Abstract
Three-dimensional (3D) bioprinting techniques can be used for the fabrication of personalized, regenerative constructs for tissue repair. The current article provides insight into the potential and opportunities of 3D bioprinting for the fabrication of cartilage regenerative constructs. Although 3D printing is already used in the orthopedic clinic, the shift toward 3D bioprinting has not yet occurred. We believe that this shift will provide an important step forward in the field of cartilage regeneration. Three-dimensional bioprinting techniques allow incorporation of cells and biological cues during the manufacturing process, to generate biologically active implants. The outer shape of the construct can be personalized based on clinical images of the patient’s defect. Additionally, by printing with multiple bio-inks, osteochondral or zonally organized constructs can be generated. Relevant mechanical properties can be obtained by hybrid printing with thermoplastic polymers and hydrogels, as well as by the incorporation of electrospun meshes in hydrogels. Finally, bioprinting techniques contribute to the automation of the implant production process, reducing the infection risk. To prompt the shift from nonliving implants toward living 3D bioprinted cartilage constructs in the clinic, some challenges need to be addressed. The bio-inks and required cartilage construct architecture need to be further optimized. The bio-ink and printing process need to meet the sterility requirements for implantation. Finally, standards are essential to ensure a reproducible quality of the 3D printed constructs. Once these challenges are addressed, 3D bioprinted living articular cartilage implants may find their way into daily clinical practice.
Keywords: regenerative medicine, additive manufacturing, bio-ink, bioprinting
Introduction
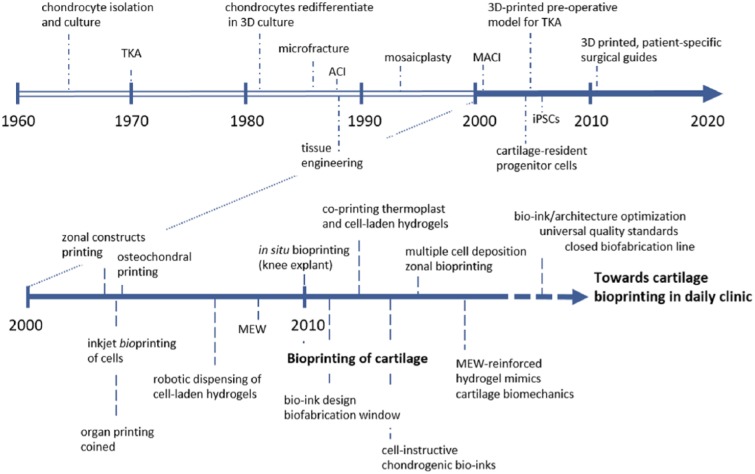
Three-dimensional (3D) bioprinting, one of the main approaches within the field of biofabrication,1 is an emerging technology that allows for the fabrication of constructs with control over spatial resolution, shape, and mechanical properties. Bioprinting facilitates the accurate positioning of biomaterials, cells, and biological cues in a layer-by-layer fashion and can, thus, be applied for the generation of personalized regenerative implants. Articular cartilage is a thin, avascular, structural organ, and it is therefore an easier potential target for treatment with bioprinted regenerative constructs compared to vascularized organs, such as the liver and kidney. Cartilage contains predominantly proteoglycans, water, collagen type II, and low numbers of chondrocytes. Due to this low cell number and the absence of vascularization, the tissue has a limited regenerative capacity.2 Consequently, most articular cartilage injuries will progress toward osteoarthritis if no interventions are taken.3 Significant improvements in reparative cartilage treatments have been achieved over the past few decades ( Fig. 1 ). However, full cartilage restoration remains a significant challenge. It is generally accepted that for stable long-term reconstruction, function repair, or even regeneration, the therapy should not only address the cartilage but also focus on reconstructing the underlying bone and reestablishing joint homeostasis. Therefore, bioprinted, personalized, regenerative constructs may provide a solution for cartilage injuries.
Figure 1.
Evolution of cartilage repair and bioprinting of cartilage. Additive manufacturing techniques and in particular bioprinting are enabling to produce patient-specific, complex architectures that mimic the composition of articular cartilage. With the development of novel bioactive bio-inks and the combination of different 3D bioprinting techniques, functional cartilage constructs will be obtained. Optimized and mature bioprinted grafts will have to meet high quality standards in order to be used as clinical devices for cartilage and joint healing. TKA = total knee arthroplasty; ACI = autologous chondrocyte implantation; MACI = matrix-induced autologous chondrocyte implantation; iPSC = induced pluripotent stem cell; MEW = melt electrospinning writing.
Although bioprinting technology is rapidly gaining interest in the field of regenerative medicine, it is still in its infancy ( Fig. 1 ). Consequently, significant steps will have to be taken before this technology can be translated to widespread clinical applications. The International Cartilage Repair Society has adopted a leading role in analyzing the current state of scientific developments for cartilage regeneration in order to, for example, provide recommendations for the execution of preclinical and clinical studies.4 The present position article can be regarded as an extension to this previous initiative and summarizes the current status of 3D bioprinting in the field of cartilage regeneration. More specifically, this article aims to address the potential and opportunities of 3D bioprinting for the fabrication of personalized regenerative articular cartilage constructs with tailored biological properties, architecture, and mechanical properties.
Current Status of 3D Printing in the Orthopedic Clinic
Three-dimensional printing technologies use 3D computer models to determine the final shape of the printed construct. Imaging techniques currently used in the clinic, for example, X-rays, magnetic resonance imaging (MRI), and computed tomography (CT), provide macroscopically detailed sequences of 2D images of patients. These sequences can relatively easily be translated into 3D images, which can serve as blueprints for 3D printing, for example, on conversion into stereolithography and additive manufacturing files.5 Currently, 3D printing technologies are already part of a number of clinical routines. Three-dimensional models of complex abnormalities are, for example, printed for educational purposes and to help surgeons in preoperative planning for challenging surgeries.6-8 Moreover, patient-specific drilling and sawing guides are printed to assist orthopedic surgeons with the placement of pedicle screws and total joint replacements respectively.9,10 Additionally, customized 3D printed implants are already commercially available, for example, for calvarial reconstruction.11 These examples indicate that the current 3D printing technologies have the capability to provide personalized implants for orthopedic defects. However, the transition from 3D printing of polymers, ceramics, and metals toward 3D bioprinting of living and biologically active constructs has not taken place in the clinical practice yet. In this research field, however, several steps have already been performed to demonstrate the possibilities and the feasibility of the clinical transition ( Table 1 ).
Table 1.
