Abstract
Objective
To evaluate a biphasic cartilage repair device (CRD) for feasibility of arthroscopic implantation, safety, biocompatibility, and efficacy for long-term repair of large osteochondral defects.
Methods
The CRD was press-fit into defects (10 mm diameter, 10 mm deep) created in the femoral trochlea of 12 horses. In the contralateral limb, 10 mm diameter full-thickness chondral defects were treated with microfracture (MFX). Radiographs were obtained pre- and postoperatively, and at 4, 12, and 24 months. Repeat arthroscopy was performed at 4 and 12 months. Gross assessment, histology, mechanical testing, and magnetic resonance imaging (MRI) were performed at 24 months.
Results
The CRD was easily placed arthroscopically. There was no evidence of joint infection, inflammation, or degeneration. CRD-treated defects had significantly more sclerosis compared to MFX early (P = 0.0006), but was not different at 24 months. CRD had better arthroscopic scores at 4 months compared to MFX (P = 0.0069). At 24 months, there was no difference in repair tissue on histology or mechanical testing. Based on MRI, CRD repair tissue had less proteoglycan (deep P = 0.027, superficial P = 0.015) and less organized collagen (deep P = 0.028) compared to MFX. Cartilage surrounding MFX defects had more fissures compared to CRD.
Conclusion
The repair tissue formed after CRD treatment of a large osteochondral lesion is fibrocartilage similar to that formed in simple chondral defects treated with MFX. The CRD can be easily placed arthroscopically, is safe, and biocompatible for 24 months. The CRD results in improved early arthroscopic repair scores and may limit fissure formation in adjacent cartilage.
Keywords: cartilage, osteochondral, biphasic graft, knee, equine
Introduction
Chondral and osteochondral defects are common lesions and pose a significant challenge due to the inherent poor repair capacity of hyaline cartilage. The majority of lesions, even if initially asymptomatic, will increase in size which hastens the loss of surrounding articular cartilage, with the risk of developing debilitating osteoarthritis.1,2 Numerous strategies have been developed in an attempt to improve healing of chondral defects; however, these methodologies cannot be directly translated and may perform poorly when applied to defects that breach the subchondral plate. The primary reason for this failure has been proposed to be a lack of subchondral bone support of repair tissue.3 Additional disadvantages of previously described procedures include the need for invasive and multiple surgeries, complexity of the surgical technique, lack of integration with host tissue, and donor site morbidity for cell-based graft strategies; lack of congruity of the repair with the joint surface, lack of integration with host tissue, donor site morbidity, risk of disease transmission when allografts are used, and lack of sufficient donor material in the case of osteochondral auto- or allografts.4-7 Therefore, development of a synthetic, off-the-shelf biphasic osteochondral device that mimics cartilage and bone, integrates well with host tissue, is biocompatible and bioresorbable, can be customized to the defect depth and diameter, and can be inserted in a minimally invasive fashion would provide a surgical solution for treatment of osteochondral defects or cartilage defects that extend into the subchondral plate.
The cartilage repair device (CRD) evaluated in this study was designed to address many of these concerns. Preclinical research and development produced a bioresorbable, acellular, biphasic scaffold for cartilage repair. Each phase was engineered to favor growth of the distinct tissues of articular cartilage and subchondral bone. The device has 100% interconnected porosity that allows movement of cells and biological fluids throughout the entire implant. The chondral phase on the surface of the scaffold is comprised of a unique Type I collagen formulation that provides a malleable scaffold that can be contoured to the surrounding joint surface. The deeper subchondral bone phase contains 80% β-tricalcium phosphate (TCP) and 20% polylactic acid (PLA) by mass. The ceramic provides an osteoconductive element, supplying a source of calcium and phosphorous ions necessary for natural bone mineralization.8 The PLA provides biomechanical support and 3-dimensional structure, and ultimately breaks down into natural body metabolites via the Krebs cycle.9,10 The ceramic granules are suspended within, not coated by, the polymer scaffold, allowing for the ceramic to be immediately available to the host on implantation.
Several studies have been performed to evaluate repair tissue of osteochondral defects with biphasic scaffold grafting. Studies have been performed in rabbit,5,11-13 mini pig,14,15 dog,16 sheep,7,17 and goat4,18 models. Due to the small size of these species, all procedures were performed by arthrotomy, and defects were relatively small ranging from 3.5 mm to 6 mm in diameter. Furthermore, duration of the investigations was relatively short ranging from 1 month to 7 months, which is far shorter than the reported time for clinical degeneration of repair cartilage following microfracture (MFX) at 24 months.19 The lack of correlation between outcomes in smaller experimental species and human patients is well known, and the horse is recognized as an appropriate model for evaluation of cartilage repair strategies prior to clinical trials.20,21 The large size of the equine joint allows creation of defects of a clinically relevant size and validation of the operative procedure.22 Furthermore, the thickness and morphology of cartilage in the equine patellofemoral joint most closely approximates that of the human knee.23 Because the horse will immediately bear weight on the construct, the equine model also presents a significant and immediate postoperative biomechanical challenge to the repair that meets, and likely exceeds, the forces that will be experienced in a human patient, thereby rigorously testing cartilage repair strategies for durability.22
The purpose of this study was to evaluate the feasibility of arthroscopic surgical delivery and the long-term safety, biocompatibility, and efficacy of the CRD hydrated with bone marrow aspirate for osteochondral repair in a single-site defect using an equine model compared to a clinical standard for cartilage repair, MFX of full-thickness chondral defects. Our hypotheses were that the CRD would be simple to implant arthroscopically, would be safe, would not elicit an immune reaction or be rejected, and would produce repair tissue in the chondral phase of an osteochondral defect that is superior to repair tissue in a full-thickness chondral defect treated with MFX.
Methods
Experimental Design and Study Population
This study was carried out according to Good Laboratory Practices (GLP) regulations and was approved by the Cornell University Institutional Animal Care and Use Committee. Twelve young adult horses aged 2 to 5 years were enrolled in the study. Prior to inclusion, horses were confirmed to be healthy and free of patellofemoral joint disease based on physical examination, lameness evaluation, and patellofemoral radiographs. The lateral trochlear ridge (LTR) of the distal femur of one limb of each horse received the osteochondral CRD treatment with the contralateral limb serving as a positive control consisting of a full-thickness chondral defect treated with MFX. Computer-generated randomization was used to assign CRD and MFX limbs and to assign horses to either mechanical testing or histology at 24 months (n = 6 horses per group). Interim repair assessment was performed at 4 and 12 months, with final outcome analysis performed at 24 months. Horses were observed daily for the duration of the study.
