Abstract
Background
Full-thickness cartilage lesions of the patella represent a common source of pain and dysfunction. Previously reported surgical treatment options include marrow stimulation, cell-based treatments, and osteochondral transfer. Minced juvenile allograft cartilage is a novel treatment option that allows for a single stage approach for these lesions.
Hypothesis
Particulated juvenile allograft cartilage (PJAC) for the treatment of chondral defects of the patella would offer acceptable lesion fill rates, mature over time, and not be associated with any negative biologic effects on the surrounding tissue.
Methods
A retrospective chart review of prospectively collected data was conducted to identify consecutive patients who were treated with PJAC for a full thickness symptomatic cartilage lesion. Qualitative (fast spin echo) and quantitative (T2 mapping) magnetic resonance imaging (MRI) was undertaken at the 6-, 12-, and 24-month postoperative mark. Numerous patient, lesion, and graft specific factors were assessed against MRI scores and percent defect fill of the graft. Graft maturation over time was also assessed.
Results
Forty-five patients total were included in the study. Average age at the time of surgery was 26.5 years (range 13-45 years), average lesion size was 208 mm2 (range 4-500 mm2), and average donor age was 49.5 months (range 3-120 months). Sixty percent of the patients were female, while 93% of all patients underwent a concomitant procedure at the time of the index operation. Six-month MRI findings revealed that no patient-, graft-, or donor-specific factors correlated with MR scores, and 82% of the knees demonstrated good to excellent fill. Twelve-month MRI findings revealed that T2 relaxation times of deep graft demonstrated negative correlation with patient age (P = 0.049) and donor age (P = 0.006), the integration zone showed a negative correlation with donor age (P = 0.026). In all, 85% of patients at 12 months displayed good to moderate fill of the graft. At 24 months, patient age demonstrated negative correlation with average T2 relaxation times of the deep and superficial graft (P = 0.005; P = 0.0029) and positive correlation with the superficial zone of the adjacent cartilage (P = 0.001). Donor age showed negative correlation with grayscale score (P = 0.004) and T2 relaxation times at deep integration zone (P = 0.018). T2 relaxation times of deep and superficial graft and integration zone improved over time (P < 0.001) and between each time point.
Conclusions
Particulated juvenile allograft tissue appears to be an acceptable reconstructive option for full-thickness cartilage lesions of the patella, offering satisfactory tissue defect fill at 6, 12, and 24 months after surgery. Imaging of the repaired cartilage demonstrates progressive graft maturation over time.
Keywords: allograft, cartilage reconstruction, patella
Introduction
Articular cartilage lesions involving the patellar articular surface can be a significant source of pain and disability, and successful treatment can often prove challenging.1,2 Lesions are frequently the sequelae of either an acute patellar dislocation or the result of excessive overload secondary to malalignment and/or maltracking; however, chondral defects may also arise from a patella fracture (posttraumatic) or from osteochondritis dissecans.3 Traditional surgical treatment options for symptomatic defects, that have failed conservative measures, include marrow stimulation techniques, cell-based treatments, and osteochondral transfer (autograft or allograft).4 Cartilage restoration techniques in this location have produced unpredictable results, at best, due to the unique cartilage thickness and contour of the patella, the relative higher density of its subchondral bone (nearly completely cortical), the high dynamic shear and compressive forces at the trochlear articulation, and the exposure difficulties inherent to the extensor mechanism.5
Particulated juvenile articular cartilage (PJAC) offers a new cellular allograft approach for the treatment of symptomatic articular defects of the patella. This material consists of minced hyaline cartilage derived from donors younger than 10 years. Currently, there are few studies reporting the results of DeNovo Natural Tissue (NT) allograft tissue implantation (Zimmer, Columbus, OH), with only 2 current series reviewing the outcomes for the treatment of patellar lesions.6,7 This procedure offers the advantage of a single stage approach to lesion treatment without violation of the subchondral plate. The allograft nature of the procedure also eliminates the donor site morbidity associated with autograft transfer techniques. Short-term results have been promising, and compare favorably to more traditional cartilage repair techniques.7,8
The purpose of this investigation was to comprehensively evaluate the magnetic resonance imaging (MRI) findings of the reconstructed cartilage in patients who had undergone transplantation of PJAC for symptomatic full thickness cartilage defects of the patella. Utilizing standard morphologic MRI and T2 mapping we sought to correlate patient and graft characteristics with lesion fill, maturation of healing tissue, and the status of the surrounding cartilage. We hypothesized that the graft would offer acceptable fill rates, that it would mature over time, and would not be associated with any negative biologic effects on the surrounding tissue.
Methods
Patient Identification and Data Collection
After institutional review board approval, a review of prospectively collected data was conducted to identify consecutive patients who had been treated at our institution with PJAC for full-thickness symptomatic patellar cartilage lesions, and subsequently underwent MRI of their knee postoperatively. We collected data related to various patient-specific factors, lesion characteristics, and allograft tissue features. The patient specific factors that were collected included age, sex, duration of knee symptoms, and other knee pathology, including the presence of patellar instability. Lesion-specific factors included lesion size, location, and etiology of the cartilage defect. Graft specific factors that were collected for analysis included donor age and number of allograft packets utilized for reconstruction.
