate development.
Materials and Methods
Mouse Strains
Pax9del/+ and Wise+/- mice have been described (Ahn et al. 2010; Zhou et al. 2011). The Pax9 and Wise genes are both located on mouse chromosome 12, separated by about 20 Mb. We crossed Pax9del/+ mice to Wise-/- mice to generate Pax9del/+; Wise+/- double-heterozygous mice. We crossed Pax9del/+;Wise+/- mice to C57BL/6J inbred mice to select for Pax9del/+;Wise+/- progeny in which the 2 mutations are in cis on the same chromosome, resulting from meiotic recombination between the 2 loci. Pax9del/+ and Pax9del/+;Wise+/- mice were subsequently maintained by backcrossing to C57BL/6J mice and intercrossed to generate homozygous mutants for analyses. For timed pregnancies, embryonic day (E) 0.5 was designated as noon of the day when a vaginal plug was identified. This study was performed in strict accordance with the recommendations in the “Guide for the Care and Use of Laboratory Animals” by the National Institutes of Health. The animal use protocol was approved by the Institutional Animal Care and Use Committee of Cincinnati Children’s Hospital Medical Center. This study conformed with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for preclinical animal studies.
Histology and In Situ Hybridization
Embryos were collected at desired developmental stages and processed for histology or in situ hybridization as described previously (Lan et al. 2001; Baek et al. 2011). For in situ hybridization, serial sections from the anterior, middle, and posterior regions of the secondary palate were processed as described previously with digoxigenin-labeled cRNA probes (Zhang et al. 1999).
Immunofluorescence Detection
For detection of phospho-Smad1/5/9, tissue sections were stained with the rabbit monoclonal antibody D5B10 (1:100; catalog 13820, Cell Signaling Technology) following standard protocols (Xu et al. 2014). For detection of hyaluronic acid (HA), tissue sections were incubated with biotin-labeled HABP (1:200; catalog 385911-50UG, Millipore) and detected with Texas red–conjugated streptavidin (1:200; SA-5006, Vector Labs). Images in red fluorescent protein (RFP) single channel were used to analyze fluorescence intensity over the palatal shelf area with ImageJ. Data were analyzed with Student’s t test, and P < 0.05 was considered statistically significant.
Quantitative RT-PCR Analysis
Quantitative RT-PCR (reverse transcription quantitative real-time polymerase chain reaction, abbreviated as RT-qPCR) was performed as previously described (Zhou et al. 2013). The sequences of gene-specific primers are provided in the Appendix Table. The relative levels of mRNAs in each sample were normalized to that of Hprt mRNAs. Student’s t test was used to analyze differential expression data.
Western Blot Assays
E13.5 palate shelves were dissected and lysed in radioimmunoprecipitation assay (RIPA) buffer (150mM NaCl, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50mM Tris, pH 8.0, and Roche Protease Inhibitor Cocktail) and sonicated 6 times (pulses: 1 s on, 1 s off). The mouse monoclonal antibodies to active β-catenin (1:1,000; clone 8E7, EMD Millipore) and β-actin (1:2,000; clone 8H10D10, Cell Signal Technologies) were used to detect the specific proteins. The intensity of detected bands was quantified with Photoshop Histogram Analysis from 3 independent experiments and the data statistically analyzed with Student’s t test.
Results
Pax9 Mutant Embryos Exhibit Decreased Canonical Wnt Signaling Activity in the Developing Palatal Shelves
Since Pax9del/del embryos exhibit significantly reduced expression of Bmp4 mRNAs in the developing palatal shelves (Zhou et al. 2013) and since BMP signaling induces Wise mRNA expression in other developmental contexts (Itasaki et al. 2003; Laurikkala et al. 2003; Lintern et al. 2009), we compared Wise mRNA expression in developing palatal shelves in Pax9del/del embryos and their control littermates. By in situ hybridization analysis, we found that Pax9del/del embryos had an obvious decrease in Wise mRNA expression in the anterior and posterior regions of the palatal shelves by E13.5 (Fig. 1A–H). RT-qPCR analysis of manually microdissected E13.5 palatal shelves confirmed that expression of Wise mRNAs was significantly reduced in Pax9del/del embryos in comparison with Pax9del/+ littermates (Fig. 1I).
Figure 1.
