Abstract
Measurement of plasma aldosterone and renin concentration, or activity, is useful for selecting antihypertensive agents and detecting hyperaldosteronism in hypertensive patients. However, it takes several days to get results when measured by radioimmunoassay and development of more rapid assays has been long expected. We have developed chemiluminescent enzyme immunoassays enabling the simultaneous measurement of both aldosterone and renin concentrations in 10 minutes by a fully automated assay using antibody-immobilized magnetic particles with quick aggregation and dispersion. We performed clinical validation of diagnostic ability of this newly developed assay-based screening of 125 patients with primary aldosteronism from 97 patients with essential hypertension. Results of this novel assay significantly correlated with the results of radioimmunoassay (aldosterone, active renin concentration, and renin activity) and liquid chromatography–tandem mass spectrometry (aldosterone). The analytic sensitivity of this particularly novel active renin assay was 0.1 pg/mL, which was better than that of radioimmunoassay (2.0 pg/mL). The ratio of aldosterone-to-renin concentrations of 6.0 (ng/dL per pg/mL) provided 92.0% sensitivity and 76.3% specificity as a cutoff for differentiating primary aldosteronism from essential hypertension. This novel measurement is expected to be a clinically reliable alternative for conventional radioimmunoassay and to provide better throughput and cost effectiveness in diagnosis of hyperaldosteronism from larger numbers of hypertensive patients in clinical settings.
Keywords: aldosterone, diagnosis, human, reaction time, renin
Primary aldosteronism (PA) is the most common causes of secondary hypertension, and unilateral PA is considered surgically curable.1 The prevalence of PA is estimated to be ≈10% of all hypertensive patients, and it is reported to account for ≈20% of patients with resistant hypertension.2–6 The estimated number of PA patients is expected to be relatively large, but the actual number of confirmed cases is more limited. This discrepancy between the estimated prevalence and the actual number of confirmed cases may be partly attributed to a diagnostic workup for PA that is composed of multiple steps and requires measurement of both plasma aldosterone and renin on multiple occasions, suggesting that these processes could be costly and time consuming.
In terms of assays of aldosterone and renin concentrations, most laboratories are still using radioimmunoassays, and availability of studies is limited using nonradioimmunoassay methods.7–10 Most of currently available radioimmunoassay systems adopt polyclonal antibodies against aldosterone, which could partly account for unsatisfactory reproducibility.9,10 On the contrary, measurement of plasma renin concentrations without using radioactive materials has been recently reported.7,8,11–13 However, currently available assays for renin concentration have relatively poor sensitivities when compared with assays for plasma renin activity (PRA). It is true that the sensitivities of PRA assay have been improved by prolonging incubation time to produce more angiotensin I, making it possible to achieve sensitivity of <1.0 ng/mL per hour,14 while producing a dilemma as to sensitivity versus rapidity. There is a need to screen patients with low renin hypertension in order to efficiently pick up those with PA, and nonradioimmunoassay measurements of renin concentrations have been refined to achieve better sensitivities without compromising a shorter reaction time.
In the present study, we characterized recently developed fully automated chemiluminescent enzyme immunoassay (CLEIA) for plasma aldosterone concentrations (PAC) and active renin concentrations (ARC), which can be measured simultaneously in 10 minutes and 20 seconds, and clinical validation of their diagnostic abilities for detecting PA from hypertensive patients was also performed in this study.
Methods
Patient Eligibility and Diagnosis of PA
Hypertensive patients referred to Tohoku University Hospital for screening of PA were included. As reported previously,15–19 we differentiated PA from essential hypertension (EH), performed subtype analysis by adrenal venous sampling (AVS), and confirmed aldosterone-producing lesions in adrenalectomized tissues by pathological evaluation (shortly described in Methods section in the online-only Data Supplement).18,19 The eligible PA patients were registered as a cohort of PA Sendai Study and gave written informed consent,17 and the present study was approved by the ethics committee of Tohoku University School of Medicine (#2011–236, #2012–365).
Development, Validation, and Characterization of CLEIA
The conventional radioimmunoassay methods for PAC, ARC, and PRA are available in Methods section in the online-only Data Supplement. Newly developed assays for PAC and ARC use antibody-immobilized magnetic particles, called MAGRAPID (Wako Pure Chemical Industries, Ltd, Osaka, Japan; Japanese unexamined patent application publication number 5977743, US patent number 9 157 911 B2), with the ability to quickly aggregate and disperse. Limits of detection, accuracy, precision, linearity, and recovery were determined for novel CLEIAs for PAC and ARC according to recommendations of the Clinical and Laboratory Standards Institute. Influence on the assays by ascorbic acid, hemoglobin, bilirubin, bilirubin-conjugate, chyle, and rheumatoid factor were analyzed for interference testing. Cross-reactivity with endogenous and synthetic steroids in addition to mineralocorticoid receptor antagonists was examined concerning the development of the aldosterone assay.
Development of the Novel Automated CLEIA for PAC
For determination of PAC, we used a highly specific antialdosterone monoclonal antibody (A2E11)20 and peroxidase-conjugated aldosterone. A detailed description of the assay protocol is presented as online-only Data Supplement; the following is a brief description of the protocol: 25 μL of plasma is mixed with 50 μL of reagent 1 including the antialdosterone monoclonal antibody and antimouse polyclonal antibody immobilized onto the magnetic particles MAGRAPID and then incubated for 180 seconds at 37°C. After the incubation, 50 μL of reagent 2 containing peroxidase-conjugated aldosterone is added and incubated for 180 seconds at 37°C. The particles can be rapidly aggregated by applying magnet, and the solution is removed. After washing the magnetic particles, these particles are quickly dispersed by removing the magnet, and 150 μL of substrate solution is added to measure chemiluminescence. The assay for PAC was calibrated in-house using synthetic aldosterone as reference material.
