Abstract
Despite availability of primary and secondary prevention measures, cervical cancer persists as one of the most common cancers among women around the world. While early stage disease can be cured with radical and even fertility-sparing surgery, patients with metastatic and recurrent cervical cancer have poor prognosis with historically limited treatment options and incurable disease. Significant advances in cervical cancer treatment have emerged as the result clinical trials seeking to determine the best therapy to prolong overall and progression-free survival. Most recently, trials involving angiogenesis blockade in addition to standard chemotherapy have demonstrated improved overall and progression free survival. This review serves to highlight pivotal trials in chemotherapy development for advanced, metastatic and recurrent cervical cancer including the paradigm-shifting work demonstrating increased overall survival with angiogenesis blockade.
Keywords: Angiogenesis inhibitors, Bevacizumab, Uterine cervical neoplasms
Introduction
Cervical cancer is diagnosed in 528,000 women annually and results in 266,000 deaths worldwide each year.1 The American Cancer Society estimates that there will be 12,900 new diagnoses and 4,100 cervical cancer-related deaths in the United States in 2015.2 Cervical cancer is one of many cancers caused by human papillomavirus (HPV) infection, but it is the only cancer for which HPV has been demonstrated to be the necessary precursor.3–5 Risk factors for cervical cancer are those associated with HPV exposure, such as increased number of sexual partners, though cigarette smoking and immunosuppression increase risk of HPV persistence.6 Despite high efficacy and availability of HPV vaccines3,7,8 and the recommendation for routine vaccination,9 completion of the vaccine series among females 13–17 years in the United States remains below 40%.10 Given difficulties achieving widespread compliance with HPV vaccination and the inability to include all oncogenic subtypes in the vaccines, the importance of continued secondary prevention remains. The vast majority of women diagnosed with cervical cancer report inability to recall when they last had a Pap smear or that it was at least 10 years earlier; however, even among women compliant with screening guidelines, cervical cancer may develop.11
Although the goals for HPV vaccination, Pap smears and HPV testing are prevention and early diagnosis, approximately 5% of women diagnosed with cervical cancer in North America present with stage IV disease12 with 5-year survival rates of 9.3–21.6%.13 Even among women with earlier stages at diagnosis, 15–61% will develop metastatic disease, usually within the first two years of completing treatment. For women diagnosed with recurrent disease, 5-year survival is less than 5%.12 This review focuses on changes in systemic treatment for women with metastatic or recurrent cervical cancer.
Development of standard chemotherapy
Single agent cisplatin was established as the backbone of chemotherapy treatment for advanced cervical cancer more than 30 years ago when a phase II trial of cisplatin 50mg/m2 demonstrated a 44% objective response rate (RR) in 25 treatment-naïve patients.14 In a Gynecologic Oncology Group (GOG) phase III study of cisplatin with or without paclitaxel for stage IVB, recurrent or persistent squamous cell carcinoma of the cervix (GOG 169), an objective response occurred in 19% of patients receiving cisplatin versus 36% of patients receiving cisplatin with paclitaxel (Table 1).15 There was a significant increase is median progression free survival (PFS), but there was no difference in overall survival (OS) and patients in the doublet arm experienced increased grade 3 to 4 anemia and neutropenia.
Table 1.
Pivotal trials contributing to chemotherapy standards for advanced, metastatic and recurrent cervical cancer
| Trial | Lead Author |
Eligibility | Arms | RR (%) |
OS (month s) |
PFS (month s) |
Conclusion |
|---|---|---|---|---|---|---|---|
|
| |||||||
| GOG 169 | Moore15 | IVB, recurrent or persistent SCC |
|
19 | 8.8 | 2.8 | Combined regimen superior for RR and PFS without detriment to QOL. No change in OS. |
| 36 | 9.7 | 4.8 | |||||
|
| |||||||
| GOG 179 | Long16 | IVB, recurrent or persistent |
|
13 | 6.5 | 2.9 | Improved OS with doublet. Results most favorable for patients with no prior radiosensitizing cisplatin. |
| 26 | 9.4 | 4.6 | |||||
| N/A | N/A | N/A | |||||
|
| |||||||
| GOG 204 | Monk17 | IVB, recurrent or persistent |
|
29 | 12.9 | 5.8 | Closed for futility. |
| 23.4 | 10.3 | 4.7 | |||||
| 22.3 | 10.3 | 4.6 | |||||
| 25.9 | 10 | 4.0 | |||||
|
| |||||||
| JCOC 0505 | Kitagawa20 | IVB, recurrent or persistent |
|
58.8 | 18.3 | 6.9 | Non-inferiority of carboplatin/paclitaxel doublet except in platinum-naïve patients. |
| 62.6 | 17.5 | 6.2 | |||||
RR response rate, OS mean overall survival, PFS mean progression free survival, QOL quality of life, d day, N/A not applicable (study arm closed early after four treatment-related deaths)
Phase II reports of high RR using methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) prompted development of GOG 179, a randomized phase III trial comparing MVAC to cisplatin plus topotecan or cisplatin alone.16 The MVAC arm was closed by the Data Safety Monitoring Board due to four treatment-related deaths among 63 patients. Among the remaining patients randomized to cisplatin or cisplatin plus topotecan, patients receiving the doublet had improved RR (27% versus 13%), median PFS (4.6 versus 2.9 months) and median OS (9.4 versus 6.5 months) as well as more grade 3 and 4 hematologic toxicity, though without detriment to quality of life. This seminal study was the first randomized phase III trial to demonstrate statistically significant increased survival with combined chemotherapy over cisplatin alone for treatment of advanced or recurrent cervical cancer.
