Abstract
Cyclosporin A (CsA) is effective at reducing pathogenic immune responses, but upon withdrawal of CsA the immune response often “rebounds” resulting in a relapse or exacerbation of disease. The mechanisms, cells and cytokines involved in the relapse or exacerbation after CsA withdrawal are unknown. We hypothesized that CsA withdrawal induces IL-17 production that could be responsible for relapse, and examined the effect of anti-IL-17A antibody on relapse induced after CsA withdrawal in mouse experimental autoimmune encephalomyelitis (EAE). CsA treatment markedly decreased the EAE disease score during the first episode, but augmented disease severity after CsA withdrawal, compared to untreated mice. After discontinuation of CsA the production of IL-17A was increased and the severity of relapse in EAE was reduced by treatment with anti-IL-17A antibody. These results suggest that the resumption of T cell immune responses after CsA withdrawal leads to a burst of IL-17A production that is at least partially responsible for relapse in EAE mice.
Abbreviations: CNS, central nervous system; CsA, cyclosporine A; EAE, Experimental autoimmune encephalomyelitis; IL, interleukin; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; NFAT, Nuclear factor of activated T-cells; PLP, proteolipid protein; ROR-γT (, RAR-related orphan receptor-γT; STAT3, signal transducer and activator of transcription 3, Tc cells: cytotoxic T cells; Th cells, helper T cells; TNF-α, tumour necrosis factor-α
Keywords: IL-17A, Experimental autoimmune encephalomyelitis (EAE), Relapse, Cyclosporin A (CsA)
Highlights
-
•
Establishment of murine rebound model induced by CsA withdrawal.
-
•
Enhanced local and systemic secretion of IL-17A in EAE mice after CsA withdrawal.
-
•
Anti-IL-17A antibody prevents EAE relapse after CsA withdrawal.
1. Introduction
Cyclosporin A (CsA) is an immunosuppressant drug that combines with cyclophilin inside cells to form an inhibitor of calcineurin, a key signalling molecule in the nuclear factor of activated T cells (NFATc) transcription activator pathway. Inhibiting calcineurin and preventing the activation of NFATc leads to a reduction in the production of growth factors and proinflammatory cytokines secreted by T cells, and dampens immune responses and inflammation [1], [2]. The immunosuppressive effects of CsA are particularly potent for effector T helper cells, and less so for other lymphocytes [3], [4], [5], [6].
Early in the study of the immunosuppressive effects of CsA, it was discovered that the drug had a strong suppressing effect on the pathogenic immune responses of induced autoimmune diseases in animals, such as rat experimental autoimmune encephalomyelitis (EAE), an animal model of human multiple sclerosis. However, paradoxically, its abrupt withdrawal tends to cause a strong “rebound” of the immune response and an enhanced exacerbation of disease [7], [8]. An explanation for this is postulated to be that T cells inhibited by CsA undergo T cell proliferation triggered by the lymphopenia that follows treatment [9]. However, the reason for the rebound effect and the activation status of immune cells after CsA withdrawal has not been thoroughly studied.
We hypothesized that stimulatory signals received during T cell suppression by CsA might accumulate and re-activate T cells after CsA withdrawal, leading to hyperresponsiveness of T cells. Therefore, in the case of CsA-suppressed interleukin (IL)-17 producing T cell-mediated diseases such as multiple sclerosis (MS) or psoriasis, the resumption of immune responses after CsA withdrawal may therefore lead to a burst of IL-17 production that may be responsible for the relapse or exacerbation of disease. Based on this hypothesis, we expect that relapse induced by CsA withdrawal may be effectively blocked with antibodies to IL-17.
2. Material and methods
2.1. Ethics statement
Mice were kept under specific pathogen-free conditions, and provided food and water ad libitum. Every effort was made to minimize suffering during injections, and all surgery was performed in humanely sacrificed mice. All animal experiments were performed in accordance with the guidelines of the Bioscience Committee of Hokkaido University and were approved by the Animal Care and Use Committee of Hokkaido University (Approval license No. 13–0131).
2.2. Induction of relapsing EAE
Female SJL/J mice (Charles River, Shizuoka, Japan), 8–10 weeks old, were immunized with the encephalitogenic proteolipid peptide (PLP)139-151 (sequence: HSLGKWLGHPDKF; purity>90%) emulsified in complete Freund's adjuvant (Difco, Detroit, MI) plus Mycobacterium tuberculosis H37Ra (BD, Sparks, MD) (5 mg/ml). Then, mice were immunized with 0.2 ml of the emulsion injected subcutaneously into two locations at the tail root. At the time of immunization and 48 h later, the mice were administered 400 ng of pertussis toxin intravenously (List Biological Laboratories, Campbell, CA). Signs and severity of disease were recorded beginning on day 7 after immunization and continuing until day 50. The following scale to measure disease severity was used: 0=no paralysis, 1=limp tail, 2=limp tail and weak gait, 3=hind limb paralysis, 4=fore and hind limb paralysis, and 5=moribund. Body weight of mice was measured daily in addition to disease severity.