Development of the Novel Automated CLEIA for ARC
For the determination of ARC, we used 2 antirenin monoclonal antibodies (Japanese patent number 2877222) to develop a 2-site sandwich immunoassay; one recognizes the nonactive site of renin by binding to both renin and prorenin, that is, total renin (monoclonal antibody 12–12, as a capture antibody),21 whereas the other binds to the active site of renin (monoclonal antibody 11–6, reported to inhibit PRA by >90%, as a detection antibody). A detailed description of the assay protocol is presented as online-only Data Supplement. The following is a brief description of the protocol: 25 μL of plasma is mixed with 50 μL of reagent 1 including antitotal renin monoclonal antibody (monoclonal antibody 12–12) immobilized to MAGRAPID particles, and then incubated for 180 seconds at 37°C. After removing the mixed solution and washing the magnetic particles, 50 μL of reagent 2 containing peroxidase-conjugated antiactive renin monoclonal antibody (monoclonal antibody 11–6) is added and then incubated for 180 seconds at 37°C. The particles are aggregated by magnet, and the solution is removed. After washing the magnetic particles, they are dispersed by removing the magnet, and then 200 μL of substrate solution is added to measure chemiluminescence. The assay for ARC was calibrated in-house using human recombinant renin (human activated renin [GenBank accession number NM_000537], amino acids 67–406 with C-terminal HIS tag, catalog number: 80200, lot number: 120228; BPS Bioscience, Inc, San Diego, CA) as reference material.
Finally, the automated immunoassay system for simultaneous measurement of PAC and ARC, named Accuraseed, was developed. Accuraseed, a system based on the newly invented MAGRAPID, and CLEIA methods, made it possible to measure PAC and ARC simultaneously from a single plasma sample in just 10 minutes and 20 seconds.
Plasma Samples Used for Validation of the Novel Assay and Comparison to Conventional Methods
All antihypertensive agents other than calcium channel blockers and α-adrenergic antagonists were withdrawn before hormonal examination. Blood samples were collected from an antecubital vein into EDTA-2Na tubes in the supine position and after bed rest for 30 minutes. To avoid cryoactivation of renin during freezing and thawing,22 blood was immediately centrifuged at room temperature, and subsequently plasma was rapidly frozen using liquid nitrogen, and finally stored at <−20°C. Samples were prepared before assay using rapid thawing in a 20°C water bath to reach room temperature, and then, these plasma samples were immediately assayed. Only one cycle of freeze-thaw process was allowed. Moreover, influence of storage temperature on the novel ARC assay was evaluated with detailed methods described in the online-only Data Supplement.
Blood samples were obtained at the baseline evaluation during the diagnostic process of PA. When the amount of preserved plasma sample obtained at the first step of the diagnostic workup was not sufficient for one-time parallel measurement, another baseline plasma sample from the sample pool of blood obtained under the same conditions, on different occasions, was used.
Validation of the Novel Assays for PAC and ARC
For the measurement of PAC, correlation and linear regression analyses and Bland–Altman plots were performed to compare the novel assay with liquid chromatography–tandem mass spectrometry (LC-MS/MS).23 The same analyses were also performed to compare the conventional radioimmunoassay, SPAC-S kit, with LC-MS/MS.
For the measurement of plasma ARC, correlation and linear regression analyses and Bland–Altman plots were performed to compare the novel assay with the conventional immunoradiometric assay (IRMA).
Clinical Evaluation of Diagnostic Usefulness Based on the Novel Assay System
The aldosterone-to-renin concentration ratio (ARRARC) determined based on the novel assay was evaluated using receiver-operating characteristics analysis to investigate the assay sensitivity and specificity for screening of PA from those with essential hypertension. Sensitivity and specificity were also calculated based on both PAC and ARC levels obtained from the novel assay to evaluate diagnostic ability of PA from patients with essential hypertension as a screening test.
Statistical Analysis
Values below lower limits of detection were assigned to analytic sensitivity values of each assay for subsequent statistical comparison. The normality of collected data was analyzed by the Kolmogorov–Smirnov test. When continuous variables showed a normal distribution, 1-way ANOVA was used for comparison. When continuous variables showed no normality of distribution, Kruskal–Wallis test with Dunn multiple comparison test, as a post hoc test, were used. Bland–Altman difference plot was used to compare 2 methods with respect to agreement. Statistical significance was set at P≤0.05, and statistical analyses were performed using JMP software (SAS Inc, Cary, NC).
Results
Laboratory Validation of the Novel Assays for PAC and ARC
Detailed results of laboratory validation experiments are presented in the online-only Data Supplement. Briefly, for the novel PAC and ARC assays, the analytic sensitivity was 5.0 ng/dL and 0.1 pg/mL, respectively, and the upper limit of detection was 160 ng/dL and 500 pg/mL, respectively, as shown in Tables S1 and S2.
