Abstract
Purpose
Anteriorly located prostate cancer (PCa) is traditionally under-diagnosed using transrectal ultrasound (TRUS)-guided biopsy, although it represents a significant proportion of all PCa. We describe the detection rate of these tumors with the addition of MR/US fusion-guided biopsy (FGB) to standard TRUS-guided biopsy.
Materials and Methods
All patients regardless of their prior biopsy history who were referred for clinical suspicion of PCa (i.e elevated PSA and abnormal DRE) underwent 3T multiparametric-MRI (MP-MRI) screening; and those with suspicious lesions in the anterior region of the prostate were identified. Patients then received a FGB of all suspicious lesions in addition to systematic 12-core extended sextant TRUS-guided biopsy. We conducted a lesion based analysis comparing cancer detection rates of anterior targets using FGB versus systematic cores taken from the same anatomic sextant within the prostate. Lengths of cancer in the most involved core were also compared between the two biopsy techniques employed. Patients with only anterior targets were analyzed separately.
Results
Of 499 patients undergoing FGB, 162 patients had a total of 241 anterior lesions. Mean age, PSA, and prostate volume in this group was 62 years, 12.7ng/dl, and 57mL, respectively. In total, PCa was diagnosed in 121 (50.2%) of anterior lesions identified on MP-MRI. Sixty-two (25.7%) of these anterior lesions were documented positive for cancer on systematic 12-core TRUS-guided biopsy cores, while 97 (40.2%) were positive on the targeted FGB cores (p=0.001). In lesions that were positive on both FGB and TRUS biopsy, the most involved core was 112% longer on FGB (3.7mm vs. 1.6mm, p≤0.01).
Forty-two patients had only anterior lesions on MP-MRI; twenty-four of them (57.1%) were found to have cancer on the FGB + TRUS biopsy platform. Six patients were positive on FGB only. Thirteen were positive on both modalities. However, 7 of 13 were upgraded by to a higher Gleason score by FGB. All 5 patients positive on TRUS biopsy only were active surveillance candidates.
Conclusion
FGB detects significantly more anteriorly located PCa than TRUS-guided biopsy alone and may serve to be an effective tool for this subset of patients.
Introduction
Prostate cancer (PCa) is found primarily in the peripheral zone of the prostate. However, there is a subset of PCa that originates anterior to the urethra. Histopathological studies of radical prostatectomy (RP) specimens show that as much as 21% of all PCa are found in this anterior region of the prostate gland(1). Furthermore, these cancers are more challenging for the urologist to accurately diagnose. Anteriorly located PCA is often missed on physical exam because of its anatomical positioning farthest from the surface of the prostate gland adjacent to the rectal wall, thereby delaying suspicion of cancer based on clinical evaluation(2–4). Even when a suspicion of PCa is found, traditional trans-rectal prostate biopsy performs poorly in diagnosis of anterior lesions(5). Patients with anterior tumors have significantly lower yield on biopsy when compared to those that are posterior, both in terms of number of cores positive and length of cancer identified per core(1). Moreover, these patients often require multiple biopsies, with increasing cumulative risk of procedure-related morbidities such as bleeding, urinary retention, infection, and occasionally urosepsis(6, 7). When anterior PCa are finally found they can carry more risk for the patient, since the lesions are larger and men are more likely to have positive surgical margins on RP specimens since these cases commonly are more progressed at the time when they are identified(8).
In the past, anteriorly directed TRUS and transperineal biopsies, as well as transurethral resection of the prostate, were used to sample the anterior aspects of prostate glands with variable results(9–12). More recently, clinicians have started utilizing multiparametric-MRI (MP-MRI) to better localize areas suspicious for cancer and subsequently aim for these areas at biopsy. Techniques for targeting lesions found on MP-MRI include cognitive localization with free-hand manipulation of the TRUS probe, real-time MRI guidance of biopsies, and both transrectal and transperineal MR/Ultrasound fusion guided biopsy (FGB)(13–16). These techniques have greatly increased the precision with which urologists can detect cancer in the prostate(15, 16). Of equal importance, mpMRI has the ability to detect clinically significant anterior tumors(17). In our study, all patients referred with a diagnosis or clinical suspicion of PCa underwent 3T mpMRI of the prostate. We aim to describe the biopsy-based detection rate and significance of anterior PCa found on MR/Ultrasound fusion-guided biopsy (FGB) following MP-MRI.