2.3. Treatment with CsA and antibody
Approximately 7–12 days after immunization, the first episode of clinical disease occurred in mice and lasted for 5–10 days. CsA (Tokyo Chemical Industry, Tokyo, Japan) (125 mg/kg) was administered intraperitoneally daily from day 8 to day 23 after PLP peptide immunization. On days 24, 27, 30, 33 and 36 after immunization, the CsA-treated group was treated with anti-IL-17A antibody (BZN035/Novartis, 10 mg/kg) or an isotype control antibody (mIgG2a, 10 mg/kg) intraperitoneally.
2.4. Measurement of cytokines
IL-6, TNF-α, IL-17A, IL-17F and IL-17A/F heterodimer concentrations in serum on day 30 or 40 after PLP peptide immunization were measured by IL-6 (BioLegend, San Diego, CA), tumor necrosis factor-α (TNF-α; BioLegend), IL-17A (BioLegend), IL-17F (eBioscience, Diego, CA) and IL-17A/F heterodimer (BioLegend) enzyme-linked immunosorbent assay (ELISA) kits as specified by the manufacturers.
2.5. Real-time PCR
Total RNA from mouse spleen and spinal cord at day 30 and 40 were extracted with TRIzol (Invitrogen, Waltham, MA) and first-strand cDNA was generated with ReverTra Ace (Toyobo, Osaka, Japan). Real-time quantitative PCR was performed using KAPA SYBR FAST qPCR Systems (KAPA Biosystems, Wilmington, MA). The specific primers used were: 5′-TCCAGAAGGCCCTCAGACTA-3′ (sense for IL-17A) and 5′-AGCATCTTCTCGACCCTGAA-3′ (antisense for IL-17A), 5′-CAAAACCAGGGCATTTCTGT-3′ (sense for IL-17F) and 5′-ATGGTGCTGTCTTCCTGACC-3′ (antisense for IL-17F), 5′-CCAAACTGGATATAATCAGGAAAT-3′ (sense for IL-6) and 5′-CTAGGTTTGCCGAGTAGATCTC-3′ (antisense for IL-6), 5′-ATGAGCACAGAAAGCATGATC-3′ (sense for TNF-α) and 5′-TCCACTTGGTGGTTTGCTACG-3′ (antisense for TNF-α), 5′-GACATTCATCATTGACCTCGTG-3′ (sense for IL-21) and 5′-TCACAGGAAGGGCATTTAGC-3′ (antisense for IL-21), 5′-TCCCTACTAGGACTCAGCCAAC-3′ (sense for IL-23) and 5′-AGAACTCAGGCTGGGCATC-3′ (antisense for IL-23), 5′-TGCATCTTGGCTTTGCAGCTCTTCCTCATG-3′ (sense for IFN-γ) and 5′-TGGACCTGTGGGTTGTTGACCTCAAACTTG-3′ (antisense for IFN-γ), 5′-GCATGTAGAGGCCATCAAAGA-3′ (sense for GM-CSF) and 5′-CGGGTCTGCACACATGTTA-3′ (antisense for GM-CSF), 5′-TGGACCGCAACAACGGCATCTATGAGAAAACC-3′ (sense for TGF-β) and 5′-TGGAGCTGAAGCAATAGTTGGTATCCAGGGCT-3′ (antisense for TGF-β), 5′- TCAGGAGCCCACCAGTACA-3′ (sense for Foxp3) and 5′- TCTGAAGGCAGAGTCAGGAGA-3′ (antisense for Foxp3), 5′-ACCACAGTCCATGCCATCAC-3′ (sense for G3PDH) and 5′-TCCACCACCCTGTTGCTGTA-3′ (antisense for G3PDH).
2.6. Isolation of CNS cells
Brains and spinal cords were homogenized and passed through a 100 μm cell strainer and collected by centrifugation. Cells were resuspended in 7 ml of RPMI 1640 medium, layered onto a Percoll density gradient (GE Healthcare Life Sciences, Waukesha, WI, USA), and centrifuged at 500 g for 30 min at room temperature. Cells were isolated by collection of the interphase fraction between 30% and 70% Percoll. After washing in HBSS, cells were analyzed by flow cytometry.