Changes in expression of Wnt signaling components in the developing palatal shelves of Pax9del/del mutant embryos. (A–D) Whole mount view of patterns of Wise mRNA expression in the palatal shelves of Pax9del/+ control and Pax9del/del mutant embryos at E12.5 and E13.5. Arrows point to the medial edge of the palatal shelves. (E–H) Frontal sections showing Wise mRNA expression patterns in the anterior and posterior regions of palate shelves in E13.5 Pax9del/+ control and Pax9del/del mutant embryos. (I) RT-qPCR analysis of the levels of expression of Wise mRNAs in E13.5 palatal shelves in Pax9del/+ control and Pax9del/del mutant embryos (n = 5). (J–M) Frontal sections showing Axin2 mRNA expression patterns in the anterior and posterior regions of palate shelves in E13.5 Pax9del/+ control and Pax9del/del mutant embryos. Green arrows point to the domains of Axin2 mRNA expression in the palatal mesenchyme. Black arrowheads point to Axin2 mRNA expression in the tooth germs. (N) RT-qPCR analysis of the levels of expression of Axin2 mRNAs in the anterior and posterior regions, respectively, of the palatal shelves in E13.5 Pax9del/+ control and Pax9del/del mutant embryos (n = 3). (O) Western blot analysis of the levels of active β-catenin proteins in the posterior halves of palatal shelves from E13.5 Pax9del/+ control and Pax9del/del mutant embryos. The levels of β-actin in each sample were detected as an internal loading control. (P) Relative active β-catenin band intensity on Western blots normalized against β-actin (n = 3 samples each for Pax9del/+ and Pax9del/del embryos). (Q, T) Whole mount view of patterns of Dkk2 mRNA expression in the palate of E12.5 Pax9del/+ control and Pax9del/del mutant embryos. (R, S, U, V) Frontal sections showing Dkk2 mRNA expression patterns in the anterior and posterior regions of palate shelves of E13.5 Pax9del/+ control and Pax9del/del mutant embryos. Black arrowheads point to Dkk2 mRNA expression in the tooth germs. (W) RT-qPCR analysis of the levels of Dkk2 mRNAs in the anterior and posterior regions, respectively, of the palatal shelves from E13.5 Pax9del/+ control and Pax9del/del mutant embryos (n = 3). E, embryonic day; ns, not significant; p, palatal shelf; t, tongue. Error bars represent SD. *P < 0.05. **P < 0.01.
Since Wise antagonizes Wnt signaling in vivo (Ahn et al. 2010), we investigated whether Wnt signaling activity is altered in the Pax9del/del palatal shelves. Surprisingly, we found that expression of Axin2, a direct target of canonical Wnt signaling (Jho et al. 2002), was reduced in the palatal mesenchyme, particularly in the posterior regions, in E13.5 Pax9del/del embryos compared with control littermates (Fig. 1J–M). RT-qPCR analysis confirmed that Axin2 mRNA expression was significantly reduced in posterior palatal tissues, whereas the levels of Axin2 mRNAs in the anterior half of the palatal shelves were modestly reduced, though the change was not statistically significant, in the E13.5 Pax9del/del embryos as compared with Pax9del/+ littermates (Fig. 1N). To further validate that canonical Wnt signaling activity in the palatal shelves is affected in Pax9del/del embryos, we took advantage of an antibody that specifically recognizes the Wnt-activated signaling form of β-catenin (van Noort et al. 2002). Western blot analysis showed that the posterior regions of palatal shelves contain significantly more accumulation of active β-catenin than anterior palatal tissues in E13.5 wild-type embryos (Appendix Fig. 1). Remarkably, the levels of active β-catenin were significantly reduced in the posterior palatal tissues in E13.5 Pax9del/del embryos as compared with Pax9del/+ littermates (Fig. 1O, P).
To understand why Pax9del/del embryos exhibit decreased canonical Wnt signaling activity in the palatal shelves despite the decrease in Wise expression, we investigated whether expression of Dkk1 and Dkk2, which encode another class of secreted antagonists of canonical Wnt signaling (reviewed by Cruciat and Niehrs 2013), was altered during palate development in Pax9del/del embryos. By in situ hybridization and RT-qPCR analyses, we found that expression of Dkk2 mRNAs was significantly increased in posterior regions of palatal mesenchyme in E13.5 Pax9del/del embryos in comparison with control littermates (Fig. 1Q–W). Thus, the decrease in canonical Wnt signaling activity in Pax9del/del palatal shelves correlated with the increase in Dkk2 expression.