Surgical Defect Creation and Device Implantation
Perioperative antimicrobial and nonsteroidal anti-inflammatory therapy was administered for 3 and 5 days, respectively. Further analgesia was provided by a preoperative epidural.24 Horses were placed under general anesthesia and a routine arthroscopic approach was made to the LTR of the distal femur. Custom instrumentation with depth control (Kensey Nash Corporation) was used to create a 10-mm diameter × 10-mm deep osteochondral defect on the mid-LTR. The device was cut to the appropriate length and placed in a well in the device packaging. Five milliliters of bone marrow were aspirated from the sternum and was used to hydrate the implant by filling the well and allowing the CRD to soak for 2 to 5 minutes. The device was press fit under arthroscopic visualization using a custom delivery tool, and a blunt probe was used to ensure the device was flush with surrounding cartilage ( Fig. 1 ). The procedure was repeated in the contralateral limb but the instrumentation was only advanced until subchondral bone was contacted, thereby creating a 10-mm diameter full-thickness chondral defect. Any remaining calcified cartilage was removed with a curette. MFX was performed using an awl to create 5 holes in the configuration of a 5-die ( Fig. 1 ).25 Horses were recovered from surgery and were immediately fully weight-bearing thereafter.
Figure 1.

Representative arthroscopic images of 10 mm diameter, 10 mm deep osteochondral defect in the distal lateral trochlear ridge of the femur grafted with a synthetic biphasic cartilage repair device (CRD) immediately following implantation (D), and at 4-month (E), and 12-month (F) recheck arthroscopies. Full-thickness 10 mm diameter chondral defect treated with microfracture in the contralateral limb served as control; defect creation (A), 4-month (B), and 12-month (C) arthroscopic images. Fissures in native cartilage surrounding defect (black arrow). *Indicates the patella.
Horses were exercise restricted for 6 weeks following surgery but were permitted free movement within their stall. Then, controlled walking was introduced starting at 5 minutes per day, and increased by an additional 5 minutes per day at weekly intervals up to a maximum of 30 minutes per day. Controlled walking exercise continued until the 4-month recheck arthroscopy.
Four- and 12-Month Recheck Arthroscopies
Horses were anesthetized and arthroscopic exploration of each joint was performed. A modified International Cartilage Repair Society (ICRS) macroscopic scoring scheme was used (Suppl. Table S1). All scoring was performed by consensus of 2 observers blinded to treatment. Horses were exercise restricted until suture removal around 14 days, and then were allowed free exercise in a pasture.
Radiographic Evaluation
Three radiographic views (lateral-medial, caudal-cranial, caudolateral-craniomedial oblique) of each patellofemoral joint were obtained at 5 time points: preoperative, postoperative, 4 months, 12 months, and 24 months. All radiographs were scored by 2 observers blinded to treatment for sclerosis, lysis, percentage of defect replaced by bone, cyst formation, osteophyte formation, and evidence of device migration (Suppl. Table S2). The sum of scores of the 2 observers was averaged to determine a final score for each parameter. Scores were not assigned when at least one observer determined they could not adequately assess the parameter based on the radiographic view, or when the parameter did not apply (e.g., percentage of defect replaced by bone and device migration were not scored on preoperative radiographs). Postoperative radiographs were to be obtained within 1 week of the implantation surgery; however, due to quarantine restrictions only 6 horses had radiographs within the designated time frame, while the remaining horses had postoperative radiographs at 9 weeks postoperatively. These subgroups were designated as 1-week and 9-week postoperative groups, respectively, for statistical analysis.
Disposition of Tissues
Subsequent to 24-month radiographs, horses were euthanized. The distal femur and proximal tibia were transected leaving all soft tissues of the patellofemoral and femorotibial joints intact. Specimens were stored chilled until magnetic resonance imaging testing was performed (within 24 hours). No abnormalities were found on general postmortem evaluation of the remaining carcass.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) was performed on a 3.0 T clinical imaging system (14.0 HDx, GE Healthcare, Milwaukee WI), using an 8-channel transmit and receive phased-array knee coil. Morphologic imaging was performed in the sagittal and axial planes using a cartilage-sensitive 2-dimensional (2D) fast spin echo (FSE) sequence that has been previously validated for cartilage assessment in preclinical models.26-28 The acquisition parameters were the following: repetition time (TR) of 5600 to 7500 ms; echo time (TE) of 20 to 30 ms (effective); field-of-view (FOV) 13 to 14 cm2; acquisition matrix (AM) 5122; number of acquisitions (NEX) 3 to 4, slice thickness (ST) 1.5 mm, no gap; and receiver bandwidth ±62.5 kHz.
Quantitative T2 mapping (GE Healthcare, Milwaukee WI) was performed using a multi-slice, multi-echo acquisition29: TR of 1000 ms, 8 sampled echoes using sequential multiples of the first echo time (7-8 ms), FOV 13 cm2; AM 384 × 256; ST 2.5 mm, no gap, NEX 2; BW ±62.5 kHz. Quantitative T1ρ imaging (GE Healthcare, Milwaukee, WI) was performed using the following parameters: spin lock frequency of 500 Hz, with 4 spin lock times (TSLs) of 0, 20, 40, and 60 ms; FOV 14 cm2; AM 256 × 160; ST3 mm; and NEX of 0.68.
Following image acquisition, all MRI studies were read by 2 experienced musculoskeletal radiologists who were blinded to treatment. The morphologic FSE images were assessed for sclerosis, synovial reaction, and percent fill of the repair site based on consensus by the 2 observers (Suppl. Table S3). T2 mapping and T1ρ imaging data sets were analyzed on a pixel-by-pixel basis with a 2-parameter weighted least-squares fit (Functool 3.1, GE Healthcare, Milwaukee WI), assuming a mono-exponential decay. Regions of interest (ROI) were obtained in a standardized fashion, within a fixed area of the cartilage over the center of the cartilage repair, separated into superficial and deep zones.
Gross Assessment
Joints were sectioned following completion of MRI. The joint surface was exposed and synovial fluid was collected and frozen for subsequent evaluation for device particles. Synovial membrane was collected and fixed in 4% paraformaldehyde for histologic assessment. The articular surface was stained with India ink to highlight fissures. Intra-articular tissues were observed for gross evidence of infection, inflammation, or foreign material. For the 6 horses designated to the histology group, osteochondral sections 3 cm long by 3 cm wide by 5 cm deep centered on the defect sites were collected and fixed in 4% paraformaldehyde. For the mechanical testing group, the LTR and patella were harvested in their entirety, soaked in Hank’s Balanced Salt Solution, and frozen at −80°C for subsequent testing.
