Abstract
The primary tauopathies are a group of neurodegenerative diseases in which tau is believed to be the major contributing factor of the neurodegenerative process. In the primary tauopathies, there is a disassociation between tau, a microtubule associated protein, and microtubules, as a result of tau hyperphosphorylation. This disassociation between tau and microtubules results in tau fibrillization and inclusion formation, and microtubule dysfunction. There are different clinical syndromes associated with different primary tauopathies, and some clinical syndromes can be associated with multiple primary tauopathies. Hence, while some clinical syndromes are highly specific and almost diagnostic of a primary tauopathy, many are not, making it difficult to diagnose a primary tauopathy. Recently, radioligands that bind to tau and can be combined with positron emission tomography to detect fibrillary tau antemortem have been developed, although preliminary data suggest that these ligands may not be very sensitive in detecting tau associated with many of the primary tauopathies. Another recent advancement in the field is evidence demonstrating that tau may show similar properties to prions although infective transmission has not been demonstrated. There have been a few clinical trials targeting tau and microtubule dysfunction, although none have had any disease modifying effects. Understanding tau biology is critical to the development of pharmacologic agents that could have disease modifying effects on the primary tauopathies.
INTRODUCTION
Tauopathy is an umbrella term that subsumes many different entities, all characterized by the abnormal deposition of tau in the brain.1–3 Many entities subsumed under the umbrella term tauopathy are diseases that can have varying clinical presentations, some of which can overlap between diseases, resulting in a complex web of clinical syndromes and tauopathy associated diseases. Some primary tauopathies do not have a clinically defined presentation, and some are considered age-related. Table 1 is a list of age related tauopathies and disease that are currently considered primary tauopathies. For those considered diseases, the abnormal tau is thought to account for the primary underlying neurodegenerative process. All diseases that are considered primary tauopathies have in common the abnormal deposition of aggregated tau in the brain. There are other diseases, in which tau deposition can be observed, but for one reason or another, tau either co-exists with another protein, or tau is not considered the primary neurodegenerative process. Diseases in this latter category include: Alzheimer’s disease in which beta-amyloid is also present,4, 5 Lewy body disease in which alpha-synuclein is also present,6 myotonic dystrophy,7 subacute sclerosing panencephalitis,7 Down’s syndrome,8 and Niemann-Pick disease-type C.7 Tau can be detected at autopsy with immunohistochemical techniques that utilize specific antibodies that recognize different epitopes of tau. One of the most recent advances in the field has been the development of radioligands that can detect tau in the brain in-vivo via positron emission tomography. In this review, I will discuss tau biology, clinical and pathological diagnosis of the primary tauopathies, and recent advances in research related to the primary tauopathies.
Table 1.
List of entities considered primary tauopathies
| Current pathological diagnoses | Type of tauopathy |
|---|---|
| Pick’s disease | 3R tauopathy |
| Progressive supranuclear palsy | 4R tauopathy |
| Corticobasal degeneration | 4R tauopathy |
| Argyrophilic grain disease | 4R tauopathy |
| Globular glial tauopathies | 4R tauopathy |
| Aging-related tau astrogliopathy | 4R tauopathy |
| Chronic traumatic encephalopathy | 3R+4R tauopathy |
| a Primary age-related tauopathy (PART) | 3R+4R tauopathy |
| Parkinsonism-Dementia complex of Guam | 3R+4R tauopathy |
| Postencephalitic Parkinsonism | 3R+4R tauopathy |
| Atypical Parkinsonism of Guadeloupe | 3R+4R tauopathy |
| Diffuse neurofilament tangles with calcification | 3R+4R tauopathy |
| Frontotemporal dementia & Parkinsonism liked to Chromosome 17 | 3R, 4R, or 3R+4R tauopathy |
This diagnosis now includes the entity previously known as tangle dominant dementia
TAU BIOLOGY
Tau is encoded by the microtubule-associated protein, tau gene, which is located on chromosome 17q21.9, 10 Tau is a microtubule-associated protein that functions in the stabilization and assembly of microtubules11. Microtubules are important for axonal transport and for maintaining the structural integrity of the cell. In the adult brain, tau is located within neurons, predominantly within axons.12 Tau is also found in oligodendrocytes and astrocytes where its function is similar to its function in neurons.13, 14 The tau amino acid sequence can essentially be divided into four compartments: the N-terminal domain, a proline rich domain, a microtubule binding domain, and the C-terminal domain2. The N-terminal domain is important to provide spacing between the microtubules. The proline rich domain is important in cell signaling and interactions with protein kinases. The microtubule binding domain is important for binding to the microtubule. The C terminal domain is important in regulating microtubule polymerization. The binding of tau to the microtubule is extremely important. In fact, binding can induce tau conformational change.15 In its normal form, tau is unfolded and phosphorylated while its abnormal form, found in the brains of patients with primary tauopathies, is characterized by hyperphosphorylated and aggregated tau that has a beta-pleated sheet conformation.16, 17 The binding of tau to microtubules is regulated by the phosphorylation/dephosphorylation equilibrium of tau.18 It is currently thought that hyperphosphorylation of tau results in a loss of tau interaction with microtubules leading to microtubule dysfunction and impaired axonal transport, and to tau fibrillarization. Recently, it has been suggested that the primary problem with hyperphosphorylated tau results from an increase in the proportion of tau sequences that are phosphorylated, as opposed to an increase in the number of phosphorylated epitopes on each tau sequence.19
Not all tau sequences are created equal. There are six isoforms of tau that are expressed in the adult brain.20 These six isoforms are derived from alternative splicing of three N-terminal exons in the tau gene: exon 2, exon 3 and exon 10.20 Three of the six isoforms are due to the splicing in of exon 10, while the other three isoforms are a result of the splicing out of exon 10. The splicing in of exon 10 results in isoforms with four repeated microtubule binding domains, while the splicing out of exon 10 results in isoforms with thee repeated microtubule binding domains. This is important, because although the healthy human brain consists of equal amounts of tau with three and four repeated binding domains, some primary tauopathies are characterized by a predominance of isoforms with four repeated binding domains (4R tauopathies), some by a predominance of isoforms with three repeated binding domains (3R tauopathies), and some by an approximately equal mix of isoforms with three and four repeated binding domains (3R + 4R tauopathies) (Table 1).