Inhibition of DKK Activity In Utero Partly Rescued Palate Morphogenesis in Pax9del/del Mice
Recent studies have shown that treatment of pregnant mice with IIIC3a, a small molecule inhibitor that competitively blocks DKK binding to the Wnt coreceptors Lrp5/6 (Li et al. 2012), could effectively inhibit Dkk2 function in embryonic tooth development (Jia et al. 2016; Kwon et al. 2017). We injected pregnant Pax9del/+ female mice intraperitoneally with IIIC3a (catalog 317701, EMD Millipore; 10 mg/kg of body weight, stock solution is 20 mg/mL in dimethyl sulfoxide [DMSO]) once a day at gestational days 12.5, 13.5, and 14.5, to test whether IIIC3a-mediated inhibition of DKK activity could rescue palate morphogenesis in Pax9del/del embryos. Whereas neither DMSO nor IIIC3a treatment had any detectable effect on palate development in the wild-type and Pax9del/+ embryos and although all Pax9del/del pups treated with DMSO still exhibited cleft palate (Fig. 2A–H), 7 of 11 (64%) IIIC3a-treated Pax9del/del pups exhibited secondary palate that was fused in the middle and posterior regions, with only a partial cleft between the anterior secondary palate and the primary palate (Fig. 2I–L). In contrast to the partial rescue of palate morphogenesis, all IIIC3a-treated Pax9del/del pups still had bud-stage tooth developmental arrest similar as untreated or DMSO-treated Pax9del/del pups (Fig. 2C, G, K).
Figure 2.
Inhibition of DKK activity in utero partly rescued palate development in Pax9del/del mutant embryos. (A, E, I) Whole mount view of the palate in WT, DMSO-treated Pax9del/del, and IIIC3a-treated Pax9del/del pups at P0. Arrowhead in panel I points to cleft between the primary and anterior secondary palates. (B–D, F–H, J–L) Representative frontal sections from the anterior, middle, and posterior regions of the secondary palate in WT, DMSO-treated Pax9del/del, and IIIC3a-treated Pax9del/del embryos at E16.5. Of 11 IIIC3a-treated Pax9del/del pups, 7 exhibited secondary palate that was fused in the middle and posterior regions, whereas the other 4 still had cleft palate. Asterisk indicates cleft palate in panels E–H. Arrows point to molar tooth germs. E, embryonic day; P0, postnatal day; p, palatal shelf; t, tongue; WT, wild type.
Genetic Inactivation of Wise Restored Wnt Signaling Activity in the Developing Palatal Shelves and Rescued Palate Development in Pax9del/del Embryos
Since Wise mutant mice exhibit increased canonical Wnt signaling activity in several developing tissues (Ahn et al. 2010; Ahn et al. 2013; Ellies et al. 2014), we investigated whether genetic inactivation of Wise could restore Wnt signaling activity in the developing palatal shelves in Pax9del/del embryos. In contrast to the complete penetrance of cleft palate in Pax9del/del embryos (Fig. 3E–H), 70% of Pax9del/del;Wise-/- embryos (14 of 20), examined after E16.5, exhibited fused secondary palate (Fig. 3I–L). All Pax9del/del;Wise-/- embryos had irregular palatal rugae in comparison with the wild-type embryos (Fig. 3A, I), which is likely due to a requirement for Wise function in ruga patterning as previously reported (Welsh and O’Brien 2009). In addition, all Pax9del/del;Wise-/- embryos exhibited bud-stage tooth developmental arrest similar to Pax9del/del embryos (Fig. 3C, G, K; Appendix Fig. 2).
Figure 3.
Genetic inactivation of Wise rescued palate morphogenesis in Pax9del/del mice. (A, E, I) Whole mount view of the palate in WT, Pax9del/del, and Pax9del/del;Wise-/- pups at P0. (B–D, F–H, J–L) Representative frontal sections from anterior, middle, and posterior regions of the secondary palate in E16.5 WT, Pax9del/del, and Pax9del/del;Wise-/- embryos. Of 20 Pax9del/del;Wise-/- pups examined after E16.5, 14 exhibited fused palate, whereas the other 6 Pax9del/del;Wise-/- pups had cleft palate. Asterisk indicates the cleft palate in panels E–H. Arrows point to the molar tooth germs. E, embryonic day; P0, postnatal day; p, palatal shelf; t, tongue; WT, wild type.