Materials and Methods
Multiparametric MRI
In this IRB approved study, we retrospectively reviewed all patients receiving mpMRI followed by FGB between August 2007 to November 2011. MpMRIs were performed using a 3.0 T scanner (Achieva, Philips Healthcare, Best, The Netherlands) in combination with an endorectal coil (BPX-30, Medrad, Pittsburgh, Pennsylvania) and a cardiac surface coil (SENSE, Philips Healthcare) positioned over the pelvis. The parameters assessed included triplanar T2 weighted, spectroscopy, and axial dynamic contrast enhanced (DCE) images, as well as axial diffusion weighted (DW) images with apparent diffusion coefficient (ADC) mapping. Well-circumscribed, round or ellipsoid areas of low intensity were considered to be suspicious for cancer on T2 weighted and ADC maps of DW imaging. On spectroscopy, any voxel with a choline to citrate ratio of greater than 3 times the mean normal value was considered positive. On DCE, a suspicion of cancer arose if the radiologist observed foci of early and intense enhancement followed by rapid washout. Turkbey et al. have described a detailed account of the MRI procedure and all parameters(18).
Each prostate MRI was evaluated in consensus by two experienced genitourinary radiologists (B.T and P.L.C). According to the number of parameters positive, lesions were graded into low, moderate, and high suspicion as previously reported. To locate each lesion, the prostate was divided into 30 distinct areas as previously outlined and included regions deemed “anterior” in both the peripheral and transition zones of the prostate(19). Both radiologists were blinded to patient serum PSA levels, previous TRUS history, and any prior biopsy histopathologic findings.
MR/Ultrasound Fusion Guided Biopsy
Patients that were deemed to have suspicious areas in the anterior zone of the prostate went on to biopsy following a Fleet® enema and antibiotic prophylaxis as per AUA guidelines. Peri-prostatic regional lidocaine was given for the procedure. All patients underwent systematic 12-core extended sextant TRUS-guided biopsy with electromagnetic tracking. Samples were taken from medial and lateral aspects of the apex, mid, and base of the prostate on both sides. The operator was blinded to the locations of the areas of suspicion found earlier on the diagnostic MP-MRI study. At the same biopsy session, the patient underwent MR/US FGB. An EM field generator (Northern Digital Inc., Ontario, Canada) was placed above the pelvis and allowed for real time tracking of a custom biopsy probe that contained an EM tracking sensor (Traxtal Inc., A Philips Healthcare Company, Toronto, Canada) which was affixed to the standard end-fire TRUS probe. A 2-D sweep of the prostate using this probe rendered a 3-D ultrasound image that was then registered and fused to the pre-biopsy MRI image. Anterior lesions suspicious for cancer were superimposed on this image and at least 2 cores were taken of each targeted lesion, one in the axial and sagittal planes.
Lesion-Based Analysis
Men with areas of suspicion in the anterior prostate were selected for our analysis. An anterior lesion was considered positive on MR/US FGB if the targeted biopsy cores revealed adenocarcinoma on pathological evaluation. The same lesion was considered positive on TRUS biopsy if one or both of the two cores from the equivalent anatomic sextant of the prostate were classified as containing adenocarcinoma (Figure 1a–b). Statistical significance between detection rates was assessed using the Fischer’s exact test. Analysis of core lengths was performed with the student’s t-test.
Figure 1.
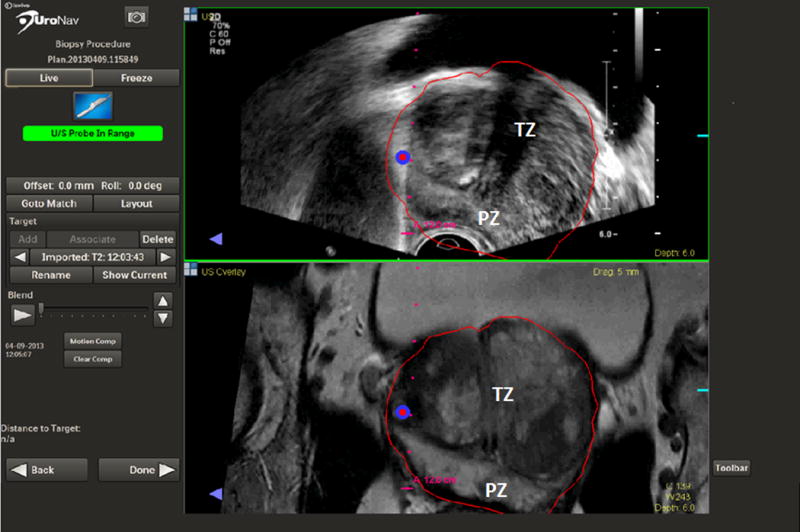
A lesion in the anterior horn of the prostate targeted by FGB (PZ - peripheral zone, TZ- transitional zone).