2.7. Flow cytometry
In flow cytometry, CD4-APC (BioLegend), IL-17-FITC (BioLegend), and Foxp3-Alexa Fluor 488 (BioLegend) were used. Single-cell suspensions obtained from spleen, brain, and spinal cords of mice were incubated with anti-CD16/CD32 (BD Biosciences, San Jose, CA, USA) to prevent Fc receptor binding and stained with anti-CD4. Intracellular cytokine staining by CD4+ cells was analyzed by monitoring the expression of IL-17. Foxp3 staining was performed according to the manufacturers’ protocol (BioLegend). For intracellular cytokine staining, cells were stimulated with 50 ng/ml PMA plus 500 ng/ml ionomycin in the presence of 1 μl/ml Brefeldin A (BioLegend) for 4 h. Samples were subjected to flow cytometric analyses using Gallios (BECKMAN COULTER). Data analysis was performed with FlowJo v10 software (Tree Star Inc., Ashland, OR, USA).
2.8. Statistical analysis
Data are presented as means±SEM. Statistically significant differences between groups were calculated by Student's t-test. Differences were considered to be significant when P<0.05 (*) or 0.005 (**).
3. Results
3.1. Induction of relapse in EAE mice after discontinuation of CsA
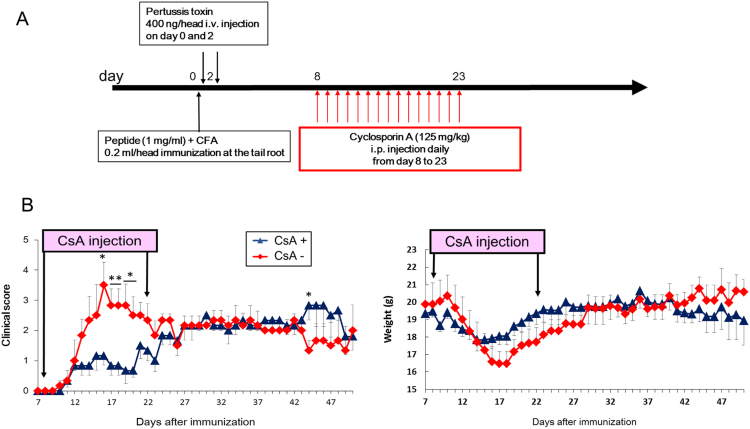
To establish a murine rebound model after CsA withdrawal, we took advantage of the EAE model of multiple sclerosis because relapse induced after CsA withdrawal was previously reported in rats [7], [8]. However, no reports of CsA-induced relapse in mice have been reported. To establish CsA-induced relapse in mice, CsA was injected into SJL/J mice immunized with PLP139-151 peptide during their first disease episode (from day 8 to day 23) (Fig. 1A). In the CsA-treated group, EAE scores in the first episode were reduced and a relapse was observed after day 40, compared to the non-CsA-treated group (Fig. 1B). Thus, we successfully established a murine rebound model induced by CsA withdrawal in PLP peptide-immunized SJL/J mice.
Fig. 1.
Establishment of a cyclosporin A-(CsA) induced EAE relapse model. (A) Protocol for CsA-induced EAE relapse model. Time points and dose of PLP peptide, pertussis toxin and CsA are shown. (B) Clinical scores and weights of EAE mice with or without CsA at the indicated time points. Error bars indicate mean±SEM (n=6 per group). *P<0.05, **P<0.005. CFA, complete Freund's adjuvant; i.v., intravenous; i.p., intraperitoneally.