Mechanical Testing
Patellofemoral joints were obtained from 6 horses euthanized for reasons unrelated to patellofemoral joint disease to serve as normal unoperated control (UC) specimens. Four test sites were chosen for mechanical evaluation (Suppl. Fig. S1). Three areas approximately 10 mm apart were indented on the LTR in the following locations: at the center of the CRD or MFX defect site or a similar location on UC LTR, adjacent to the defect edge, and remote (>1 cm) to the defect site. One area on the patella was also tested in a region that approximately corresponded with the contact point with the LTR defect site.
Each trochlea was placed in a rotating cup, tightened with screws positioned on the testing machine (ELF 3200, BOSE), and secured in place with a C-clamp. The LTR was oriented so that the cartilage area-of-interest was perpendicular to the indentation fixture. A porous indenter (diameter 1.25 mm) was attached to the upper actuator and a compressive load was applied at a rate of 5 g/s to a maximum of 20 g. Load and displacement were recorded throughout testing. At the completion of testing the indentation fixture was replaced with a needle to measure cartilage thickness. The needle was displaced from the surface at a rate of 0.05 mm/s until a rapid increase in load was observed indicating that bone had been contacted. The distance between the surface of the articular cartilage and the location at which the peak occurred was taken as the thickness. The test was repeated for the patella.
Displacement-time data were numerically fit to the biphasic indentation creep solution to determine 3 intrinsic material coefficients at each test site: aggregate modulus (Ha), Poisson’s ratio (ν) and hydraulic permeability (k) as described previously.30,31 Values for the shear modulus (µ) were calculated from the parameters Ha and ν. The early creep response was not used in the determination of the material coefficients because of generally poor agreement between the experimental data and theoretical model at short times after loading.30 The dynamic modulus (Edyn) was defined as the modulus during initial loading with the porous, free-draining indenter. It was calculated as the slope of the linear portion of the loading portion of the test.
Histologic Processing and Scoring
Following fixation, osteochondral blocks were cut in the sagittal plane to decrease their surface area prior to decalcification in cacodylate buffered 10% EDTA. Decalcified osteochondral blocks and fixed synovium specimens were then processed, embedded in paraffin, and sectioned at 8 µm. Sections were stained with hematoxylin and eosin, safranin O and fast green, and type II collagen immunohistochemistry was performed. Osteochondral histology sections were scored using the ICRS II for Osteochondral Repair Scoring System.32 Synovial membrane sections were stained with hematoxylin and eosin and scored for villus architecture, subintimal fibrosis, intimal thickening, vascularity, and inflammatory cell infiltrate on a scale from 0 to 15 (adapted from Fortier et al.33), with 0 representing normal synovial architecture. All scoring was performed by 2 observers blinded to treatment.
Statistical Analysis
Significance was set at P < 0.05 for all tests. For each radiographic score category and for measures of arthroscopic graft healing, a mixed effect model was fitted to the data with horse as a random effect, time and treatment as fixed effects, and an interaction term for treatment * time. Time was treated as a discrete variable to allow for potential nonlinear effects. Tukey’s post hoc tests were used to determine differences between treatments over time. Statistical analysis was performed using JMP (SAS Institute).
For mechanical testing data, a linear mixed model was applied to the data. Location and treatment were modeled as fixed effects. Bonferroni post hoc testing was then conducted to allow for pairwise comparisons of each treatment at each location. Statistical analysis was done using SAS version 9.3.
Statistical analysis for MRI was performed with 2-tailed paired t tests for the superficial and deep zones to detect differences of T2 and T1ρ values among the evaluated ROIs. Two-tailed sign tests were performed between treatment and control limbs to detect differences of the subjective grading of sclerosis, synovial reaction, and percent fill. Statistical analysis was done using SAS version 9.3.
For histologic data 2-tailed Wilcoxon signed-rank test was used to analyze total scores for the synovial membrane scoring scheme and the osteochondral scoring scheme to determine differences between treatment groups. Statistical analysis was performed using JMP (SAS Institute).
Results
Surgical Procedure and Clinical Assessment
Implantation of the CRD was successful in all horses using a minimally invasive arthroscopic technique. In one horse, the first attempt at device placement failed. The device had been advanced part-way beyond the edge of the delivery tool and misalignment of the device with the defect resulted in buckling of the device during deployment. The damaged device was successfully removed and a new device was placed without consequence. The device was not advanced until the edges of the delivery tool were in close proximity to and aligned with the defect edge in all subsequent horses.
One horse was euthanized 2 weeks after implantation surgery due to a pelvic fracture (unrelated to the surgical procedure) and a replacement horse was enrolled in the study. The only data points included in statistical analysis for the horse that sustained the pelvic fracture were pre- and 1 week postoperative radiographic scores.
Radiography
All horses had radiographically normal patellofemoral joints on preoperative radiographs. There was no evidence of lysis, bone cysts, or osteophytes at any time. Therefore, only sclerosis, percentage replaced by bone, and device migration categories were used for statistical analysis.
There was no sclerosis on preoperative radiographs. Sclerosis peaked at 4 months in both CRD- and MFX-treated joints, with CRD having significantly more sclerosis compared to MFX ( Table 1 ; P = 0.0006). Sclerosis at 12 months in MFX- and at 24 months in CRD-treated joints returned to normal and was not different from preoperative scores. There was no difference in the percent replaced by bone score from 1 week to 4 months postoperatively, but scores improved significantly at 12 months, and further improved in CRD joints at 24 months so that there was no difference between CRD and MFX at the end point of the 24-month study ( Table 1 ; P < 0.0001).
Table 1.