Diagnosis of PA and EH
Endocrinologic diagnosis was performed by conventional radioimmunoassays. A total of 222 hypertensive patients were referred for evaluation. Of them, 125 were diagnosed as having PA, 75 (60%) had unilateral disease and were finally diagnosed to have aldosterone-producing adenoma (APA) after adrenalectomy (Table), 50 (40%) had bilateral hyperaldosteronism (BHA), and the remaining 97 patients were diagnosed to have EH (Table). Conventional radioimmunoassay showed that PAC, aldosterone-to-renin activity ratio (ARR), and captopril-challenged ARR were significantly higher in patients with unilateral APA than in those with BHA or EH (P<0.05). PRA was significantly higher in patients with EH than in those with unilateral APA or BHA (P<0.05), whereas comparison between patients with unilateral APA and those with BHA showed no significant difference in baseline PRA levels.
Table.
Demographic and Baseline Data
| Parameters | Unilateral PA | Bilateral PA | Essential Hypertension | P Values |
|---|---|---|---|---|
| Number | 75 | 50 | 97 | |
| Age, y | 55.3±10.5 | 53.9±10.9 | 48.4±9.84 | <0.05*,† |
| Sex (male, female) | 51, 24 | 19, 31 | 64, 33 | NS |
| Baseline plasma aldosterone, ng/dL | 39.7 (22.6–54.8) | 15.9 (11.6–19.7) | 11.6 (10.0–17.0) | <0.05*,†,‡ |
| Baseline plasma renin activity, ng/mL/h | 0.20 (0.10–0.40) | 0.30 (0.18–0.52) | 0.80 (0.60–1.3) | <0.05*,† |
| Baseline ARR, ng/dL per ng/mL/h | 179 (78.5–332) | 78.0 (46.9–124) | 19.3 (15.6–49.8) | <0.05*,†,‡ |
| Captopril-challenged ARR, ng/dL per ng/mL/h | 128 (44.5–255) | 61.0 (36.3–83.1) | 13.0 (5.9–15.8) | <0.05*,†,‡ |
| Cortisol with 1 mg DST, μg/dL | 1.2±0.6 | 1.0±0.3 | 0.9±0.3 | <0.05*,‡ |
| No. of cases with adrenal nodules (Uni, Bi, None) | 53, 9, 13 | 18, 1, 31 | 28, 16, 53 | NS |
| Size of adrenal nodules, mm | 15.4±7.1 | 13.8±5.7 | 12.4±5.0 | <0.05* |
| SBP, mm Hg | 155.7±18.7 | 145.6±18.7 | 145.2±18.4 | < 0.05*,‡ |
| DBP, mm Hg | 97.2±15.1 | 90.6±12.5 | 94.3±13.3 | NS |
| HR, bpm | 72.6±10.1 | 77.5±13.2 | 78.9±11.3 | <0.05* |
| No. of antihypertensive agents per day | 2.7±1.6 | 1.8±1.3 | 1.9±1.5 | <0.05*,‡ |
| eGFR, mL/min/1.73 m2 | 75.6±21.5 | 76.0±15.4 | 79.0±19.5 | NS |
| Na, mmol/L | 142.2±1.9 | 141.9±1.7 | 141.3±1.9 | <0.05* |
| K, mmol/L | 3.4±0.6 | 4.0±0.4 | 4.0±0.4 | <0.05*,‡ |
| Cl, mmol/L | 103.0±2.6 | 104.9±2.1 | 103.9±1.8 | <0.05*,† |
| K replacement, mmol/d | 7.0±13.1 | 1.7±5.9 | 0 | <0.05*,‡ |
Data were shown as mean±SD or median (25th–75th percentile), unless otherwise stated. ARR indicates aldosterone-to-renin activity ratio; Bi, bilateral; DBP, diastolic blood pressure; DST, dexamethasone suppression test; eGFR, estimated glomerular filtration rate; HR, heart rate; NS, not significant; PA, primary aldosteronism; SBP, systolic blood pressure; and Uni, unilateral.
Statistical significance in comparison between unilateral PA and essential hypertension groups.
Statistical significance in comparison between bilateral PA and essential hypertension groups.
Statistical significance in comparison between unilateral and bilateral PA groups.
Clinical Validation of the Novel Assay for PAC
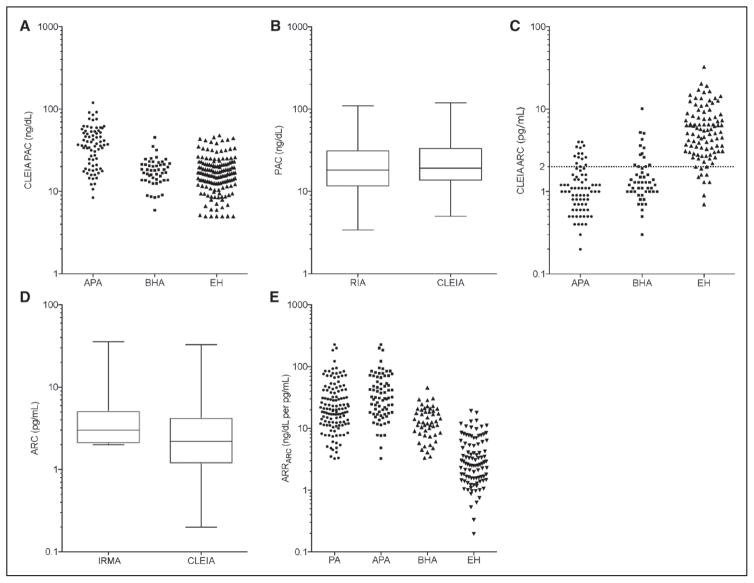
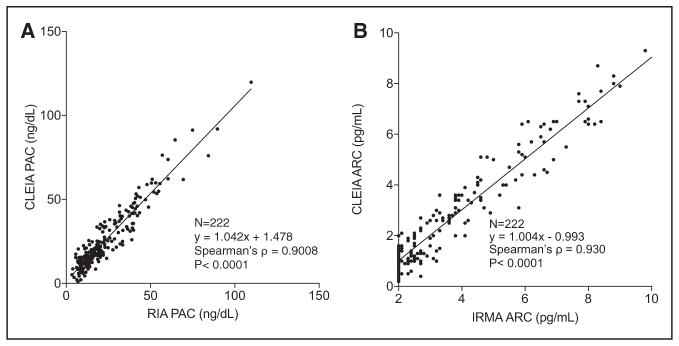
Plasma aldosterone concentration was measured using CLEIA in the whole study population, and it was significantly higher in those with unilateral APA when compared with those with BHA or EH (Figure 2A; Table S18 in the online-only Data Supplement), as well as in patients with BHA compared with those with EH (Figure 2A; Table S18). Measurements of PAC by CLEIA significantly correlated with those by the conventional radioimmunoassay (ρ =0.9008; P<0.0001; Figure 1A).