Surgical Technique
The PJAC transplantation was performed by 1 of 4 operating surgeons using a consistent surgical technique. All patients initially underwent a diagnostic knee arthroscopy to document the status of all compartments of the knee, verifying that the diseased articular cartilage was confined to the patellofemoral articulation. Reconstruction of the patellar cartilage was then undertaken following the arthroscopic portion of the procedure. There were several different etiologies of patellar cartilage lesions in this study and thus the majority of patients underwent concomitant procedures to address underlying pathologies during the index patellar cartilage reconstruction. Secondary procedures were dependent on the pathoanatomy of the patient at the time of surgery and included lateral retinacular release, tibial tubercle transfer, loose body removal, trochlear cartilage reconstruction, and/or medial patellofemoral ligament (MPFL) reconstruction.
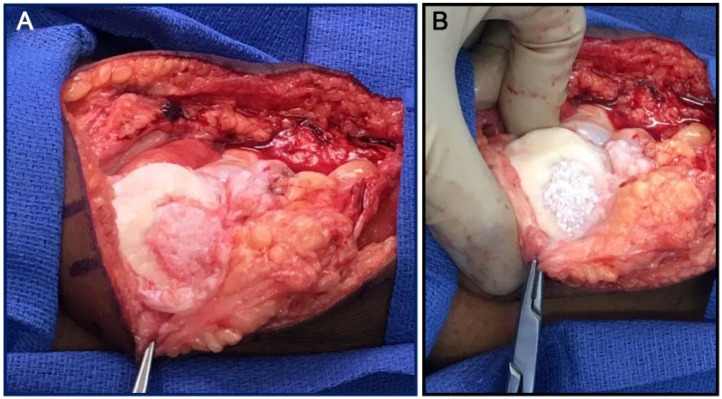
Patella cartilage reconstruction with PJAC was performed in an open fashion in all patients through a small medial parapatellar arthrotomy. The decision to perform a cartilage reconstruction procedure with this graft type was made preoperatively based on the presence of a full thickness cartilage lesion on the undersurface of the patella, as demonstrated by preoperative MRI. The recipient bed was identified and gently debrided to ensure that the subchondral plate was not violated and that no bleeding bone was exposed. Every attempt was made to create a well-shouldered lesion with vertical walls at the site of the patella cartilage defect. Allograft tissue was then implanted using one of two methods and secured with fibrin glue. The first method consisted of placing the particulate tissue directly in the lesion with an allograft to lesion size ratio of 1 packet for every 2.5 cm2 of defect area. This method allowed for a single layer of minced allograft to be placed in the defect bed and then secured with fibrin glue. An alternate method for defect reconstruction involved first templating the geometry of the lesion with sterile foil. Once the template was obtained allograft tissue was placed inside the template and secured with fibrin glue, effectively making an allograft implant to be placed into the defect and then sealed with fibrin glue. This technique allowed for complete lesion fill with the implant ( Fig. 1 ).
Figure 1.
(A) An intraoperative photography of a patellar chondral lesion prior to particulated juvenile allograft cartilage (PJAC) transplantation. (B) The lesion following PJAC transplantation.
Postoperatively, all patients were placed in a hinged knee brace locked in extension for 4 to 6 weeks, and passive range of motion with the use of a continuous passive motion device was initiated at day 2 to 5. Initially, passive motion was restricted to 0° to 30° and progressed as tolerated, and patients were initially allowed toe-touch weightbearing in the brace, progressing to partial weightbearing with the brace locked in full extension. Continuous passive motion was used for 4 to 6 hours per day for the first 6 weeks. Eccentric quadriceps exercises were initiated within the first week of surgery. After the initial healing phase strengthening exercises were started and the patients began sport specific training at the 3- to 4-month time point. The rehabilitation protocol was modified as indicated by other associated procedures (tibial tubercle transfer or MPFL reconstruction).
MRI Acquisition and Grading9
A 1.5-T (450 model) or a 3.0-T (750 model) imaging system (General Electric Healthcare, Milwaukee, WI) was utilized for MRI purposes. Fast spin echo images were obtained in 3 planes to allow assessment of articular cartilage using a previously validated cartilage-sensitive pulse sequence.10 The moderate echo time (TE) used to acquire images allowed for high-contrast resolution between articular cartilage, subchondral bone, and joint fluid, while avoiding the susceptibility artifacts of the postoperative knee seen in gradient echo imaging techniques. A repetition time (TR) of 3500 to 6000 ms, TE of 34 ms (effective), field of view of 13 to 16 cm2, and matrix of 512 × 256 to 416, providing minimum in-plane resolution of 254 μm in the frequency direction by 312 μm in the phase direction by slice resolution of 3 to 3.5 mm with no gap. A wider receiver bandwidth of 31.2 to 62.5 kHz was used over the entire frequency range for minimization of chemical shift misregistration. A fat-suppressed pulse sequence in the sagittal plane was used to evaluate for the presence of subchondral bone marrow edema.
T2 relaxation time mapping was performed using a multislice, multiecho modified CPMG (Carr-Purcell-Meiboom-Gill) pulse sequence, which uses interleaved slices and tailored refocusing pulses to minimize contribution from simulated echoes.11 Standard T2 mapping pulse sequence parameters used were a TR of 800 ms, 8 echoes sampled using sequential multiples of the first TE (9-10 ms), field of view of 16 cm2, and matrix of 256 to 384 × 256, providing a minimum in-plane resolution of 254 μm in the frequency direction by 312 μm in the phase direction, by slice resolution of 2.0 to 3.0 mm with no gap, and a receiver bandwidth of 62.5 kHz. After image acquisition, data sets were analyzed on a pixel-by-pixel basis with a 2-parameter weighted least-squares fit algorithm, assuming a monoexponential fit (Functool 3.1, General Electric Healthcare). Quantitative T2 values were calculated by taking the natural logarithm of the signal decay curve in a selected region of interest. Regions of interest were obtained in a standardized fashion, from the treated area of articular cartilage, at the interface with adjacent uninvolved tissue, as well as of the adjacent and opposite articular cartilage surfaces. All T2 relaxation times in the article will be reported in milliseconds.