Results
499 patients underwent 3T mpMRI of the prostate and subsequent FGB. Of these, 162 patients were found to have a total of 241 anteriorly located suspicious lesions. In patients with anterior lesions identified, there were the mean/median of 1.5± 0.9/1 respectively, anteriorly located lesions per patient (range 1–6) and a mean of 2.1 (2–4) targeted FGB cores were sampled from each lesion. Mean age in this group was 62±7.2 years (44–82), mean PSA was 12.7±13.7 ng/mL (0.3–95.8), and mean prostatic volume was 57±30.6 mL (16–187) as measured on MRI. Demographic data is depicted in Table 1. Of all patients having a positive biopsy on our FGB platform with the biopsy protocol as previously described including a systematic 12-core extended sextant TRUS-guided biopsy in addition to targeted biopsies of suspicious lesions identified on MP-MRI, 57% had at least one previous negative standard-of-care TRUS-guided biopsy. Furthermore, ten percent of our cohort had undergone 4 or more prior negative biopsy sessions and 3 patients had previous negative saturation biopsies. These extensive efforts were undertaken in attempts to diagnose clinically suspected PCa based on PSA levels or dynamics despite prior negative standard-of-care biopsy sampling efforts. (Figure 1)
Table 1.
Patient Demographics
| Patients with Anterior Lesions | Patients with Only Anterior Lesions | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| N | 162 | 42 | ||||
| Number with Previously Negative Biopsies | 92 (57%) | 30 (71%) | ||||
|
|
||||||
| Mean | Median | Range | Mean | Median | Range | |
|
|
||||||
| Age (years) | 62.4 | 63 | 44–80 | 64.1 | 64 | 44–79 |
| PSA (ng/dl) | 12.7 | 8.4 | 0.3–95.8 | 22.6 | 12.6 | 1.57–95.8 |
| Prostate Volume on MRI (mL) | 57.1 | 48 | 19–187 | 61.1 | 53.5 | 27–187 |
| PSA density (ng/dl/mL) | 0.25 | 0.15 | .02–1.68 | 0.39 | 0.28 | .04–1.49 |
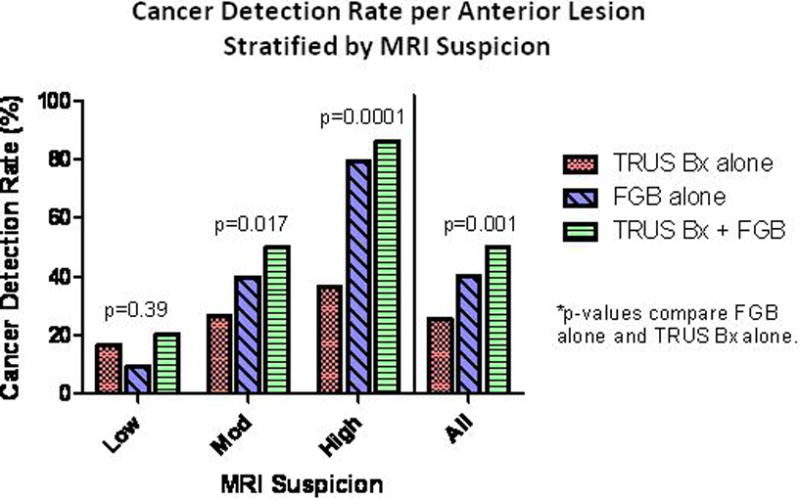
Of the 237 anterior lesions found on MP-MRI, 121 (50.2%) were positive on our biopsy platform combining biopsy pathology results both extended sextant TRUS-guided biopsy with targeted FGB cores. Of those positive for PCa, 62 (25.7%) were positive on TRUS-guided biopsy cores and 97 (40.3%) were positive of targeted FGB cores (p=0.001). A total of 92 patients underwent prior biopsy with negative results. The detection rate in this subgroup of patients for TRUS cores was 29.3% versus 42.4% for FGB cores (p= 0.065). In the subgroup of patients with no prior history of prostate biopsy (n=21) the detection rate for TRUS was 47.6% versus 61.9% for FGB cores (p=0.35). When stratified by high and moderate suspicion, FGB increased cancer detection by 118 (p=0.0001) and 50 percent (p=0.017), respectively, when compared to TRUS-guided biopsy results alone. FGB detected 4 fewer low suspicion lesions than TRUS-guided biopsy. Detection rates stratified by level of MRI suspicion are depicted in Figure 2.