Figure 2.
A, Distribution of chemiluminescent enzyme immunoassay (CLEIA) plasma aldosterone concentration (PAC) measurements in aldosterone-producing adenoma (APA), bilateral hyperaldosteronism (BHA), and essential hypertension (EH). B, Comparison of PAC measurements between CLEIA and RIA. C, Distribution of CLEIA active renin concentration (ARC) measurements in APA, BHA, and EH. D, Comparison of ARC measurements between CLEIA and immunoradiometric assay (IRMA). E, Distribution of ARRARC in PA, APA, BHA, and EH.
Figure 1.
Correlation of plasma aldosterone concentration (PAC) measurements between chemiluminescent enzyme immunoassay (CLEIA) and radioimmunoassay (RIA; A) and correlation of ARC measurements between CLEIA and immunoradiometric assay (IRMA) (B). ARC indicates active renin concentration.
In terms of aldosterone assay using LC-MS/MS, 102 patients’ samples were not available to LC-MS/MS because of shortage of plasma material, and this resulted in limited analysis of the remaining 120 plasma samples (n=120, accounting for n=61 in APA, n=37 in BHA, and n=22 in EH). Plasma aldosterone concentrations measured by CLEIA significantly correlated with PAC measured by LC-MS/MS (ρ =0.977; P<0.0001; Figure S7A). Plasma aldosterone concentrations measured by radioimmunoassay were correlated with those by LC-MS/MS (ρ =0.949; P<0.0001; Figure S7B), and the degree of correlation between the novel CLEIA and LC-MS/MS was superior to the conventional radioimmunoassay and LC-MS/MS.
The influence on the novel PAC assay for those with renal impairment was investigated. Those with paired results of aldosterone measurements obtained from the novel assay and LC-MS/MS (n=120) were divided into the following 2 groups: one group without chronic kidney disease (meaning estimated glomerular filtration rate ≥60 mL/min per 1.73 m2; n=107) and the other with eGFR <60 mL/min per 1.73 m2 (N=13). Both the CLEIA and LC-MS/MS, measurements of plasma aldosterone concentrations revealed no significant differences whether they had renal insufficiency or not (Table S19). The 2 assays showed significant correlation with acceptable linear fit (ρ values were 0.984 and 0.970 in those with and without renal insufficiency), as shown in Figure S9A and S9B, respectively.
Clinical Validation of the Novel Assay for ARC
Active renin concentrations measured by CLEIA significantly correlated with those by the immunoradiometric assay (ρ =0.930; P<0.0001; Figure 1B). Active renin concentrations measured by CLEIA correlated with measurements of PRA by radioimmunoassay (ρ =0.912; P<0.0001; Figure S10). Based on measurements obtained by CLEIA, plasma ARC was significantly lower in those with unilateral APA and BHA, when compared with those with EH (Figure 2C; Table S18). Comparison between those with unilateral APA and those with BHA showed no significant difference in ARC measurements (Figure 2C; Table S18).
Comparison of ARC Assays Between the Novel and Conventional Methods
The ARC measurements of the same plasma samples by the conventional immunoradiometric assay and CLEIA were as shown in Figure 2D. Of interest, 48.8% of those with PA (52.0% and 44.0% of those with APA and BHA, respectively) and 3.1% of those with EH, accounting for 28.8% of all patients, showed that their ARC measurements by the immnoradiometric assay were below its analytic sensitivity (2.0 pg/mL), and these measurements were set to 2.0 pg/mL (Figure 2D). With the novel CLEIA of ARC, these measurements were distributed between 0.2 and 2.0 pg/mL (Figure 2C), and no measurements were found to be below the analytic sensitivity of the CLEIA (0.1 pg/mL).
Screening of PA Based on the Novel Simultaneous Assay for PAC and ARC
The aldosterone-to-renin concentration ratio were calculated as follows: 45.0±41.7, 14.3±8.4, and 4.1±3.4 ng/dL per pg/ mL, respectively, in patients with unilateral APA, BHA, and EH (mean±SD; Figure 2E; Table S18). Values of ARRARC were significantly elevated in those with unilateral APA when compared with BHA or EH, whereas those with BHA showed significantly higher ARRARC than those with EH (Figure 2E; Table S18).