3.2. Enhanced production of IL-17A after discontinuation of CsA
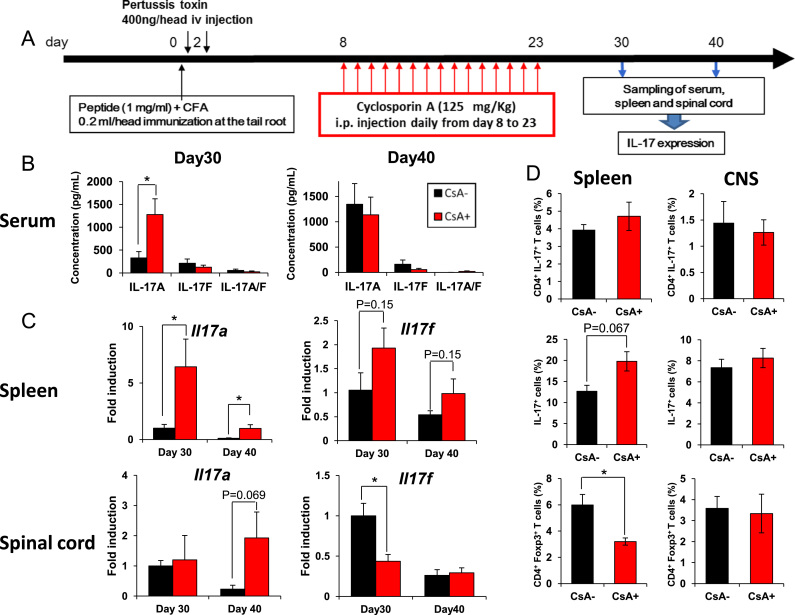
IL-17-producing T cells (Th17 and Tc17 cells) are key immune cells for EAE development [10], [11]. Among the IL-17 cytokine family, Th17 cells express IL-17A and IL-17F [12], which bind to a shared IL-17 receptor, IL-17RA/RC [13], [14], suggesting that these cytokines have similar biological functions. The IL-17A/F heterodimer is also expressed in Th17 cells [15], [16]. Therefore, we determined whether IL-17A, IL-17F, or IL-17A/F heterodimer expressions were affected after CsA withdrawal. Serum, spleens and spinal cord from EAE mice on days 30 and 40 were collected and IL-17 levels were analyzed by ELISA for serum or by real-time PCR for spleen and spinal cord (Fig. 2A). Enhanced IL-17A protein levels were observed in serum on day 30 after discontinuation of CsA-treatment compared to non-CsA-treatment, although there was no such increase in IL-17F and IL-17A/F (Fig. 2B). Real-time PCR revealed that IL-17A production was significantly upregulated in spleens on days 30 and 40 and tended to be increased in the spinal cord on day 40 (P=0.069) following CsA withdrawal (Fig. 2C). There was no significant difference in splenic IL-17F expressions between CsA-treatment and non-treatment groups. However, IL-17F expressions were downregulated in the spinal cord after CsA withdrawal on day 30 (P=0.022).
Fig. 2.
Expression of IL-17 after discontinuation of cyclosporin A (CsA). (A) Protocol for sampling of serum, spleen and spinal cord after discontinuation of CsA. (B) Levels of IL-17A, IL-17F, or IL-17A/F heterodimer in serum. Serum on day 30 or 40 was collected and used for IL-17A, IL-17F, or IL-17A/F heterodimer measurement by ELISA in triplicate wells and is shown as the mean±SEM. (C) Analysis of gene expression of IL-17A or IL-17F in spleen or spinal cord on days 30 or 40. Total RNA extracted from the spleen or spinal cord of individual mice on days 30 or 40 was subjected to quantitative real-time PCR. Expression levels are normalized to G3PDH. (D) At day 30 of EAE, spleen cells and CNS infiltrating cells were evaluated for secretion of IL-17 and for expression of Foxp3 by CD4+ cells. Error bars represent mean±SEM (B–C, n=6; D, n=5–7 per group). *P<0.05. CFA, complete Freund's adjuvant; i.v., intravenous; i.p., intraperitoneally.
Next, we evaluated the percentage of Th17/Treg cells in spleen and spinal cord by intracellular staining using flow cytometry. Although we observed increased proportion of splenic IL-17A-producing cells in CsA-treatment mice, the population of IL-17A-producing CD4+ T cells in spleen was not altered between CsA-treatment and non-treatment groups (Fig. 2D). On the other hand, the percentage of CD4+ Foxp3+ Treg cells in spleen was decreased after discontinuation of CsA. In CNS-infiltrating lymphocytes, the percentage and number of Th17 and Treg cells were not significantly altered by CsA treatment (Fig. 2D). These results suggest that there were IL-17A-inducing immune cells besides Th17 cells upon CsA withdrawal in EAE.