Following phase II trials showing promise for a doublet of vinorelbine plus cisplatin, a phase III trial (GOG 204) was planned with two arms comparing paclitaxel-cisplatin (PC) to vinorelbine-cisplatin (VC); however, two additional arms comparing gemcitabine-cisplatin (GC) and topotecan-cisplatin (TC) were added when phase II data for GC and phase III data for TC became available. After a planned interim analysis, the study was closed for futility. This phase III trial showed that VC, GC and TC were not superior to PC in RR, OS or PFS and that there was no difference in quality of life between study arms.17
Despite improvements in cisplatin-based combination chemotherapy, RR remained low for advanced cervical cancer, prompting a multivariate logistic regression analysis of data from GOG 110, 169, and 179 that identified five risk factors for poor response to therapy: black race, performance status > 0, pelvic disease, prior radiosensitizer and time interval from diagnosis to first recurrence less than 1 year. The authors developed a simple prognostic index combining risk factors to create three groups: low risk (0–1 risk factor), mid-risk (2–3 risk factors) and high-risk (4–5 risk factors) and validated the index using data from GOG 149.18
It has been suggested that therapeutic equivalency of cisplatin-paclitaxel (PT) and carboplatin-paclitaxel (CT) demonstrated in ovarian cancer may be extrapolated to cervical cancer. To evaluate this hypothesis, the Japanese Clinical Oncology Group developed a multicenter, open label, randomized phase III trial to evaluate efficacy, safety and quality of life of CT compared with PT.19 Median OS was 18.3 months for PT versus 17.5 months for CT (HR 0.994; 90%CI, 0.79–1.25), demonstrating non-inferiority of CT with significantly longer proportion of non-hospitalization periods for patients receiving CT (p<0.001). Median PFS was 6.9 months for PT versus 6.2 months for CT (HR 1.041, 95% CI, 0.803–1.351). Among patients with no prior cisplatin treatment, OS was shorter with CT (13.0 versus 23.2 months; HR 1.571; 95% CI, 1.06–2.32), indicating that cisplatin remains superior for platinum-naïve patients.20
Over the past 30 years, cisplatin-based combination chemotherapy has been shown to produce the best PFS15,16 and OS16,20 for the majority of patients with advanced and recurrent cervical cancer, with exceptions for those with high-risk for non-response to cisplatin based on Moore’s criteria.18 Despite extensive research to improve chemotherapy for advanced and recurrent cervical cancer, OS continues to be measured in months. For this reason, investigations in recent years have delved into other pathways in hopes of eliciting improved response to treatment with prolongation of survival.
Angiogenesis blockade
Historically, options have been limited for patients with persistent or recurrent cervical cancer after platinum-based chemotherapy.14–18,21,22 Angiogenesis, the process of new blood vessel formation, is essential for growth of new tissue, wound healing and embryogenesis but is also fundamental for tumor proliferation. Vascular endothelial growth factor (VEGF) is the major mediator of tumor angiogenesis.23 Neovascularization correlates directly with disease spread and inversely with survival. Ferrara et al developed bevacizumab, a humanized anti-VEGF monoclonal antibody that bound with an affinity comparable to that of the original antibody.24 Bevacizumab was the first angiogenesis inhibitor to be approved by the Food and Drug Administration for cancer treatment.23
Bevacizumab
In a retrospective case series of 6 patients with heavily pretreated recurrent cervical cancer, five of the six patients received 5-fluorouracil in combination with bevacizumab and one of the six patients received capecitabine with bevacizumab (Table 2).25 Among these six patients, complete response (17%, n=1), partial response (17%, n=1) or stable disease (33%, n=2) was seen among 67% (n=4), demonstrating encouraging anti-tumor activity with minimal grade 4 adverse events (1 patient developed neutropenic sepsis). Among the four patients who demonstrated clinical benefit, median PFS was 4.3 months.
Table 2.