On the basis of the measurements of ARRARC, receiver-operating characteristics analysis was performed to investigate a diagnostic ability of ARRARC as a screening test for PA from those with EH. The receiver-operating characteristics plot with AUC of 0.951 revealed that ARRARC cutoff of 6.0 ng/dL per pg/mL yielded sensitivity of 92.0% and specificity of 76.3% to discriminate PA from EH (Figure S11A; Table S20A).
Furthermore, sensitivity and specificity to diagnose PA from those with EH were also extensively investigated based on combination of 2 items, namely PAC and ARC measurements (Table S21A), rather than the single calculated index of ARRARC. On the basis of the measurements by Accuraseed assays, combined cutoff values of PAC 12.0 ng/dL and ARC 4.0 pg/mL revealed sensitivity of 90.4% and specificity of 70.1%. Adopting the cutoff values of ARC 5.0 pg/mL with the same aldosterone cutoff 12 ng/dL raises the sensitivity to 91.2%. On the contrary, higher cutoff values of plasma aldosterone 15 ng/dL with the same ARC cutoff 5.0 pg/mL improves specificity to 73.2% from 67.0%, when compared with the aldosterone cutoff 12 ng/dL.
Additionally, we investigated the diagnostic ability of ARRARC in the screening of patients with APA from those with EH. The receiver-operating characteristics with AUC of 0.978 showed that ARRARC cutoff of 7.7 ng/dL per pg/mL revealed sensitivity of 96.0% and specificity of 83.5% to discriminate APA from EH (Figure S11B; Table S20B). Another analysis based on combination of PAC and ARC cutoff values revealed that a pair of PAC 12.0 ng/dL and ARC 4.0 pg/mL showed sensitivity of 97.4% and specificity of 70.2%, with detailed data shown in Table S21B.
Discussion
This study reports the clinical validation of the recently developed CLEIA of PAC and ARC, which can be simultaneously measured in 10 minutes. Subsequently, we also evaluated diagnostic abilities of the newly developed assay-based clinical indexes as a screening test to diagnose those with PA from those with EH. Patients with PA have an increased risk of cerebral, cardiovascular, and renal events than those with EH, and unilateral PA has potential to be surgically curable.1 In addition, the prevalence of PA is reported to be elevated in those with resistant hypertension when compared with regular hypertensives.5 In terms of pharmacological treatment of hypertension, the use of mineralocorticoid receptor antagonists has been increasing, because this class of agents has been reported to be effective for adequate control of high blood pressure in patients with mild-to-moderate hypertension,24 as well as the effective add-on medication for those with resistant hypertension25,26 and the specific pharmacological treatment for those with PA.1,27 An increasing number of reports demonstrated that aldosterone-to-renin ratio and low PRA (or ARC) can predict the response to MR antagonists.28–30 Accordingly, there might be substantial and increasing needs to measure PAC and PRA (or ARC) for treating patients with mineralocorticoid receptor antagonists. In other words, clinical demands for more efficient and cost-effective way of screening of hypertensive patients with high ARR and low PRA (or ARC) have been increasing. In this regard, the newly developed CLEIA system might be expected to make a substantial contribution to developing a faster way of analysis, because the assay system only needs ≈10 minutes to measure both PAC and ARC simultaneously from a single small amount of sample (50 μL).
We validated the CLEIA system in terms of its precision, correlation, and sensitivity in the context of diagnostic processes in patients with PA and EH. First, precision of the new assay for aldosterone was considered to be mainly attributed to the highly specific monoclonal antibody it uses.20 The previous report on another automated CLEIA for PAC and ARC showed that the same antibody against aldosterone was used.7 As reported in that previous study,7 the present study also showed reliable precision across its assay range.
Second, the correlation of assays for PAC and ARC were thoroughly examined. CLEIA aldosterone measurements revealed significantly high correlation coefficients with both the LC-MS/MS measurements and those obtained with the currently widely used radioimmunoassay kit. In addition, Bland–Altman plot analysis of CLEIA aldosterone measurements with LC-MS/MS also demonstrated that the novel 10-minute assay could be a clinically reasonable substitute for conventional radioimmunoassay. In terms of ARC, CLEIA also showed significantly high correlations coefficients with the conventional IRMA kit.
Third, the analytic sensitivity of ARC assay was improved from 2.0 to 0.1 pg/mL. In the present study, the ARC levels of nearly half (48.8%) of the PA patients that could be measured by the novel CLEIA were below the limit of analytic sensitivity (2.0 pg/mL) of the conventional IRMA ARC kit. This might imply that values of ARRARC obtained by IRMA might be falsely calculated as smaller than their actual values. The novel CLEIA for ARC made it possible to detect 0.1 pg/mL, and this improvement might result in changes of ARRARC values for diagnostic discrimination of PA from EH as a screening index.
Although the sensitivity of the novel ARC assay was improved to 0.1 pg/mL, that of the PAC assay showed no improvement compared with the conventional radioimmunoassay (5.0 versus 2.5 ng/dL). We considered, however, that the analytic sensitivity of the novel PAC assay, 5.0 ng/dL, might be clinically acceptable in the diagnostic processes of PA because of the following 2 reasons. First, in the process of screening, PAC of 5.0 ng/dL should be enough to detect PA cases, although there are rare cases of normal PAC (eg, <10 ng/dL), almost if not all are above the level of sensitivity of the assay. Second, in the setting of confirmatory tests, measurement of relatively low range of PAC might be important, especially in 2 major suppression tests, for example, saline infusion test and fludrocortisone suppression test. According to the current guideline published by the Endocrine Society,1 optimal cutoff values have been reported to range >5.0 ng/dL to confirm PA in interpretation of these confirmatory suppression tests.