All MRI studies were read by a single experienced musculoskeletal radiologist without knowledge of the patient or treating surgeon.10 The images were scored according to a previously described cartilage repair criteria: signal intensity of the repaired area relative to the surrounding cartilage (hypointense, isointense, or hyperintense), subchondral edema (none, mild, moderate, or severe), geometry, bony overgrowth (absence or presence), interface with adjacent cartilage (absence, presence, size of fissure), percentage of fill based on both coronal and sagittal images (0%-33%, 34%-66%, or 67%-100%), integrity of adjacent cartilage (Outerbridge grading), fat-pad scarring (mild, moderate, or severe), synovial reaction, graft hypertrophy, and graft displacement.12,13 This will be referred to as the “grayscale” score for the remainder of the article.
Statistical Analysis
Descriptive statistics were calculated for patient and clinical characteristics for the study cohort. For continuous data, means with standard deviations and ranges are reported while frequencies and percentages are reported for discrete variables. Bivariate correlations were performed to assess the relationship between patient characteristics versus various MRI readings that were performed at 6, 12, and 24 months. Pearson correlation coefficients were used to assess the magnitude of the correlation at 6 and 12 months, while Spearman’s rho coefficients were used for correlations at 24 months. Because the assumption of normality was not met, non-parametric Kruskal-Wallis tests were used to compare the differences in patient and MRI T2 data between the different levels of lesion deficit fill. To control for the clustered nature of the longitudinal data, generalized estimating equation (GEE) models were used to evaluate MRI changes that were used to assess graft maturation over time. All analyses were performed using SPSS version 22.0 (IBM Corp, Armonk, NY).
Results
Patient Demographics
From the years 2010 to 2014, 45 patients underwent transplantation of particulate juvenile allograft cartilage for the treatment of a symptomatic cartilage lesion of the patella. In total, 38 patients were available for MRI acquisition at the 6-month postoperative time point and 34 patients were available at the 12-month time point. Eleven patients were identified with 24-month MRI follow-up. Forty percent of the patient population (n = 18) were male, while 60% were female (n = 27). Patient characteristics included an average age at the time of surgery of 26.5 years (range 13-45 years), and the average duration of symptoms prior to undergoing cartilage reconstruction was 56.6 months (range 2 weeks to 45 years). Average lesion size on the undersurface of the patella was 208 mm2 (range 4-500 mm2). The average number of allograft packets delivered to the cartilage lesion was 1.7 (range 1-3), and the average donor age of the allograft tissue was 49.5 months (range 3-120 months). In total, 42 of the 45 (93%) patients underwent a concomitant procedure at the time of their index patellar cartilage reconstruction. The mean number of concomitant procedures was 1.3, with a range of 0 to 3 ( Table 1 ).
Table 1.
Patient Characteristics.
| Patient Characteristic | Mean or n | SD or % | Minimum | Maximum |
|---|---|---|---|---|
| Patient age, years | 26.5 | 9.0 | 13.0 | 45.0 |
| Donor age, months | 49.5 | 38.1 | 3.0 | 120.0 |
| No. of packets | 1.7 | 0.6 | 1.0 | 3.0 |
| 1 | 16 | 36.4 | ||
| 2 | 25 | 56.8 | ||
| 3 | 3 | 6.8 | ||
| Lesion area, mm2 | 208.2 | 121.5 | 4.0 | 500.0 |
| Symptom duration, months | 56.6 | 51.7 | 0.5 | 180.0 |
| Patient sex | ||||
| Male | 18 | 40.0% | ||
| Female | 27 | 60.0% | ||
| Diagnosis | ||||
| Trauma | 5 | 11.1% | ||
| OA | 14 | 31.1% | ||
| Instability | 26 | 57.8% | ||
| Concomitant procedure | 1.6 | 0.9 | 0 | 3 |
| Osteotomy | 19 | 42.2% | ||
| MPFL | 19 | 42.2% | ||
| Lateral release | 14 | 31.1% | ||
| OCD/OATS | 5 | 11.1% | ||
| Loose bodies | 5 | 11.1% | ||
| Debridement | 3 | 6.7% | ||
| Anterior release | 3 | 6.7% | ||
| Microfracture | 2 | 4.4% | ||
| Arthrotomy | 2 | 4.4% | ||
| Chondroplasty | 1 | 2.2% | ||
| Elmslie | 1 | 2.2% | ||
| None | 3 | 6.7% | ||
OA = osteoarthritis; MPFL= medial patellofemoral ligament; OCD = osteochondritis dissecans; OATS = osteochondral autograft transfer system.
MRI Findings at 6 Months
MRI follow-up at 6 months demonstrated that patient age, donor age, lesion size, and preoperative symptom duration did not correlate with T2 mapping results or any of the factors within the grayscale scoring system (including overall score). The overall MRI grayscale score mirrored the defect percent fill in a nearly linear fashion. Specifically, with regard to percentage of defect fill by the graft, 7 patients had poor fill (0%-33%), 10 had moderate fill (34%-66%), and 21 demonstrated good fill (67%-100%). Two patients were noted to have developed graft hypertrophy and only 1 patient displayed significant graft displacement, as measured on MRI. Subchondral edema at the lesion site and adjacent synovitis were minimal, as only 3 and 2 knees were affected, respectively. ( Tables 2 and 3 )
Table 2.