PATHOLOGICAL DIAGNOSIS OF THE PRIMARY TAUOPATHIES
The pathological diagnosis of a primary tauopathy is complex. It not only depends on the immunohistochemical demonstration of abnormal tau deposition in the brain, but also on the presence/absence and amount of other non-tau proteins in the brain, the distribution of the abnormal tau that is deposited, and the morphological characteristics of the tau in different regions of the brain. Further, diagnosis may also depend on the predominant tau isoform that is present, although this is not always straightforward. For example, Pick disease is typically thought of as a 3R primary tauopathy because neuronal tau in Pick disease is primarily 3R tau.21 However, glial pathology in Pick disease is predominantly 4R tau.22 Hence, one has to be careful when sampling tissue for biochemical tau analyses for diagnosis. It should also be stressed that although we consider these diseases to be primary tauopathies, in most instances there are pathologies present in addition to the primary tau pathology. In some instances, three or more pathologies may co-exist. It is not uncommon, for example, to have a primary tauopathy such as progressive supranuclear palsy or primary age related tauopathy23 co-existing with argyrophilic grains disease, another tauopathy.24 In some instances there may also be beta-amyloid deposition in addition to the primary tauopathy which may not necessarily signify Alzheimer’s disease. Furthermore, protein pathology may also be accompanied by vascular pathology. It is, therefore, not surprising that the clinical phenotypes do not always match perfectly with what one expects based on pathological diagnosis which tends to focus on the so-called, “leading pathology”.
AGE RELATED TAUOPATHIES
Before discussing diseases that are considered primary tauopathies, it is worth mentioning that the presence of tau and hence a tauopathy is not always considered a disease process. Three age related tauopathy are worth further discussion: argyrophilic grain disease; primary age-related tauopathy and aging related tau astrogliopathy. Argyrophilic grain disease as the name implies indicates that its presence is not normal or solely due to aging. Argyrophilic grain disease is characterized by the presence of silver positive grain like structures identified primarily in the medial temporal lobe. To date, there is no definitive clinical feature associated with the presence of this pathology. Hence, it remains to determine whether argyrophilic grain disease is truly a neurodegenerative disease. The term primary age-related tauopathy was recently coined.23 It refers to the presence of tau deposition in neurons within limbic structures of the brain, in the absence of, or minimally present, beta-amyloid deposition. Primary age-related tauopathy is considered by most, although not all, distinct from Alzheimer’s disease. Recently it was demonstrated that primary age-related tauopathy is associated with subtle cognitive slowing and executive dysfunction, as well as atrophy of the left anterior hippocampus.25 Hence it appears that this pathology may not truly be indicative of tau deposition solely from normal aging. Unlike primary age-related tauopathy in which tau is deposited in neurons, aging related tau astrogliopathy is characterized by tau deposition in astrocytes. Currently, there is no clinical correlate of aging related tau astrogliopathy.
CLINICAL DIAGNOSIS OF DISEASES CONSIDERED PRIMARY TAUOPATHIES
Without specific biomarkers, it is difficult to make a diagnosis that is 100% predictive of an underlying tauopathy. Table 2 is a list of common presenting signs and symptoms and whether their presence is suggestive of an underlying tauopathy. As can be seen, most signs and symptoms by themselves are not going to be helpful in predicting an underlying tauopathy with any degree of certainty. Instead, it has become clear that recognition of a profile, or constellation of signs and symptoms, is more helpful than linking a specific sign or symptom, in predicting an underlying tauopathy. This profile or constellation of signs and symptoms is better known as a syndrome. Hence, in order to best predict an underlying tauopathy, in the absence of a specific biomarker, we have now come to rely on the recognition of specific syndromes that are highly suggestive of a tauopathy. The following three syndromes are highly suggestive of, although not pathognomonic for a tauopathy diagnosis: Richardson’s syndrome; primary progressive apraxia of speech; and corticobasal syndrome.
Table 2.
List of clinical signs and symptoms and their association with an underlying tauopathy
| Specific signs and symptoms | Association with tauopathy |
|---|---|
| Motor | |
| Vertical supranuclear gaze palsy | + |
| Axial rigidity | + |
| Unexplained falls | + |
| Speech apraxia | + |
| Limb apraxia | ± |
| Dysarthria | ± |
| Stiffness of muscles | ± |
| Dystonia | ± |
| Action myoclonus | ± |
| Early gait freezing (freezing while trying to walk) | ± |
| Resting tremor | − |
| Ataxia | − |
| Weakness of limb | − |
| Cognitive & Behavioral | |
| Memory loss | ± |
| Behavioral and/or personality change | ± |
| Limb apraxia | ± |
| Spatial/perceptual deficitsa | − |
| Aphasia in the absence of speech apraxia | − |
| Problems with calculations | − |
| Loss of word or object knowledge | − |
| Loss of facial recognition | − |
| Other | |
| Depression/Anxiety | ± |
| Head trauma | ± |
| Constipation | ± |
| Loss of smell | ± |
| Urinary incontinence | ± |
| Orthostatic hypotension | − |
| Fasciculation | − |
| Rapid eye movement sleep behavior disorder | − |
| Delusions (e.g. Capgras, Othello’s) | − |
| Visual/auditory/tactile hallucinations | − |
+ Highly suggestive of underlying primary tauopathy, ± Equivocal; −argues against underlying primary tauopathy
Only rarely associated with a primary tauopathy
Richardson’s syndrome
Richardson’s syndrome is the classic presenting syndrome suggestive of a pathological diagnosis of progressive supranuclear palsy,26 and hence suggestive of an underlying primary tauopathy. This syndrome is characterized by the insidious onset and progression of gait and balance problems leading to unexplained falls. Typically, patients with Richardson’s syndrome will have additional symptoms present at onset including sensitivity to bright light, dizziness, a hoarse raspy voice, neck stiffness, an unusual facial appearance with the eye brows elevated, and a general slowing down of movements. Patients may be described as having a loss of general interest in people about them or apathy. A resting tremor and loss of memory are not present arguing against a diagnosis of Parkinson’s and Alzheimer’s disease, respectively. Neurological examination reveals the presence of executive dysfunction (evidence of disorganization and poor planning) and a relatively symmetric akinetic rigid syndrome. There is a loss of postural reflexes, axial rigidity (neck and trunk rigidity) and loss of, or slowness of, vertical eye movements to commands but relatively preserved eye movements with the dolls eye maneuver (supranuclear gaze palsy). Treatment with high doses of carbidopa/levodopa (> 600mg) and similar agents are typically unhelpful with an absence of any clinically significant response.