With respect to assay sensitivity, we also considered possible underlying mechanisms for the difference in improvement of analytic sensitivity between the novel ARC and PAC assays. As compared with conventional assays, both assays used newly developed magnetic particles, MAGRAPID, on which antibodies were immobilized, and this method might be considered to make substantial contribution in shortening of immunoreaction, because, in addition to increased surface areas compared with conventional beads, the magnetic particles have advantage to be freely dispersed and quickly aggregated with magnetic power in reaction tube, meaning increased likelihood to capture renin or antialdosterone antibody in shorter reaction times. Moreover, this effect provided by the magnetic particles might be more prominent in the ARC assay, because the assay was based on the 2-antibody sandwich method, in which the increased amount of immobilized antibody would increase trapping of renin and contribute to the increased sensitivity, and this was not applicable to the competitive PAC assay.
Although the ratio of ARR and ARRARC have been widely accepted as a screening index of PA,1,31,32 conventional assays for ARC have not necessarily provided a sensitivity high enough to differentiate patients with PA from those with low renin hypertension. Under these circumstances, the novel ARC assay with better analytic sensitivity (0.1 pg/mL) might improve its diagnostic ability of ARRARC as a screening test, and a screening method using ARRARC might be preferred because of its rapidity and convenience, that is, PAC, ARC, and the resultant ARRARC can be obtained in 10 minutes by simultaneous assay of a single specimen. In the present study, we considered that evaluation of clinical usefulness of both indexes, ARR and ARRARC, and a third screening method based on individual measurement of PAC and ARC.
With respect to ARRARC, some cutoff values have been advocated with various sensitivity and specificity; for instance, the Endocrine Society clinical practice guideline listed 20 different cutoff values with combination of different units in measurements of renin and aldosterone.1 We considered that we needed to standardize the novel ARC assay to make as accurate comparison as possible in the pursuit for optimal cutoff values. Among the 3 major studies on recently developed enzyme assays of ARC.7,12,13 Manolopoulou et al reported that their iSYS ARC assay was traceable to World Health Organization international standard renin (code 68/356) with its conversion factor of 1.67 (unit conversion from pg/mL to μIU/mL, meaning one unit of standard renin was measured as 600 ng using iSYS ARC assay). We used the same World Health Organization international standard of renin and determined that one unit of the renin was measured as 591 ng using Accuraseed ARC assay with conversion factor of 1.692 (unit conversion from pg/mL to μIU/mL; as shown in Methods and Results in the online-only Data Supplement). On the basis of these findings, we considered that accuracy of the novel ARC assay was clinically acceptable and results obtained from these ARC assays could be directly compared.
On the basis of the consideration above, ARRARC cutoff of 6.0 ng/dL per pg/mL corresponds to 5.7 (ng/dL per ng/L), which was shown as one of the advocated cutoff values,1 and we suggest cutoff values <6.0 when we intend to raise the sensitivity to ≈100%, as is also shown in iSYS assay study showing sensitivity of 98.9% with cutoff of 1.12 ng/dL per μIU/mL (equivalent to 1.87 ng/dL per pg/mL).7
The present study has some limitations as follows: disproportionate enrollment of subjects compared with an actual prevalence of PA in general hypertensive cohorts, the relatively small sample size for assessment of diagnostic ability, retrospective way of evaluation, and the nature of a single-center study, all making it difficult to offer a definitive proposal. Future multicenter studies with more appropriate design of subject enrollment in a prospective fashion are warranted. Based on these limitations, utility of both ARRARC-based and PAC and ARC-based approaches will need to be prospectively studied in a prevalence study.
Finally, we demonstrated that the novel CLEIA system has clinically acceptable accuracy and improved analytic sensitivity to diagnose PA patients. With the invention of antibody-immobilized magnetic particles with abilities of quick aggregation and dispersion, called MAGRAPID, and the adoption of the highly specific antibodies, this CLEIA system made it possible to measure both PAC and ARC at the same time from a single small amount of sample (50 μL), and it needed only 10 minutes and 20 seconds in the fully automated system. The utilization of this assay system might be expected to contribute not only for facilitating widespread screening of PA in a more rapid and cost-effective manner.
Perspectives
Conventional radioimmunoassays for PAC and ARC have been considered as relatively time-consuming and sometimes unrewarding in everyday clinical practice of hypertension, having most clinicians somewhat unwilling to aggressively perform screening for PA. In contrast, clinical need to perform screening for PA has been still increasing based on the current knowledge that its prevalence might be even higher than previously expected, for instance as high as 20% in those with resistant hypertension. The newly developed CLEIA provided high sensitivities and rapidity clinically enough to be a better alternative to the conventional assays and is expected to provide more efficient, more cost-effective diagnostic framework in the management of PA. Additionally, and more importantly, rapid and onsite availability of PAC and ARC examinations might provide clinical advantage for clinicians in evaluation of pathophysiology and making treatment plans, not only in settings for PA but also in management for larger population of hypertensive patients.
Supplementary Material
Novelty and Significance.
What Is New?
We developed a novel 10-minute simultaneous assay system for plasma aldosterone and active renin concentrations using highly sensitive and specific enzyme-linked assays.