Quantitative MRI Characteristics with Patient-Specific Factors.
| MRI Characteristic | MRI Follow-up | Patient Age | Donor Age | Lesion Area | Symptom Duration |
|---|---|---|---|---|---|
| MRI Total | 6Ma | 0.021 | −0.186 | −0.212 | −0.128 |
| 12Ma | −0.223 | −0.287 | −0.316 | −0.311 | |
| 24Mb | −0.252 | −0.628* | −0.374 | −0.257 | |
| T2 average deep normal cartilage | 6Ma | 0.019 | −0.139 | 0.052 | −0.081 |
| 12Ma | −0.129 | −0.030 | 0.034 | 0.109 | |
| 24Mb | −0.108 | 0.086 | 0.185 | 0.810* | |
| T2 average deep graft | 6Ma | −0.226 | −0.170 | −0.090 | 0.092 |
| 12Ma | −0.369* | −0.511** | 0.094 | −0.319 | |
| 24Mb | −0.874** | −0.528 | 0.037 | 0.024 | |
| T2 average deep integration zone | 6Ma | −0.048 | −0.218 | −0.186 | 0.142 |
| 12Ma | −0.045 | −0.419* | −0.130 | 0.135 | |
| 24Mb | −0.611 | −0.798* | 0.519 | −0.381 | |
| T2 average superficial normal cartilage | 6Ma | −0.146 | −0.116 | −0.221 | 0.016 |
| 12Ma | −0.336 | −0.310 | −0.029 | 0.063 | |
| 24Mb | 0.934** | 0.307 | 0.037 | −0.143 | |
| T2 average superficial graft | 6Ma | −0.147 | 0.011 | 0.090 | 0.088 |
| 12Ma | −0.075 | −0.055 | 0.062 | −0.254 | |
| 24Mb | −0.759* | −0.482 | 0.262 | 0.156 | |
| T2 average superficial integration zone | 6Ma | 0.249 | 0.018 | −0.178 | 0.173 |
| 12Ma | −0.200 | −0.144 | −0.134 | −0.022 | |
| 24Mb | 0.096 | 0.233 | 0.408 | 0.405 |
MRI = magnetic resonance imaging; 6M = 6 months; 12M = 12 months; 24M = 24 months.
Pearson correlation coefficient.
Spearman’s rho coefficient.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Table 3.
Differences in Patient Characteristics and Quantitative MRI Measures of Defect Fill.
| Characteristic | MRI Follow-Up | Poor Fill (0%-33%) | Moderate Fill (34%-66%) | Good Fill (67%-100%) | P |
|---|---|---|---|---|---|
| Patient age | 6M | 23.3 (7.0) | 30.8 (8.1) | 24.5 (8.7) | 0.106 |
| 12M | 25.0 (6.9) | 32.2 (5.9) | 23.2 (8.3) | 0.015 | |
| 24M | 24.5 (14.8) | 34.0 (0.0) | 25.8 (11.4) | 0.866 | |
| Donor age | 6M | 60.8 (40.9) | 52.9 (39.0) | 43.3 (39.0) | 0.593 |
| 12M | 68.2 (45.2) | 48.8 (36.4) | 43.9 (41.4) | 0.504 | |
| 24M | 66.0 (42.4) | 84.0 (0.0) | 35.8 (42.1) | 0.274 | |
| Lesion area | 6M | 314.0 (184.3) | 209.6 (117.9) | 190.3 (94.7) | 0.091 |
| 12M | 233.5 (174.0) | 167.1 (103.3) | 176.4 (93.0) | 0.573 | |
| 24M | 350.0 (212.1) | 49.0 (0.0) | 146.6 (48.3) | 0.078 | |
| Symptom duration | 6M | 60.7 (55.2) | 58.4 (56.5) | 50.1 (45.4) | 0.849 |
| 12M | 46.6 (43.5) | 62.3 (56.6) | 40.4 (37.0) | 0.459 | |
| 24M | 84.0 (101.8) | 180.0 (0.0) | 55.3 (53.6) | 0.249 | |
| MRI total | 6M | 16.3 (2.2) | 19.8 (2.0) | 22.7 (2.4) | <0.001 |
| 12M | 15.4 (1.5) | 21.3 (3.3) | 22.9 (2.3) | 0.000 | |
| 24M | 15.0 (2.8) | 22.0 (0.0) | 22.9 (3.4) | 0.104 | |
| T2 average deep normal cartilage | 6M | 26.6 (4.9) | 23.4 (3.5) | 27.3 (3.6) | 0.057 |
| 12M | 26.6 (4.5) | 22.4 (3.5) | 24.5 (3.6) | 0.160 | |
| 24M | 0.0 (0.0) | 28.8 (0.0) | 27.0 (1.7) | 0.317 | |
| T2 average deep graft | 6M | 50.5 (12.8) | 44.6 (9.9) | 54.5 (10.1) | 0.090 |
| 12M | 46.9 (9.8) | 41.4 (12.9) | 42.8 (7.8) | 0.665 | |
| 24M | 0.0 (0.0) | 30.5 (0.0) | 31.4 (7.6) | 1.000 | |
| T2 average deep integration zone | 6M | 35.8 (1.3) | 40.5 (7.3) | 43.0 (5.0) | 0.097 |
| 12M | 37.4 (10.0) | 36.5 (5.2) | 37.4 (4.9) | 0.931 | |
| 24M | 0.0 (0.0) | 27.2 (0.0) | 31.2 (5.1) | 0.317 | |
| T2 average superficial normal cartilage | 6M | 32.7 (3.7) | 30.2 (4.7) | 34.1 (4.2) | 0.122 |
| 12M | 32.7 (5.9) | 30.6 (5.3) | 30.7 (2.8) | 0.673 | |
| 24M | 0.0 (0.0) | 32.4 (0.0) | 33.7 (5.4) | 1.000 | |
| T2 average superficial graft | 6M | 52.2 (6.7) | 52.3 (10.5) | 55.1 (11.8) | 0.786 |
| 12M | 52.7 (7.3) | 51.4 (11.9) | 48.2 (12.4) | 0.708 | |
| 24M | 0.0 (0.0) | 29.6 (0.0) | 33.8 (6.5) | 0.801 | |
| T2 average superficial integration zone | 6M | 42.4 (5.8) | 41.9 (5.7) | 44.5 (5.5) | 0.512 |
| 12M | 41.9 (7.7) | 39.1 (6.1) | 41.5 (4.8) | 0.546 | |
| 24M | 0.0 (0.0) | 31.1 (0.0) | 35.8 (5.2) | 0.617 |
MRI = magnetic resonance imaging; 6M = 6 months; 12M = 12 months; 24M = 24 months.