Primary progressive apraxia of speech
Primary progressive apraxia of speech is also characterized by an insidious onset and worsening of symptoms over time.27 The main clinical features are a slow effortful speech sometimes associated with difficulty articulating words, leading to the production of either distorted sounds or the substitution of normal sounds with distorted sounds or speech output with lengthened intersegment durations between syllables, words, or phrases.28 Sometimes one may observe groping movements of the tongue and mouth, and multiple trials in order to produce the intended sounds. Currently, two variants of primary progressive apraxia of speech are recognized: a phonetic variant in which articulatory errors dominate (Type 1) and a prosodic variant in which a slowed speech output is typical (Type 2).29 Language characteristics including syntax, grammar, comprehension, and naming are intact. Hence, the patient easily understands spoken and written sentences and word meaning. Over time primary progressive apraxia of speech evolves and after 6–7 years many patients develop features that begin to look more like Richardson’s syndrome.30 In other patients aphasia develops and progressively gets worse in the absence of features typical of Richardson’s syndrome. Regardless, eventually all patients with primary progressive apraxia of speech become mute, although communication by other means such as writing, gesticulating, typing, texting or signing remains in intact.
Corticobasal syndrome
The corticobasal syndrome is the third syndrome that is also strongly associated with an underlying tauopathy,31 although of all the three syndromes discussed it may be the least specific to an underlying primary tauopathy.1, 32 The corticobasal syndrome is characterized by the presence of asymmetric clinical features that suggest a combination of cortical and subcortical (basal ganglia) pathology. Cortical dysfunction can manifest as the alien limb phenomenon33 (in which the patient has lost control over a limb) attributed to involvement of sensory motor cortices and connections. Patients may personify their limb and sometimes will refer to their limb as “my little friend”. Another typical feature is the presence of limb apraxia in which the patient may not be able to perform a task that previously could be performed in the absence of motor weakness. For example, a patient may not know how to use a screw driver to drive a screw having done so for decades before. Some patients may manifest unwanted movements of other body parts (e.g. opening and closing of the mouth with alternating movements of the hand) and may have cortical sensory loss and agraphesthesia (difficulty recognizing a number or a letter that is traced in the palm of the hand). Myoclonus (quick involuntary jerks) and dystonia (abnormal posturing) may be observed. Basal ganglia related features must also be present, and may include asymmetric limb rigidity and/or akinesia (decreased speed of movement), with little significant or sustained improvement from levodopa therapy. Although not always present, cortical dysfunction of the frontal and temporal lobes may manifest as executive dysfunction, behavioral or personality change, or aphasia (language impairment).
Other clinical features and syndromes
Other clinical syndromes can also be associated with a primary tauopathy. However, many of these other clinical syndromes are less specific and hence are equally likely, or even more likely, to be associated with another neurodegenerative process in which tau is not consider the primary problem. These include the behavioral variant of frontotemporal dementia (in which patients present with behavioral and personality change)34, the logopenic variant of primary progressive aphasia (in which patients present with language and other problems affecting naming, word retrieval, working memory and calculations)35 and semantic dementia (in which patients present with a loss of object knowledge, for example not knowing that a zebra has stripes or that a carrot is orange in color).36 Other classic clinical syndromes such as dementia with Lewy bodies (in which dementia, Parkinsonism and psychoses occur in any combination)6 are rarely associated with a primary tauopathy. One clinical feature that merits further discussion is that of head trauma. Chronic head trauma has been associated with the primary tauopathy, chronic traumatic encephalopathy. This pathology was recently characterized as the accumulation of abnormal tau in neurons and glial cells that are located predominantly around small blood vessels at the depths of cortical sulci and in an irregular pattern.37 Currently there are many unanswered question regarding chronic traumatic encephalopathy, and other than head injury “at some point in time”, there is little clinical data associated with this primary tauopathy.
CLINICALLY AVAILABLE DIAGNOSTIC TESTS
At the present time, there is no clinically available test that is specific to an underlying tauopathy. There are, however, some tests that may be more suggestive of any underlying tauopathy than others that are worth discussing. There are no blood tests that can determine whether a patient has an underlying tauopathy or not. Some studies have suggested that measuring tau levels, total tau levels and phosphorylated tau levels in cerebrospinal fluid may provide support for an underlying tauopathy38. Others report no association between cerebrospinal fluid tau levels and the presence or absence of an underlying tauopathy39.