Bevacizumab in cervical cancer treatment
| Trial | Lead Author |
Pathology | Arms | RR (%) |
OS (months) |
PFS (months) |
Conclusion |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Wright25 | SCC, AS |
|
33 | 5.1 | NR | Bevacizumab is well-tolerated and displays anti-tumor activity in recurrent cervical cancer. | |
|
| |||||||
| GOG 227C | Monk26 | SCC, AS | Bevacizumab 15mg/kg IV Q3 weeks | 35 | 7.3 | 3.4 | Bevacizumab is well-tolerated and active as second- and third-line therapy for recurrent cervical cancer, warrants phase III trial. |
|
| |||||||
| RTOG 0417 | Schefter27,28 | SCC | Cisplatin 40mg/m2 + RT + brachytherapy + bevacizumab 10mg/kg Q2 weeks for 3 cycles | NR | 3-year | 3-year | Bevacizumab in addition to standard chemoradiation for locally advanced cervical cancer is feasible and safe. |
| 81.3% | 68.7% | ||||||
|
| |||||||
| Zighelboim29 | SCC, adenocarcinoma | Cisplatin 50mg/m2 d1 + Topotecan 0.75mg/m2 d1–3 + Bevacizumab 15mg/kg d1 Q3weeks | 35 | 13.2 | 7.1 | Addition of bevacizumab to cisplatin and topotecan produces an active by highly toxic regimen. | |
|
| |||||||
| GOG 240 | Tewari30 | SCC, AS, or adenocarcinoma |
|
14.3 | 5.9 | Bevacizumab resulted in 3.7 month increased OS (17 months compared to 13.3 months) | |
| 17.5 | 8.2 | ||||||
| 12.7 | |||||||
| 16.2 | |||||||
RR response rate, OS mean overall survival, PFS mean progression free survival, NR no reported, QOL quality of life, d day
Based on the preliminary results reported by Wright et al, GOG 227C, a phase II trial to assess efficacy and tolerability of bevacizumab in treatment of recurrent cervical cancer was opened.26 Among the 46 women enrolled, 82.6% (n=38) had received prior radiation and one (n=34, 73.9%) or two (n=12, 26.1%) prior cytotoxic regimens for recurrent disease. Eleven of the 46 patients (23.9%, 2-sided 90% CI, 14–37%) achieved PFS for at least 6 months and another 5 patients (10.9%, 2-sided 90% CI, 4–22%) achieved partial response. Median PFS of 3.40 months (95%CI, 2.53–4.53 months) and OS of 7.29 months (95% CI, 6.11–10.41 months) with bevacizumab compared favorably to other phase II trials for persistent or recurrent disease, prompting development of a phase III trial.26
Since bevacizumab had demonstrated clinical activity in pretreated populations, the Radiation Therapy Oncology Group (RTOG) designed a phase II single-arm study (protocol 0407) of bevacizumab in addition to standard chemoradiation for bulky stage IB-IIIB cervical cancer to investigate efficacy and safety.27 Among 60 patients enrolled, 49 were evaluable and had a median follow-up of 12.4 months (range, 4.6–31.4 months) with no serious adverse events.27 This study was not powered for PFS or OS analysis, but in a report of secondary endpoints, over a median follow-up time of 3.8 years (range, 0.8–6.0 years) the 3-year OS was 81.3% (95% CI, 67.2–89.8%) and PFS was 68.7% (95% CI, 53.5–79.8%).28 This phase II trial indicates that further study of bevacizumab for treatment of locally advanced disease is warranted.
In a multicenter phase II trial evaluating a regimen of topotecan, cisplatin and bevacizumab for persistent or recurrent cervical cancer, 27 patients with no prior chemotherapy for recurrence received a median of 3 treatment cycles (range, 1–19 cycles) and a median of 10 months (range, 1.7–33.4 months) of follow-up.29 Among the 26 evaluable patients, 59% (80% CI, 46–70%) experienced 6-month PFS; one (4%, 80% CI, 0.4–14%) experienced complete response and 8 (31%, 80% CI, 19–45%) experienced partial response lasting a median of 4.4 months. Median PFS was 7.1 months (80% CI, 4.7–10.1 months) and median OS was 13.2 months (80% CI, 8.0–15.4 months). Unfortunately, grade 3–4 hematologic toxicity was common with high incidence (78%) of unanticipated hospitalizations.