Figure 2.
FGB detected an additional 59 cancers that would not have been identified by systematic 12-core extended sextant TRUS-guided biopsy alone. Twenty-five lesions (42%) harbored Gleason (Gl) 6 cancer, 20 (34%) had Gl 7(3+4), 3 (5%) had Gl 7(4+3), 8 (14%) had Gl 8, 1 (2%) had Gl 9(4+5), and 2 (3%) had Gl 10. TRUS biopsy detected 24 lesions that were not picked up by FGB. Eighteen of the 24 lesions were Gl 6 cancer, 5 were Gl 7(3+4), and 1 was Gl 8. The FGB and TRUS biopsy were concordant for 38 lesions. However, the average length of cancer in FGB cores was 112% longer than in correlative TRUS-guided extended sextant biopsy cores (3.7mm versus 1.6mm, respectively, p<0.01) (Table 2a).
Table 2a.
Gleason grade distribution of anterior prostate cancer lesions identified exclusively based upon TRUS-guided versus fusion-guided biopsies.
| Gleason Grade | Detected only on TRUS | Detected only on FGB |
|---|---|---|
| 6 (3+3) | 18 | 25 |
| 7 (3+4) | 5 | 20 |
| 7 (4+3) | – | 3 |
| 8 (4+4) | 1 | 8 |
| 9 (4+5) | – | 1 |
| 10 (5+5) | – | 2 |
Forty-two patients in our cohort had only anterior lesions identified on MP-MRI; twenty-four (57.1%) of these patients had PCa detected after undergoing FGB + TRUS biopsy. Of the 24 patients with positive biopsy findings, 6 patients had cancer that was detected only on FGB – four with primary Gleason pattern 3 (3+3=6 or 3+4=7) and two with primary Gleason pattern 4 disease (≥4+3=7). Five patients were detected by TRUS only; all with Gleason 6 disease who qualified for active surveillance as per established NCCN guidelines. Thirteen of twenty-four patients were positive on both FGB and TRUS biopsies. However, FGB upgraded 7 of these patients to a higher Gleason score (Table 2b).
Table 2b.
Distribution of anterior prostate cancer lesions based upon TRUS-guided vs fusion-guided biopsies.
| Anterior Lesions | Detected only on TRUS | Detected only on FGB | Detected by both TRUS & FGB |
|---|---|---|---|
| Positive for PCa | 5/24 | 6/24 | 13/24 |
| Gleason Score | 6 (3+3) | 3 pts Gl ≤7 (3+4) 4 pts Gl ≥7 (4+3) |
FGB upgraded 7/13 pts. |
Discussion
Cancers originating in the anterior portion of the prostate gland present a unique diagnostic challenge for the urologist using standard DRE, PSA, and TRUS-guided biopsy means of screening and PCa detection. These tumors represent the majority of cancers missed on initial 12-core transrectal biopsy(5) and are largely to blame when patients lose active surveillance candidacy.20 Numerous methods have been attempted to address the inadequacy of systematic 12-core extended sextant TRUS-guided biopsy in sampling the anterior aspects of the prostate gland including adding extreme anterior apical cores,9 extended transperineal template biopsy(10, 11, 20), and saturation biopsy(21).