What Is Relevant?
The newly developed assay system was clinically validated as compared with conventional radioimmunoassays and liquid chromatography–tandem mass spectrometry and is considered useful for rapid assessment of the renin–angiotensin–aldosterone system in hypertensive patients.
Summary
The present chemiluminescent enzyme immunoassay system is expected to improve medical care of hypertensive patients based on rapid assessment of renin–angiotensin–aldosterone system and to facilitate screening of secondary hypertension including primary aldosteronism in clinical settings.
Acknowledgments
We sincerely appreciate the following healthcare professionals and investigators who dedicated their expertise in the present study: Kei Omata, MD; Masahiro Nezu, MD; Yoshitsugu Iwakura, MD; and Yasuhiro Igarashi, MD, from Tohoku University Hospital, and Wataru Akahane and Yoshiko Kawamura from Wako Pure Chemical Industries, Ltd. We also thank Yasuko Tsukada, Akane Sugawara, Mika Ainoya, Kumi Kikuchi, and Hiroko Kato for their secretarial assistance.
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.117.09078/-/DC1.
References
- 1.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF., Jr The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 2.Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF., Jr Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–1050. doi: 10.1210/jc.2003-031337. [DOI] [PubMed] [Google Scholar]
- 3.Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 4.Stowasser M, Taylor PJ, Pimenta E, Ahmed AH, Gordon RD. Laboratory investigation of primary aldosteronism. Clin Biochem Rev. 2010;31:39–56. [PMC free article] [PubMed] [Google Scholar]
- 5.Hannemann A, Bidlingmaier M, Friedrich N, et al. Screening for primary aldosteronism in hypertensive subjects: results from two German epidemiological studies. Eur J Endocrinol. 2012;167:7–15. doi: 10.1530/EJE-11-1013. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun DA. Hyperaldosteronism as a common cause of resistant hypertension. Annu Rev Med. 2013;64:233–247. doi: 10.1146/annurev-med-042711-135929. [DOI] [PubMed] [Google Scholar]
- 7.Manolopoulou J, Fischer E, Dietz A, Diederich S, Holmes D, Junnila R, Grimminger P, Reincke M, Morganti A, Bidlingmaier M. Clinical validation for the aldosterone-to-renin ratio and aldosterone suppression testing using simultaneous fully automated chemiluminescence immunoassays. J Hypertens. 2015;33:2500–2511. doi: 10.1097/HJH.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 8.Perschel FH, Schemer R, Seiler L, Reincke M, Deinum J, Maser-Gluth C, Mechelhoff D, Tauber R, Diederich S. Rapid screening test for primary hyperaldosteronism: ratio of plasma aldosterone to renin concentration determined by fully automated chemiluminescence immunoassays. Clin Chem. 2004;50:1650–1655. doi: 10.1373/clinchem.2004.033159. [DOI] [PubMed] [Google Scholar]
- 9.Stowasser M, Gordon RD. Aldosterone assays: an urgent need for improvement. Clin Chem. 2006;52:1640–1642. doi: 10.1373/clinchem.2006.073460. [DOI] [PubMed] [Google Scholar]
- 10.Schirpenbach C, Seiler L, Maser-Gluth C, Beuschlein F, Reincke M, Bidlingmaier M. Automated chemiluminescence-immunoassay for aldosterone during dynamic testing: comparison to radioimmunoassays with and without extraction steps. Clin Chem. 2006;52:1749–1755. doi: 10.1373/clinchem.2006.068502. [DOI] [PubMed] [Google Scholar]
- 11.Dorrian CA1, Toole BJ, Alvarez-Madrazo S, Kelly A, Connell JM, Wallace AM. A screening procedure for primary aldosteronism based on the Diasorin Liaison automated chemiluminescent immunoassay for direct renin. Ann Clin Biochem. 2010;47:195–199. doi: 10.1258/acb.2010.009230. [DOI] [PubMed] [Google Scholar]
- 12.Burrello J, Monticone S, Buffolo F, Lucchiari M, Tetti M, Rabbia F, Mengozzi G, Williams TA, Veglio F, Mulatero P. Diagnostic accuracy of aldosterone and renin measurement by chemiluminescent immunoassay and radioimmunoassay in primary aldosteronism. J Hypertens. 2016;34:920–927. doi: 10.1097/HJH.0000000000000880. [DOI] [PubMed] [Google Scholar]
- 13.Rossi GP, Ceolotto G, Rossitto G, Seccia TM, Maiolino G, Berton C, Basso D, Plebani M. Prospective validation of an automated chemiluminescence-based assay of renin and aldosterone for the work-up of arterial hypertension. Clin Chem Lab Med. 2016;54:1441–1450. doi: 10.1515/cclm-2015-1094. [DOI] [PubMed] [Google Scholar]
- 14.Sealey JE. Plasma renin activity and plasma prorenin assays. Clin Chem. 1991;37:1811–1819. [PubMed] [Google Scholar]
- 15.Iwakura Y, Morimoto R, Kudo M, Ono Y, Takase K, Seiji K, Arai Y, Nakamura Y, Sasano H, Ito S, Satoh F. Predictors of decreasing glomerular filtration rate and prevalence of chronic kidney disease after treatment of primary aldosteronism: renal outcome of 213 cases. J Clin Endocrinol Metab. 2014;99:1593–1598. doi: 10.1210/jc.2013-2180. [DOI] [PubMed] [Google Scholar]