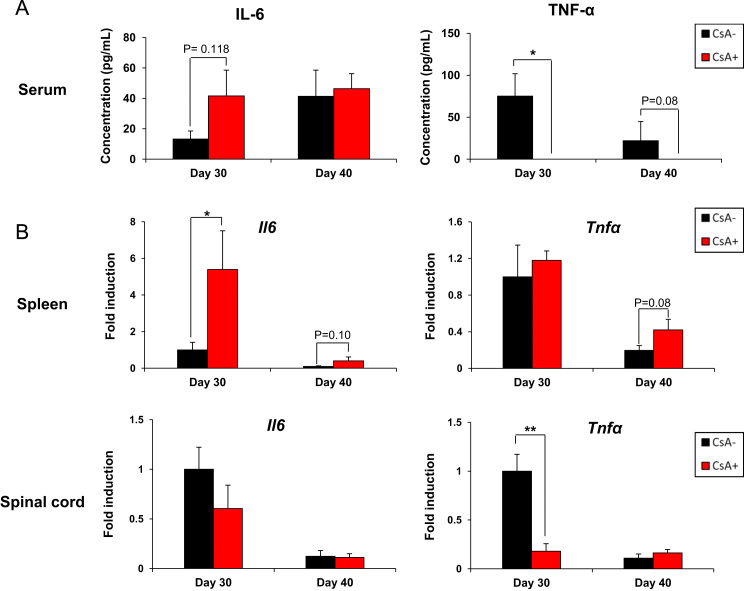
To determine whether IL-17A levels were selectively induced by discontinuation of CsA in EAE, the levels of other proinflammatory cytokines was evaluated by ELISA or real-time PCR. IL-6 and TNF-α are important proinflammatory cytokines in the induction of EAE and pathogenesis of MS [17], [18], [19], [20], [21], [22], [23], [24]. Splenic IL-6 levels at day 30 were upregulated by discontinuation of CsA, whereas IL-6 levels in the serum and spinal cord were not significantly different compared to controls (Fig. 3A and B). In contrast, the discontinuation of CsA significantly reduced TNF-α levels in the serum and spinal cord (Fig. 3A and B). To further investigate the balance between Th17 and Treg cells by discontinuation of CsA, we also examined mRNA expressions of other inflammatory/regulatory cytokines. We found that mRNA expression of IL-21, IL-23 and GM-CSF in the spleen on day 30 following CsA withdrawal were upregulated, whereas we detected no significant differences in the expressions of IFN-γ mRNA between CsA-treatment and non-CsA-treatment groups (Fig. S1). Moreover, Foxp3 mRNA expressions at day 30 were downregulated after CsA withdrawal in both spleen and spinal cord. These data suggest that CsA withdrawal induces the selective production of IL-17A and its related genes systemically and locally.
Fig. 3.
Expression of IL-6 and TNF-α after discontinuation of cyclosporin A (CsA). (A) Expression of IL-6 and TNF-α in serum on days 30 or 40 by ELISA in triplicate wells are shown as the mean±SEM. (B) Analysis of IL-6 and TNF-α gene expression in the spleen or spinal cord on days 30 or 40 by quantitative real-time PCR. Expression levels are normalized to G3PDH. Error bars indicate the mean±SEM (n=6 per group). *P<0.05, **P<0.005.
3.3. Anti-IL-17A antibody reduced severity of EAE relapse after CsA treatment
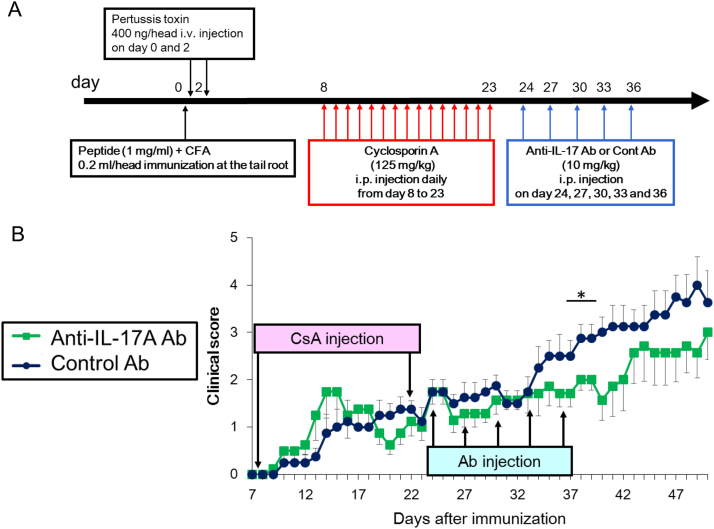
To determine whether increased IL-17A levels are involved in the augmented severity of EAE after CsA withdrawal, neutralizing IL-17A antibodies were injected on day 24, 27, 30, 33, and 36 after CsA withdrawal (Fig. 4A). Treatment with anti-IL-17A antibodies effectively lowered the severity of relapse in EAE after CsA withdrawal (P=0.036 on day 38 and 39, P=0.018 on day 40) (Fig. 4B). We also noted that clinical EAE score was elevated on day 43. Since the elevation occurred 7 days after the final anti-IL-17A antibody treatment, it may be due to insufficient level of anti-IL-17A antibody for neutralizing recurred IL-17A production. Therefore, the data suggest that the upregulation of IL-17A production upon CsA withdrawal is involved in EAE relapse.
Fig. 4.