Radiographic Scores for Sclerosis and Percentage Replaced by Bone.
| Sclerosis |
Percentage Replaced by Bone |
|||
|---|---|---|---|---|
| CRD | MFX | CRD | MFX | |
| Preoperative (n = 13) | 0 ± 0.21e | 0 ± 0.21e | n/a | n/a |
| 1-Week (n = 6) | 0.02 ± 0.38e | 0.01 ± 0.34e | 5.93 ± 0.53a | 6.00 ± 0.50a |
| 9-Week (n = 7) | 2.12 ± 0.34ab | 0.64 ± 0.31cde | 5.39 ± 0.41a | 5.20 ± 0.41a |
| 4-Month (n = 12) | 3.03 ± 0.24a | 1.54 ± 0.25bcd | 4.45 ± 0.28a | 4.86 ± 0.29a |
| 12-Month (n = 12) | 1.58 ± 0.22bc | 0.58 ± 0.22de | 2.26 ± 0.29b | 1.30 ± 0.30bc |
| 24-Month (n = 12) | 0.42 ± 0.20e | 0.08 ± 0.20e | 0.92 ± 0.24c | 0.46 ± 0.24c |
A 10-mm diameter, 10-mm deep osteochondral defect was created in the distal lateral femoral trochlear ridge of horses and a biphasic cartilage repair device (CRD) was placed to fill the defect. A full-thickness chondral defect treated with microfracture served as positive control (MFX) in the contralateral joint. Radiographic views of the joints were obtained preoperatively, 1 week, 9 weeks, 4 months, 12 months, and 24 months postoperatively. Sclerosis score 0 = none to 3 = severe. Percentage replaced by bone score based on percent relative radiopacity compared to remote normal bone 0 = 100%, 1 = 75% to 99%, 2 = 50% to 75%, 3 ≤50%. Scores from 2 observers were summed to reach a final score. Different superscript letters indicate significant differences between groups within scoring category using a mixed effect model and Tukey’s post hoc test, P < 0.05. n/a = not applicable.
Two horses had a small free radiopaque particle evident on radiographs and scored positively for device migration. This resulted in CRD having a higher migration score (0.5 ± 0.34) than MFX (0) at 1 week (P = 0.0052). In the horse that sustained the pelvic fracture 2 weeks postoperatively, the patellofemoral joint was opened during necropsy and no gross free material was evident. No other horse had evidence of device migration at any time.
Second Look Arthroscopic Assessment (4 and 12 Months)
Cartilage repair device treated defects had significantly better (lower) modified ICRS macroscopic scores at 4 months compared to MFX (P = 0.0069; Table 2 ). There was no evidence of infection, device migration, synovial inflammation, or blood within any joint at any time ( Fig. 1 ).
Table 2.
Modified International Cartilage Repair Society Macroscopic Scores Obtained at Recheck Arthroscopy Performed at 4 and 12 Months Postoperatively.
A 10-mm diameter, 10-mm deep osteochondral defect was created in the distal lateral femoral trochlear ridge of horses (n = 12) and a biphasic cartilage repair device (CRD) was placed to fill the defect. A full-thickness chondral defect treated with microfracture (MFX) served as positive control in the contralateral joint. Recheck arthroscopy and macroscopic scoring was performed at 4 and 12 months. Mean ± SD; different superscript letters indicate significant differences between treatment and time groups using a mixed effect model and Tukey’s post hoc test, P < 0.05.
Some additional subjective observations were made during second-look arthroscopy that were not scored for statistical analysis. On palpation with a blunt probe, repair tissue in the CRD defects at 4 months was consistently noted to be soft, but thicker and more like normal remote articular cartilage than the thin layer of repair tissue in the MFX defect. A striking difference at 12 months was the presence and extent of cartilage fissures. In the CRD defects, there were none to few short (2-3 mm) full- or partial-thickness fissures radiating from the perimeter of the defects in the adjacent articular cartilage. In contrast, there were frequently several (6-10) long (1-4 cm) primarily full-thickness cartilage fissures radiating from the edges of the MFX defects.
Gross Observations (24 month)
There was no gross evidence of infection, inflammation, or fibrosis of the surrounding soft tissues. Insufficient synovial fluid was present for collection from 2 treated and 2 control joints although the synovial fluid appeared to have normal color and viscosity. No foreign material was identified on microscopic assessment of synovial fluid smears. India ink staining revealed fissures in host cartilage surrounding all MFX defects, with fissures in the repair tissue in 4 joints. In contrast, fissures were evident in the surrounding host cartilage in only 5 CRD joints, and within the repair tissue of 2 defects. There were patellar lesions in the form of worn and thinned cartilage opposite the defect site in 2 MFX joints and 1 CRD treated joint.
Mechanical Testing
There were a number of mechanical tests performed for which the theoretical fits did not yield useable data. The sites for which unusable test results were obtained occurred in 2 animals. In one animal, 1 site was adjacent to the device, while in the same animal, but on the contralateral limb, an additional site was remote to the MFX. In another animal, there were 2 sites remote and on the patella on the limb that received MFX. For these test sites, the indenter continued to displace and never reached equilibrium during testing. It is unclear why this happened, but the data from these test sites were excluded from the pooled analysis because no meaningful data could be obtained from the tests.
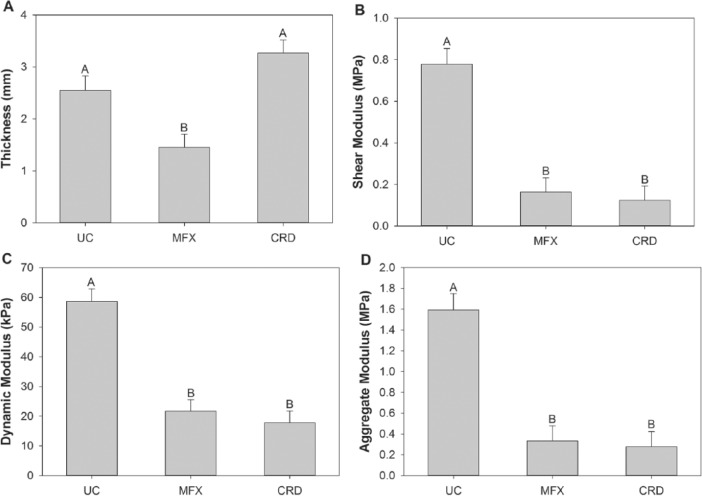
Poisson’s ratio and permeability were not significantly different across each treatment group in each location. There was also no significant difference between treatment group for the adjacent, remote, or patella sites for all parameters. The CRD site of implantation was significantly thicker than the MFX site and not different from UC cartilage. The UC was also significantly thicker than MFX tissue ( Fig. 2A ). There was no significant difference between the dynamic, shear, or aggregate modulus values at the level of the defect of the CRD compared to MFX, which were both significantly less than the UC site ( Fig. 2B , C , and D , respectively).
Figure 2.