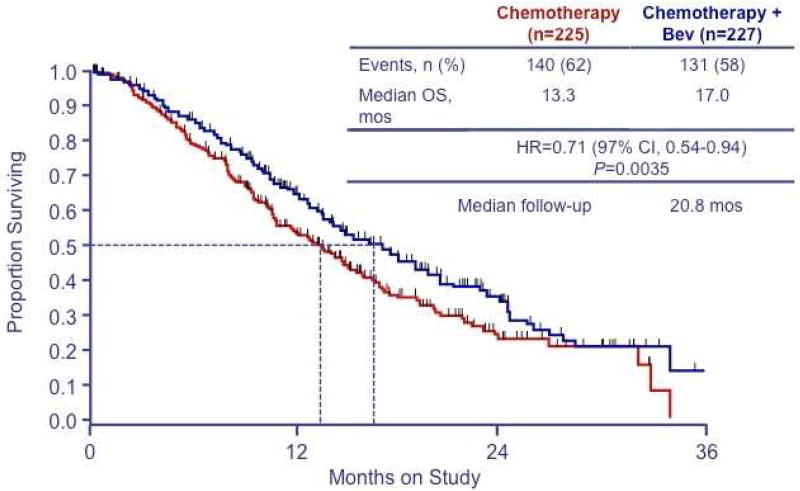
The first phase III randomized trial (GOG 240) of bevacizumab for advanced cervical cancer randomized women to one of 4 arms: 1) cisplatin plus paclitaxel, 2) cisplatin, paclitaxel and bevacizumab, 3) topotecan plus paclitaxel, 4) topotecan, paclitaxel and bevacizumab.30 Inclusion criterial included adequate hepatic, bone marrow and renal function as well as good nutritional status. Most (75%) of the study group had previously received platinum and were evenly distributed between the two backbones. Addition of bevacizumab to chemotherapy resulted in a 3.7 month increase in median OS (17.0 versus 13.3 months, Figure 1) and higher RR (48% versus 36%, p=0.008). Sub-analysis showed beneficial effects of bevacizumab in patients previously exposed to platinum and among those with recurrent or persistent disease. Additionally, benefits of bevacizumab were demonstrated in patients with recurrent disease in a previously irradiated field. Grade 2 or higher hypertension, grade 3 or higher gastrointestinal or genitourinary fistulas and grade 3 or higher thromboembolic events were all significantly higher among patients receiving bevacizumab, but quality of life scores indicated that addition of bevacizumab did not adversely affect health-related quality of life. In the final protocol-specified OS analysis, bevacizumab improved OS to 16.8 months versus 13.3 months for chemotherapy alone (HR 0.765; 95% CI: 0.62, 0.95; p=0.0068).31
Figure 1. Overall survival in GOG 240 according to chemotherapy regimen.
Overall survival among patients assigned to cisplatin-paclitaxel (CP) chemotherapy with or without bevacizumab and those assigned to topotecan-paclitaxel (TP) chemotherapy with or without bevacizumab. (©Massachusetts Medical Society. Reprinted with permission from Tewari KS et al. N Engl J Med 2014;370(8):734–743.30)
One exploratory objective of GOG 240 was to prospectively validate pooled clinical prognostic factors (Moore criteria). High-risk patients (4–5 factors) had significantly worse OS (p<0.0001). Hazard ratios of death for treating with topotecan in low-risk (0–1 factors), mid-risk (2–3 factors), and high-risk (4–5 factors) subsets were 1.18 (95% CI 0.63–2.24), 1.11 (95% CI 0.82–1.5), and 0.84 (95% CI 0.50–1.42), respectively, while hazard ratios of death for treating with bevacizumab in low-risk, mid-risk, and high-risk subsets were 0.96 (95% CI 0.51–1.83; p=0.9087), 0.673 (95% CI 0.5–0.91; p=0.0094), and 0.536 (95% CI 0.32–0.905; p=0.0196), respectively. Toxicity concerns and lack of statistically significant survival benefit in the low-risk group of patients may justify reservation of bevacizumab for mid-risk and high-risk populations unless larger studies demonstrate benefit for the low-risk population.32
Other anti-angiogenesis agents
Other anti-angiogenic agents under study include sunitinib, pazopanib, lapatinib and cediranib (Table 3). Sunitinib malate is an orally bioavailable small molecule that inhibits members of the split-kinase domain family of receptor tyrosine kinases, including VEGF and platelet-derived growth factor.33 Sunitinib is FDA approved for patients with metastatic renal cell carcinoma and gastrointestinal stromal tumors. A multicenter phase II trial was performed to evaluate sunitinib in women with locally advanced or metastatic cervical carcinoma who had received up to one prior line of chemotherapy with a primary endpoint of objective RR.34 Among 19 patients enrolled in the study, 16 had stable disease but no objective response after a median duration of 4.4 months. Five patients (26.3%) developed fistula, although 4 of these patients had received prior radiation, making it difficult to determine the contribution of sunitinib to fistula development. Regardless, this study showed that sunitinib does not have sufficient activity as a single agent in cervical cancer.
Table 3.