- 16.Iwakura Y, Ito S, Morimoto R, Kudo M, Ono Y, Nezu M, Takase K, Seiji K, Ishidoya S, Arai Y, Funamizu Y, Miki T, Nakamura Y, Sasano H, Satoh F. Renal resistive index predicts postoperative blood pressure outcome in primary aldosteronism. Hypertension. 2016;67:654–660. doi: 10.1161/HYPERTENSIONAHA.115.05924. [DOI] [PubMed] [Google Scholar]
- 17.Satoh F, Morimoto R, Seiji K, Satani N, Ota H, Iwakura Y, Ono Y, Kudo M, Nezu M, Omata K, Tezuka Y, Kawasaki Y, Ishidoya S, Arai Y, Takase K, Nakamura Y, McNamara K, Sasano H, Ito S. Progress in primary aldosteronism: is there a role for segmental adrenal venous sampling and adrenal sparing surgery in patients with primary aldosteronism? Eur J Endocrinol. 2015;173:465–477. doi: 10.1530/EJE-14-1161. [DOI] [PubMed] [Google Scholar]
- 18.Ono Y, Nakamura Y, Maekawa T, Felizola SJ, Morimoto R, Iwakura Y, Kudo M, Seiji K, Takase K, Arai Y, Gomez-Sanchez CE, Ito S, Sasano H, Satoh F. Different expression of 11β-hydroxylase and aldosterone synthase between aldosterone-producing microadenomas and macroadenomas. Hypertension. 2014;64:438–444. doi: 10.1161/HYPERTENSIONAHA.113.02944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura Y, Kitada M, Satoh F, Maekawa T, Morimoto R, Yamazaki Y, Ise K, Gomez-Sanchez CE, Ito S, Arai Y, Dezawa M, Sasano H. Intratumoral heterogeneity of steroidogenesis in aldosterone-producing adenoma revealed by intensive double- and triple-immunostaining for CYP11B2/B1 and CYP17. Mol Cell Endocrinol. 2016;422:57–63. doi: 10.1016/j.mce.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Sanchez CE, Foecking MF, Ferris MW, Chavarri MR, Uribe L, Gomez-Sanchez EP. The production of monoclonal antibodies against aldosterone. Steroids. 1987;49:581–587. doi: 10.1016/0039-128x(87)90097-3. [DOI] [PubMed] [Google Scholar]
- 21.Higaki J, Morishita R, Ogihara T, Nishiura M. Effects of aging on human plasma renin: simultaneous multiple assays of enzyme activity and immunoactivity of plasma renin. Acta Endocrinol (Copenh) 1989;120:81–86. doi: 10.1530/acta.0.1200081. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto K, Matsunaga M, Hara A, Kawai C. Studies on the activation and molecular weight of inactive renin in human plasma. Hypertension. 1980;2:680–685. doi: 10.1161/01.hyp.2.5.680. [DOI] [PubMed] [Google Scholar]
- 23.Satoh F, Morimoto R, Ono Y, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65:1096–1102. doi: 10.1161/HYPERTENSIONAHA.114.04453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelliccia F, Patti G, Rosano G, Greco C, Gaudio C. Efficacy and safety of eplerenone in the management of mild to moderate arterial hypertension: systematic review and meta-analysis. Int J Cardiol. 2014;177:219–228. doi: 10.1016/j.ijcard.2014.09.091. [DOI] [PubMed] [Google Scholar]
- 25.Williams B, MacDonald TM, Morant S, Webb DJ, Sever PG, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazo-sin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068. doi: 10.1016/S0140-6736(15)00257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudenbostel T, Calhoun DA. Use of aldosterone antagonists for treatment of uncontrolled resistant hypertension. Am J Hypertens. 2017;30:103–109. doi: 10.1093/ajh/hpw105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pechère-Bertschi A, Herpin D, Lefebvre H. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 7: medical treatment of primary aldosteronism. Ann Endocrinol (Paris) 2016;77:226–234. doi: 10.1016/j.ando.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Nishizaka MK, Calhoun DA. The role of aldosterone antagonists in the management of resistant hypertension. Curr Hypertens Rep. 2005;7:343–347. doi: 10.1007/s11906-005-0067-3. [DOI] [PubMed] [Google Scholar]
- 29.Hood SJ, Taylor KP, Ashby MJ, Brown MJ. The spironolactone, amiloride, losartan, and thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation. 2007;116:268–275. doi: 10.1161/CIRCULATIONAHA.107.690396. [DOI] [PubMed] [Google Scholar]
- 30.Funder JW. Primary aldosteronism and low-renin hypertension: a continuum? Nephrol Dial Transplant. 2013;28:1625–1627. doi: 10.1093/ndt/gft052. [DOI] [PubMed] [Google Scholar]
- 31.Tiu SC, Choi CH, Shek CC, Ng YW, Chan FK, Ng CM, Kong AP. The use of aldosterone-renin ratio as a diagnostic test for primary hyperal-dosteronism and its test characteristics under different conditions of blood sampling. J Clin Endocrinol Metab. 2005;90:72–78. doi: 10.1210/jc.2004-1149. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Madrazo S, Padmanabhan S, Mayosi BM, Watkins H, Avery P, Wallace AM, Fraser R, Davies E, Keavney B, Connell JM. Familial and phenotypic associations of the aldosterone renin ratio. J Clin Endocrinol Metab. 2009;94:4324–4333. doi: 10.1210/jc.2009-1406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.