Anti-IL-17A antibody reduces severity of the EAE relapse after the discontinuation of cyclosporin A (CsA). (A) Time point and dose of PLP peptide, pertussis toxin, CsA, and anti-IL-17A or control antibody. (B) Clinical scores of EAE mice treated with neutralizing anti-IL-17A or control antibody after discontinuation of CsA at the indicated time points. Error bars indicate the mean±SEM (n=7/anti-IL-17A antibody group, n=8/control antibody group). *P<0.05. CFA, complete Freund's adjuvant; i.v., intravenous; i.p., intraperitoneally.
4. Discussion
CsA is commonly used in current medical practice as an effective treatment for autoimmune diseases. Although its effectiveness is sustainable with continued treatment, the use of the drug in many patients is associated with side effects such as hypertension and renal damage that limit the length of time that it can be used. Therefore, most patients stop using the drug within 2 years in the US [1]. Cyclosporine is a strong immunosuppressant and deteriorates immunocompromized conditions, thereby increasing the risk of complications by serious infections. Therefore, as a general rule, when switching to biologics which attenuate immunoreactions, complete withdrawal of cyclosporine before switching is recommended [25]. However it is generally true that the direct switch to biologics causes the relapse of symptoms when the biologic therapy takes time to be effective. Therefore, the transition from CsA to a subsequent therapeutic can be difficult to manage for both physicians and patients. A therapeutic switching methodology that can be accomplished with a lower risk of exacerbation during the transition period would be of great benefit to autoimmune patients.
EAE is an animal model of human multiple sclerosis caused by inflammation of the central nervous system in mice and rats immunized with encephalitogenic peptides such as PLP or MOG peptide [26]. In this study, CsA treatment and its discontinuation induced EAE relapse in mice indicating EAE to be a suitable model for studying CsA withdrawal-dependent rebound including relapse or exacerbation of disease. IL-17 deficient mice or treatment with neutralizing anti-IL-17A antibody inhibited the severity of EAE disease scores, suggesting IL-17A is a critical cytokine for the development of EAE [10], [27], [28]. Therefore, we measured IL-17 levels in a CsA withdrawal-dependent rebound model on days 30 and 40, the time points after CsA treatment and before relapse, respectively. IL-17A levels were increased locally and systemically in EAE mice after CsA withdrawal.
IL-17A and IL-17F are highly homologous members of the IL-17 family [29], [30] and are a common ligand for IL-17RA/RC [13], [14]. Previous studies reported that IL-17A and IL-17F are produced by Th17 cells [12] and are both regulated by cytokines such as IL-23, IL-6 and transforming growth factor (TGF)-β as well as transcription factors such as RAR-related orphan receptor-γT (ROR-γT) and signal transducer and activator of transcription 3 (STAT3) [27], [31], [32], [33], suggesting IL-17A and IL-17F fulfil similar functions. We demonstrated that CsA withdrawal induced the selective local and systemic production of IL-17A and blockade with neutralizing IL-17A antibodies protected mice from EAE relapse caused by CsA withdrawal. This suggested that IL-17A is a key cytokine that mediates disease rebound after CsA treatment. These results are consistent with a previous study demonstrating that IL-17A and IL-17F have a differential role in autoimmune diseases, although they are important for host defence against pathogenic bacteria [34]. IL-17A is critical for the development of autoimmune diseases including collagen-induced arthritis and EAE, whereas IL-17F is less important. However, the signals involved in selective IL-17A production after CsA withdrawal is unknown. Possible explanations for selective IL-17A production might be the resumption of Th17 cells after CsA withdrawal accompanied by hyperresponsiveness to stimulation because of accumulated stimulatory signals received while T cells were suppressed by CsA. In EAE, T cells receive Th17-favoring stimulatory signals such as IL-6 and TGF-β that are involved in Th17 cell differentiation. Although both IL-17A and IL-17F are produced by Th17 cells, the production of IL-17A is much higher than IL-17F [35], suggesting the greater involvement of IL-17A production by T cells in the CsA withdrawal-dependent rebound effect.
IL-6 and TNF-α are important cytokines for the development of EAE. Upon CsA withdrawal, IL-6 expression was increased in the spleen, but not in the spinal cord. Inflammation in the spinal cord, the target tissue in EAE, was related to the severity of EAE. No change in IL-6 levels in the spinal cord suggests that IL-6-expressing T cells do not infiltrate into the spinal cord. TNF-α levels were significantly decreased after CsA withdrawal, suggesting that CsA may facilitate the sustained inhibition of TNF-α production from T cells and that anti-TNF-α antibody treatment is not a candidate for rebound after CsA withdrawal. Augmented IL-17A production in the spinal cord and spleen at day 40 indicated that IL-17A-producing T cells infiltrate into the spinal cord just before relapse. Taken together with the cytokine analysis, T cells producing IL-17A might be critical for EAE relapse after CsA withdrawal. We also found that the population of IL-17A-producing CD4+ T cells was not changed after CsA withdrawal. This result suggests that IL-17A may be produced by different kind of immune cells including CD8+ T cells and γδT cells. Further studies are needed to elucidate the cellular and molecular mechanisms after CsA withdrawal.