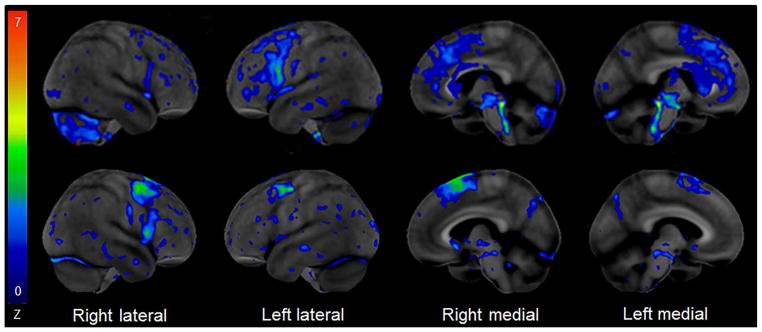
Neuroimaging modalities on-the-other-hand may provide some help when considering a diagnosis of a tauopathy. There are a handful of clinically useful findings on magnetic resonance imaging (MRI) and on molecular imaging that although not specific, can provide some help in making a diagnosis of a tauopathy. MRI head scan is typically performed to exclude the presence of structural lesions that could account for presenting syndromes suggestive of an underlying tauopathy. However, MRI also reveals anatomic patterns of involvement that are somewhat useful for diagnosing a tauopathy. One such feature is the presence of midbrain atrophy, particularly in the absence of atrophy of the pons,40, 41 although this is not a sensitive marker of pathology42. This is sometimes referred to as the hummingbird sign due to a reduction in the anterior-posterior diameter of the midbrain43 (Figure 1). Atrophy, seen as flattening of the superior colliculi, and atrophy of the superior cerebellar peduncles44, 45 are also strongly associated with, and hence suggestive of, the presence of an underlying tauopathy. Striking atrophy (referred to as knife edge atrophy) of the frontal and temporal lobes on MRI (Figure 1) can be a feature of Pick disease,46 and hence when this characteristic pattern of atrophy is present it is suggestive of an underlying 3R tauopathy. Asymmetric frontoparietal atrophy (Figure 1) is somewhat suggestive of underlying corticobasal degeneration pathology. In addition to MRI, [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) may also provide clues to the presence of an underlying tauopathy.47 Atrophy of the midbrain results in a focal signal of hypometabolism in the midbrain (Figure 2) known as the pimple sign.48 In addition, sometimes there is a subtle hypometabolic track between the midbrain and the cerebellum, likely reflecting atrophy of the superior cerebellar peduncles that may also be present (Figure 2). Other features suggestive of an underlying tauopathy include focal hypometabolism of the lateral premotor and supplementary motor cortices47 (Figure 2), as well as frontoparietal and caudate hypometabolism occurring together49 (Figure 2). It must be pointed out, however, that all of the abnormalities discussed relating to MRI or FDG-PET are less than 100% sensitive and specific for diagnosing an underlying primary tauopathy.
Figure 1.
T1 weighted MRI features suggestive of an underlying primary tauopathy include the humming bird sign resulting from atrophy of the dorsal midbrain and preserved pons (A, bottom image) suggestive of progressive supranuclear palsy, asymmetric parietal atrophy, right greater than left, suggestive of corticobasal degeneration (B, bottom image) and striking atrophy of the prefrontal cortex and anterior temporal lobe with secondary ventricular enlargement, worse of the left (C, bottom image) suggestive of Pick’s disease. Top images are normal MRI scans for comparison.
Figure 2.
[18F] Fluorodeoxyglucose PET scan using the Cortex Suite software reveals mild hypometabolism of the left posterior frontal cortex, bilateral supplemental motor cortices, midbrain, superior cerebellar peduncle and right cerebellum in a case of Richardson’s syndrome (top row) and mild hypometabolism in bilateral posterior frontal cortices, and right supplemental motor cortex in a patient with primary progressive apraxia of speech (bottom row) suggestive of an underlying primary tauopathy.
MANAGEMENT OF TAUOPATHIES
At present there are no disease modifying therapies for treating tauopathies. Treatment of tauopathies focuses on alleviating or ameliorating symptoms for which treatment exists. Unfortunately, many symptoms and signs albeit debilitating are untreatable. Medications are ineffective to ameliorate Parkinsonism and hence patients do not typically respond to dopamine targeted treatments. Management is complex, however, and one has to target whatever symptom is most bothersome to the patient and their careers. For example, a patient with an underlying tauopathy and a Richardson syndrome presentation may be most bothered by bright lights (photosensitivity).50 Management would simply be having the patient wear dark sun glasses that prevent exposure to bright light. Hence, management for photosensitivity is not specific to photosensitivity in tauopathies but photosensitivity in general. Since almost any symptom can be associated with an underlying tauopathy detailed and specific management of each and every symptom and sign is beyond the scope of this review. Table 3 provides a list of symptoms commonly observed in the primary tauopathy and general guidance on management of such symptoms. Some symptoms that are typically encountered in tauopathies that may respond relatively well to pharmacologic treatments include depression, anxiety, myoclonus, pathological crying/laughing, and insomnia. Others, including vertigo, diplopia (double vision), dystonia, Parkinsonism, poor appetite and weight loss, are difficult to treat and may not respond to any available treatment. There are also non-pharmacologic options that should be offered to patients with suspected tauopathy.51 Physical therapy for gait and balance problems is useful to prevent a faster decline in motor function but will not reverse any loss of function. Speech therapy is helpful in patients with progressive apraxia of speech and a swallow evaluation is critical any patient who is having trouble swallowing. A simple maneuver such as tucking the chin when swallowing can help reduce the risk of aspiration. Patients who are mute can benefit from the usage of devices that allow for communication in the absence of marked to severe aphasia or motor dysfunction of the limbs which typically can occur later in the disease course.
Table 3.