We next compared expression of Axin2 mRNAs in the developing palatal shelves in Pax9del/del;Wise-/- mutant and Pax9del/+;Wise+/- control littermates. By in situ hybridization, we found that expression of Axin2 mRNAs was consistently increased in the anterior and posterior regions of the palatal mesenchyme in E13.5 Pax9del/del;Wise-/- embryos as compared with their Pax9del/+;Wise+/- littermates (Fig. 4A–D). RT-qPCR analysis of manually microdissected palatal shelves also showed a modest, albeit statistically not significant, increase in Axin2 mRNA levels in E13.5 Pax9del/del;Wise-/- embryos (Fig. 4E). Furthermore, in contrast to the significantly reduced levels of active β-catenin protein in E13.5 Pax9del/del palatal shelves versus Pax9del/+ littermates (Fig. 1O, P), we found that the palatal shelves of E13.5 Pax9del/del;Wise-/- embryos and their Pax9del/+;Wise+/- littermates had similar amounts of active β-catenin protein (Fig. 4F, G). Together, these results indicate that complete loss of Wise function restored canonical Wnt signaling in Pax9del/del palatal mesenchyme to the level comparable to that in Pax9del/+;Wise+/- control samples.
Figure 4.
Genetic inactivation of Wise restored canonical Wnt signaling activity in the developing palatal shelves in Pax9del/del embryos. (A–D) Frontal sections of E13.5 palatal shelves showing Axin2 mRNA expression pattern in the anterior and posterior regions of palate shelves in Pax9del/+;Wise+/- control and Pax9del/del;Wise-/- double-mutant embryos. Green arrows point to the domains of Axin2 mRNA expression in the anterior palatal mesenchyme. Black arrowheads point to Axin2 mRNA expression in the tooth germs. (E) RT-qPCR analysis of the levels of expression of Axin2 mRNAs in the anterior and posterior regions of the palatal shelves in E13.5 Pax9del/+;Wise+/- control and Pax9del/del;Wise-/- double-mutant embryos (n = 3). (F) Western blot detection of active β-catenin in the posterior palatal shelves in E13.5 Pax9del/+;Wise+/- control and Pax9del/del;Wise-/- mutant embryos. The levels of β-actin were detected as an internal loading control. (G) Relative band intensity of active β-catenin protein on Western blots was analyzed by ImageJ (n = 3) and normalized to that of β-actin. (H–M) Frontal sections showing Bmp4 mRNA expression patterns in the anterior and posterior regions of palatal shelves in E13.5 WT, Pax9del/del, and Pax9del/del;Wise-/- embryos. (N) RT-qPCR analysis of the levels of expression of Bmp4 mRNAs in E13.5 palatal shelves in Pax9del/+ control and Pax9del/del mutant embryos, as well as Pax9del/+;Wise+/- control and Pax9del/del;Wise-/- mutant embryos (n = 5 for each genotype). (O–T) Immunofluorescence detection of pSmad1/5/9 proteins in the posterior and anterior regions of the palatal shelves from E13.5 WT, Pax9del/del, and Pax9del/del;Wise-/- embryos. Yellow arrows point to comparable regions of the posterior palatal mesenchyme, whereas white arrows point to comparable regions of anterior palatal mesenchyme. Error bars represent SD. E, embryonic day; ns, not significant; p, palatal shelf; t, tongue; WT, wild type. ***P < 0.001.
Since Wise can antagonize Bmp and Wnt signaling (Itasaki et al. 2003; Laurikkala et al. 2003; Lintern et al. 2009), we analyzed whether loss of Wise function affected BMP signaling in Pax9del/del palatal shelves. By in situ hybridization and RT-qPCR analyses, we found that Bmp4 mRNA expression was significantly reduced in Pax9del/del;Wise-/- palatal shelves versus Pax9del/+;Wise+/- littermates, similar to the reduction in Bmp4 expression in Pax9del/del palatal shelves (Fig. 4H–N). Moreover, we found that the E13.5 Pax9del/del and Pax9del/del; Wise-/- embryos exhibit similarly reduced levels of pSmad1/5/9 in the palatal mesenchyme (Fig. 4O–T). Together, these results suggest that Wise does not have a detectable effect on canonical BMP signaling in the developing palatal mesenchyme.