Thickness (A), shear modulus (B), dynamic modulus (C), and aggregate modulus (D) results derived from mechanical testing of repair tissue of osteochondral defects treated with cartilage repair device (CRD, n = 6) and full-thickness chondral defect treated with microfracture (MFX, n = 6), and unoperated normal articular cartilage controls (UC, n = 6) from equine distal lateral femoral trochlear ridges 24 months after defect creation. Mixed effect model with Bonferonni post hoc test; different letters indicate significant differences between treatment groups, P < 0.05.
Magnetic Resonance Imaging and Histologic Assessment
Significant differences of T1ρ were found in the deep (P = 0.027) and superficial zones (P = 0.015) between the CRD and MFX joints. A significant difference of T2 was found in the deep zone (P = 0.028) between the CRD and MFX joints, but not the superficial zone (P = 0.074). The CRD group had prolonged T1ρ and T2 values in all regions evaluated. No differences in sclerosis, synovial reaction, or percent fill were detected between CRD and MFX joints (P = 0.13, P = 1.0, and P = 1.0, respectively) ( Fig. 3 ).
Figure 3.
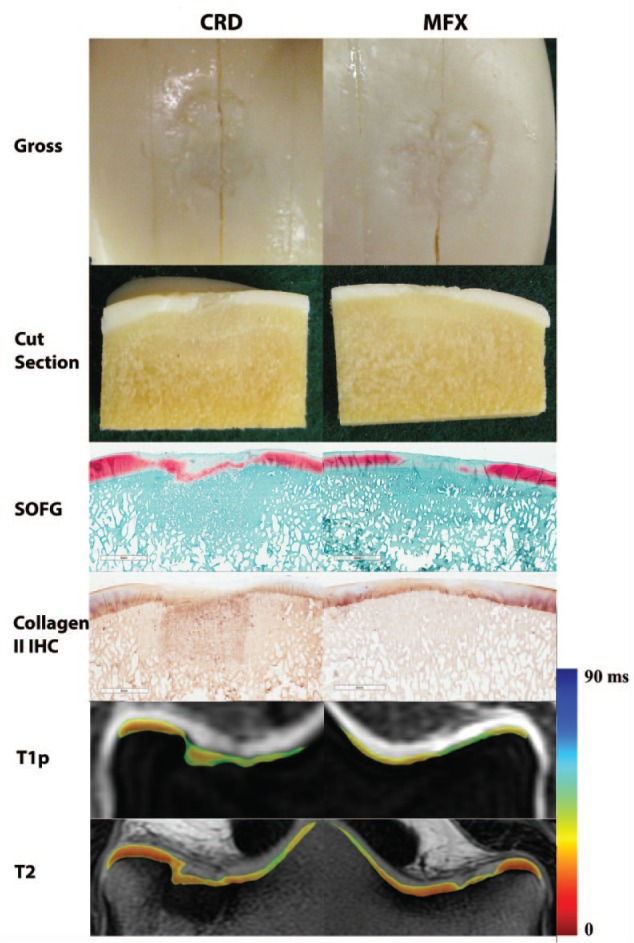
Representative images from a single horse of a 10-mm diameter, 10-mm deep osteochondral defect in the distal lateral femoral trochlear ridge grafted with a synthetic biphasic cartilage repair device (CRD) and the positive control contralateral limb with a 10-mm diameter full-thickness chondral defect treated with microfracture (MFX). Gross, histologic, and MRI assessment of repair tissue was performed 24 months postoperatively. Images include the articular surface of sagittally sectioned osteochondral blocks (Gross), the cut edge from the center of the defect of the osteochondral blocks (Cut section), safranin-O fast green histology (SOFG), collagen type II immunohistochemistry (IHC), quantitative T1ρ MRI axial plane image indicating proteoglycan content, and quantitative T2 MRI axial plane images indicating collagen organization.
There was no significant difference between treatment groups for histologic scores of osteochondral or synovial tissue sections ( Table 3 ).
Table 3.
International Cartilage Repair Society (ICRS) II Osteochondral Repair Scoring System Histology Scores and Synovial Membrane Histology Scores.
| CRD | MFX | P | |
|---|---|---|---|
| ICRS II Osteochondral Repair Scorea | 3361.67 ± 125.84 | 3529.00 ± 41.79 | 0.18 |
| Synovial Membrane Scoreb | 0.25 ± 0.18 | 0.50 ± 0.27 | 0.66 |
A 10-mm diameter, 10-mm deep osteochondral defect was created in the distal lateral femoral trochlear ridge of horses (n = 12) and a biphasic cartilage repair device (CRD) was placed to fill the defect. A full-thickness chondral defect treated with microfracture (MFX) served as positive control in the contralateral joint. Animals were sacrificed and tissues processed for histology 24 months postoperatively. Mean ± SD; 2-tailed Wilcoxon signed-rank test, P < 0.05.
Score range from 0 to 4,400, with 4,400 representing normal cartilage and bone.
Score range 0 to 15, with 0 representing normal synovial membrane.
Discussion
A bioresorbable, acellular, biphasic CRD hydrated with bone marrow aspirate was evaluated over 2 years in an equine model for feasibility of arthroscopic implantation, safety, biocompatibility, and efficacy for repairing large osteochondral defects in a GLP study. Overall, the CRD resulted in osseous repair tissue supporting a superficial layer of cartilage tissue similar to, but thicker than repair tissue formed following MFX of full-thickness cartilage defects with an intact subchondral plate. The use of a control group with intact subchondral bone is a rigorous model compared to an unfilled osteochondral defect. This is an interesting surgical treatment concept, where osteochondral defect repair, using a CRD, could lead to similar bone and cartilage repair tissue as that formed following MFX of a simpler defect that was contained to cartilage only, and did not breech the underlying subchondral bone plate. The CRD was easily placed using a minimally invasive arthroscopic technique. There was no evidence of infection, inflammation, or rejection of the CRD at any time. Therefore, this study supports the feasibility of CRD implantation using a simple minimally invasive technique. Furthermore, the CRD appears to be safe and biocompatible for intra-articular use for at least 2 years.