MRI Findings at 12 Months
At the 12-month time point, the T2 average relaxation times of the deep graft segments demonstrated a negative correlation with both patient age (r = −0.369; P = 0.049) and donor age (r = −0.511; P = 0.006). T2 average relaxation times of the deep integration zone of the graft also demonstrated a negative correlation with donor age of the graft (r = −0.419; P = 0.026). No other patient or graft characteristics affected the postoperative MRI scores at the 12-month time interval. The overall MRI grayscale score mirrored the defect percent fill in a nearly linear fashion. Specifically, with regards to percentage of defect fill by the graft, 5 patients had poor fill (0%-33%), 10 had moderate fill (34%-66%), and 19 demonstrated good fill (67%-100%). Two patients were noted to have developed graft hypertrophy and only 1 patient at the 6-month time point displayed significant graft displacement as measured on MRI. One patient demonstrated moderate subchondral edema at the lesion site and one patient demonstrated severe edema. 3/31 knees displayed mild synovitis ( Tables 2 and 3 ).
MRI Findings at 24 Months
Age of the patient demonstrated a negative correlation with average T2 relaxation times of the deep and superficial graft (r = −0.874; P = 0.005 and r = −0.759; P = 0.0029) and a positive correlation with the superficial zone of the adjacent normal cartilage (r = 0.934; P = 0.001). Donor age displayed a negative correlation with both the overall grayscale score (r = −0.628; P = 0.0039) and the average T2 relaxation times at the deep integration zone (r = −0.798; P = 0.018). Duration of clinical symptoms demonstrated a positive correlation with the average T2 relaxation times of the adjacent deep normal cartilage. No other patient or graft characteristics affected the postoperative MRI scores at this time point. Percentage of defect fill by the graft demonstrated similar results to the other time points: 2 patients had poor fill (0%-33%), 1 had moderate fill (34%-66%), and 8 demonstrated good fill (67%-100%). Percentage of defect filled at the 24-month MRI follow-up was not significantly related to any patient, lesion, or graft characteristics. One patient was noted to have developed graft hypertrophy and no patients displayed graft displacement as measured on MRI. Subchondral edema was absent at this time point, and only 1 knee demonstrated a reactive synovial response ( Tables 2 and 3 ).
Graft Maturation over Time
Final scoring results based on grayscale imaging showed no significant differences between 6, 12, and 24 months (average score of 20.9 points at 6 months, 21.5 points at 12 months, and 21.5 points at 24 months). Average T2 relaxation times of both deep and superficial portions of the graft improved (decreased) over time (P < 0.001) and between each time point in a statistically significant fashion (see appendix) ( Figs. 2 and 3 ).
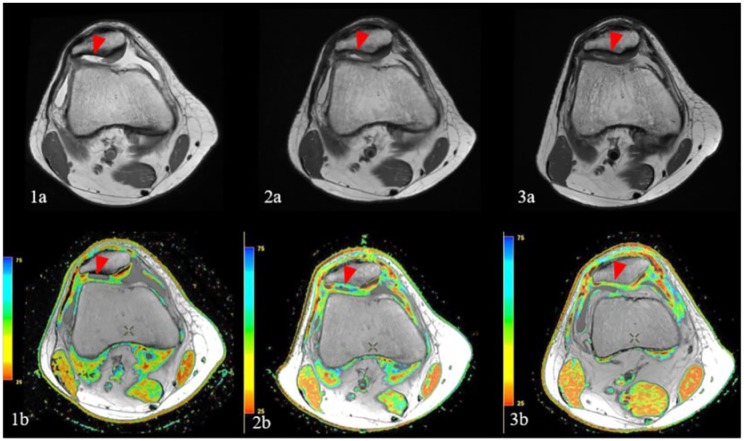
Figure 2.
Sequential magnetic resonance images demonstrate a shouldered chondral defect of the lateral patellar facet on initial fast spin echo proton density (FSE PD) weighted images (1a, red arrowhead), with corresponding defect on quantitative T2 cartilage mapping sequence (1b, red arrowhead). Subsequent FSE PD image (2a) 6 months following patellar particulated juvenile allograft cartilage (PJAC) transplant demonstrates good fill of the defect by hyperintense repair tissue (red arrowhead), with corresponding prolongation of relaxation times and lack of normal chondral stratification on corresponding T2 map (2b, red arrowhead). FSE PD image (3a) obtained 12 months following repair demonstrates decreased relative hyperintensity of the graft, with corresponding decrease in T2 relaxation values and early chondral stratification on T2 mapping (3b, red arrowhead), indicative of ongoing graft maturation.