Bott et al.(10) and Dimmen et al.(22) used extended transperineal biopsies in men with previous negative TRUS biopsies and found cancer in 38% and 55% of these men, respectively. In both studies, the majority of cancers were located anteriorly which was unsampled or undersampled using the standard 12-core biopsy which is known to be limited in detecting these cancer foci. Taira et al.(11) similarly used transperineal biopsies in patients with zero to four or greater previous negative biopsy sessions and cited high detection rates. Although transperineal biopsy does relatively well in finding anterior PCa, this technique requires the use of general anesthesia, which likely precludes it from being used in a widespread, office-based setting. Also, transperineal biopsy may require upwards of 50 biopsy cores be taken, increasing patient discomfort and the risk of morbid events(10, 11, 22). Importantly, the risk of serious infection post prostate biopsy has increased four-fold in the last decade(23).
MRI of the prostate and subsequent biopsy has been explored as a relatively novel option for anterior PCa detection(13, 17). A recent Italian study conducted by Ouzzane et. al.(13) describes the benefit of using freehand targeting of anterior lesions following prostate MRI. In a population of patients with areas suspicious for PCa in the anterior portion of the prostate, they report an 18% overall positivity rate using freehand targeted biopsies in addition to systematic biopsies. In concordance with the elusive nature of anterior tumors, 21 of 46 positive lesions were not detected by systematic biopsies in their study. This translates to an improvement in anterior cancer detection of 84% with the addition of freehand MRI targeted biopsies. We achieved a slightly higher improvement rate of 95% with the addition of MR/US fusion-guidance of biopsies in our study, but we had a substantially higher overall positivity rate per lesion of 50.2% using our biopsy protocol.
For anterior lesions that were characterized as highly suspicious, FGB performed extremely well, detecting cancer in 80 percent of cases. This represents a greater than twofold increase in the detection rate over TRUS-guidance alone. There was a large drop off in the percentage of moderately suspicious lesions that were found to have PCa, but the detection rate of 40 percent achieved by FGB was still significantly higher than the 27 percent attained by TRUS-guided biopsy. When used as a combined platform integrating FGB and systematic 12-core TRUS guided biopsy, high and moderate suspicion lesions were found to have cancer 86 and 50 percent of the time, respectively. For anterior lesions classified under “low suspicion”, FGB provided minimal added benefit in our study as only two additional lesions were detected. This is not surprising since low suspicion lesions are commonly representative of benign hyperplasic changes or inflammation within the prostate rather than cancer(24).
Of the anterior lesions biopsy-proven to be PCa, FGB allowed identification of 59 that would not have been detected on systematic TRUS biopsy alone. Thirty-four of these contained Gleason 3+4=7 or greater disease, which, depending on age and functional status, would qualify as clinically significant lesions that warrant intervention. Importantly, FGB missed 25 anterior lesions that were found to have cancer on TRUS-guided biopsy. However, the majority of these lesions (18) contained Gleason 6 cancer. The remainder, 5 consisted of Gleason 3+4=7 lesions and only a single was Gleason 8 focus. These results emphasize that while FGB is a promising new tool, systematic TRUS-guided biopsy still maintains an important role in detecting PCa.
In our study, we found that on average, if a lesion was positive on both FGB and TRUS biopsies, the length of cancer in the FGB core was 112% longer than on TRUS biopsy. We suspect that the ability of FGB to obtain a longer cancer-containing core may be largely responsible for the 7 cases which were upgraded to a higher Gleason score in our patient-based analysis. Supporting this hypothesis is the work done by Corcoran and colleagues who demonstrated that incomplete tumor sampling plays a role in the under-staging of PCa(25). The length of cancer in biopsy cores has also been shown to be important by Dong and colleagues(26), who performed a multivariate regression analysis in patients with Gleason 6 disease and concluded that those with longer segments involved with PCa in biopsy cores were more likely to be pathologically upgraded. Furthermore, our data supports the recent work of Hambrock and colleagues, who demonstrated that MR guided biopsies are more accurate for pre-operative risk stratification by Gleason grade than are systematic TRUS biopsies(27). Using our FGB system to more accurately target the center of a suspicious lesion, we hope there will be a higher concordance between biopsy results and prostate pathology results, thus translating to a decrease in management decisions that are based on incomplete or inaccurate clinical data.