Overview of Publications on the (Bio)fabrication of (Articular) Cartilage Regenerative Constructs.
| Bio-Ink |
(Bio)printed Constructs |
|||||
|---|---|---|---|---|---|---|
| Reference | Printing Technique | Material(s) | Cells and/or Biological Cues | Mechanical Properties | Tissue Formation | Remarks |
| Cohen et al. (2006)18 | Extrusion bioprinting | Alginate | • Full thickness bovine articular chondrocytes | • Compressive modulus (unconfined) ~1.8 ± 0.1 kPa |
In vitro • Viability, ~94% • Cartilage-like tissue formation similar in printed constructs as in casted alginate or alginate beads |
• Printed meniscus and intervertebral disk shapes* • Demonstrated that viability and differentiation behavior of chondrocytes were not influenced by the printing procedure |
| Schuurman et al. (2012)61 | Extrusion printing | Alginate reinforced with PCL | • C20A4 cell-line* | • Compressive modulus (unconfined) ~6000 kPa |
In vitro • Viability, ~70-90% |
• First demonstration of simultaneous deposition of thermoplastic polymer and hydrogel for the fabrication of reinforced constructs |
| Cui et al. (2012)19 | Inkjet bioprinting | PEGDMA | • Full thickness human articular chondrocytes• TGF-β1 and FGF-2 in medium during culture | • Compressive modulus (unconfined) ~30-37 kPa |
In vitro • More cell proliferation and more total cartilage-like tissue formation in constructs cultured with both TGF-β1 and FGF-2 after 4 weeks of differentiation |
• Demonstrated that culturing printed constructs with both TGF-β1 and FGF-2 increases proliferation and on longer term (21 days) cartilage-like tissue formation |
| Cui et al. (2012)70 | Inkjet bioprinting | PEGDMA | • Full thickness human articular chondrocytes | • Compressive modulus (unconfined) ~38-320 kPa |
Ex vivo • Viability, ~89% • Cartilage-like tissue formation increased during the first 4 weeks of culture and remained stable after (6 weeks total) • Stable construct integration in the defect after 6 weeks |
• Printing directly into defects in porcine osteochondral plugs • Demonstrated the importance of rapid gelation for maintaining initial cell distribution |
| Schuurman et al. (2013)17 | Extrusion bioprinting | GelMA with hyaluronic acidPCL reinforcement* | • Full thickness equine articular chondrocytes• Hyaluronic acid | • Compressive modulus (unconfined) for gelMA constructs: ~10-175 kPa (dependent on concentration) |
In vitro • Viability, ~73-83% • Similar cartilage-like tissue formation in constructs with and without hyaluronic acid after 4 weeks differentiation |
• Addition of hyaluronic acid increased the printability |
| Boere et al. (2014)64 | Extrusion printing | GelMA (cast) reinforced with pHMGCL/PCL (printed) | • Full thickness human articular chondrocytes | • Construct failure (unconfined compression) ~2.7 N (~7.7 N when covalent bonds between gelMA and pHMGCL/PCL) |
In vitro • Covalent bonds between hydrogel and reinforcement to increase construct stability and mechanical strength In vivo • Rats, subcutaneous; more collagen type II but less GAGs after 8 weeks compared to in vitro study |
• Interconnected cartilage-like tissue network after 6 weeks of differentiation |
| Kundu et al. (2015)83 | Extrusion bioprinting | Alginate reinforced with PCL | • Nasal human chondrocytes• TGF-β | • Not reported |
In vitro • Viability, ~85%• More cartilage-like tissue formation in constructs with TGF-β after 4 weeks of differentiation In vivo • Immunodeficient mice, subcutaneous; moderate cartilage-like tissue formation in constructs with TGF-β after 4 weeks |
• Printed constructs with a cell and growth factor-laden bio-ink, reinforced with a printed thermoplastic polymer |
| Kesti et al. (2015)84 | Extrusion bioprinting | HAMA with HA-pNIPAAM | • Full thickness bovine articular chondrocytes | • Not reported |
In vitro • Viability, low when HA-pNIPAAM remains in the culture, high when HA-pNIPAAM was eluted |
• HA-pNIPAAM supports printing and can be eluted from the final construct after printing and crosslinking |
| Kesti et al. (2015)39 | Extrusion bioprinting | Gellan with alginate | • Full thickness bovine articular chondrocytes • Hydroxyapatite particles • Cartilage extracellular matrix particles |
• Tensile modulus ~116-230 kPa |
In vitro • Viability, ~80-96% (60% in the center of large constructs) • Cartilage extracellular matrix particles increased cartilage-like tissue formation during 8 weeks of differentiation. However, constructs cultured in TGF-β3 supplemented medium contained most cartilage matrix |
• Printed ear, nose, meniscus, and vertebral disk shapes by using support structures* |
| Markstedt et al. (2015)85 | Extrusion bioprinting | Nanofibrillated cellulose with alginate | • Nasal human chondrocytes | • Compressive modulus (unconfined) ~75-250 kPa (depending on the ratio of both components) |
In vitro • Viability, ~73-86% |
• Printed ear and meniscus shapes* |
| Visser et al. (2015)66 | Melt electrospinning | Electrospun PCL infused with gelMA | • Human articular chondrocytes in gelMA• Hyaluronic acid• Mechanical stimulation (14 days without followed by 14 days with) | • Compressive modulus (unconfined) ~80-400 kPa for hybrid construct depending on mesh porosity• Similar stress/strain curve for gelMA reinforced with a 93% porous mesh as for native articular cartilage |
In vitro • Viability, ~73-86% • Expression of chondrogenic genes increased in mechanical stimulated constructs during 4 weeks of culture. No differences were found at protein level |
• With low content of thermoplastic polymer fibers (7%), mechanical properties could be achieved within the range of articular cartilage |
| Izadifar et al. (2016)86 | Extrusion bioprinting | Alginate reinforced with PCL | • Full thickness embryonic chick chondrocytes (“rounded” and “fibroblastic” subpopulations) | • Not reported |
In vitro • Viability, ~77-85% • More proliferation and cartilage-like tissue formation in constructs with the “fibroblastic” chondrocyte subpopulation compared to the “rounded’ subpopulation |
• Printed constructs with a cell-laden bio-ink, reinforced with a printed thermoplastic polymer • Demonstrated rapid cooling of PCL strands after printing • Different chondrocyte subpopulations give differences in cartilage-like tissue formation |
| Abbadessa et al. (2016)87 | Extrusion bioprinting | polyHPMA-lac-PEG | • Full thickness equine articular chondrocytes• HAMA or CSMA | • Compressive modulus (unconfined) ~13-16 kPa for all combinations |
In vitro • Viability, ~85-95% (printed) • Similar levels of cartilage-like tissue formation in polyHPMA-lac-PEG hydrogels as in fibrin controls after 4 weeks of culture (cast) |
• Incorporation of HAMA and to a lesser extent CSMA decreased the degradation rate and improved the thermosensitive profile and printability |
| Mouser et al. (2016)88 | Extrusion bioprinting | gelMA with gellan gum | • Full thickness equine articular chondrocytes | • Compressive modulus (unconfined) ~2.7-186 kPa depending on concentration and ratio of gelMA and gellan gum |
In vitro • All evaluated concentrations supported cartilage-like tissue formation during 6 weeks of culture. Relatively high gellan gum concentrations compromised chondrogenesis and high total polymer concentrations hampered matrix distribution |
• Identified yield stress as dominant factor for bioprintability • Addition of gellan gum improved filament deposition, increased construct stiffness, and supported chondrogenesis |
Proof of concept study.
PCL = polycaprolactone; PEGDMA = poly(ethylene) glycol dimethacrylate; TGF = transforming growth factor; FGF = fibroblast growth factor; GelMA = gelatin-methacryloyl; pHMGCL/PCL = poly(hydroxylmethylglycolide-co-ϵ-caprolactone)/poly(ϵ-caprolactone); HAMA = methacrylated hyaluronic acid; HA-pNIPAAM = poly(N-isopropylacrylamide) grafted hyaluronan; polyHPMA-lac-PEG = polyethylene glycol midblock flanked by two poly[N-(2-hydroxypropyl) methacrylamide mono/dilactate]; CSMA = methacrylated chondroitin sulfate; PEGT-PBT = poly(ethylene glycol)-terephthalate-poly(butylene terephthalate).