Other anti-angiogenesis agents in cervical cancer treatment
| Trial | Lead Author |
Pathology | Arms | RR (%) |
OS (weeks) |
PFS (weeks) |
Conclusion |
|---|---|---|---|---|---|---|---|
|
| |||||||
| NCIC CTG IND.184 | Mackay34 | SCC, AS, or adenocarcinoma |
|
0 | NR | 24.6 | Higher rate of fistula formation (26.3%) than expected. Insufficient activity as single agent. |
|
| |||||||
| Monk35,36 | SCC, AS, or adenocarcinoma |
|
9 | 50.7 | 18.1 | Pazopanib improved PFS and OS. | |
| 5 | 39.1 | 17.1 | |||||
|
| |||||||
| CRUK/10/001 | Symonds37 | SCC, AS, or adenocarcinoma |
|
66 | 59 | 35 | Addition of cediranib to carboplatin and paclitaxel results in prolonged PFS with no change in OS. |
| 42 | 63 | 30 | |||||
RR response rate, OS mean overall survival, PFS mean progression free survival, NR no reported
A phase II open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy confirmed activity of anti-angiogenesis agents in advanced and recurrent cervical cancer. Pazopanib is an oral angiogenesis inhibitor that targets VEGF receptor, platelet-derived growth factor receptor, and c-Kit and is FDA approved for use in metastatic soft tissue sarcomas and metastatic renal cell carcinoma. Lapatinib is an oral small-molecule dual tyrosine kinase inhibitor that targets epidermal growth factor receptor and human epidermal growth factor receptor 2 (HER2/neu) and is FDA approved for use in combination with capecitabine for patients with advanced or metastatic breast cancer. The combined arm in this study was closed for futility after interim analysis, but the trial demonstrated improved PFS for pazopanib monotherapy compared to lapatinib monotherapy.35 Interim analysis data indicted improved OS in the pazopanib arm; however, the study was not powered for OS and final analysis failed to show any significant difference.36
A randomized double blind phase II trial (CIRCCa) of carboplatin-paclitaxel plus cediranib versus carboplatin-paclitaxel plus placebo in metastatic and recurrent cervical cancer was performed in the United Kingdom.37 Cediranib is a tyrosine kinase inhibitor of VEGF receptors 1, 2 and 3 and has been formulated as an oral medication. The CIRCCa trial randomized patients to receive cediranib 20mg or placebo daily in addition to carboplatin AUC5 and paclitaxel 175mg/m2 every 21 days for a maximum of 6 cycles. Median PFS was 30 weeks with placebo versus 35 weeks with cediranib (HR 0.61; 80% CI, 0.41–0.89; p=0.046). Median OS was 63 weeks with placebo versus 59 weeks with cediranib (HR 0.93; 80% CI, 0.64–1.36; p=0.401). Response rate was higher in those receiving cediranib (66% versus 42%; p=0.030) as was toxicity, with 19% of patients experiencing grade 2–4 toxicity compared with 9% in the placebo group.
Anti-angiogensis agents other than bevacizumab have failed to demonstrate statistically significant benefit in OS for advanced and recurrent cervical cancer. A phase II study of sunitinib monotherapy failed to show an objective response.34 Other phase II trials of anti-angiogenesis agents have demonstrated improved PFS with no benefit to OS. Pazopanib monotherapy showed improved PFS compared to lapatinib monotherapy but was not powered to assess OS,35,36 while cediranib added to a carboplatin-paclitaxel chemotherapy backbone showed improved PFS compared to placebo (35 versus 30 weeks) but no statistically significant difference in OS.37 Additional phase II trials are needed to determine which anti-angiogenesis agents may produce an OS benefit for patients with advanced or recurrent cervical cancer.
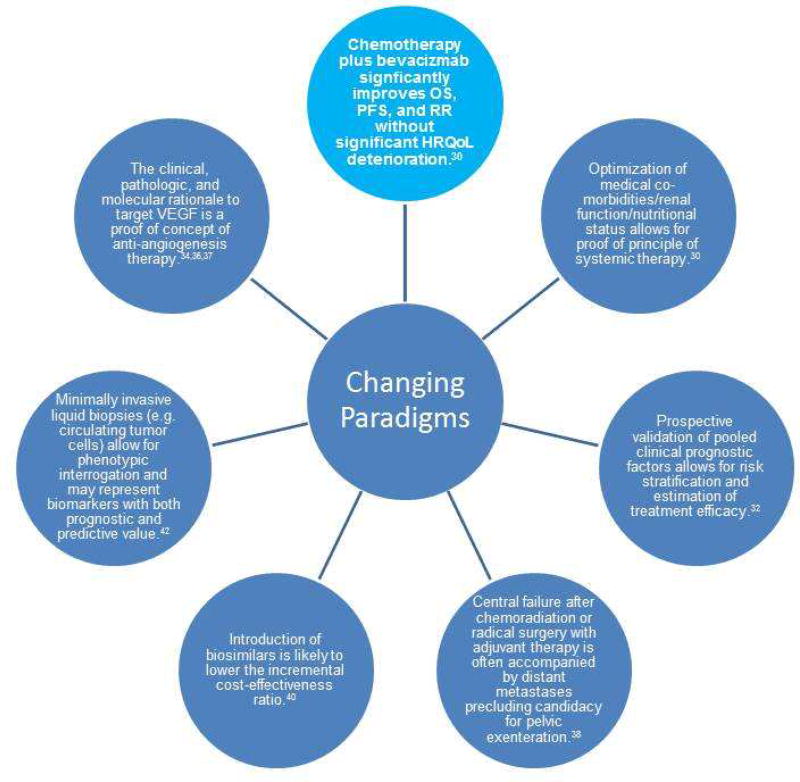
Changing Paradigms
Although women with advanced cervical cancer are at high risk for persistence and recurrence, major paradigm shifts have occurred in recent years that have changed the outlook these women (Figure 2). The clinical, pathologic, and molecular rationale to target VEGF is a proof of concept of anti-angiogenesis therapy. While a phase II trial of sunitinib failed to show significant single-agent activity for advanced or metastatic cervical cancer,34 a phase II open-label study of pazopanib and lapatinib confirmed activity of anti-angiogenesis agents in advanced and recurrent cervical cancer.36 Addition of cediranib to carboplatin and paclitaxel resulted in 5 week prolongation of PFS, though it had no significant impact on OS.37 These studies demonstrated potential benefit of anti-angiogenesis therapy in treatment of advanced and recurrent disease.