Quantitative MRI using T2 and T1ρ correlate with collagen organization and water content, and proteoglycan content, respectively, with a prolongation of either value suggestive of a greater degree of collagen disorganization and proteoglycan loss, respectively. Repair cartilage in both CRD- and MFX-treated defects had a variable appearance on MR images, ranging from hyperintense early repair tissue to hypointense fibrocartilage. Significant prolongation of T2 and T1ρ values was seen centrally in the repair, reflecting an increase of mobile water in the immature tissue. The prolonged values with wider standards of deviation located centrally indicate nonuniformity of the repair tissue relative to the native articular cartilage. The reduction of T1ρ and T2 values at the interface may indicate incorporation with the boundary of the native tissue. Quantitative MRI suggested improved collagen fibril orientation (T2 mapping) and proteoglycan content (T1ρ) in the deep but not superficial layers of the MFX compared to CRD group.34-36 The wide standard deviations of T1ρ and T2 values of the CRD group suggests greater heterogeneity of reparative fibrocartilage at the repair sites as compared to the MFX group ( Table 4 ). Specific histologic type or functional capacity of the repair tissue cannot be inferred by any specific T2 or T1ρ value.
Table 4.
Quantitative T1ρ and T2 MRI values.
| C-CRD | N-CRD | C-MFX | N-MFX | P a | P b | |
|---|---|---|---|---|---|---|
| Deep T1ρ | 64.1 ± 21.8 | 32.7 ± 63.9 | 50.1 ± 11.8 | 33.7 ± 4.2 | 0.027 | 0.0005 |
| Superficial T1ρ | 74.8 ± 27.1 | 41.7 ± 3.2 | 58.1 ± 13.0 | 42.3 ± 6.7 | 0.015 | 0.0016 |
| Deep T2 | 45.2 ± 17.5 | 16.1 ± 2.1 | 30.3 ± 7.5 | 16.8 ± 3.2 | 0.028 | 0.0001 |
| Superficial T2 | 50.9 ± 18.2 | 27.1 ± 3.6 | 36.9 ± 10.5 | 28.5 ± 3.9 | 0.074 | 0.0004 |
A 10-mm diameter, 10-mm deep osteochondral defect was created in the distal lateral femoral trochlear ridge of horses (n = 12) and a biphasic cartilage repair device (CRD) was placed to fill the defect. A full-thickness chondral defect treated with microfracture (MFX) served as positive control in the contralateral joint. Animals were sacrificed at 24 months postoperatively and MRI of intact joints performed. Values were obtained from superficial and deep at the center of the defect repair tissue (C-CRD, C-MFX) and at remote intact cartilage (N-CRD, N-MFX). Mean ± SD; 2-tailed paired t test, P < 0.05.
Indicates comparison between C-CRD and C-MFX.
Indicates comparison between center of defect and remote intact cartilage within treatment group.
To date, a repair technique that predictably results in regeneration of hyaline cartilage remains elusive. Microfracture relies on formation of a superclot within the cartilage defect and migration of mesenchymal cells from the marrow cavity to participate in the formation of fibrocartilage repair tissue, which has poor long-term durability.19,37,38 The CRD investigated was designed to promote cellular migration and was hydrated with bone marrow aspirate prior to implantation. Penetration of the subchondral plate when creating the osteochondral defect allows migration of marrow elements similar to MFX; saturation of the device with bone marrow simply accelerated this process. Bone marrow serves as a source of mesenchymal cells and growth factors. However, only 0.001% to 0.01% of mononuclear cells in bone marrow aspirate are mesenchymal stem cells.39,40 Hydration of the CRD with a concentration of bone marrow or another source of cells and growth factors might achieve a more hyaline-like cartilage repair tissue. Further investigation of the optimal hydration medium for bone and cartilage repair is warranted. The device may also be hydrated with saline; however, given that the device was hydrated with bone marrow in this study, it is not possible to comment on how the CRD would perform if hydrated in this manner.
Evaluation of the impact of an osteochondral defect repair technique on the adjacent and opposing cartilage is also important in addition to assessment of the repair tissue itself. An increasing number and depth of fissures radiating from the defect edge into the surrounding articular cartilage was noted in MFX joints, with all MFX joints affected at 24 months, while only 5/12 CRD-treated joints had fissures. Other studies have also observed the phenomenon of degeneration of cartilage adjacent to an empty defect.33,41 One of these studies also observed fewer fissures in cartilage adjacent to grafted defects consistent with the results of the current study.33 This may reflect the early mechanical support to the surrounding cartilage by a graft material that may mitigate fissure formation. Despite the gross lesions detected in the adjacent cartilage surfaces, the cartilage at these locations appears to have retained mechanical properties that did not differ from normal cartilage; however, mechanical testing was not performed in the area of a crack. As previously stated, most cartilage defects will increase in size over time and result in degeneration of the joint.1,2 A longer duration study may be required to detect degeneration and loss of the mechanical properties of the abnormal surrounding cartilage. A further limitation is that determination of fissures and patellar cartilage lesions was performed subjectively. Development of a method to objectively quantify the damage to surrounding cartilage would be beneficial.
The longer duration of sclerosis above preoperative levels in the CRD-treated joints compared to MFX is expected given the degree of bone invasion with the 10-mm deep osteochondral defect. Importantly, sclerosis resolved radiographically and was not different from MFX on MRI at the 2-year study end-point, and therefore likely represents a normal healing response rather than pathologic change. The lack of difference in radiopacity of the CRD and MFX defects up to 4 months likely reflects the contribution of the radiopaque β-TCP to the overall opacity of bone on radiographs. Two horses were noted to have a small radiopaque particle free in the joint on postoperative radiographs. The radiopacity may have been a small portion of the device or may have been native bone inadvertently left in the joint following creation of the osteochondral defect. The radiopaque particle was not identified on any subsequent evaluation methodology (radiographs, arthroscopy, MRI, or postmortem evaluation) in any horse, or 2 weeks postoperative in the horse that sustained a pelvic fracture and was euthanized. One of the limitations of the study was that radiographs were not performed intra-operatively to ensure removal of all particulate matter prior to closure and recovery. Therefore, it cannot be ascertained whether the one small radiopaque particle identified on 1 week postoperative radiographs in the 2 horses were migration from the device or a consequence of inadequate flushing of debris from the joint at the conclusion of surgery. In addition, surgery to remove and determine the source of the particle was not attempted once it was identified. The surface of the device, which is entirely radiolucent material, was intact in both horses at their next evaluation, which would suggest that no deep radiopaque device material migrated out of the defect after the device was placed.
Historically, concerns were raised with the potential detrimental effects of decreased local tissue pH due to release of lactic acid during degradation of large-volume PLA implants. Numerous factors influence the rate of PLA degradation and therefore lactic acid release.42 A study of implants made of 50% PLA and 50% polyglycolic acid with 3 different porosities (0%, 33%, and 75%) found that increasing porosity resulted in a slower degradation rate and lesser decrease in pH.43 Greater fluid clearance is thought to be responsible for the lack of acid accumulation with highly porous implants. The CRD has an 80% void volume, and of the remaining volume of the synthetic bone phase of the device only a small percentage is PLA. Therefore, the low volume of PLA, high porosity, and significant blood flow in the bone underlying the subchondral plate makes significant acid accumulation unlikely. In keeping with this, there were no adverse effects on tissue adjacent to the PLA containing portion of the CRD.