The Potential of 3D Bioprinting for Regenerative Cartilage Constructs
Cell Laden and Bioactive Inks
Bioprinting techniques provide many possibilities for the fabrication of personalized cartilage constructs. A key factor for the success of these regenerative constructs is to make them biologically active. One strategy to accomplish this is by incorporating cells. It has been widely demonstrated that the bioprinting process when using extrusion, inkjet, or laser-based printing technologies does not hamper the viability or long-term performance of the deposited cells.12-19 Extrusion-based printing allows the deposition of cell-laden filaments and is regarded as the most suitable technique for the 3D bioprinting of viable constructs of several centimeters in size and with high cell densities.20 Consequently, for the printing of cartilage constructs, extrusion-based printing techniques are most often considered (85% of the publications; Table 1 - 3 ). However, the resolution of the fiber thickness is limited by the extrusion process to ~100 µm. In contrast, inkjet and laser-based printing allow the deposition of smaller volumes and are, thus, more suitable for the accurate deposition of micropatterns, down to the level of single cells. Currently, the most promising carrier materials, or “bio-inks,” for cell-based 3D bioprinting are based on hydrogels, as they facilitate homogeneous cell encapsulation in a highly hydrated and mechanically supportive 3D environment.
Table 2.
Overview of Publications on the (Bio)fabrication of Zonally Organized Cartilage Regenerative Constructs.
| Bio-Ink |
(Bio)printed Constructs |
|||||
|---|---|---|---|---|---|---|
| Reference | Printing Technique | Material(s) | Cells and/or Biological Cues | Mechanical Properties | Tissue Formation | Remarks |
| Woodfield et al. (2004)89 And Woodfield et al. (2005)90 | Extrusion printing | PEGT/PBT | • Full thickness human articular chondrocytes (seeded after printing)• Differences in pore size | • Equilibrium compressive modulus (unconfined) ~200-2500 kPa • Dynamic compressive modulus (unconfined) ~200-4300 kPa |
In vitro • Viability, no quantification • Cartilage-like tissue formation after 3 weeks of differentiationIn vivo (homogenous pore size) • Immunodeficient mice, subcutaneous; more cartilage-like tissue formation after 3 weeks compared to in vitro. Fibrous capsule around construct |
• Goal was to study zonal differentiation • Demonstrated that pore size gradients promote inhomogeneous cell distribution. However no differences in cartilage-like tissue formation was found when normalized to the cell density |
| Schuurman et al. (2013)91 | Extrusion printing | PEGT-PBT | • Superficial zone equine articular chondrocyte pellet• Deep zone equine articular chondrocyte pellets | • Not reported |
In vitro • Abundant cartilage-like tissue formation during 1 month of differentiation. However, zonal characteristics could not be maintained |
• Demonstrated the feasibility of placing aggregates based on cells in a printed scaffold |
Table 3.
Overview of Publications on the (Bio)fabrication of Osteochondral Regenerative Constructs.
| Bio-Ink |
(Bio)printed Constructs |
|||||
|---|---|---|---|---|---|---|
| Reference | Printing Technique | Material(s) | Cells and/or Biological Cues | Mechanical Properties | Tissue Formation | Remarks |
| *Lee et al. (2010)44 | Extrusion printing | PCL with hydroxyapatite | • Cell free • PCL network infused with a TGF-β3 laden collagen type I hydrogel (cartilage) • Different pore size for bone and cartilage |
• Not reported for the construct prior to implantation • Compressive modulus (unconfined) ~5500 kPa at explanation |
In vivo • Rabbits, orthotopic; cells migrated into the scaffolds and produced cartilage-like tissue after 4 months in the presence of TGF-β3 |
• Demonstrated the feasibility of cartilage regeneration via cell-free scaffolds that attract host cells |
| *Cohen et al. (2010)69 | Extrusion printing | Alginate (cartilage)Gelatin (bone) | • Cell free • Demineralized bone matrix (bone) |
• Not reported |
Ex vivo • Printing directly into an (osteo)chondral defect in a bovine femur |
• First demonstration of printing directly into a defect with automatic geometric feedback during printing |
| *Shim et al. (2012)92 | Extrusion bioprinting | Alginate reinforced with PCL | • Nasal human chondrocytes (cartilage)• Human osteoblast cell line (MG63, bone) | • Not reported |
In vitro • Viability, ~90-95% • Cells proliferated during 7 days of culture |
• Proof of concept for printing with multiple cell types that remain in their separate compartments during culture |
| Fedorovich et al. (2012)48 | Extrusion bioprinting | Alginate | • Full thickness human articular chondrocytes (cartilage)• Human mesenchymal stromal cells (bone)• Biphasic calcium phosphate particles (bone) | • Compressive modulus (unconfined) ~4-15 kPa (dependent on construct porosity) |
In vitro • Viability, ~89% • Bone-like and cartilage-like tissue formation in the separate regions of the constructs after 3 weeks of differentiation In vivo• Immunodeficient mice, subcutaneous; bone-like and cartilage-like tissue formation in the separate regions after 6 weeks |
• Demonstrated the feasibility of printing heterogeneous tissue constructs with distinctive tissue formation in osteo and chondral regions |
| Levato et al. (2014)49 | Extrusion bioprinting | GelMA with gellan gum | • Murine mesenchymal stromal cells (cartilage/bone)• Polylactic acid microcarriers (bone) | • Compressive modulus (unconfined) ~25-50 kPa (dependent on concentration of microcarriers) | In vitro• Viability, ~60-90% | • Demonstrated the feasibility to extrude larger aggregates (cells on microcarriers)• Focus paper on bone compartment, osteochondral constructs as proof of concept |
Proof of concept study.
PCL = polycaprolactone; PEGDMA = poly(ethylene) glycol dimethacrylate; TGF = transforming growth factor; FGF = fibroblast growth factor; GelMA = gelatin-methacryloyl; pHMGCL/PCL = poly(hydroxylmethylglycolide-co-ϵ-caprolactone)/poly(ϵ-caprolactone); HAMA = methacrylated hyaluronic acid; HA-pNIPAAM = poly(N-isopropylacrylamide) grafted hyaluronan; polyHPMA-lac-PEG = polyethylene glycol midblock flanked by two poly[N-(2-hydroxypropyl) methacrylamide mono/dilactate]; CSMA = methacrylated chondroitin sulfate; PEGT-PBT = poly(ethylene glycol)-terephthalate-poly(butylene terephthalate).