Figure 2. Changing Paradigms in Advanced, Metastatic and Recurrent Cervical Cancer.
OS overall survival, PFS progression free survival, HRQoL health related quality of life, VEGF vascular endothelial growth factor. Shown are the paradigm changes described in the text.
Chemotherapy plus bevacizumab significantly improves OS, PFS, and RR without significant deterioration in health-related quality of life. Single-agent cisplatin was established as the backbone of chemotherapy treatment for cervical cancer many years ago, but more recent trials have shown benefit for chemotherapy doublets and more recently with angiogenesis inhibitors. While many trials have shown improved RR or PFS for one chemotherapy regimen compared to another, rarely has improved OS be demonstrated. Recently, GOG 240 transformed treatment for advanced, recurrent and metastatic cervical cancer by demonstrating that targeted agents can significantly improve survival. The findings of GOG 240 revealing a 3.7 month increase in OS with no significant deterioration in quality of life serve as proof of principle in the value of systemic therapy and proof of concept of the efficacy of angiogenesis blockade therapy.30 The increase in OS with the addition of bevacizumab creates a therapeutic window through which other novel drugs such as immunotherapy, other classis of anti-angiogenic agents, PARP and mTOR inhibitors may be active to further extend survival.
Optimization of medical co-morbidities, renal function and nutritional status allows for proof of principle of systemic therapy. Patients who were previously thought to be too sick to benefit from systemic therapy suffered rapid deterioration in their quality of life and short survival after diagnosis. In GOG 204, the cisplatin-paclitaxel (CP) and topotecan-paclitaxel (TP) arms had OS of 12.9 and 10.3 months, respectively.17 In GOG 240, after optimization of medical comorbidities, the CP and TP arms without bevacizumab had OS of 14.3 an 12.7 months, respectively.30 Reducing medical comorbidities such as improving renal function with use of stents and nephrostomy tubes, improvement of performance status through optimization of pain control, and correction of malnutrition can make patients eligible for systemic therapy and contribute to prolonged OS.
Prospective validation of pooled clinical prognostic factors allows for risk stratification and estimation of treatment efficacy.32 One of the objectives of GOG 240 was to prospectively validate the five risk factors for poor response to cisplatin-based therapy identified by Moore et al.18 Median OS was not significantly different for low-risk patients receiving bevacizumab in addition to chemotherapy, but among high-risk patients, median OS was 6.3 months for chemotherapy alone versus 12.1 months for chemotherapy with bevacizumab. While there was a clinical benefit for receipt of bevacizumab in all groups, those with highest risk for poor response to cisplatin-based therapy derived the greatest benefit from inclusion of bevacizumab in their treatment regimens.
Central failure after chemoradiation or radical surgery with adjuvant therapy is often accompanied by distant metastases, precluding candidacy for pelvic exenteration.38 Isolated central pelvic recurrences that lend themselves to pelvic exenteration are becoming increasingly rare in the era of concurrent chemoradiation plus brachytherapy. While feasible and potentially curative, pelvic exenteration has high morbidity rates even in the hands of an experienced gynecologic oncologist.39 However, since the original introduction of the procedure by Brunschwig in 1948, technical advances such as the intestinal conduit for urinary diversion and end-to-end anastomosis using the intestinal stapling device for preservation of fecal stream have produced significant improvements in morbidity.38
Introduction of biosimilars is likely to lower the incremental cost-effectiveness ratio. Bevacizumab therapy adds $73,791 per 3.5 months of life gained, or $5,775 per month of added life and $24,597 per quality adjusted life month. A Markov model created based on GOG 240 indicates that cost reductions through availability of biosimilars result in declines in the incremental cost-effectiveness ratio, since increased costs are largely direct costs due to the drug rather than indirect costs for management of bevacizumab-induced complications.40 As biosimilars are introduced to the market, the use of bevacizumab in advanced and recurrent cervical cancer will gain cost efficacy; however, it may be many years before bevacizumab is affordable for women in low- and middle-income countries where the overwhelming majority of advanced cervical cancer cases occur.
Minimally invasive liquid biopsies allow for phenotypic interrogation and may represent biomarkers with both prognostic and predictive value.41,42 The primary translational research objective of GOG 240 was to determine whether circulating tumor cells (CTCs) could be isolated from patients and if CTC counts would be associated with hazard of death. Median CTC count was 7 CTCs/8.5 mL whole blood (range, 0–18) pre-cycle 1 and 4 CTCs/8.5 mL whole blood (range, 0–17) 36 days post-cycle 1. The hazard of death for pre-treatment CTC counts was 0.9 (95% CI 0.81, 0.99) within the cisplatin-paclitaxel-bevacizumab group and patients with greater declines had a lower hazard of death (HR 0.87; 95% CI 0.79, 0.95).