Differences in Palatal Shelf Morphogenesis in Pax9del/del and Pax9del/del;Wise-/- Embryos
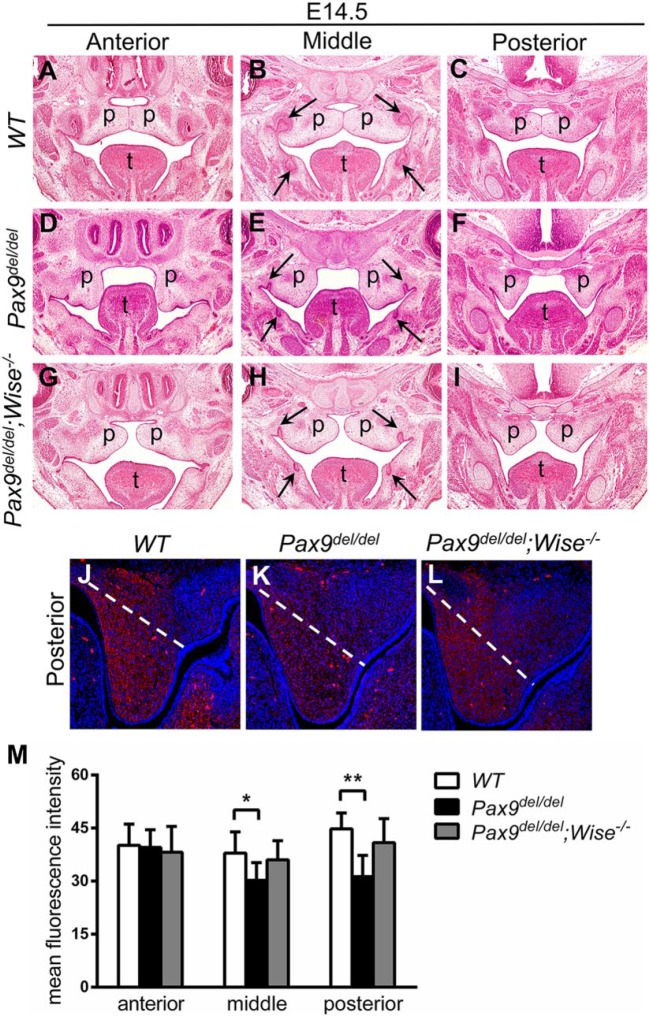
Previous studies have shown that mouse embryos homozygous for each of 2 distinct Pax9-null alleles exhibit delay in palatal shelf elevation/reorientation to the horizontal position above the tongue (Peters et al. 1998; Zhou et al. 2013). By E14.5, the palatal shelves had reoriented to the horizontal position and often initiated fusion at the midline in wild-type and Pax9del/+ embryos (Fig. 5A–C), whereas the palatal shelves in Pax9del/del littermates were still oriented vertically (Fig. 5D–F). Remarkably, both palatal shelves had reoriented to the horizontal position in 4 of 5 E14.5 Pax9del/del;Wise-/- embryos (Fig. 5G–I), suggesting that reduction in Wnt signaling is at least part of the mechanism underlying the impairment of palatal shelf morphogenesis in Pax9del/del embryos.
Figure 5.
Inactivation of Wise rescued palatal shelf elevation/reorientation in Pax9del/del embryos. (A–I) Representative frontal sections from anterior, middle, and posterior regions of the secondary palate in E14.5 WT, Pax9del/del, and Pax9del/del;Wise-/- embryos. Arrows point to the molar tooth germs. (J–L) Representative frontal sections from the posterior region of the palatal shelves of E13.5 WT, Pax9del/del, and Pax9del/del;Wise-/- embryos showing fluorescent staining of hyaluronic acid (red color). White dashed line marks the proximal boundary of the palatal shelf area used for quantification of fluorescence intensity. (M) Quantification of mean fluorescence intensity of hyaluronic acid staining in the WT, Pax9del/del, and Pax9del/del;Wise-/- samples (n = 4 for each genotype). Fluorescence intensity of hyaluronic acid staining was normalized against the area of palatal shelves. Error bars represent SD. p, palatal shelf; t, tongue; WT, wild type. *P < 0.05. **P < 0.01.