Multiple cell types have been explored for their application in bioactive cartilage implants. Autologous chondrocytes or chondrons, chondrocytes with their pericellular matrix, can be harvested form a non–load-bearing cartilage surface or the perimeter of a cartilage defect in the patient.21-23 So far, in cartilage bioprinting research, the focus has predominantly been on the use of chondrocytes ( Table 1 - 3 ). Nevertheless, when using autologous chondrocytes, obtaining sufficient cell numbers remains a challenge, especially since expansion in monolayer culture causes dedifferentiation of the cells toward a more fibroblastic phenotype.24 Additionally, complications such as donor site morbidity are likely to occur. An alternative cell type is the multipotent mesenchymal stromal cell (MSC) population, which can be derived from multiple tissues, for example, bone marrow, adipose tissues, and muscles.25 These cells can be differentiated into chondrocyte-like cells in the presence of specific growth factors, such as the transforming growth factor beta family. However, adequate cues to control MSC fate have to be provided, as these cells have the tendency to progress into hypertrophic chondrogenesis and to give rise to bone formation via the endochondral pathway once implanted in vivo.26 Combining MSCs with chondrocytes or chondrons has shown promising results both in vitro and in vivo, in which it seems that the MSCs stimulate and direct the chondrocytes/chondrons to synthesize new cartilage-like tissue.27-29 Furthermore, alternative cell populations with regenerative potential are being investigated, including subpopulations of chondroprogenitor cells, which can be harvested from mature cartilage and can be expanded in mono-layer culture without losing their chondrogenic phenotype.30 Also, the induced pluripotent stem cells that show unlimited self-renewal and can be generated from numerous, easily accessible cell types (i.e., keratinocytes), constitute an interesting cell source for cartilage regeneration, provided that the safety concerns about their usage are cleared.31,32 Multiple research groups are focusing on these different cell populations for cartilage regeneration purposes and on enhancing the cell performance by, for example, culturing with specific growth factors, biological cues, and mechanical loading regimes; however, these detailed strategies are not within the scope of the present opinion article and have been reviewed elsewere.33-35
An alternative strategy to generate bioactive constructs based on 3D printing technologies involves the embedding of biological cues that stimulate encapsulated cells or attracts and/or stimulates cells from the host. Hydrogel-based bio-inks allow for the incorporation of growth factors, bioactive proteins, peptides, chemicals, and matrix components,36 and printing procedures have so far not shown any negative effects on the activity of these biological cues17,37-39 ( Table 1 - 3 ). Large molecules that are constituents of the native cartilage matrix, such as hyaluronic acid,40,41 can be used as promising biological cues for cartilage regeneration. Addition of these components also have an impact on the overall rheological properties, often increasing the printability of a hydrogel bio-ink for extrusion printing.17,42 When printing with thermoplastic polymers, on the other hand, biological molecules are exposed to relatively high temperatures during the extrusion process. Therefore, while thermostable compounds can be loaded during printing, labile compounds need to be incorporated afterwards. For example, dexamethasone still exhibits osteoinductive properties in vitro after being printed in a polycaprolactone (PCL)/poloxamine polymer blend at 110°C.43 Contrarily, transforming growth factor beta cannot be heated to this temperature but can be coated on printed PCL scaffolds and was demonstrated to attract and stimulate cells from surrounding tissues in vivo when incorporated with this approach.44 Furthermore, other recently developed regenerative strategies, that include, for example, the incorporation of cell-secreted exosomes45 or microRNAs, can easily be combined with bioprinting technologies, since all these moieties can be preserved in the highly hydrated environment provided by hydrogel bio-inks.
Shape, Architecture, and Multiphasic Organization
For the generation of personalized regenerative implants, precise control over shape and internal architecture is essential. As discussed above, the outer shape of a construct can be personalized by using medical imaging as the foundation of the print template. Additionally, multiple “inks” consisting of different biomaterials, bioactive factors, and/or cells can be loaded in a bioprinter to fabricate complex anatomical architectures with multiple tissue types. Irregular shapes and overhangs can also be obtained via the printing of support structures with a sacrificial material, such as alginate, agarose, PCL, polyvinyl alcohol (PVA), polyethylene glycol (PEG), and pluronics.12-14,46,47
Printing with multiple bio-inks also allows for the inclusion of multiple tissues and tissue interfaces in a single construct. This is of particular importance in the orthopedic field, where tissue interfaces play a significant role in the underlying (patho)biology. Osteochondral constructs have, for example, been successfully generated with either osteoblasts in the bone part and chondrocytes in the cartilage part,48 or stem cells in both layers with additional biological cues to induce bone differentiation in the one part and cartilage formation in the other.26,49 However, in the latter study further evaluation of biological cues is necessary, as the MSCs generated bone via the endochondral pathway in the cartilage layer after in vivo implantation.26 Furthermore, to mimic the bone compartment, bio-inks that can both carry cells and harden over time have been developed, to achieve a stiffness within the same range of those of cancellous bone.38 Such bio-inks could be combined with hydrogels described above to obtain fully printed, cellular composites with mechanical properties that appropriately match bone and cartilage regions. Additionally, vessel-like structures can be printed in the bone compartment to support vascularization by using sacrificial materials.13,14,46
Printing with multiple bio-inks also provides a platform to mimic the zonal organization of articular cartilage. Articular cartilage exhibits distinct depth dependence in composition and mechanical performance,50,51 which have been notably difficult to reproduce with conventional approaches to cartilage repair.52 It is believed that restoration of this zonal organization will improve integration and performance of the construct at the defect site.53,54 Three-dimensional bioprinting may be a unique tool to achieve the appropriate zone-specific compositional and mechanical heterogeneity present in articular cartilage. Increasing resolution of 3D bioprinters might even allow for imitation of the specific fiber arrangement of the split line patterns found at the articular surface, as well as the more complex Benninghoff arcade orientation of collagen fibers emanating from the subchondral bone.55 Additionally, depth-dependent differences in cell densities can be replicated via gradient bioprinting, in which a cell-free and a cell-laden bio-ink are mixed at the nozzle of the bioprinter to accurately change the final cell density during printing.56 The zonal cellular phenotypes of embedded/seeded cells can be stimulated by the incorporation of biological cues and matrix components.57,58 For example, it was demonstrated that the incorporation of chondroitin sulfate and matrix metalloproteinase–sensitive peptides in a PEG-based hydrogel stimulates MSCs to produce superficial zone-specific matrix components, while incorporating chondroitin sulfate alone or hyaluronic acid stimulates intermediate and deep zone matrix production, respectively.59 Another option for the fabrication of zonally organized constructs is to incorporate zonally harvested chondrocytes, which have been shown to maintain their zone-specific biosynthetic activities during culture in PEG-based hydrogels.60
Mechanical Properties
Finally, 3D bioprinting can generate constructs composed of both hydrogels and thermoplastic polymers with mechanical properties that suit the challenging mechanical environment of the joint. It has been demonstrated that these hybrid constructs exhibit mechanical characteristics similar to the thermoplastic polymer frame without the hydrogel.47,61 Therefore, by changing the architecture of the thermoplastic polymer frame, the mechanical properties of the construct can be tailored.62,63 To prevent disruption of the interface between the two materials during mechanical loading, covalent binding of the thermoplastic polymer and the hydrogel was shown feasible and effective.64 Additionally, the hybrid printing and covalent binding of both materials did not affect the viability of cells incorporated in the hydrogel.47,61
An alternative approach to reinforce hydrogel constructs is using melt electrospun meshes. Melt electrospinning writing allows for the controlled deposition of thermoplastic polymer filaments with a thickness in the order of 1 to 20 µm, while bioprinting generates filaments with a thickness in the order of 100 µm.65 By incorporating electrospun meshes in a cast hydrogel construct, the mechanical behavior of the hybrid construct can approach the bulk mechanical properties of native articular cartilage.66 Therefore, if accurate melt electrospinning writing could be combined with 3D bioprinting technologies, organized constructs with heterogeneous mechanical characteristics similar to native articular cartilage could be fabricated. The first setups to combine melt electrospinning with bioprinting are already being developed. For example, the feasibility of generating constructs with alternating layers of inkjet-printed hydrogels and random electrospun thermoplastic polymer meshes has already been demonstrated, emphasizing the prospect of the fabrication of organized, custom-made constructs with native mechanical characteristics.67
Automation of Implant Production Process
Bioprinting can also contribute to the automation of the implant production process. Besides printing into a wells-plate, constructs could also be printed directly into a bioreactor, minimizing handling and, thus, infection risks. Also, successful co-printing of a bioreactor simultaneously with a construct has been demonstrated.68 Constructs fabricated with these approaches require a 2-step surgical procedure for clinical implantation. During a first surgery autologous cells are harvested, which then need to be bioprinted and precultured in a laboratory. Later, during a second surgery, the bioprinted construct can be implanted into the patient’s cartilage defect. As bioprinting is a relatively fast process (minutes), a 1-step surgical approach might also be feasible, in which the cell-laden construct is fabricated in the operation theater and directly implanted. Potentially, cartilage defects could also be filled in situ, by printing the implant directly into the lesion. This approach has been exemplified by the direct ex vivo printing into osteochondral plugs or femurs.69,70 In line with this, steps exploring the feasibility of in situ bioprinting for other tissues have been taken, for example, for calvarial defects in living mice.16 Additionally, a bio-pen is being developed to simplify the in situ print procedure.71 Although 1-step surgical procedures and printing directly into a defect are exciting concepts and foster the idea of further automation in surgery, it would add the challenge to initiate the neocartilage formation within the harsh environment of a diseased joint.