Caris life sciences evaluated 592 cervical cancer specimens in their repository using next-generation sequencing (NGS), in situ hybridization (ISH) and immunohistochemistry.48 Mutational hotspots were identified corresponding to PI3KCA (26%), BRCA2 (21%), BRCA1 (10%), KRAS (10%), TP53 (10%), and FBXW7 (10%) using NGS on 224 specimens. They also observed gene amplification (ISH) of EGFR (20/174, 11%), and HER2 (32/395, 8%). Immunohistochemistry studies showed overexpression of estrogen receptor (118/590, 20%), progesterone receptor (48/589, 8%), and androgen receptor (22/578, 4%) in addition to other protein signatures. These data suggest that theranostic biomarkers may help guide therapy for patients who fail anti-angiogenesis therapy. The NGS, ISH and IHC results suggest that PI3K/AKT/mTOR pathway inhibitors, EGFR- and HER2-directed therapy, immunotherapy and hormonal therapy may be promising areas for future research.
Additional work is needed to develop and test molecularly targeted drugs and immune system modulation to achieve improved outcomes for women with persistent, metastatic and recurrent cervical cancer. Further study of theranostic biomarkers may help guide therapy for patients who progress on antiangiogenesis therapy or who are otherwise incurable.41 With continued exploration of these avenues, new therapeutic paradigms are likely to emerge that further improve survival and quality of life in this vulnerable population.
Acknowledgments
Supported by National Institutes of Health T-32 training grant (Ruth L. Kirschstein National Research Service Award Institutional Training Research Grant, 2T32 CA-060396-11).
Dr. Tewari has reported working as a consultant for Roche/Genentech, Caris, and Advaxis; serving on the advisory board of Roche/Genentech, Caris, Advaxis, Vermillion; serving on the speaker’s bureau of Vermillion; and performing contracted research for Genentech, Amgen, Endocyte, and Astra-Zeneca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement:
Dr. Pfaendler has no conflicts of interest.
Contributor Information
Krista S. Pfaendler, The Division of Gynecologic Oncology, University of California, Irvine, 101 The City Drive South, Building 56, Orange, CA 92868, USA
Krishnansu S. Tewari, The Division of Gynecologic Oncology, The Gynecologic Oncology Group at University of California, Irvine, Irvine Medical Center, University of California, 101 The City Drive South, Building 56, Orange, CA 92868, USA
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. [Accessed 4/8/2015];GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet] 2013 http://globocan.iarc.fr.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015 doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.McCormack PL. Quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine (gardasil((R))): a review of its use in the prevention of premalignant anogenital lesions, cervical and anal cancers, and genital warts. Drugs. 2014;74(11):1253–1283. doi: 10.1007/s40265-014-0255-z. [DOI] [PubMed] [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of pathology. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.zur Hausen H, de Villiers EM. Human papillomaviruses. Annual review of microbiology. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]
- 6.ACOG. ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstetrics and gynecology. 2012;120(5):1222–1238. doi: 10.1097/aog.0b013e318277c92a. [DOI] [PubMed] [Google Scholar]
- 7.Joura EA, Kjaer SK, Wheeler CM, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26(52):6844–6851. doi: 10.1016/j.vaccine.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 8.Malagon T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. The Lancet. Infectious diseases. 2012;12(10):781–789. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2014;63(Rr-05):1–30. [PubMed] [Google Scholar]
- 10.CDC. Centers for Disease Control and Prevention (CDC) 2013 NIS-Teen Vaccination Coverage Table Data. [Accessed 11 November 2014];2014 http://www.cdc.gov/vaccines/imz-managers/coverage/nis/teen/data/tables-2013.html.