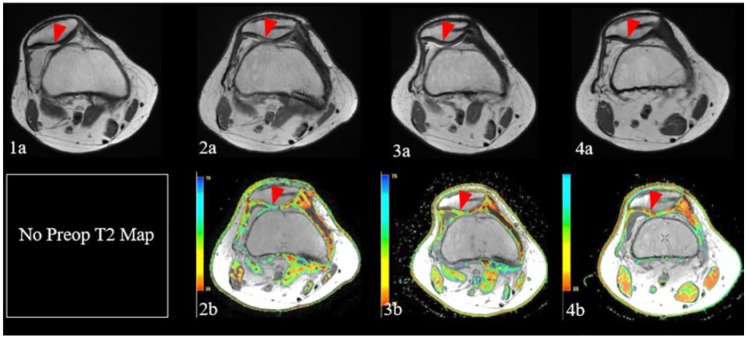
Figure 3.
Sequential magnetic resonance images demonstrate chondral defect over the lateral patellar facet on initial fast spin echo proton density (FSE PD) weighted images (1a, red arrowhead), in the setting of lateral patellar subluxation. Subsequent FSE PD image (2a) following patellar particulated juvenile allograft cartilage (PJAC) transplantation, medial patellofemoral ligament (MPFL) reconstruction, and tibial tubercle osteotomy demonstrates restoration of normal patellofemoral alignment, as well as good fill of the chondral defect by hyperintense repair tissue (red arrowhead), with corresponding prolongation of relaxation times and lack of normal chondral stratification on corresponding T2 map (2b, red arrowhead). FSE PD image (3a) obtained approximately 1 year following repair demonstrates decreased relative hyperintensity of the graft, with corresponding decrease in T2 relaxation values and early chondral stratification on T2 mapping (3b, red arrowhead), indicative of ongoing graft maturation. Follow up images obtained approximately 2.5 years following the initial surgery demonstrate further normalization of graft signal on FSE PD weighted images (4a, red arrowhead), with further decrease in relaxation times and progressive stratification of repair tissue on T2 maps (4b, red arrowhead), reflecting further graft maturation.
Average T2 relaxation times of both deep and superficial portions of the integration zone also improved over time (P < 0.001) and between each time point in a statistically significant fashion (see appendix). Average T2 relaxation times of the adjacent normal cartilage did not change over time or between time points in statistically significant fashion (see appendix) ( Fig. 4 ).
Figure 4.
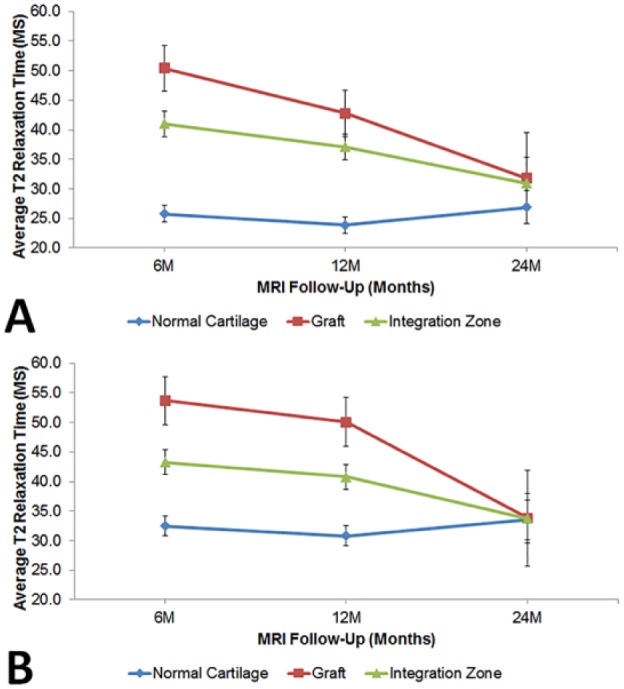
(A) Average relaxation times of the deep portion of the graft, integration zone, and adjacent normal cartilage, where significant change in T2 relaxation time was found in the graft and integration zone (P < 0.001 for both). (B) Average relaxation times of the superficial portion of the graft, integration zone, and adjacent normal cartilage, where similar significant changes were seen in the graft (P < 0.001) and integration zone (P = 0.001).
Discussion
Patellar chondral lesions have historically been treated with a myriad of reconstructive techniques.9 Currently, no gold standard exists, and previous authors have focused on techniques such as microfracture,14 autologous chondrocyte implantation,11 osteochondral autograft transplantation,9 and osteochondral allograft transplantation.15 The goal of the present investigation was to comprehensively evaluate tissue regeneration in the treated cartilage lesion with the use of postoperative MRI. By utilizing both a cartilage-sensitive pulse sequence and T2 mapping, we were able to describe the allograft maturation process. Basic morphologic grading (“grayscale scoring”) demonstrated only incremental, nonsignificant improvements between 6, 12, and 24 months. Percent fill at each time point demonstrated favorable results with 82% of patients having moderate to good fill at the 6-month mark, 85% of patients having moderate to good fill at the 12-month mark, and 75% of patient demonstrating moderate to good fill at 24 months following surgery.