Current Challenges in Bringing 3D Bioprinting to Clinical Applications
Bioprinting requires the combination of multiple elements in a bio-ink, for example, printability, shape stability after printing, cell therapies, biological cues, and mechanical strength. Taking all these aspects into account often results in a tradeoff, in which the separate elements are suboptimal in the bio-ink or final construct.20 Future research should, for example, focus on new methods to improve the printability of hydrogel-based bio-inks without negatively influencing the cell behavior.17,72 Additional research should focus on new methods for the formulation and processing of bio-inks prior to printing,73 in order to generate a larger range of biomaterials that can successfully be employed in bioprinting technologies. Besides optimizing the printing procedures and bio-inks, there is still a need for deeper understanding of cartilage regeneration in general, in order to determine, for example, what cell types, biological cues, and organization are required in the final construct for successful cartilage regeneration.
For the clinical translation of bioprinted living cartilage implants, the bio-ink has to meet the same regulations and safety requirements, concerning, for example, sterility, (endo)toxin content, reproducibility, and, if cell-laden, Advanced Therapy Medicinal Product regulations as biomaterials used for other implantable devices.74 To ensure sterility after 3D printing, the printing process needs to be incorporated in a Good Manufacturing Practice facility, and the printer itself and all its components should be sterile and able to operate in a sterile environment. Furthermore, the whole fabrication process should preferably involve minimal manual handling and postprocessing steps in closed systems. Bioprinters that can fulfill these requirements are already commercially available. However, most commercial printers nowadays work on open-loop and have no feedback on the quality of the print, thus requiring monitoring from skilled operators. Implementation of automated, reliable quality control during the printing process will facilitate adaptation of printers into clinics. Ideally, these systems also would have an integrated bioreactor system to allow for in vitro culture prior to implantation without extra handling of the construct. During this culture the constructs can relatively easily be stimulated with, for example, growth factors or mechanical loading, to stimulate the encapsulated cells to differentiate into the desired lineage. Additionally, cartilage-like tissue formation is stimulated in this period to provide an initial matrix, which will increase the construct stiffness and might improve integration in the defect. A bioprinter-bioreactor setup, or closed biofabrication line, is not commercially available yet; however, the first steps toward these kind of setups are currently being taken.75
To ensure safety of 3D bioprinted living medical implants for clinical use and to help organizations qualify and validate the printing process and bio-inks, standards are required. Standards are already available for additive manufacturing in general, under American Society for Testing and Materials (ASTM) F2792. Additionally, standards for tissue-engineered constructs have been published by the ASTM international committee F04, the International Organization for Standardization technical committee 150/SCZ, and the British Standards Institute. However, currently no such standard is available for bioprinting technologies.76,77 There are a number of attempts to develop validation protocols for relatively simple bioprint-related analyses, such as the quantification of the shape fidelity of a 3D printed construct. Such analysis are based on the length, width, and height of a printed filament compared to the nozzle diameter,78,79 the shape of the pores in a 3D construct compared to the theoretical shape,80 or on the deviation between the printed construct and the 3D computer model.39,81 Notably, multiple studies have demonstrated that optical scanning for validation can be incorporated in 3D bioprinters.69,82 This offers the prospect of real-time assessment of print fidelity and immediate quality control/quality analysis that may be useful for regulatory compliance. Ultimate clinical translation will also have to include strategies and regulations for cell sourcing, whether autologous or allogeneic, cell incorporation in the bio-ink or seeding on the printed construct, and implantation techniques to implant and fix the printed construct into the defect side. One universal standard to assess the quality of a 3D printed living construct in terms of sterility, shape-fidelity, biological properties, cell incorporation, surgical implantation techniques, and safety, is still lacking and needs to be set up in order to smoothen the transition from bench to bedside.
Conclusion
The 3D printing techniques that are currently used in the orthopedic clinic are just a glimpse of how this technology might contribute to future patient treatments, especially for articular cartilage regenerative therapies. Bioprinting techniques allow for the fabrication of personalized constructs with accurately positioned cells and biological cues to mimic the osteochondral interface and/or the zonal organization of articular cartilage. Mechanical properties can be tailored by hybrid printing or reinforcement strategies to match those of the cartilage area that needs replacement. Finally, bioprinting fosters the prospect of automation of the implant production process.
Although bioprinting provides many opportunities to generate cartilage regenerative constructs, which closely resemble the native tissue, there are still multiple challenges that need to be overcome. The main challenge is to make the transition in the clinic from nonliving personalized 3D printed implants toward biologically active and living implants. In order to accomplish this, some additional challenges need to be overcome first. Bio-inks have to be further optimized and the required construct architecture and mechanical properties need to be established. Additionally, the printing process, as well as the bio-inks, need to meet the specific sterility requirements to allow for implantation. Finally, universal quality standards are necessary to smoothen the clinical translation of new bio-inks and printing technologies. Tackling these challenges will foster the shift of biologically active and living bioprinted implants from the laboratory toward the daily clinical practice.
Footnotes
Acknowledgment and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: V. H. M. Mouser and J. Malda received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under Grant Agreement No. 309962 (HydroZONES). R. Levato and J. Malda received funding from the Dutch Arthritis Foundation, and J. Malda received funding from the European Research Council under Grant Agreement No. 647426 (3D-JOINT). L. J. Bonassar received funding from Histogenics, Inc., 3D BioCorp, Inc., General Electric, Inc., New York State Advanced Research Fund, and NIH F31AR064695-01. D. D. D’Lima received funding from the National Institutes of Health (P01 AG007996), the California Institute of Regenerative Medicine (PC1-08128), and the Shaffer Family Foundation. D. Grande received funding from the Lora and Craig Treiber Family Foundation, American Foundation for Surgery of the Hand, and the Department of Orthopedic Surgery Northwell Health System. T. Klein received funding from the Australian Research Council (FT110100166) and the National Health and Medical Research Council (1067108). M. Zenobi-Wong received funding from the Swiss National Science Foundation (CR32I3_146338).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Lawrence J. Bonassar is a cofounder, equity holder, and consultant for 3D BioCorp, Inc., and a consultant for Histogenics, Inc.