It has been hypothesized that HA, an extracellular glycosaminoglycan that is capable of binding a large amount of water, accumulates at higher levels in specific regions of the palatal mesenchyme and generates osmotic pressure to drive palatal shelf reorientation (Brinkley and Morris-Wiman 1987; reviewed by Ferguson 1988; Li et al. 2017). We found that Pax9del/del embryos had significantly reduced HA accumulation in the middle and posterior regions of palatal mesenchyme at E13.5 when compared with control littermates (Fig. 5J, K, M). Remarkably, the palatal mesenchyme in E13.5 Pax9del/del; Wise-/- embryos had HA accumulation restored to similar levels as in the Pax9del/+;Wise+/- control embryos (Fig. 5L, M), although they still showed a palatal shelf shape defect as in Pax9del/del embryos—that is, the lack of the lateral indentation between the palatal shelf and maxillary molar tooth germ (Fig. 5J–L). These results indicate that Pax9 plays a crucial role in palatal shelf elevation/reorientation through regulation of Wnt signaling activity in the palatal mesenchyme.
Discussion
Mice lacking Pax9 function exhibit multiple defects in palate development, including aberrant shape of palatal shelves, reduced palatal mesenchyme proliferation, and defect in palatal shelf elevation/reorientation (Peters et al. 1998; Zhou et al. 2013), which provide an excellent model for studying molecular and cellular mechanisms of palatogenesis. In this study, we found that Pax9del/del mouse embryos exhibit decreased canonical Wnt signaling activity in developing palatal shelves and that modulating Wnt signaling through pharmacologic inhibition of DKK activity or genetic inactivation of Wise partly rescued palate morphogenesis in Pax9del/del mice. Our data significantly improve understanding of the roles of canonical Wnt signaling in palatogenesis and identify a previously unknown link between Pax9 and Wnt signaling in palate development.
Previous studies have established that palate development involves differential molecular patterning and regionalized control of growth along the anterior-posterior axis (recently reviewed by Lan et al. 2015). It has been shown that expression of the BATGAL transgenic reporter of canonical Wnt signaling activity is restricted to the anterior palatal mesenchyme and absent from the posterior palatal mesenchyme (He et al. 2011; Liu et al. 2015). We found that Axin2, a known endogenous target of canonical Wnt signaling (Jho et al. 2002), is expressed in both anterior and posterior palatal mesenchyme in wild-type mouse embryos and its expression is significantly reduced in posterior regions of the palatal mesenchyme in Pax9del/del embryos (Fig. 1J–N). Moreover, we found that the signaling form of β-catenin protein was accumulated at much higher levels in the posterior than anterior regions of the palatal shelves in E13.5 wild-type embryos (Appendix Fig. 1) and was significantly reduced in the posterior palatal tissues in E13.5 Pax9del/del embryos (Fig. 1O, P). The decrease in expression of Axin2 mRNAs and active β-catenin protein in E13.5 Pax9del/del palatal shelves correlated with a significant increase in expression of Dkk2 mRNAs in the posterior palatal mesenchyme (Fig. 1Q–W). Remarkably, IIIC3a treatment in utero rescued development and fusion of the palatal shelves in the middle and posterior regions in Pax9del/del embryos (Fig. 2). Together, these results indicate that canonical Wnt signaling is active in the anterior and posterior regions of the developing palatal shelves and that Pax9-regulated canonical Wnt signaling is crucial for palate morphogenesis, particularly in the middle and posterior regions.
During preparation of this manuscript, another laboratory independently found that the expression levels of several Wnt antagonists were greatly increased in the Pax9 mutant embryos. These authors were able to rescue embryonic palatogenesis in vivo using 2 DKK antagonists (R. D’Souza, personal communication). Results from both our studies are hence encouraging and suggest that the WNT pathway is a good target for future therapeutic interventions.
Uniquely in this study, we also used a genetic approach to delete another WNT antagonist, Wise (Ahn et al. 2010). Wise mRNAs were downregulated by 26% was significantly downregulated in the developing palatal tissues in Pax9del/del embryos (Fig. 1I). However, this decrease in expression was not enough to prevent clefting. As we found, the full deletion of Wise was necessary to offset the inhibition of canonical Wnt signaling in Pax9del/del palatal mesenchyme, likely caused by the increase in Dkk2 expression. The germline inactivation of both Wise alleles restored canonical Wnt signaling in the palatal tissues and rescued palate morphogenesis in Pax9del/del embryos.