T2 relaxation time mapping is a powerful tool that allows clinicians to assess collagen organization as a measure of tissue maturation, and has been shown to correlate with collagen orientation within the articular cartilage.16 Overall, following allograft transplantation, our results demonstrated a decrease (improvement) in average T2 relaxation times for the graft from 6, 12, and 24 months, with the 24-month results producing T2 relaxation times similar to adjacent (normal) cartilage. This phenomenon was true for T2 relaxation times in both the deep and superficial portions of the graft and integration zone. The graft itself is a biologically active tissue and it is not surprising that it matures and integrates with adjacent normal tissue in a temporal fashion. These results are consistent with a recently reported study evaluating particulated juvenile allograft cartilage for the treatment of femoral and/or tibial cartilage lesions, in which Farr et al.8 observed a linear decrease in T2 relaxation times, and concluded that tissue maturation and reorganization was still occurring over a time period of 24 months.
Further study should focus on determining the relationship between donor age (and possibly other donor characteristics) and graft biology, with the aim to identify optimal donor characteristics. Our study demonstrated that younger donor age was correlated with increasing T2 relaxation time, which might be accounted for by the high synthetic capacity in very young donors, and the rapid synthesis of extracellular matrix proteins potentially leading to a matrix with early poor collagen organization. It is also difficult to understand the finding of increasing patient age being correlated with lower T2 relaxation times, and this fact might be explained by the heterogeneity of the minced graft material. Every attempt was made to sample a region of interest most representative of the graft, however at early time points the graft may still be in its particulated phase and therefore causing heterogeneity (both prolonged and shortened) within relaxation time measurements.
The graft itself demonstrated excellent stability with minimal adverse effects on local tissue. Only 1 patient in our series sustained graft displacement, and only 2 patients demonstrated graft hypertrophy. Hypertrophy was defined based on the appearance of the graft at the defect site and whether or not the tissue volume was greater in comparison to the surrounding cartilage. Subchondral edema at the lesion was minimal at all 3 time points, and improved over time. In a similar fashion only a small percentage of knee displayed synovitis after grafting. Based on T2 relaxation times of adjacent cartilage and the integration zone, we can conclude that placement of the graft did not have any adverse effects on the native local tissue.
Our study has several weaknesses. We are not reporting our clinical data here, as our purpose was to objectively evaluate structural healing of the lesions and to evaluate the characteristics of the allograft tissue in a temporal fashion. The majority of these patients underwent a concomitant procedure at the time of allograft transplantation, which ultimately confounds clinical results when assessing improvement in knee function following operative treatment of complex knee pathology. Therefore, to isolate the results of the transplanted allograft tissue, we chose to comprehensively evaluate its appearance on postoperative MRI, and not investigate clinical outcomes. Of note, we do acknowledge that the status of the trochlear cartilage is very important to the overall effect of the graft. Only 2 patients displayed significant disease of the trochlea requiring microfracture at the time of their PJAC procedure ( Table 1 ). A second weakness of the study would be the age ranges of the patients treated as there is likely a physiologic healing difference between adolescent patients and the older patients. A third weakness of our study is the lack of serial MRIs in all patients. However, the added strengths of a relatively large number of patients at each time point and the addition of T2 mapping enhances the usefulness of the information reported herein.
In conclusion, particulated juvenile allograft tissue appears to be an acceptable reconstructive option for full-thickness cartilage lesions of the patella, offering satisfactory tissue defect fill at 6, 12, and 24 months after surgery. Imaging of the repaired cartilage demonstrates progressive graft maturation over time.
Appendix
Table A1.
Results Comparing Longitudinal Change in Normal, Graft, and Integration Zone in Deep and Superficial MRI Measures.a
| MRI Zone | MRI Follow-up (Months) | Mean (ms) | Standard Error | 95% Confidence Interval |
P | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Deep portion | Normal cartilage | |||||
| 6M | 25.8 | 0.7 | 24.4 | 27.2 | 0.069 | |
| 12M | 23.9 | 0.7 | 22.5 | 25.3 | ||
| 24M | 26.9 | 1.4 | 24.1 | 29.7 | ||
| Graft | ||||||
| 6M | 50.4 | 1.9 | 46.6 | 54.2 | <0.001 | |
| 12M | 42.8 | 2.0 | 38.9 | 46.7 | ||
| 24M | 31.8 | 3.8 | 24.1 | 39.5 | ||
| Integration zone | ||||||
| 6M | 41.0 | 1.1 | 38.9 | 43.2 | <0.001 | |
| 12M | 37.1 | 1.1 | 34.8 | 39.3 | ||
| 24M | 30.9 | 2.2 | 26.6 | 35.3 | ||
| Superficial portion | Normal cartilage | |||||
| 6M | 32.48 | 0.84 | 30.79 | 34.17 | 0.214 | |
| 12M | 30.79 | 0.84 | 29.10 | 32.47 | ||
| 24M | 33.51 | 1.66 | 30.20 | 36.83 | ||
| Graft | ||||||
| 6M | 53.69 | 2.02 | 49.65 | 57.74 | <0.001 | |
| 12M | 50.07 | 2.06 | 45.95 | 54.18 | ||
| 24M | 33.80 | 4.04 | 25.72 | 41.88 | ||
| Integration zone | ||||||
| 6M | 43.29 | 1.04 | 41.21 | 45.37 | 0.001 | |
| 12M | 40.80 | 1.06 | 38.68 | 42.92 | ||
| 24M | 33.76 | 2.08 | 29.59 | 37.92 | ||
Table A2.