References
- 1. Groll J, Boland T, Blunk T, Burdick JA, Cho DW, Dalton PD, et al. Biofabrication: reappraising the definition of an evolving field. Biofabrication. 2016;8(1):013001. doi: 10.1088/1758-5090/8/1/013001. [DOI] [PubMed] [Google Scholar]
- 2. Almarza AJ, Athanasiou KA. Design characteristics for the tissue engineering of cartilaginous tissues. Ann Biomed Eng. 2004;32(1):2-17. [DOI] [PubMed] [Google Scholar]
- 3. Prakash D, Learmonth D. Natural progression of osteo-chondral defect in the femoral condyle. Knee. 2002;9(1):7-10. doi: 10.1016/S0968-0160(01)00133-8. [DOI] [PubMed] [Google Scholar]
- 4. Buschmann MD, Saris DBF. Introduction to the International Cartilage Repair Society Recommendation Papers. Cartilage. 2011;2(2):99. doi: 10.1177/1947603511402625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ventola CL. Medical applications for 3D printing: current and projected uses. P T. 2014;39(10):704-11. doi: 10.1016/j.infsof.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spottiswoode BS, van den Heever DJ, Chang Y, Engelhardt S, Du Plessis S, Nicolls F, et al. Preoperative three-dimensional model creation of magnetic resonance brain images as a tool to assist neurosurgical planning. Stereotact Funct Neurosurg. 2013;91(3):162-9. doi: 10.1159/000345264. [DOI] [PubMed] [Google Scholar]
- 7. Windisch G, Salaberger D, Rosmarin W, Kastner J, Exner GU, Haldi-Brändle V, et al. A model for clubfoot based on micro-CT data. J Anat. 2007;210(6):761-6. doi: 10.1111/j.1469-7580.2007.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen J, Reyes SA. Creation of a 3D printed temporal bone model from clinical CT data. Am J Otolaryngol. 2015;36(5):619-24. doi: 10.1016/j.amjoto.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 9. Noble JW, Moore CA, Liu N. The value of patient-matched instrumentation in total knee arthroplasty. J Arthroplasty. 2012;27(1):153-5. doi: 10.1016/j.arth.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 10. Lu S, Xu YQ, Zhang YZ, Li YB, Xie L, Shi JH, et al. A novel computer-assisted drill guide template for thoracic pedicle screw placement: a cadaveric and clinical study. Int J Med Robot. 2009;5(2):184-91. doi: 10.1007/s00402-011-1383-5. [DOI] [PubMed] [Google Scholar]
- 11. Probst FA, Hutmacher DW, Müller DF, Machens HG, Schantz JT. Rekonstruktion der Kalvaria durch ein präfabriziertes bioaktives Implantat. Handchirurgie Mikrochirurgie Plast Chir. 2010;42(6):369-73. doi: 10.1055/s-0030-1248310. [DOI] [PubMed] [Google Scholar]
- 12. Visser J, Peters B, Burger TJ, Boomstra J, Dhert WJ, Melchels FP, et al. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication. 2013;5(3):035007. doi: 10.1088/1758-5082/5/3/035007. [DOI] [PubMed] [Google Scholar]
- 13. Skardal A, Zhang J, Prestwich GD. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31(24):6173-81. doi: 10.1016/j.biomaterials.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 14. Lee W, Lee V, Polio S, Keegan P, Lee JH, Fischer K, et al. On-demand three-dimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnol Bioeng. 2010;105(6):1178-86. doi: 10.1002/bit.22613. [DOI] [PubMed] [Google Scholar]
- 15. Koch L, Gruene M, Unger C, Chichkov B. Laser assisted cell printing. Curr Pharm Biotechnol. 2013;14(1):91-7. doi: 10.2174/138920113804805368. [DOI] [PubMed] [Google Scholar]
- 16. Keriquel V, Guillemot F, Arnault I, Guillotin B, Miraux S, Amédée J, et al. In vivo bioprinting for computer- and robotic-assisted medical intervention: preliminary study in mice. Biofabrication. 2010;2(1):014101. doi: 10.1088/1758-5082/2/1/014101. [DOI] [PubMed] [Google Scholar]
- 17. Schuurman W, Levett PA, Pot MW, van Weeren PR, Dhert WJ, Hutmacher DW, et al. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol Biosci. 2013;13(5):551-61. doi: 10.1002/mabi.201200471. [DOI] [PubMed] [Google Scholar]
- 18. Cohen DL, Malone E, Lipson H, Bonassar LJ. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006;12(5):1325-35. doi: 10.1089/ten.2006.12.1325. [DOI] [PubMed] [Google Scholar]
- 19. Cui X, Breitenkamp K, Lotz M, D’Lima D. Synergistic action of fibroblast growth factor-2 and transforming growth factor-beta1 enhances bioprinted human neocartilage formation. Biotechnol Bioeng. 2012;109(9):2357-68. doi: 10.1002/bit.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJ, et al. 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater. 2013;25(36):5011-28. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 21. Bekkers JE, Tsuchida AI, van Rijen MH, Vonk LA, Dhert WJ, Creemers LB, et al. Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells: comparison with microfracture. Am J Sports Med. 2013;41(9):2158-66. doi: 10.1177/0363546513494181. [DOI] [PubMed] [Google Scholar]
- 22. Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage: I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci. 1988;90:635-43. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Z. Chondrons and the pericellular matrix of chondrocytes. Tissue Eng Part B Rev. 2015;21(3):267-77. doi: 10.1089/ten.teb.2014.0286. [DOI] [PubMed] [Google Scholar]
- 24. Ma B, Leijten JC, Wu L, Kip M, van Blitterswijk CA, Post JN, et al. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthritis Cartilage. 2013;21(4):599-603. doi: 10.1016/j.joca.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 25. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 26. Visser J, Gawlitta D, Benders KE, Toma SM, Pouran B, van Weeren PR, et al. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials. 2015;37:174-82. doi: 10.1016/j.biomaterials.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 27. Acharya C, Adesida A, Zajac P, Mumme M, Riesle J, Martin I, et al. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Physiol. 2012;227(1):88-97. doi: 10.1002/jcp.22706. [DOI] [PubMed] [Google Scholar]
- 28. Mo XT, Guo SC, Xie HQ, Deng L, Zhi W, Xiang Z, et al. Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone. 2009;45(1):42-51. doi: 10.1016/j.bone.2008.07.240. [DOI] [PubMed] [Google Scholar]
- 29. de Windt TS, Saris DB, Slaper-Cortenbach IC, van Rijen MH, Gawlitta D, Creemers LB, et al. Direct cell-cell contact with chondrocytes is a key mechanism in multipotent mesenchymal. Tissue Eng Part A. 2015;21(19-20):2536-47. doi: 10.1089/ten.tea.2014.0673. [DOI] [PubMed] [Google Scholar]
- 30. Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015;11(4):206-12. doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Medvedev SP, Grigor’eva EV, Shevchenko AI, Malakhova AA, Dementyeva EV, Shilov AA, et al. Human induced pluripotent stem cells derived from fetal neural stem cells successfully undergo directed differentiation into cartilage. Stem Cells Dev. 2011;20(6):1099-112. doi: 10.1089/scd.2010.0249. [DOI] [PubMed] [Google Scholar]