Symptoms that commonly occur in the primary tauopathies and associated management likely to provide some benefit
| Symptoms | Management |
|---|---|
| Postural tremor | Beta-blockers |
| Slowness of movements | Dopamine |
| Muscle stiffness | Dopamine |
| Sensitivity to bright lights | Wearing dark sunshades |
| Involuntary eye closure | Botox |
| Neck pain associated with dystonia | Botox |
| Drooling | Botox of salivary glands |
| Trouble with balance and falls | Physical and occupational therapy |
| Trouble swallowing | Swallow evaluation |
| Choking while eating or drinking | Swallow evaluation |
| Dysarthria or apraxia of speech | Speech therapy |
| Excessive tearing (lacrimation) | Artificial tears multiple times daily |
| Difficulty falling or staying asleep | Proper sleep hygiene, behavioral and pharmacologic management |
| Excessive daytime sleepiness | Caffeine, proper sleep hygiene |
| Depression | Antidepressants |
| Anxiety | Anxiolytics |
| Emotional incontinence (laughs/cries excessively) | Antidepressants and Nuedexta |
| Loss of sex drive (libido) | Exercise, antidepressants |
GENETIC FACTORS
There is some evidence that the primary tauopathies may have genetic links. The chromosomal region containing the microtubule associated protein tau gene includes two major haplotypes, H1 and H2, which are essentially defined by linkage disequilibrium between several polymorphisms over the entirety of the gene.52 The inheritance of the H1 haplotype and the H1/H1 genotype in the western world is a risk factor for the development of a primary tauopathy.52, 53 Linkage disequilibrium fine -mapping analysis has also further demonstrated an association between the primary tauopathies and the microtubule associated protein tau H1c haplotype, which is a variant of the H1 haplotype.54, 55 This variant has been shown to be associated with increased deposition of the 4R tau isoforms.54–56 Interestingly, the H2 haplotype has been suggested to be associated with a protective effect against the development of a primary tauopathy.57 A large genome wide association study of the primary tauopathy progressive supranuclear palsy discovered previously unidentified signals associated with progressive supranuclear palsy,58 although none have been subsequently shown to have any relevance.
RECENT ADVANCES IN TAU RESEARCH
Tau-PET
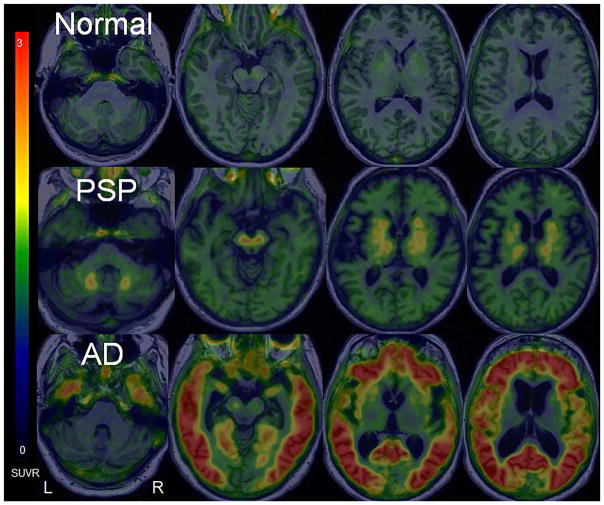
The determination of whether a patient has one of the primary tauopathies or not typically occurs at the time of autopsy after the patient has dies. Recently, however, there has been the development of in-vivo PET radiotracers that allows for the detection of tau in the brain in vivo. Previously, there were only tracers that allowed for the in-vivo detection of beta-amyloid.59 Over the past 5-years there have been many radiotracers developed with the intention of selectively detecting tau in the brain60. Unfortunately, due to many unwanted side-effects and other problems with these tracers such as toxic metabolites, many of these tracers have not been successfully translated into research. Of the tracers that have been tested, one tracer that has been very successfully integrated into research is [18F]AV-1451.61, 62 Autoradiographic studies have demonstrated that [18F]AV-1451 selectively binds to tau, does not bind to other proteins such as beta-amyloid, alpha-synuclein and others, and is safe for human studies.61, 63–65 Many studies have now demonstrated that AV-1451 can detect 3R+ 4R tau isoforms and hence is a very good biomarker to study Alzheimer’s disease which is characterized by the presence of 3R+4R tau.66, 67 Unfortunately, AV-1451 does not look as promising to detect isolated 3R, or 4R tau. There is relatively little observed binding to tau in the primary tauopathies, compared to Alzheimer’s disease49, 68–70 (Figure 3). Furthermore, there appears to be off target binding in the basal ganglia, midbrain and elsewhere, with AV-1451, regions that are critically involved in 4R tauopathies63, 65 (Figure 3). Therefore, AV-1451 may not be a good biomarker for the primary tauopathies. Two other tau tracers have also been utilized in research. Unfortunately, none of them have proven to be superior to AV-1451 for studying the primary tauopathies. With-that-said, PBB3, may have the ability to detect a wider range of the primary tauopathies due to more robust binding to 4R tau isoforms71. The second, THK5351, has also been performed in patients suspected of having an underlying primary tauopathy with similar results to AV-145172 although binding may be targeting dopamine related receptors and not tau?
Figure 3.
[18F] AV-1451 tau-PET shows minimal uptake in a normal control patient (top row), mild-moderate uptake in the dentate nucleus of the cerebellum, midbrain and basal ganglia in a patient with progressive supranuclear palsy (PSP), a primary 4R tauopathy (2nd row), and striking uptake in the cortex in a patient with typical Alzheimer’s disease (AD), a 3R + 4R tauopathy, for comparison (bottom row).