The Comparison Here Is Used to See if There Were Differences between the Normal, Graft, and Integration Zone at Each Time Point, within the Deep and Superficial MRI Reads.
| MRI Zone | Average T2 Relaxation Time (ms)—Deep Portion |
||||||
|---|---|---|---|---|---|---|---|
| MRI Follow-up (Months) | MRI Group | Mean (ms) | Standard Error | 95% Confidence Interval |
P | ||
| Lower Bound | Upper Bound | ||||||
| Deep portion | 6M | Normal cartilage | 25.8 | 0.7 | 24.4 | 27.2 | <0.001 |
| 6M | Graft | 50.4 | 1.9 | 46.6 | 54.2 | ||
| 6M | Integration zone | 41.0 | 1.1 | 38.9 | 43.2 | ||
| 12M | Normal cartilage | 23.9 | 0.7 | 22.5 | 25.3 | <0.001 | |
| 12M | Graft | 42.8 | 2.0 | 38.9 | 46.7 | ||
| 12M | Integration zone | 37.1 | 1.1 | 34.8 | 39.3 | ||
| 24M | Normal cartilage | 26.9 | 1.4 | 24.1 | 29.7 | 0.427 | |
| 24M | Graft | 31.8 | 3.8 | 24.1 | 39.5 | ||
| 24M | Integration zone | 30.9 | 2.2 | 26.6 | 35.3 | ||
| Superficial Portion | 6M | Normal cartilage | 32.48 | 0.84 | 30.79 | 34.17 | <0.001 |
| 6M | Graft | 53.69 | 2.02 | 49.65 | 57.74 | ||
| 6M | Integration zone | 43.29 | 1.04 | 41.21 | 45.37 | ||
| 12M | Normal cartilage | 30.79 | 0.84 | 29.10 | 32.47 | <0.001 | |
| 12M | Graft | 50.07 | 2.06 | 45.95 | 54.18 | ||
| 12M | Integration zone | 40.80 | 1.06 | 38.68 | 42.92 | ||
| 24M | Normal cartilage | 33.51 | 1.66 | 30.20 | 36.83 | 0.997 | |
| 24M | Graft | 33.80 | 4.04 | 25.72 | 41.88 | ||
| 24M | Integration zone | 33.76 | 2.08 | 29.59 | 37.92 | ||
MRI = magnetic resonance imaging; 6M = 6 months; 12M = 12 months; 24M = 24 months.
Footnotes
Acknowledgment and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Informed Consent: Written informed consent was obtained from all subjects before the study.
Ethical Approval: Not applicable.
Trial registration: Not applicable.
References
- 1. Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage of the knee. J Bone Joint Surg Am. 2010;92:994-1009. [DOI] [PubMed] [Google Scholar]
- 2. Noyes F, Barber-Westin SD. Advanced patellofemoral cartilage lesions in patients younger than 60 years of age: is there an ideal operative option? Arthroscopy. 2013;29:1423-36. [DOI] [PubMed] [Google Scholar]
- 3. van Jonbergen HP, Poolman RW, van Kampen A. Isolated patellofemoral osteoarthritis. A systematic or treatment options using the GRADE approach. Acta Orthop. 2010;81:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mouzopoulos G, Borbon C, Siebold R. Patellar chondral defects: a review of a challenging entity. Knee Surg Sports Traumatol Arthroosc. 2011;19:1990-2001. [DOI] [PubMed] [Google Scholar]
- 5. Griffin JW, Gilmore J, Miller MD. Treatment of a patellar chondral defect using juvenile articular cartilage allograft implantation. Arthroscopy. 2013;2:e351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buckwalter JA, Bowman GN, Albright JP, Wolf BR, Bollier M. Clincial outcomes of patellar chondral lesions treated with juvenile particulated cartilage allografts. Iowa Orthop J. 2014;34:44-49. [PMC free article] [PubMed] [Google Scholar]
- 7. Tompkins M, Hamann JC, Diduch DR, Bonner KF, Hart JM, Gwathmey W, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29:1661-70. [DOI] [PubMed] [Google Scholar]
- 8. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage. Am J Sports Med. 2014;42:1417-25. [DOI] [PubMed] [Google Scholar]
- 9. Nho SJ, Foo LF, Green DM, Shindle MK, Warren RF, Wickiewicz TL, et al. Magnetic resonance imaging and clinical evaluation of patellar resurfacing with press-fit osteochondral autograft plugs. Am J Sports Med. 2008;36:1101-9. [DOI] [PubMed] [Google Scholar]
- 10. Potter HG, Linklater LM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage of the knee: an evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80:1276-84. [DOI] [PubMed] [Google Scholar]
- 11. Peterson L. Articular cartilage injuries treated with autologous chondrocyte transplantation in the human knee. Acta Orthop Belg. 1996;62(suppl 1):196-200. [PubMed] [Google Scholar]
- 12. Brown WE, Potter HG, Marx RG, Wickiewicz TL, Warren RF. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin Orthop Relat Res. 2004;422:214-33. [DOI] [PubMed] [Google Scholar]
- 13. Maier CF, Tan SG, Hariharan H, Potter HG. T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging. 2003;17:358-64. [DOI] [PubMed] [Google Scholar]
- 14. Steadman J, Rodkey W, Singleton S, Briggs K. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop. 1997;7:300-4. [Google Scholar]
- 15. Garret JC. Fresh osteochondral allografts for the treatment of articular defects in osteochondritis dissecans of the lateral femoral condyle in adults. Clin Orthop Relat Res. 1994;303:33-7. [PubMed] [Google Scholar]
- 16. Xia Y, Moody JB, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage. 2001;9:393-406. [DOI] [PubMed] [Google Scholar]