- 32. Wei Y, Zeng W, Wan R, Wang J, Zhou Q, Qiu S, et al. Chondrogenic differentiation of induced pluripotent stem cells from osteoarthritic chondrocytes in alginate matrix. Eur Cell Mater. 2012;23:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vonk LA, de Windt TS, Slaper-Cortenbach ICM, Saris DBF. Autologous, allogeneic, induced pluripotent stem cell or a combination stem cell therapy? Where are we headed in cartilage repair and why: a concise review. Stem Cell Res Ther. 2015;6:94. doi: 10.1186/s13287-015-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Panadero JA, Lanceros-Mendez S, Ribelles JLG. Differentiation of mesenchymal stem cells for cartilage tissue engineering: Individual and synergetic effects of three-dimensional environment and mechanical loading. Acta Biomater. 2016;33:1-12. doi: 10.1016/j.actbio.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 35. Mardones R, Jofré CM, Minguell JJ. Cell therapy and tissue engineering approaches for cartilage repair and/or regeneration. Int J Stem Cells. 2015;8(1):48-53. doi: 10.15283/ijsc.2015.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vermonden T, Censi R, Hennink WE. Hydrogels for protein delivery. Chem Rev. 2012;112(5):2853-88. doi: 10.1021/cr200157d. [DOI] [PubMed] [Google Scholar]
- 37. Poldervaart MT, Wang H, van der Stok J, Weinans H, Leeuwenburgh SC, Öner FC, et al. Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats. PLoS One. 2013;8(8):e72610. doi: 10.1371/journal.pone.0072610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sawkins MJ, Mistry P, Brown BN, Shakesheff KM, Bonassar LJ, Yang J. Cell and protein compatible 3D bioprinting of mechanically strong constructs for bone repair. Biofabrication. 2015;7(3):035004. doi: 10.1088/1758-5090/7/3/035004. [DOI] [PubMed] [Google Scholar]
- 39. Kesti M, Eberhardt C, Pagliccia G, Kenkel D, Grande D, Boss A, et al. Bioprinting complex cartilaginous structures with clinically compliant biomaterials. Adv Funct Mater. 2015;25(48):7406-17. doi: 10.1002/adfm.201503423. [DOI] [Google Scholar]
- 40. Ishida O, Tanaka Y, Morimoto I, Takigawa M, Eto S. Chondrocytes are regulated by cellular adhesion through CD44 and hyaluronic acid pathway. J Bone Miner Res. 1997;12(10):1657-63. doi: 10.1359/jbmr.1997.12.10.1657. [DOI] [PubMed] [Google Scholar]
- 41. Levett PA, Melchels FPW, Schrobback K, Hutmacher DW, Malda J, Klein TJ. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014;10(1):214-23. doi: 10.1016/j.actbio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 42. Levett PA, Hutmacher DW, Malda J, Klein TJ. Hyaluronic acid enhances the mechanical properties of tissue-engineered cartilage constructs. PLoS One. 2014;9(12):e113216. doi: 10.1371/journal.pone.0113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Costa PF, Puga AM, Díaz-Gomez L, Concheiro A, Busch DH, Alvarez-Lorenzo C. Additive manufacturing of scaffolds with dexamethasone controlled release for enhanced bone regeneration. Int J Pharm. 2015;496(2):541-50. doi: 10.1016/j.ijpharm.2015.10.055. [DOI] [PubMed] [Google Scholar]
- 44. Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376(9739):440-8. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malda J, Boere J, van de Lest CHA, van Weeren RP, Wauben MHM. Extracellular vesicles—new tool for joint repair and regeneration. Nat Rev Rheumatol. 2016;12(4):243-9. doi: 10.1038/nrrheum.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11(9):768-74. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee JS, Hong JM, Jung JW, Shim JH, Oh JH, Cho DW. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6(2):024103. doi: 10.1088/1758-5082/6/2/024103. [DOI] [PubMed] [Google Scholar]
- 48. Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, van Weeren PR, Malda J, et al. Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C Methods. 2012;18(1):33-44. doi: 10.1089/ten.tec.2011.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Levato R, Visser J, Planell JA, Engel E, Malda J, Mateos-Timoneda MA. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication. 2014;6(3):035020. doi: 10.1088/1758-5082/6/3/035020. [DOI] [PubMed] [Google Scholar]
- 50. Buckley MR, Gleghorn JP, Bonassar LJ, Cohen I. Mapping the depth dependence of shear properties in articular cartilage. J Biomech. 2008;41(11):2430-7. doi: 10.1016/j.jbiomech.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 51. Silverberg JL, Barrett AR, Das M, Petersen PB, Bonassar LJ, Cohen I. Structure-function relations and rigidity percolation in the shear properties of articular cartilage. Biophys J. 2014;107(7):1721-30. doi: 10.1016/j.bpj.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Griffin DJ, Bonnevie ED, Lachowsky DJ, Hart JC, Sparks HD, Moran N, et al. Mechanical characterization of matrix-induced autologous chondrocyte implantation (MACI®) grafts in an equine model at 53 weeks. J Biomech. 2015;48(10):1944-9. doi: 10.1016/j.jbiomech.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 53. Klein TJ, Malda J, Sah RL, Hutmacher DW. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev. 2009;15(2):143-57. doi: 10.1089/ten.teb.2008.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hollander AP, Dickinson SC, Kafienah W. Stem cells and cartilage development: complexities of a simple tissue. Stem Cells. 2010;28(11):1992-6. doi: 10.1002/stem.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Benninghoff A. Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion. Z Anat Entwicklungsgesch. 1925;76(1-3):43-63. doi: 10.1007/BF02134417. [DOI] [Google Scholar]
- 56. Carlier A, Skvortsov GA, Hafezi F, Ferraris E, Patterson J, Koç B, et al. Computational model-informed design and bioprinting of cell-patterned constructs for bone tissue engineering. Biofabrication. 2016;8(2):025009. doi: 10.1088/1758-5090/8/2/025009. [DOI] [PubMed] [Google Scholar]
- 57. Klein TJ, Rizzi SC, Reichert JC, Georgi N, Malda J, Schuurman W, et al. Strategies for zonal cartilage repair using hydrogels. Macromol Biosci. 2009;9(11):1049-58. doi: 10.1002/mabi.200900176. [DOI] [PubMed] [Google Scholar]
- 58. Tatman PD, Gerull W, Sweeney-Easter S, Davis JI, Gee AO, Kim DH. Multiscale biofabrication of articular cartilage: bioinspired and biomimetic approaches. Tissue Eng Part B Rev. 2015;21(6):543-59. doi: 10.1089/ten.teb.2015.0142. [DOI] [PubMed] [Google Scholar]
- 59. Nguyen LH, Kudva AK, Saxena NS, Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32(29):6946-52. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 60. Kim TK, Sharma B, Williams CG, Ruffner MA, Malik A, McFarland EG, et al. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage. 2003;11(9):653-64. doi: 10.1016/S1063-4584(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 61. Schuurman W, Khristov V, Pot MW, van Weeren PR, Dhert WJA, Malda J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication. 2011;3(2):021001. doi: 10.1088/1758-5082/3/2/021001. [DOI] [PubMed] [Google Scholar]
- 62. Moroni L, de Wijn JR, van Blitterswijk CA. Three-dimensional fiber-deposited PEOT/PBT copolymer scaffolds for tissue engineering: influence of porosity, molecular network mesh size, and swelling in aqueous media on dynamic mechanical properties. J Biomed Mater Res Part A. 2005;75A(4):957-65. doi: 10.1002/jbm.a.30499. [DOI] [PubMed] [Google Scholar]