- 11.Subramaniam A, Fauci JM, Schneider KE, et al. Invasive cervical cancer and screening: what are the rates of unscreened and underscreened women in the modern era? Journal of lower genital tract disease. 2011;15(2):110–113. doi: 10.1097/LGT.0b013e3181f515a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waggoner SE. Cervical cancer. Lancet. 2003;361(9376):2217–2225. doi: 10.1016/S0140-6736(03)13778-6. [DOI] [PubMed] [Google Scholar]
- 13.N H, AM N, M K, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; Apr, 2014. pp. 1975–2011. posted to the SEER web site. [Google Scholar]
- 14.Thigpen T, Shingleton H, Homesley H, LaGasse L, Blessing J. cis-Dichlorodiammineplatinum(II) in the treatment of gynecologic malignancies: phase II trials by the Gynecologic Oncology Group. Cancer treatment reports. 1979;63(9–10):1549–1555. [PubMed] [Google Scholar]
- 15.Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(15):3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 16.Long HJ, 3rd, Bundy BN, Grendys EC, Jr, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(21):4626–4633. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(28):4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore DH, Tian C, Monk BJ, Long HJ, Omura GA, Bloss JD. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecologic oncology. 2010;116(1):44–49. doi: 10.1016/j.ygyno.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito I, Kitagawa R, Fukuda H, et al. A phase III trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage IVB, persistent or recurrent cervical cancer: Gynecologic Cancer Study Group/Japan Clinical Oncology Group Study (JCOG0505) Japanese journal of clinical oncology. 2010;40(1):90–93. doi: 10.1093/jjco/hyp117. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel Plus Carboplatin Versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 doi: 10.1200/JCO.2014.58.4391. [DOI] [PubMed] [Google Scholar]
- 21.Bloss JD, Blessing JA, Behrens BC, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: a gynecologic oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(7):1832–1837. doi: 10.1200/JCO.2002.07.045. [DOI] [PubMed] [Google Scholar]
- 22.Omura GA, Blessing JA, Vaccarello L, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(1):165–171. doi: 10.1200/JCO.1997.15.1.165. [DOI] [PubMed] [Google Scholar]
- 23.Kerbel RS. Tumor angiogenesis. The New England journal of medicine. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature reviews. Drug discovery. 2004;3(5):391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 25.Wright JD, Viviano D, Powell MA, et al. Bevacizumab combination therapy in heavily pretreated, recurrent cervical cancer. Gynecologic oncology. 2006;103(2):489–493. doi: 10.1016/j.ygyno.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(7):1069–1074. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schefter TE, Winter K, Kwon JS, et al. A phase II study of bevacizumab in combination with definitive radiotherapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma: preliminary results of RTOG 0417. International journal of radiation oncology, biology, physics. 2012;83(4):1179–1184. doi: 10.1016/j.ijrobp.2011.10.060. [DOI] [PubMed] [Google Scholar]
- 28.Schefter T, Winter K, Kwon JS, et al. RTOG 0417: efficacy of bevacizumab in combination with definitive radiation therapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma. International journal of radiation oncology, biology, physics. 2014;88(1):101–105. doi: 10.1016/j.ijrobp.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Zighelboim I, Wright JD, Gao F, et al. Multicenter phase II trial of topotecan, cisplatin and bevacizumab for recurrent or persistent cervical cancer. Gynecologic oncology. 2013;130(1):64–68. doi: 10.1016/j.ygyno.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. The New England journal of medicine. 2014;370(8):734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tewari KS, Sill MW, Penson RT, et al. Proffered Paper Session presented at European Society for Medical Oncology. Madrid, Spain: Sep 28, 2014. Final overall survival analysis of the phase III randomized trial of chemotherapy with and without bevacizumab for advanced cervical cancer: A NRG Oncology-Gynecologic Oncology Group Study. 2014. [Google Scholar]
- 32.Tewari KS, Sill M, Monk BJ, et al. Prospective validation of pooled clinical prognostic factors in patients with recurrent and advanced cervical cancer: A Gynecologic Oncology Group (GOG) study. Gynecologic oncology. 2014;133:59–60. Supplement 1(0) [Google Scholar]
- 33.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(7):884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 34.Mackay HJ, Tinker A, Winquist E, et al. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG Trial IND.184. Gynecologic oncology. 2010;116(2):163–167. doi: 10.1016/j.ygyno.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Monk BJ, Mas Lopez L, Zarba JJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(22):3562–3569. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 36.Monk BJ, Pandite LN. Survival data from a phase II, open-label study of pazopanib or lapatinib monotherapy in patients with advanced and recurrent cervical cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(36):4845. doi: 10.1200/JCO.2011.38.8777. [DOI] [PubMed] [Google Scholar]
- 37.Symonds P, Gourley C, Davidson S, et al. LBA25_PRCIRCCA: A randomised double blind phase II trial of carboplatin-paclitaxel plus cediranib versus carboplatin-paclitaxel plus placebo in metastatic/recurrent cervical cancer.(CRUK grant ref: C1256/A11416) Annals of Oncology. 2014;25(suppl 4) mdu438. 426. [Google Scholar]
- 38.Tewari KS, Monk BJ. In: Clinical Gynecologic Oncology: Expert Consult-Online. DiSaia PJ, Creasman WT, editors. Elsevier Health Sciences; 2012. pp. 107–112. [Google Scholar]
- 39.Yoo HJ, Lim MC, Seo SS, et al. Pelvic exenteration for recurrent cervical cancer: ten-year experience at National Cancer Center in Korea. Journal of gynecologic oncology. 2012;23(4):242–250. doi: 10.3802/jgo.2012.23.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minion LE, Bai J, Monk BJ, et al. A Markov model to evaluate cost-effectiveness of antiangiogenesis therapy using bevacizumab in advanced cervical cancer. Gynecologic oncology. 2015 doi: 10.1016/j.ygyno.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 41.Feldman R, Gatalica Z, Reddy SK, Tewari KS. Paving the road to personalized medicine in cervical cancer: Theranostic biomarker evaluation in a 592-specimen library. Gynecologic oncology. 2015;137:141. Supplement 1(0) [Google Scholar]
- 42.Tewari KS, Sill MW, Penson RT, et al. Society of Gynecologic Oncology. Chicago IL: Mar 29, 2015. Impact of circulating tumor cells (CTCs) on overall survival among patients treated with chemotherapy plus bevacizumab for advanced cervical cancer: A NRG Oncology - Gynecologic Oncology Group study. 2015. [Google Scholar]