One of the challenges and limitations of this study is that repair of an osteochondral defect was compared to a full-thickness chondral defect treated with MFX and there was no empty osteochondral defect control. There is no established control for evaluating osteochondral repair. A systematic review of cartilage repair strategies including MFX, autologous chondrocyte implantation, osteochondral autograft, and matrix-induced autologous chondrocyte implantation published at the time of study design concluded that no technique was convincingly superior.37 An osteochondral autograft would have prevented use of the contralateral joint as control and doubled the number of horses sacrificed to 24. Others have previously investigated spontaneous healing of large untreated osteochondral defects in goats at 1 year and found that nontreated defects did not heal; rather, the lesions expanded and were incompletely filled with fibrous tissue and central cysts with only a thin layer of fibrocartilage at the defect periphery.41 Given insufficient evidence justifying increasing horse numbers for a control that was not convincingly superior to MFX (osteochondral autograft), and the obvious poor performance of an empty osteochondral defect, a full-thickness chondral defect treated with MFX was thought to be a more clinically relevant and rigorous control than an empty osteochondral defect.
In conclusion, the CRD underwent rigorous evaluation in a long-term large animal model with a positive control treatment for comparison. This synthetic, bioresorbable device is immediately available for arthroscopic implantation and was safe and biocompatible for up to 2 years in an equine single-site osteochondral defect model. Unlike previous evidence of lack of adequate repair of empty osteochondral defects, the cartilage repair tissue produced following CRD implantation in this study was similar to that produced following the current clinical standard for cartilage repair, MFX of full-thickness chondral defects. However, the results of the study did not support our hypothesis that the repair tissue produced by the CRD would be superior to that produced by MFX. While there was no difference between histologic assessment of CRD and MFX repair tissue, MRI suggested some improved values in the MFX group compared to CRD for proteoglycan content and collagen organization. Ultimately, histology is considered the gold standard for cartilage repair assessment; however, this discordance of results warrants further investigation as to what may cause such a difference to occur.
Supplementary Material
Footnotes
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
Authors’ Note: Surgery, radiographs, lameness evaluation, postmortem analysis, synovial fluid analysis, and synovial membrane and osteochondral histology were performed at Cornell University College of Veterinary Medicine. Magnetic resonance imaging, gross assessment and tissue collection from femoropatellar joints, and mechanical testing were performed at Hospital for Special Surgery.
Acknowledgments and Funding: The authors would like to acknowledge Ms. Denise Archer for quality control oversight of the study; Dr. Kathleen Quinlan for assistance with histology scoring; Dr. Jon Cheetham for statistical analysis of radiographic, arthroscopic, and histologic scores; Dr. Hollis G. Potter for assistance with MRI evaluation; Drs. Peter Torzilli and Suzanne Maher for mechanical testing consultation; and Joseph Nguyen for assistance with statistical analysis on the mechanical testing data. Funding was provided by Kensey Nash Corporation.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Fortier was on the Board of Scientific Advisors to Kensey Nash during the time this project was completed. Castiglione, Bradica, and Saska are employees of Kensey Nash Corporation. The HSS MRI Laboratory (Pownder and Koff) receives institutional research support from General Electric Healthcare. McCarrel and Gilbert have no conflicts of interest to report.
Ethical Approval: Ethical approval was not sought for the present study because only research animals were used under approval from the Cornell University Institutional Care and Use Committee.
Supplementary Material: Supplementary material for this article is available online.
References
- 1. Cicuttini F, Ding C, Wluka A, Davis S, Ebeling PR, Jones G. Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum. 2005;52:2033-9. [DOI] [PubMed] [Google Scholar]
- 2. Davies-Tuck ML, Wluka AE, Wang Y, Wang Y, Teichtahl AJ, Jones G, et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:337-42. [DOI] [PubMed] [Google Scholar]
- 3. Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18:434-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Getgood AM, Kew SJ, Brooks R, Aberman H, Simon T, Lynn AK, et al. Evaluation of early-stage osteochondral defect repair using a biphasic scaffold based on a collagen-glycosaminoglycan biopolymer in a caprine model. Knee. 2012;19:422-30. [DOI] [PubMed] [Google Scholar]
- 5. Ahn JH, Lee TH, Oh JS, Kim SY, Kim HJ, Park IK, et al. Novel hyaluronate-atelocollagen/beta-TCP-hydroxyapatite biphasic scaffold for the repair of osteochondral defects in rabbits. Tissue Eng Part A. 2009;15:2595-604. [DOI] [PubMed] [Google Scholar]
- 6. Chang CH, Lin FH, Lin CC, Chou CH, Liu HC. Cartilage tissue engineering on the surface of a novel gelatin-calcium-phosphate biphasic scaffold in a double-chamber bioreactor. J Biomed Mater Res B Appl Biomater. 2004;71:313-21. [DOI] [PubMed] [Google Scholar]
- 7. Kandel RA, Grynpas M, Pilliar R, Lee J, Wang J, Waldman S, et al. Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a sheep model. Biomaterials. 2006;27:4120-31. [DOI] [PubMed] [Google Scholar]
- 8. Bostman OM. Absorbable implants for the fixation of fractures. J Bone Joint Surg Am. 1991;73:148-53. [PubMed] [Google Scholar]
- 9. Hollinger JO, Battistone GC. Biodegradable bone repair materials. Synthetic polymers and ceramics. Clin Orthop Relat Res. 1986;207:290-305. [PubMed] [Google Scholar]
- 10. Vaccaro AR. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics. 2002;25:s571-8. [DOI] [PubMed] [Google Scholar]