The increased Dkk2 expression in the palatal mesenchyme caused significant inhibition of canonical Wnt signaling in Pax9del/del embryos despite the decrease in Wise expression. These results suggest that Dkk2 is a more potent inhibitor of canonical Wnt signaling than Wise. Alternatively, but not exclusively, the increase in Dkk2 expression and decrease in canonical Wnt signaling might have occurred earlier in palate development in Pax9del/del embryos whereas the downregulation of Wise occurred later due to changes in expression of other genes, such as Bmp4. Indeed, our data show that Wise mRNAs were still robustly expressed in the Pax9del/del palatal shelves at E12.5 (Fig. 1B) but were significantly downregulated in the Pax9del/del palatal shelves by E13.5 (Fig. 1C–I). Further studies are needed to elucidate the detailed molecular mechanisms involving Pax9-mediated regulation of canonical Wnt signaling during palate development.
Author Contributions
C. Li contributed to design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; Y. Lan contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; R. Krumlauf contributed to design and critically revised the manuscript; R. Jiang contributed to conception, design, data analysis and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank Rena D’Souza for discussions during this study.
Footnotes
A supplemental appendix to this article is available online.
This work was supported by National Institutes of Health / National Institute of Dental and Craniofacial Research grants DE013681 and DE018401 to R. Jiang and by support from the Stowers Institute to R. Krumlauf.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ahn Y, Sanderson BW, Klein OD, Krumlauf R. 2010. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development. 137(19):3221–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn Y, Sims C, Logue JM, Weatherbee SD, Krumlauf R. 2013. Lrp4 and Wise interplay controls the formation and patterning of mammary and other skin appendage placodes by modulating Wnt signaling. Development. 140(3):583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek JA, Lan Y, Liu H, Maltby KM, Mishina Y, Jiang R. 2011. Bmpr1a signaling plays critical roles in palatal shelf growth and palatal bone formation. Dev Biol. 350(2):520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley LL, Morris-Wiman J. 1987. Computer-assisted analysis of hyaluronate distribution during morphogenesis of the mouse secondary palate. Development. 100(4):629–635. [DOI] [PubMed] [Google Scholar]
- Bush JO, Jiang R. 2012. Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development. 139(2):231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet BT, Blanton SH, Burt A, Ma D, Stal S, Mulliken JB, Hecht JT. 2008. Variation in WNT genes is associated with non-syndromic cleft lip with or without cleft palate. Hum Mol Genet. 17(14):2212–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciat CM, Niehrs C. 2013. Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 5(3):a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MJ, Marazita ML, Beaty TH, Murray JC. 2011. Cleft lip and palate: understanding genetic and environmental influences. Nature Rev Genet. 12(3):167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellies DL, Economou A, Viviano B, Rey JP, Paine-Saunders S, Krumlauf R, Saunders S. 2014. Wise regulates bone deposition through genetic interactions with Lrp5. PLoS One. 9(5):e96257 Erratum in: PLoS One 9(7): e104467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MW. 1988. Palate development. Development. 103 Suppl:41–61. [DOI] [PubMed] [Google Scholar]
- He F, Xiong W, Wang Y, Li L, Liu C, Yamagami T, Taketo MM, Zhou C, Chen Y. 2011. Epithelial Wnt/β-catenin signaling regulates palatal shelf fusion through regulation of Tgfβ3 expression. Dev Biol. 350(2):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, Nishita M, Suzuki K, Yamada G, Minami Y, et al. 2008. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development. 135(23):3871–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. 2003. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 130(18):4295–4305. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. 2002. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 22(4):1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Kwon HE, Lan Y, Zhou J, Liu H, Jiang R. 2016. Bmp4-Msx1 signaling and Osr2 control tooth organogenesis through antagonistic regulation of secreted Wnt antagonists. Dev Biol. 420(1):110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HE, Jia S, Lan Y, Liu H, Jiang R. 2017. Activin and Bmp4 signaling converge on Wnt activation during odontogenesis. J Dent Res [epub ahead of print 1 June 2017] in press. doi: 10.1177/0022034517713710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Jia S, Jiang R. 2014. Molecular patterning of the mammalian dentition. Semin Cell Dev Biol. 25–26:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Jiang R. 2009. Sonic hedgehog signaling regulates reciprocal epithelial-mesenchymal interactions controlling palatal outgrowth. Development. 136(8):1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Kingsley PD, Cho ES, Jiang R. 2001. Osr2, a new mouse gene related to Drosophila odd-skipped, exhibits dynamic expression patterns during craniofacial, limb, and kidney development. Mech Dev. 107(1–2):175–179. [DOI] [PubMed] [Google Scholar]
- Lan Y, Ovitt CE, Cho ES, Maltby KM, Wang Q, Jiang R. 2004. Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development. 131(13):3207–3216. [DOI] [PubMed] [Google Scholar]
- Lan Y, Xu J, Jiang R. 2015. Cellular and molecular mechanisms of palatogenesis. Curr Top Dev Biol. 115:59–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurikkala J, Kassai Y, Pakkasjärvi L, Thesleff I, Itoh N. 2003. Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev Biol. 264(1):91–105. [DOI] [PubMed] [Google Scholar]
- Li C, Lan Y, Jiang R. 2017. Molecular and cellular mechanisms of palate development. J Dent Res. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Shan J, Chang W, Kim I, Bao J, Lee HJ, Zhang X, Samuel VT, Shulman GI, Liu D, et al. 2012. Chemical and genetic evidence for the involvement of Wnt antagonist Dickkopf2 in regulation of glucose metabolism. Proc Natl Acad Sci U S A. 109(28):11402–11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintern KB, Guidato S, Rowe A, Saldanha JW, Itasaki N. 2009. Characterization of wise protein and its molecular mechanism to interact with both Wnt and BMP signals. J Biol Chem. 284(34):23159–23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang M, Zhao W, Yuan X, Yang X, Li Y, Qiu M, Zhu XJ, Zhang Z. 2015. Gpr177-mediated Wnt signaling is required for secondary palate development. J Dent Res. 94(7):961–967. [DOI] [PubMed] [Google Scholar]
- Menezes R, Letra A, Kim AH, Küchler EC, Day A, Tannure PN, da Motta LG, Paiva K, Granjeiro JM, Vieira AR. 2010. Studies with Wnt genes and nonsyndromic cleft lip and palate. Birth Defects Res A Clin Mol Teratol. 88(11):995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H, Neubüser A, Kratochwil K, Balling R. 1998. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 12(17):2735–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R, Spencer-Dene B, Connor EC, Gritli-Linde A, McMahon AP, Dickson C, Thesleff I, Rice DP. 2004. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J Clin Invest. 113(12):1692–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata I, Maas R. 1994. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 6(4):348–356. [DOI] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. 2002. Wnt signaling controls the phosphorylation status of β-catenin. J Biol Chem. 277(20):17901–17905. [DOI] [PubMed] [Google Scholar]
- Welsh IC, O’Brien TP. 2009. Signaling integration in the rugae growth zone directs sequential SHH signaling center formation during the rostral outgrowth of the palate. Dev Biol. 336(1):53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu H, Park JS, Lan Y, Jiang R. 2014. Osr1 acts downstream of and interacts synergistically with Six2 to maintain nephron progenitor cells during kidney organogenesis. Development. 141(7):1442–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhao X, Hu Y, St Amand T, Zhang M, Ramamurthy R, Qiu M, Chen Y. 1999. Msx1 is required for the induction of Patched by Sonic hedgehog in the mammalian tooth germ. Dev Dyn. 215(1):45–53. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lan Y, Chai Y, Jiang R. 2009. Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into a single row. Science. 323(5918):1232–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y. 2002. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 129(17):4135–4146. [DOI] [PubMed] [Google Scholar]
- Zhou J, Gao Y, Lan Y, Jia S, Jiang R. 2013. Pax9 regulates a molecular network involving Bmp4, Fgf10, Shh signaling and the Osr2 transcription factor to control palate morphogenesis. Development. 140(23):4709–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Gao Y, Zhang Z, Zhang Y, Maltby KM, Liu Z, Lan Y, Jiang R. 2011. Osr2 acts downstream of Pax9 and interacts with both Msx1 and Pax9 to pattern the tooth developmental field. Dev Biol. 353(2):344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.