Abstract
Purpose:
Autologous chondrocyte implantation (ACI) is a treatment option even in early osteoarthritis (OA). Surgical preparation for ACI should avoid penetration of the subchondral bone plate to prevent hemorrhage, fibrin clot formation, and subsequent activation of the inflammatory response.
Hypothesis:
Current surgical procedures with ring curettes preserve the integrity of the subchondral bone plate, even in patients with OA.
Methods:
Subchondral femoral bone plates (n = 40) of OA knees undergoing total knee arthroplasty were prepared in vivo using standard, non–brute-force debridement for ACI. To approach regular wear/early OA, only cartilage with maximally grade 3A International Cartilage Repair Society score was prepared. Effects were analyzed by light microscopy.
Results:
In 87.5% of the specimens (35/40), standard debridement did not violate the tide mark, except for occasional minor openings with a smooth edge (diameter approximately 20 µm). In contrast, 5/40 samples (12.5%) showed one large area with a missing bone plate and an open bone marrow space. Twenty-eight specimens (70%) showed at least remnants of uncalcified cartilage.
Conclusion:
On the basis of size/fine structure, the occasional minor openings are likely due to increased vascular penetration through the tide mark in the pathologically altered bone-cartilage interface in OA. The consequences of limited hemorrhage through minor openings or selected large defects following in vivo debridement are still unknown. Thus, standard debridement appears suitable for cartilage regeneration even in OA defects.
Keywords: surgical preparation for ACI, subchondral bone plate, osteoarthritic knee, articular cartilage regeneration, autologous chondrocyte implantation
Introduction
Autologous chondrocyte implantation (ACI) is a common procedure for articular cartilage regeneration. It has emerged as an effective and durable solution for the treatment of large, full-thickness cartilage and osteochondral lesions of the knee joint, suggesting that the clinical and functional outcome remains high until 10 or 20 years after implantation.1 Tallheden et al. already addressed the demand for the resurfacing of large areas in patients with osteoarthritis (OA) and described autologous chondrocytes from OA patients as a potential source for their biological treatment.2 The classic indication for the ACI is a traumatic cartilage lesion, but this method is also increasingly used for regenerative cartilage procedures of OA lesions or combined cartilage degeneration/trauma, for example, in high-performance athletes. OA is the most common musculoskeletal disease in the elderly, and it could become the fourth leading cause of disability by the year 2020.3 This is aggravated by the fact that even asymptomatic cartilage defects (only identified by magnetic resonance imaging) may accelerate the future loss of knee cartilage volume in affected individuals,4 a finding confirmed by another study demonstrating disease progression in 81% of chondral defects over only 2 years.5 For these reasons, ACI has recently been considered as a treatment option even in patients with early OA.6
If in the case of microfracturing or osteochondral grafting the subchondral bone plate is penetrated, a still unknown composition of cells and mediators with incompletely characterized effects on cartilage healing is released from the bone marrow. The repair outcome after ACI is clearly influenced by the preparation of the defects.7 Surgical preparation for traditional ACI and other resurfacing methods includes a debridement of the subchondral bone plate with a ring curette, detailed by Peterson as “carefully down to the subchondral bone without causing any bleeding.”8
A gold standard for the assessment of articular cartilage quality after regeneration attempts still remains to be established. The goal of our study was thus to microscopically determine whether surgical debridement of the subchondral bone plate for ACI and other resurfacing methods in the OA knee is possible without penetrating the bone marrow space. This may be crucial for the resulting properties of the healing cartilage (including the attachment of cells or matrix) and for the development of new techniques.
Materials and Methods
In Vivo Samples
Informed consent was obtained from 40 OA patients undergoing total knee arthroplasty (TKA; mean age 70 years; age range 50-82 years; 24 females, 16 males). The study was approved by the ethics committee of the Jena University Hospital (Registration Number: 1789-05/06). Patients with other known diseases affecting the skeleton, including osteoporosis or rheumatoid arthritis, were excluded. To avoid additional operating time, the preparations were performed on the patients’ intact condyles while a second surgeon proceeded with the regular TKA. The condyles were then cut and the fragments sent for processing.
During surgery, defects with a size of approximately 2 × 1 cm were created on one femoral condyle using a surgical ring curette also applied for ACI (inner ring diameter 5 mm; 15° offset; Richard Wolf, Knittlingen, Germany). In order to simulate the force utilized during standard ACI debridement, standardized forces were applied for curetting as previously described. To quantify the forces applied for standard force, a machine to measure the pressure-strength was used (FPG 7/20-5-010 Koegel, Leipzig, Germany). Force was applied by pressing the tip of the curette against the measuring block of the machine similarly to the procedure during debridement. The respective forces were measured 20 times on 3 consecutive days (at different times during the day to avoid a circadian bias) and the results expressed as means ± standard errors of the mean. A normal distribution (Gauss) was found. The force for traditional debridement was 15.2 ± 0.4 N (n = 60).7 For the purpose of comparability, all specimens were prepared by the same surgeon (JM).