- 63. Moroni L, de Wijn JR, van Blitterswijk CA. 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials. 2006;27(7):974-85. doi: 10.1016/j.biomaterials.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 64. Boere KW, Visser J, Seyednejad H, Rahimian S, Gawlitta D, van Steenbergen MJ, et al. Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater. 2014;10(6):2602-11. doi: 10.1016/j.actbio.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 65. Hochleitner G, Jüngst T, Brown TD, Hahn K, Moseke C, Jakob F, et al. Additive manufacturing of scaffolds with sub-micron filaments via melt electrospinning writing. Biofabrication. 2015;7(3):035002. doi: 10.1088/1758-5090/7/3/035002. [DOI] [PubMed] [Google Scholar]
- 66. Visser J, Melchels FP, Jeon JE, van Bussel EM, Kimpton LS, Byrne HM, et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat Commun. 2015;6:6933. doi: 10.1038/ncomms7933. [DOI] [PubMed] [Google Scholar]
- 67. Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5(1):015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 68. Costa PF, Vaquette C, Baldwin J, Chhaya M, Gomes ME, Reis RL, et al. Biofabrication of customized bone grafts by combination of additive manufacturing and bioreactor knowhow. Biofabrication. 2014;6(3):035006. doi: 10.1088/1758-5082/6/3/035006. [DOI] [PubMed] [Google Scholar]
- 69. Cohen DL, Lipton JI, Bonassar LJ, Lipson H. Additive manufacturing for in situ repair of osteochondral defects. Biofabrication. 2010;2(3):035004. doi: 10.1088/1758-5082/2/3/035004. [DOI] [PubMed] [Google Scholar]
- 70. Cui X, Breitenkamp K, Finn MGG, Lotz M, D’Lima DD. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18(11-12):1304-12. doi: 10.1089/ten.tea.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. O’Connell CD, Di Bella C, Thompson F, Augustine C, Beirne S, Cornock R, et al. Development of the Biopen: a handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication. 2016;8(1):015019. doi: 10.1088/1758-5090/8/1/015019. [DOI] [PubMed] [Google Scholar]
- 72. Melchels FPW, Dhert WJA, Hutmacher DW, Malda J. Development and characterisation of a new bioink for additive tissue manufacturing. J Mater Chem B. 2014;2(16):2282-9. doi: 10.1039/c3tb21280g. [DOI] [PubMed] [Google Scholar]
- 73. Cohen DL, Lo W, Tsavaris A, Peng D, Lipson H, Bonassar LJ. Increased mixing improves hydrogel homogeneity and quality of three-dimensional printed constructs. Tissue Eng Part C Methods. 2011;17(2):239-48. doi: 10.1089/ten.tec.2010.0093. [DOI] [PubMed] [Google Scholar]
- 74. Halme DG, Kessler DA. FDA regulation of stem-cell–based therapies. N Engl J Med. 2006;355(16):1730-5. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- 75. Mironov V, Kasyanov V, Markwald RR. Organ printing: from bioprinter to organ biofabrication line. Curr Opin Biotechnol. 2011;22(5):667-73. doi: 10.1016/j.copbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 76. Hutmacher DW. Bioprinting, biofabrication, biomanufacturing? The need for definitions and norms in additive manufacturing in the biomedical sciences. MRS Bull. 2015;40(6):113. [Google Scholar]
- 77. Chhaya MP, Poh PSP, Balmayor ER, van Griensven M, Schantz JT, Hutmacher DW. Additive manufacturing in biomedical sciences and the need for definitions and norms. Expert Rev Med Devices. 2015;12(5):537-43. doi: 10.1586/17434440.2015.1059274. [DOI] [PubMed] [Google Scholar]
- 78. Kang KH, Hockaday LA, Butcher JT. Quantitative optimization of solid freeform deposition of aqueous hydrogels. Biofabrication. 2013;5(3):035001. doi: 10.1088/1758-5082/5/3/035001. [DOI] [PubMed] [Google Scholar]
- 79. Wüst S, Godla ME, Müller R, Hofmann S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014;10(2):630-40. doi: 10.1016/j.actbio.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 80. Billiet T, Gevaert E, De Schryver T, Cornelissen M, Dubruel P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35(1):49-62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 81. Murphy SV, Skardal A, Atala A. Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A. 2013;101A(1):272-84. doi: 10.1002/jbm.a.34326. [DOI] [PubMed] [Google Scholar]
- 82. Ballyns JJ, Cohen DL, Malone E, Maher SA, Potter HG, Wright T, et al. An optical method for evaluation of geometric fidelity for anatomically shaped tissue-engineered constructs. Tissue Eng Part C Methods. 2010;16(4):693-703. doi: 10.1089/ten.tec.2009.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kundu J, Shim JH, Jang J, Kim SW, Cho DW. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med. 2015;9(11):1286-97. doi: 10.1002/term.1682. [DOI] [PubMed] [Google Scholar]
- 84. Kesti M, Müller M, Becher J, Schnabelrauch M, D’Este M, Eglin D, et al. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015;11(1):162-72. doi: 10.1016/j.actbio.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 85. Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16(5):1489-96. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 86. Izadifar Z, Chang T, Kulyk W, Chen X, Eames BF. Analyzing biological performance of 3D-printed, cell-impregnated hybrid constructs for cartilage tissue engineering. Tissue Eng Part C Methods. 2016;22(3):173-88. doi: 10.1089/ten.tec.2015.0307. [DOI] [PubMed] [Google Scholar]
- 87. Abbadessa A, Mouser VH, Blokzijl MM, Gawlitta D, Dhert WJ, Hennink WE, et al. A synthetic thermosensitive hydrogel for cartilage bioprinting and its biofunctionalization with polysaccharides. Biomacromolecules. 2016;17(6):2137-47. doi: 10.1021/acs.biomac.6b00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mouser VH, Melchels FP, Visser J, Dhert WJ, Gawlitta D, Malda J. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication. 2016;8(3):035003.doi: 10.1088/1758-5090/8/3/035003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Woodfield TBF, Malda J, de Wijn J, Péters F, Riesle J, van Blitterswijk CA. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials. 2004;25(18):4149-61. doi: 10.1016/j.biomaterials.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 90. Woodfield TBF, Van Blitterswijk CA, De Wijn J, Sims TJ, Hollander AP, Riesle J. Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-engineered cartilage constructs. Tissue Eng. 2005;11(9-10):1297-311. doi: 10.1089/ten.2005.11.1297. [DOI] [PubMed] [Google Scholar]
- 91. Schuurman W, Harimulyo EB, Gawlitta D, Woodfield TB, Dhert WJ, van Weeren PR, et al. Three-dimensional assembly of tissue-engineered cartilage constructs results in cartilaginous tissue formation without retainment of zonal characteristics. J Tissue Eng Regen Med. 2016;10(4):315-24. doi: 10.1002/term.1726. [DOI] [PubMed] [Google Scholar]
- 92. Shim JH, Lee JS, Kim JY, Cho DW. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J Micromech Microeng. 2012;22(8):085014. doi: 10.1088/0960-1317/22/8/085014. [DOI] [Google Scholar]