Prion-like properties and propagation of tau
Over the past decade one area of tau research that has dominated the field, is whether tau has prion like properties and can propagate from cell to cell and beyond.73, 74 More specifically, does tau behave like a prion and can it be transmitted like an infection. The term prion was first coined by Prusiner in 1982 to describe the infectious transmissibility of a proteinatious particle.75 The concept of tau being prion-like may date back to the idea that tau, in the form of neurofibrillary tangles, has a stereotypic pattern of spread throughout the brain in Alzheimer’s disease when the Braak staging scheme was published.4 This staging scheme, although developed from cross-sectional analysis, suggests that tau first deposits in the transentorhinal cortex before spreading to the hippocampus proper and then multimodal and unimodal cortices. Adding fuel to the fire was the demonstration that hyperphosphorylated tau could recruit or seed normal tau to assemble into filamentous aggregates.76 More recently, three important areas of study have been published that further promotes this idea. The first provided some evidence that extracellular tau may be able to enter cells and promote tau aggregation inside the cell.77 The second was the demonstration that tau when injected into mice may promote tau filamentous aggregation.78 The third provided some evidence that tau can jump from cell to cell.79 This notion of prion-like behavior of tau is very contentious however, given the lack of transmission like an infection, with many researchers opposing the notion that the behavior of tau mirrors that of the prion proteins of spongiform encephalopathy.80
TREATMENT TRIALS AND FUTURE DIRECTIONS
There are many different approaches being directed at treating tauopathies. These approaches include stabilizing microtubules, decreasing hyperphosphorylated tau, inhibiting protein kinases, inhibiting aggregation of tau fibrils, and enhancing intracellular tau degradation. Four different agents have been tested so far in human Phase I–III trials including methylene blue, riluzole,81 the octapeptide NAP,82 and tideglusib.83 None of these compounds have shown any evidence for efficacy. In addition, many different tau immunotherapies are currently being assessed84. Such immunotherapies involve both active and passive vaccine approaches with antibodies being developed that targets full-length tau, tau fragments or specific epitopes of tau.84
Two areas of active tauopathy research that will likely define the near future are the continued development of biomarkers that can detect in-vivo tau, and the development of compounds or antibodies for clinical trials that target tau. In addition, one would expect the continued development of mouse models to better represent the primary tauopathies and genetic studies to identify genetic influences that either account for disease or provide a model to study disease. Most of these research endeavors will likely focus on Alzheimer’s disease given the higher prevalence of this disease, growing elderly population, heightened awareness, financial burden to society and potential financial gains associated with discoveries, although there is some research that is now focused on the primary tauopathies, particularly progressive supranuclear palsy. Focusing on the primary tauopathies is critical, as it is unclear whether any biomarker or treatment developed targeting non-primary tauopathies such as Alzheimer’s disease will also be equally applicable to the primary tauopathies.
CONCLUSION
The primary tauopathies represent a group of pathological entities in which most, but not all, are considered a type of neurodegenerative disease. All primary tauopathy are associated with the deposition of abnormal hyperphosphorylated tau protein. There are a few clinical features that are highly suggestive of an underlying primary tauopathy but there are no perfect clinical or neuroimaging biomarkers that are able to accurately, and robustly, detect and differentiate the different types of primary tauopathies. Basic research related to the primary tauopathies include mouse and fly models, as well as studies at the cellular level with some researchers suggesting tauopathies are prion-like diseases. At present there are no disease modifying treatments, although clinical trials have begun to focus more on the primary tauopathies.
Acknowledgments
Financial support: KAJ is funded by NIH grants R01 NS89757 (PI), R01 AG037491 (PI) and R21 NS94684 (PI).
I would like to acknowledge Jennifer L. Whitwell, PhD, Mayo Clinic, Department of Radiology, Rochester, MN, for creating and providing figures 1–3.
LIST OF ABBRVIATIONS
- 3R
tau isoform with 3 repeats in the microtubule binding domain
- 4R
tau isoform with 4 repeats in the microtubule binding domain
- 3R + 4R
mixed 3 and 4 repeat tau isoforms
- AV-1451
a type of ligand that selectively binds to tau
- FDG-PET
fleurodeoxyglucose positron emission tomography
- MRI
magnetic resonance imaging
- PBB3
a type of ligand that selectively binds to tau
- THK5351
a type of ligand that selectively binds to tau
Footnotes
Conflict of interest disclosure: The author reports no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Josephs KA, Hodges JR, Snowden J, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 2011;122(2):137–153. doi: 10.1007/s00401-011-0839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arendt T, Stieler JT, Holzer M. Tau and tauopathies. Brain Res Bull. 2016;126(Pt 3):238–292. doi: 10.1016/j.brainresbull.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR, Ghetti B. Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci U S A. 1997;94(8):4113–4118. doi: 10.1073/pnas.94.8.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 5.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 7.Spillantini MG, Tolnay M, Love S, Goedert M. Microtubule-associated protein tau, heparan sulphate and alpha-synuclein in several neurodegenerative diseases with dementia. Acta Neuropathol. 1999;97(6):585–594. doi: 10.1007/s004010051034. [DOI] [PubMed] [Google Scholar]
- 8.Bussiere T, Hof PR, Mailliot C, et al. Phosphorylated serine422 on tau proteins is a pathological epitope found in several diseases with neurofibrillary degeneration. Acta Neuropathol. 1999;97(3):221–230. doi: 10.1007/s004010050978. [DOI] [PubMed] [Google Scholar]