The debridement was performed until no more articular cartilage was visible and until a hit of the curette on the bone plate created a sound like metal on stone (in analogy to the check performed during the surgical procedure). In order to obtain samples representative of regular wear/early OA conditions, only OA samples with maximally grade 3A (International Cartilage Repair Society score) were used. Subsequently, the condyle was resected and preserved for histologic examination.
Histology
Regular paraffin embedding was performed after decalcification of the tissue.9 This technique completely preserved all bony structures, including the subchondral bone plate and the tide mark, and was therefore considered as a valid comparison for the samples.7 At least 8 longitudinal cuts were prepared in the middle of the greatest convexity of each condyle. For light microscopy analysis, methylene blue and Masson’s trichrome-Goldner stains were used.
Results
Standard debridement did not violate the tide mark in 87.5% of the cases (35/40 specimens). In contrast, 5 samples (12.5%) showed one large area with a missing bone plate and an open bone marrow space ( Fig. 1 ). Except for the large openings, the samples displayed only occasional minor openings with a smooth edge and a diameter of approximately 20 µm ( Figs. 2 and 3 ). Twenty-eight specimens (70%) showed at least remnants of uncalcified cartilage.
Figure 1.
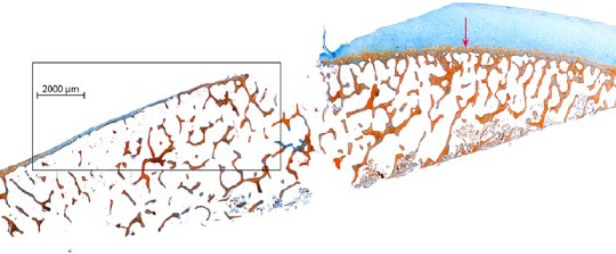
In vivo preparation (standard debridement) of chondral defects in osteoarthritic knees. Representative section of the debrided surface in 1 of the 5 samples (12.5%) displaying one large area with a missing bone plate and an open bone marrow space (Masson’s trichrome-Goldner stain). Red arrow: tide mark line.
Figure 2.

Partial magnification of the marked area in Figure 1 . Representative section of the debrided surface with a large area of nonviolated tide mark, only interrupted by occasional minor openings with a smooth edge of approximately 20 µm in diameter (black arrows) and one large area with a missing bone plate and an open bone marrow space (green arrow).
Figure 3.
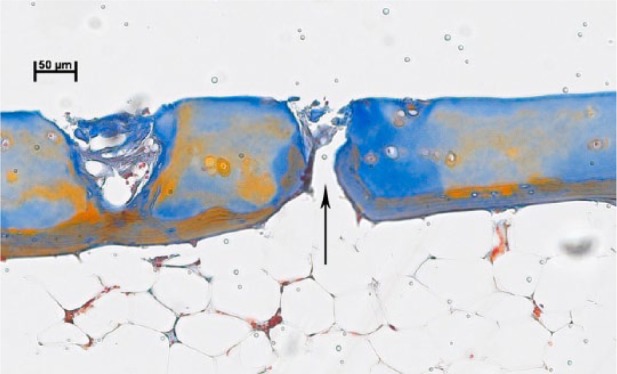
Further magnification of the area marked in Figure 2 (left black arrow) showing one of the occasional minor openings with a smooth edge and a diameter of approximately 20 µm (arrow).
Discussion
This study confirmed in a large number of samples from OA knee joints (n = 40) that, under standard debridement of the subchondral bone plate in vivo with a surgical ring curette, there is generally no violation of the tide mark line, and the bone marrow space remains separated from the joint space. However, in contrast to own previous results7 5/40 samples (12.5%) showed one large area with a missing bone plate and an open bone marrow space following standard debridement.
Since thus far all human OA in vivo specimens were prepared by the same investigator (JM) under standardized non–brute-force conditions,7 the differences are unlikely due to preparation artifacts. Thus, in the OA knee joint an opening of the bone marrow space following debridement cannot be completely excluded and in the clinical setting commonly requires an attempt to close the defect with different techniques, for example, fibrin glue, coagulation or others.
In both the present study and our above-mentioned recent study,7 in vivo OA samples (total of n = 45) revealed occasional, randomly distributed, minor openings of approximately 20 µm in diameter with a smooth edge (0-2 minor openings/section on average). We previously described them as “marginal violation with isolated bleeding points” or “limited alterations,” presumably representing the equivalent of the selected bleeding points observed in situ.7 On the basis of size and anatomical features, these openings likely represent increased vascular penetration through the tide mark in the pathologically altered bone-cartilage interface in OA,10 as externally confirmed by a pathologist (GRFK; see acknowledgement). However, it is currently unknown whether such minor openings have any influence on either the quality of the regenerated cartilage or the clinical outcome of ACI.