In summary, we report the establishment of a murine rebound EAE model induced by CsA withdrawal, the enhanced production of IL-17A after CsA withdrawal, and reduction of disease rebound by neutralizing anti-IL-17A antibodies. Therefore, neutralizing anti-IL-17A antibodies might be a potential switching drug after CsA therapy.
Conflict of interest
S.K. and T.M. are supported in part by a collaborative research grant from Novartis Pharma K.K. The remaining authors declare no competing financial interests.
Acknowledgements
This study was supported in part by JSPS KAKENHI Grant no. JP24590072 and JP16K08221, The Fugaku Trust for Medical Research, The Research Foundation for Pharmaceutical Sciences, The Nakatomi Foundation, and Japan Rheumatism Foundation. We thank Y. Tani, A. Ueki (Novartis Pharma KK) and F. Kolbinger (Novartis AG) for critical comments on the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.08.021.
Contributor Information
Shigeyuki Kon, Email: kon@pharm.hokudai.ac.jp.
Tadashi Matsuda, Email: tmatsuda@pharm.hokudai.ac.jp.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Matsuda S., Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 2.Prud'homme G.J., Vanier L.E. Cyclosporine, tolerance, and autoimmunity. Clin. Immunol. Immunopathol. 1993;66:185–192. doi: 10.1006/clin.1993.1024. [DOI] [PubMed] [Google Scholar]
- 3.Amor K.T., Ryan C., Menter A. The use of cyclosporine in dermatology: (Part I) J. Am. Acad. Dermatol. 2010;63:925–946. doi: 10.1016/j.jaad.2010.02.063. quiz 947-928. [DOI] [PubMed] [Google Scholar]
- 4.Strauss G., Osen W., Debatin K.M. Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clin. Exp. Immunol. 2002;128:255–266. doi: 10.1046/j.1365-2249.2002.01777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minguillon J., Morancho B., Kim S.J., Lopez-Botet M., Aramburu J. Concentrations of cyclosporin A and FK506 that inhibit IL-2 induction in human T cells do not affect TGF-beta1 biosynthesis, whereas higher doses of cyclosporin A trigger apoptosis and release of preformed TGF-beta1. J. Leukoc. Biol. 2005;77:748–758. doi: 10.1189/jlb.0904503. [DOI] [PubMed] [Google Scholar]
- 6.Ferraccioli G.F., Tomietto P., De Santis M. Rationale for T cell inhibition by cyclosporin A in major autoimmune diseases. Ann. N. Y. Acad. Sci. 2005;1051:658–665. doi: 10.1196/annals.1361.110. [DOI] [PubMed] [Google Scholar]
- 7.Chabannes D., Ryffel B., Borel J.F. SRI 62-834, a cyclic ether analogue of the phospholipid ET-18-OCH3, displays long-lasting beneficial effect in chronic relapsing experimental allergic encephalomyelitis in the Lewis rat. Comparison with cyclosporin and (Val2)-dihydrocyclosporin effects in clinical, functional and histological studies. J. Autoimmun. 1992;5:199–211. doi: 10.1016/0896-8411(92)90200-a. [DOI] [PubMed] [Google Scholar]
- 8.Kovarik J., Chabannes D., Borel J.F. Immunoregulation and drug treatment in chronic relapsing experimental allergic encephalomyelitis in the Lewis rat. Int. J. Immunopharmacol. 1995;17:255–263. doi: 10.1016/0192-0561(95)00012-q. [DOI] [PubMed] [Google Scholar]
- 9.Theofilopoulos A.N., Dummer W., Kono D.H. T cell homeostasis and systemic autoimmunity. J. Clin. Invest. 2001;108:335–340. doi: 10.1172/JCI12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komiyama Y., Nakae S., Matsuki T., Nambu A., Ishigame H., Kakuta S., Sudo K., Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 11.Pollinger B. IL-17 producing T cells in mouse models of multiple sclerosis and rheumatoid arthritis. J. Mol. Med. (Berl.) 2012;90:613–624. doi: 10.1007/s00109-011-0841-4. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L., Ivanov R., II, Spolski R., Min K., Shenderov T., Egawa D.E., Levy W.J., Leonard D.R. Littman, IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 13.Toy D., Kugler D., Wolfson M., Vanden Bos T., Gurgel J., Derry J., Tocker J., Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 14.Kuestner R.E., Taft D.W., Haran A., Brandt C.S., Brender T., Lum K., Harder B., Okada S., Ostrander C.D., Kreindler J.L., Aujla S.J., Reardon B., Moore M., Shea P., Schreckhise R., Bukowski T.R., Presnell S., Guerra-Lewis P., Parrish-Novak J., Ellsworth J.L., Jaspers S., Lewis K.E., Appleby M., Kolls J.K., Rixon M., West J.W., Gao Z., Levin S.D. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang S.H., Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 16.Wright J.F., Guo Y., Quazi A., Luxenberg D.P., Bennett F., Ross J.F., Qiu Y., Whitters M.J., Tomkinson K.N., Dunussi-Joannopoulos K., Carreno B.M., Collins M., Wolfman N.M. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J. Biol. Chem. 2007;282:13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 17.Mendel I., Katz A., Kozak N., Ben-Nun A., Revel M. Interleukin-6 functions in autoimmune encephalomyelitis: a study in gene-targeted mice. Eur. J. Immunol. 1998;28:1727–1737. doi: 10.1002/(SICI)1521-4141(199805)28:05<1727::AID-IMMU1727>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Okuda Y., Sakoda S., Bernard C.C., Fujimura H., Saeki Y., Kishimoto T., Yanagihara T. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int. Immunol. 1998;10:703–708. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- 19.Okuda Y., Sakoda S., Fujimura H., Saeki Y., Kishimoto T., Yanagihara T. IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glucoprotein 35-55 induced experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1999;101:188–196. doi: 10.1016/s0165-5728(99)00139-3. [DOI] [PubMed] [Google Scholar]
- 20.Samoilova E.B., Horton J.L., Hilliard B., Liu T.S., Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- 21.Campbell I.L. Structural and functional impact of the transgenic expression of cytokines in the CNS. Ann. N. Y. Acad. Sci. 1998;840:83–96. doi: 10.1111/j.1749-6632.1998.tb09552.x. [DOI] [PubMed] [Google Scholar]
- 22.Dal Canto R.A., Shaw M.K., Nolan G.P., Steinman L., Fathman C.G. Local delivery of TNF by retrovirus-transduced T lymphocytes exacerbates experimental autoimmune encephalomyelitis. Clin. Immunol. 1999;90:10–14. doi: 10.1006/clim.1998.4653. [DOI] [PubMed] [Google Scholar]
- 23.Douni E., Akassoglou K., Alexopoulou L., Georgopoulos S., Haralambous S., Hill S., Kassiotis G., Kontoyiannis D., Pasparakis M., Plows D., Probert L., Kollias G. Transgenic and knockout analyses of the role of TNF in immune regulation and disease pathogenesis. J. Inflamm. 1995;47:27–38. [PubMed] [Google Scholar]
- 24.Selmaj K., Raine C.S., Farooq M., Norton W.T., Brosnan C.F. Cytokine cytotoxicity against oligodendrocytes. Apoptosis induced by lymphotoxin. J. Immunol. 1991;147:1522–1529. [PubMed] [Google Scholar]
- 25.Ohtsuki M., Terui T., Ozawa A., Morita A., Sano S., Takahashi H., Komine M., Etoh T., Igarashi A., Torii H., Asahina A., Nemoto O., Nakagawa H. Japanese guidance for use of biologics for psoriasis. J. Dermatol. 2013;40:683–695. doi: 10.1111/1346-8138.12239. [DOI] [PubMed] [Google Scholar]
- 26.Stromnes I.M., Goverman J.M. Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 27.Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. eJ. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofstetter H.H., Ibrahim S.M., Koczan D., Kruse N., Weishaupt A., Toyka K.V., Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Kolls J.K., Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Moseley T.A., Haudenschild D.R., Rose L., Reddi A.H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 31.Ivanov II, McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 32.Yang X.O., Panopoulos A.D., Nurieva R., Chang S.H., Wang D., Watowich S.S., Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 33.Yang X.O., Pappu B.P., Nurieva R., Akimzhanov A., Kang H.S., Chung Y., Ma L., Shah B., Panopoulos A.D., Schluns K.S., Watowich S.S., Tian Q., Jetten A.M., Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishigame H., Kakuta S., Nagai T., Kadoki M., Nambu A., Komiyama Y., Fujikado N., Tanahashi Y., Akitsu A., Kotaki H., Sudo K., Nakae S., Sasakawa C., Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Haak S., Croxford A.L., Kreymborg K., Heppner F.L., Pouly S., Becher B., Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material