- 9.Neve RL, Harris P, Kosik KS, Kurnit DM, Donlon TA. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986;387(3):271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- 10.Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988;85(11):4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempf M, Clement A, Faissner A, Lee G, Brandt R. Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament-dependent manner. J Neurosci. 1996;16(18):5583–5592. doi: 10.1523/JNEUROSCI.16-18-05583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papasozomenos SC, Binder LI. Phosphorylation determines two distinct species of Tau in the central nervous system. Cell Motil Cytoskeleton. 1987;8(3):210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- 14.LoPresti P, Szuchet S, Papasozomenos SC, Zinkowski RP, Binder LI. Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc Natl Acad Sci U S A. 1995;92(22):10369–10373. doi: 10.1073/pnas.92.22.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woody RW, Clark DC, Roberts GC, Martin SR, Bayley PM. Molecular flexibility in microtubule proteins: proton nuclear magnetic resonance characterization. Biochemistry. 1983;22(9):2186–2192. doi: 10.1021/bi00278a020. [DOI] [PubMed] [Google Scholar]
- 16.Uversky VN. What does it mean to be natively unfolded? Eur J Biochem. 2002;269(1):2–12. doi: 10.1046/j.0014-2956.2001.02649.x. [DOI] [PubMed] [Google Scholar]
- 17.Jeganathan S, von Bergen M, Mandelkow EM, Mandelkow E. The natively unfolded character of tau and its aggregation to Alzheimer-like paired helical filaments. Biochemistry. 2008;47(40):10526–10539. doi: 10.1021/bi800783d. [DOI] [PubMed] [Google Scholar]
- 18.Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984;259(8):5301–5305. [PubMed] [Google Scholar]
- 19.Morris M, Knudsen GM, Maeda S, et al. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat Neurosci. 2015;18(8):1183–1189. doi: 10.1038/nn.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 21.Delacourte A, Sergeant N, Wattez A, Gauvreau D, Robitaille Y. Vulnerable neuronal subsets in Alzheimer’s and Pick’s disease are distinguished by their tau isoform distribution and phosphorylation. Ann Neurol. 1998;43(2):193–204. doi: 10.1002/ana.410430209. [DOI] [PubMed] [Google Scholar]
- 22.Hogg M, Grujic ZM, Baker M, et al. The L266V tau mutation is associated with frontotemporal dementia and Pick-like 3R and 4R tauopathy. Acta Neuropathol. 2003;106(4):323–336. doi: 10.1007/s00401-003-0734-x. [DOI] [PubMed] [Google Scholar]
- 23.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta neuropathologica. 2014;128(6):755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Togo T, Sahara N, Yen SH, et al. Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol. 2002;61(6):547–556. doi: 10.1093/jnen/61.6.547. [DOI] [PubMed] [Google Scholar]
- 25.Josephs KA, Murray ME, Tosakulwong N, et al. Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: a clinico-imaging-pathological study of primary age-related tauopathy (PART) Acta neuropathologica. 2017 doi: 10.1007/s00401-017-1681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams DR, de Silva R, Paviour DC, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain. 2005;128(Pt 6):1247–1258. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]
- 27.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(Pt 5):1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duffy J. Apraxia of Speech in degenerative neurologic disease. Aphasiology. 2006;20(6):511–527. [Google Scholar]
- 29.Josephs KA, Duffy JR, Strand EA, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81(4):337–345. doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Josephs KA, Duffy JR, Strand EA, et al. The evolution of primary progressive apraxia of speech. Brain. 2014;137(Pt 10):2783–2795. doi: 10.1093/brain/awu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling H, O’Sullivan SS, Holton JL, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain. 2012;133(Pt 7):2045–2057. doi: 10.1093/brain/awq123. [DOI] [PubMed] [Google Scholar]
- 33.Hassan A, Josephs KA. Alien Hand Syndrome. Current neurology and neuroscience reports. 2016;16(8):73. doi: 10.1007/s11910-016-0676-z. [DOI] [PubMed] [Google Scholar]
- 34.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol. 1975;27(4):635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- 37.McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta neuropathologica. 2016;131(1):75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borroni B, Gardoni F, Parnetti L, et al. Pattern of Tau forms in CSF is altered in progressive supranuclear palsy. Neurobiol Aging. 2009;30(1):34–40. doi: 10.1016/j.neurobiolaging.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Kuiperij HB, Verbeek MM. Tau forms in CSF as a reliable biomarker for progressive supranuclear palsy. Neurology. 2011;76(16):1443. doi: 10.1212/WNL.0b013e318210e671. author reply 1443. [DOI] [PubMed] [Google Scholar]
- 40.Massey LA, Micallef C, Paviour DC, et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord. 2012;27(14):1754–1762. doi: 10.1002/mds.24968. [DOI] [PubMed] [Google Scholar]
- 41.Oba H, Yagishita A, Terada H, et al. New and reliable MRI diagnosis for progressive supranuclear palsy. Neurology. 2005;64(12):2050–2055. doi: 10.1212/01.WNL.0000165960.04422.D0. [DOI] [PubMed] [Google Scholar]
- 42.Whitwell JL, Jack CR, Jr, Parisi JE, et al. Midbrain atrophy is not a biomarker of progressive supranuclear palsy pathology. Eur J Neurol. 2013;20(10):1417–1422. doi: 10.1111/ene.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groschel K, Kastrup A, Litvan I, Schulz JB. Penguins and hummingbirds: midbrain atrophy in progressive supranuclear palsy. Neurology. 2006;66(6):949–950. doi: 10.1212/01.wnl.0000203342.77115.bf. [DOI] [PubMed] [Google Scholar]
- 44.Paviour DC, Price SL, Stevens JM, Lees AJ, Fox NC. Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. 2005;64(4):675–679. doi: 10.1212/01.WNL.0000151854.85743.C7. [DOI] [PubMed] [Google Scholar]
- 45.Tsuboi Y, Slowinski J, Josephs KA, Honer WG, Wszolek ZK, Dickson DW. Atrophy of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. 2003;60(11):1766–1769. doi: 10.1212/01.wnl.0000068011.21396.f4. [DOI] [PubMed] [Google Scholar]
- 46.Whitwell JL, Josephs KA, Rossor MN, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol. 2005;62(9):1402–1408. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- 47.Zalewski N, Botha H, Whitwell JL, Lowe V, Dickson DW, Josephs KA. FDG-PET in pathologically confirmed spontaneous 4R-tauopathy variants. J Neurol. 2014;261(4):710–716. doi: 10.1007/s00415-014-7256-4. [DOI] [PubMed] [Google Scholar]