In the studies conducted so far, in vivo–prepared OA samples displayed a varying degree of remnants of uncalcified cartilage on the subchondral bone plate, especially at the edges of the debrided area7 (compare with Fig. 1 ). In the current study, this affected as much as 70% of the analyzed samples. Although such cartilage remnants may represent a problem for the insertion of cell-free or cell-seeded matrix implants, clinical application (may it be press-fit or sutured) is usually not hindered by these microscopically detected remnants.7
To the best of our knowledge, this is the first study examining the subchondral bone plate after standard-force in vivo debridement for ACI in OA in a large series. In vitro studies have also addressed the quality of cartilage lesion debridement using different open and arthroscopic techniques; however, exclusively postmortem material and only normal human osteochondral blocks were used.11 In agreement with the present study, Drobnic et al. also found that the predominant depth of the debridement in adult normal human samples was at the level of the calcified cartilage or the subchondral end plate (i.e., in the region of the tide mark), with only occasional minor access to the subchondral sinusoids or opening of deep bone.11 Although the latter in vitro results may not be completely comparable with the present in vivo studies, both studies underline that it is possible to preserve the integrity of the subchondral bone plate using standard-force debridement with ring curettes.
In conclusion, standard debridement of the subchondral bone plate in vivo in OA with a surgical ring curette under standardized non–brute-force conditions generally did not violate the subchondral bone plate, except for occasional minor openings. Therefore, the standard surgical preparation technique for ACI appears suitable for cartilage regeneration even in cases of OA defects.
However, in a minority of cases (12.5%) one large area with a missing bone plate and an open bone marrow space was exposed following standard debridement. In conjunction with the cartilage remnants frequently observed in these samples, the current debridement techniques can thus still be optimized in terms of (1) application of more standardized pressure7 or specially designed precision punches12 to more reliably preserve the integrity of the subchondral bone plate; and (2) development of more sophisticated techniques and tools for complete removal of the noncalcified cartilage.
Footnotes
Acknowledgments and Funding: We are very thankful to Mrs. C. Mueller for her outstanding performance regarding the histology, Mr. H. Hauk for his excellent work regarding the photographs, and to Prof. G. R. F. Krueger for his support.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of the Jena University Hospital (Registration Number: 1789-05/06).
Informed Consent: Written informed consent was obtained from all individual participants included in the study.
References
- 1. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-24. [DOI] [PubMed] [Google Scholar]
- 2. Tallheden T, Bengtsson C, Brantsing C, Sjoegren-Jansson E, Carlsson L, Peterson L, et al. Proliferation and differentiation of chondrocytes from osteoarthritic patients. Arthritis Res Ther. 2005;7(3):R560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei Y, Zeng W, Wan R, Wang J, Zhou Q, Qiu S, et al. Chondrogenic differentiation of induced pluripotent stem cells from osteoarthritic chondrocytes in alginate matrix. Eur Cell Mater. 2012;23:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cicuttini FM, Ding C, Wluka AE, Davis S, Ebeling PR, Jones G. Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum. 2005;52:2033-9. [DOI] [PubMed] [Google Scholar]
- 5. Davies-Tuck ML, Wluka AE, Wang Y, Teichtahl AJ, Jones G, Ding C, et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(3):337-42. [DOI] [PubMed] [Google Scholar]
- 6. Minas T, Gomoll AH, Solhpour S, Rosenberger R, Probst C, Bryant T. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin Orthop Relat Res. 2010;468(1):147-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mika J, Clanton TO, Pretzel D, Schneider G, Ambrose CG, Kinne RW. Surgical preparation for articular cartilage regeneration without penetration of the subchondral bone plate: in vitro and in vivo studies in humans and sheep. Am J Sports Med. 2011;39(3):624-31. [DOI] [PubMed] [Google Scholar]
- 8. Peterson L. ACI surgical technique and results at 2-10 years. In: Zanasi S, Brittberg M, Marcacci M, editors. Basic science, clinical repair and reconstruction of articular cartilage defects: current status and prospects. Bologna, Italy: Timeo Editore; 2006. p. 325-32. [Google Scholar]
- 9. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheilm E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004; 86-A(3):455-64. [DOI] [PubMed] [Google Scholar]
- 10. Bullough P. Noninflammatory arthritides. In: Bullough P, editor. Orthopaedic pathology. New York: Mosby; 2004. p. 11:263. [Google Scholar]
- 11. Drobnic M, Radosavljevic D, Cör A, Brittberg M, Strazar K. Debridement of cartilage lesions before autologous chondrocyte implantation by open or transarthroscopic techniques: a comparative study using post-mortem materials. J Bone Joint Surg Br. 2010;92(4):602-8. [DOI] [PubMed] [Google Scholar]
- 12. Jubel A, Andermahr J, Schiffer G, Fischer J, Rehm KE, Stoddart MJ, et al. Transplantation of de novo scaffold-free cartilage implants into sheep knee chondral defects. Am J Sports Med. 2008;36(8):1555-64. [DOI] [PubMed] [Google Scholar]