- 11. Da H, Jia SJ, Meng GL, Cheng JH, Zhou W, Xiong Z, et al. The impact of compact layer in biphasic scaffold on osteochondral tissue engineering. PLoS One. 2013;8:e54838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernandez FB, Shenoy S, Suresh Babu S, Varma HK, John A. Short-term studies using ceramic scaffolds in lapine model for osteochondral defect amelioration. Biomed Mater. 2012;7:035005. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka T, Komaki H, Chazono M, Fujii K. Use of a biphasic graft constructed with chondrocytes overlying a beta-tricalcium phosphate block in the treatment of rabbit osteochondral defects. Tissue Eng. 2005;11:331-9. [DOI] [PubMed] [Google Scholar]
- 14. Jiang CC, Chiang H, Liao CJ, Lin YJ, Kuo TF, Shieh CS, et al. Repair of porcine articular cartilage defect with a biphasic osteochondral composite. J Orthop Res. 2007;25:1277-90. [DOI] [PubMed] [Google Scholar]
- 15. Betsch M, Schneppendahl J, Thuns S, Herten M, Sager M, Jungbluth P, et al. Bone marrow aspiration concentrate and platelet rich plasma for osteochondral repair in a porcine osteochondral defect model. PLoS One. 2013;8:e71602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Q, Peng J, Lu SB, Guo QY, Zhao B, Zhang L, et al. Evaluation of an extracellular matrix-derived acellular biphasic scaffold/cell construct in the repair of a large articular high-load-bearing osteochondral defect in a canine model. Chin Med J (Engl). 2011;124:3930-8. [PubMed] [Google Scholar]
- 17. Schleicher I, Lips KS, Sommer U, Schappat I, Martin AP, Szalay G, et al. Biphasic scaffolds for repair of deep osteochondral defects in a sheep model. J Surg Res. 2013;183:184-92. [DOI] [PubMed] [Google Scholar]
- 18. Getgood A, Henson F, Brooks R, Fortier LA, Rushton N. Platelet-rich plasma activation in combination with biphasic osteochondral scaffolds-conditions for maximal growth factor production. Knee Surg Sport Traumatol Arthrosc. 2011;19:1942-7. [DOI] [PubMed] [Google Scholar]
- 19. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-63. [DOI] [PubMed] [Google Scholar]
- 20. McIlwraith CW, Fortier LA, Frisbie DD, Nixon AJ. Equine models of articular cartilage repair. Cartilage. 2011;2:317-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hurtig MB, Buschmann MD, Fortier LA, Hoemann CD, Hunziker EB, Jurvelin JS, et al. Preclinical studies for cartilage repair: recommendations from the International Cartilage Repair Society. Cartilage. 2011;2:137-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahern BJ, Parvizi J, Boston R, Schaer TP. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage. 2009;17:705-13. [DOI] [PubMed] [Google Scholar]
- 23. Frisbie DD, Cross MW, McIlwraith CW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006;19:142-6. [PubMed] [Google Scholar]
- 24. Goodrich LR, Nixon AJ, Fubini SL, Ducharme NG, Fortier LA, Warnick LD, et al. Epidural morphine and detomidine decreases postoperative hindlimb lameness in horses after bilateral stifle arthroscopy. Vet Surg. 2002;31:232-9. [DOI] [PubMed] [Google Scholar]
- 25. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(Suppl.):S362-9. [DOI] [PubMed] [Google Scholar]
- 26. Kelly BT, Potter HG, Deng XH, Pearle AD, Turner AS, Warren RF, et al. Meniscal allograft transplantation in the sheep knee: evaluation of chondroprotective effects. Am J Sports Med. 2006;34:1464-77. [DOI] [PubMed] [Google Scholar]
- 27. Glenn RE, McCarty EC, Potter HG, Juliao SF, Gordon JD, Spindler KP. Comparison of fresh osteochondral autografts and allografts: a canine model. Am J Sports Med. 2006;34:1084-93. [DOI] [PubMed] [Google Scholar]
- 28. Nho SJ, Foo LF, Green DM, Shindle MK, Warren RF, Wickiewicz TL, et al. Magnetic resonance imaging and clinical evaluation of patellar resurfacing with press-fit osteochondral autograft plugs. Am J Sports Med. 2008;36:1101-9. [DOI] [PubMed] [Google Scholar]
- 29. Maier CF, Tan SG, Hariharan H, Potter HG. T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging. 2003;17:358-64. [DOI] [PubMed] [Google Scholar]
- 30. Mak AF, Lai WM, Mow VC. Biphasic indentation of articular cartilage—I. Theoretical analysis. J Biomech. 1987;20:703-14. [DOI] [PubMed] [Google Scholar]
- 31. Mow VC, Gibbs MC, Lai WM, Zhu WB, Athanasiou KA. Biphasic indentation of articular cartilage—II. A numerical algorithm and an experimental study. J Biomech. 1989;22:853-61. [DOI] [PubMed] [Google Scholar]
- 32. Getgood A, Bradica G, Castiglione E, Frenkel S, Kandel RA, Fortier L. Validation of the ICRS osteochondral histology score (ICRS-OCHS). In: Orthopedic Research Society Annual Meeting; New Orleans, LA; 2014. [Google Scholar]
- 33. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927-37. [DOI] [PubMed] [Google Scholar]
- 34. Wheaton AJ, Casey FL, Gougoutas AJ, Dodge GR, Borthakur A, Lonner JH, et al. Correlation of T1rho with fixed charge density in cartilage. J Magn Reson Imaging. 2004;20:519-25. [DOI] [PubMed] [Google Scholar]
- 35. Xia Y, Moody JB, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage. 2001;9:393-406. [DOI] [PubMed] [Google Scholar]
- 36. Liess C, Lüsse S, Karger N, Heller M, Glüer CG. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10:907-13. [DOI] [PubMed] [Google Scholar]
- 37. Magnussen RA, Dunn WR, Carey JL, Spindler KP. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008;466:952-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gill TJ, McCulloch PC, Glasson SS, Blanchet T, Morris EA. Chondral defect repair after the microfracture procedure: a nonhuman primate model. Am J Sports Med. 2005;33:680-5. [DOI] [PubMed] [Google Scholar]
- 39. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-7. [DOI] [PubMed] [Google Scholar]
- 40. Martin DR, Cox NR, Hathcock TL, Niemeyer GP, Baker HJ. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol. 2002;30:879-86. [DOI] [PubMed] [Google Scholar]
- 41. Jackson DW, Lalor PA, Aberman HM, Simon TM. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J Bone Joint Surg Am. 2001;83:53-64. [DOI] [PubMed] [Google Scholar]
- 42. Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review. Adv Drug Deliv Rev. Epub 2016. June 26. [DOI] [PubMed] [Google Scholar]
- 43. Athanasiou KA, Schmitz JP, Agrawal CM. The effects of porosity on in vitro degradation of polylactic acid-polyglycolic acid implants used in repair of articular cartilage. Tissue Eng. 1998;4:53-63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.