- 48.Botha H, Whitwell JL, Madhaven A, Senjem ML, Lowe V, Josephs KA. The pimple sign of progressive supranuclear palsy syndrome. Parkinsonism Relat Disord. 2014;20(2):180–185. doi: 10.1016/j.parkreldis.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Josephs KA, Whitwell JL, Tacik P, et al. [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol. 2016 doi: 10.1007/s00401-016-1618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper AD, Josephs KA. Photophobia, visual hallucinations, and REM sleep behavior disorder in progressive supranuclear palsy and corticobasal degeneration: a prospective study. Parkinsonism Relat Disord. 2009;15(1):59–61. doi: 10.1016/j.parkreldis.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Tilley E, McLoughlin J, Koblar SA, et al. Effectiveness of allied health therapy in the symptomatic management of progressive supranuclear palsy: a systematic review. JBI Database System Rev Implement Rep. 2016;14(6):148–195. doi: 10.11124/JBISRIR-2016-2002352. [DOI] [PubMed] [Google Scholar]
- 52.Baker M, Litvan I, Houlden H, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8(4):711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- 53.Houlden H, Baker M, Morris HR, et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56(12):1702–1706. doi: 10.1212/wnl.56.12.1702. [DOI] [PubMed] [Google Scholar]
- 54.Myers AJ, Pittman AM, Zhao AS, et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol Dis. 2007;25(3):561–570. doi: 10.1016/j.nbd.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Rademakers R, Melquist S, Cruts M, et al. High-density SNP haplotyping suggests altered regulation of tau gene expression in progressive supranuclear palsy. Hum Mol Genet. 2005;14(21):3281–3292. doi: 10.1093/hmg/ddi361. [DOI] [PubMed] [Google Scholar]
- 56.Kouri N, Murray ME, Hassan A, et al. Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain. 2011;134(Pt 11):3264–3275. doi: 10.1093/brain/awr234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caffrey TM, Joachim C, Wade-Martins R. Haplotype-specific expression of the N-terminal exons 2 and 3 at the human MAPT locus. Neurobiol Aging. 2008;29(12):1923–1929. doi: 10.1016/j.neurobiolaging.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoglinger GU, Melhem NM, Dickson DW, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43(7):699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 60.Dani M, Brooks DJ, Edison P. Tau imaging in neurodegenerative diseases. Eur J Nucl Med Mol Imaging. 2016;43(6):1139–1150. doi: 10.1007/s00259-015-3231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chien DT, Bahri S, Szardenings AK, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34(2):457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- 62.Xia CF, Arteaga J, Chen G, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement. 2013;9(6):666–676. doi: 10.1016/j.jalz.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4(1):58. doi: 10.1186/s40478-016-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sander K, Lashley T, Gami P, et al. Characterization of tau positron emission tomography tracer [F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement. 2016 doi: 10.1016/j.jalz.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Marquie M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78(5):787–800. doi: 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho H, Choi JY, Hwang MS, et al. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology. 2016 doi: 10.1212/WNL.0000000000002892. [DOI] [PubMed] [Google Scholar]
- 67.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whitwell JL, Lowe VJ, Tosakulwong N, et al. [18 F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov Disord. 2016 doi: 10.1002/mds.26834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hammes J, Bischof GN, Giehl K, et al. Elevated in vivo [18F]-AV-1451 uptake in a patient with progressive supranuclear palsy. Mov Disord. 2016 doi: 10.1002/mds.26727. [DOI] [PubMed] [Google Scholar]
- 70.Smith R, Schain M, Nilsson C, et al. Increased basal ganglia binding of 18 F-AV-1451 in patients with progressive supranuclear palsy. Mov Disord. 2016 doi: 10.1002/mds.26813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ono M, Sahara N, Kumata K, et al. Distinct binding of PET ligands PBB3 and AV-1451 to tau fibril strains in neurodegenerative tauopathies. Brain: a journal of neurology. 2017 doi: 10.1093/brain/aww339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishiki A, Harada R, Okamura N, et al. Tau imaging with [18 F]THK-5351 in progressive supranuclear palsy. European journal of neurology. 2017;24(1):130–136. doi: 10.1111/ene.13164. [DOI] [PubMed] [Google Scholar]
- 73.Clavaguera F, Hench J, Goedert M, Tolnay M. Prion-like transmission and spreading of tau pathology. Neuropathol Appl Neurobiol. 2014 doi: 10.1111/nan.12197. [DOI] [PubMed] [Google Scholar]
- 74.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11(3):155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 76.Alonso Adel C, Li B, Grundke-Iqbal I, Iqbal K. Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc Natl Acad Sci U S A. 2006;103(23):8864–8869. doi: 10.1073/pnas.0603214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284(19):12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11(7):909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanders DW, Kaufman SK, DeVos SL, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82(6):1271–1288. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hyman BT. Tau propagation, different tau phenotypes, and prion-like properties of tau. Neuron. 2014;82(6):1189–1190. doi: 10.1016/j.neuron.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 81.Bensimon G, Ludolph A, Agid Y, et al. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain. 2009;132(Pt 1):156–171. doi: 10.1093/brain/awn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boxer AL, Lang AE, Grossman M, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 2014;13(7):676–685. doi: 10.1016/S1474-4422(14)70088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tolosa E, Litvan I, Hoglinger GU, et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 2014;29(4):470–478. doi: 10.1002/mds.25824. [DOI] [PubMed] [Google Scholar]
- 84.Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer’s disease. Trends Mol Med. 2015;21(6):394–402. doi: 10.1016/j.molmed.2015.03.003. [DOI] [PubMed] [Google Scholar]