It is important to consider those patients with only anterior lesions who were positive on 12-core TRUS biopsy but were missed on FGB. One of two technical issues is likely to be at play. The first involves the limitations of MRI in detecting all foci of PCa. Despite the enhanced resolution of 3T MRI and the experience of our genitourinary radiologists, small lesions (<5mm) and those within heterogeneous appearing prostates may be exceedingly difficult to recognize(28). Consequently, these types of lesions may not have been targeted but may still have turned up as positive by virtue of the semi-random nature of standard TRUS-guided biopsy.
The alternative explanation for patients positive solely on TRUS biopsy involves the technical limitations of the fusion biopsy system. Though great care is taken to ensure an accurate fusion of ultrasound data to the MRI images, a margin of error of approximately 2.4 mm still exists(29). This implies that low volume lesions, even if identified on MRI, may still be missed on fusion biopsy targeting due to the inherent spatial errors in dual modality imaging coregistration. Of note, the inability of fusion biopsy to detect small foci of cancer may in truth benefit the patient, as these lesions are likely to be of low clinical significance(5, 30, 31). The fact that all of these patients harbored Gleason 6 cancer and were active surveillance candidates further supports the notion that MR/US fusion-guided biopsy does not miss cases of aggressive PCa.
Our study has some limitations. Although used in previously published studies, the method employed for correlating anterior lesions sampled by FGB to TRUS cores in the same anatomic location would be best validated with RP specimens. However, due to the unique referral nature of our center, many patients are referred back to their primary urologists for definitive therapy. Also, some of our patients with low volume, low risk PCa chose to avoid RP altogether and enter into an active surveillance protocol. A second limitation of our study is the high risk nature of our population based on concerning PSA levels or dynamics despite prior negative biopsies or low volume disease on standard-of-care biopsies, which is also reflective of the referral nature of majority of the patients accrued into our imaging and image-guided biopsy research protocols.
A limitation of any study incorporating MRI-based image-guided biopsy is the high cost and time requirement of performing MP-MRI to identify suspicious lesions. To limit the cost and time necessary for this level of imaging, our group has begun investigating the accuracy of MP-MRI without an endorectal coil(32). Also, it is important to consider that many patients with anterior cancer will typically undergo multiple previous negative biopsy sessions. Instead of continuing to biopsy these patients with conventional methods, it may be cost effective to employ MP-MRI and subsequent fusion-guided biopsies in efforts to obviate excess biopsy sessions and the associated patient anxiety and potential morbidity from repeated procedures. Furthermore, it is obvious that properly selected patients could benefit from avoiding the morbidity and oncological risks of high grade cancers missed by TRUS-guided biopsy alone which often delays definitive diagnosis.
Conclusion
There exists a cohort of patients who harbor anterior PCa lesions that are challenging to diagnose with the traditional biopsy methods. MR imaging and subsequent MR-US fusion biopsy provide accurate sampling of these lesions that are often otherwise missed by systematic TRUS biopsy.
Footnotes
Conflicts of Interest
None disclosed.
References
- 1.Bott SR, Young MP, Kellett MJ, et al. Anterior prostate cancer: is it more difficult to diagnose? BJU international. 2002 Jun;89(9):886–9. doi: 10.1046/j.1464-410x.2002.02796.x. [DOI] [PubMed] [Google Scholar]
- 2.Greene DR, Fitzpatrick JM, Scardino PT. Anatomy of the prostate and distribution of early prostate cancer. Seminars in surgical oncology. 1995 Jan-Feb;11(1):9–22. doi: 10.1002/ssu.2980110104. [DOI] [PubMed] [Google Scholar]
- 3.Demura T, Hioka T, Furuno T, et al. Differences in tumor core distribution between palpable and nonpalpable prostate tumors in patients diagnosed using extensive transperineal ultrasound-guided template prostate biopsy. Cancer. 2005 May 1;103(9):1826–32. doi: 10.1002/cncr.21020. [DOI] [PubMed] [Google Scholar]
- 4.Chen ME, Johnston DA, Tang K, et al. Detailed mapping of prostate carcinoma foci: biopsy strategy implications. Cancer. 2000 Oct 15;89(8):1800–9. doi: 10.1002/1097-0142(20001015)89:8<1800::aid-cncr21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Numao N, Kawakami S, Sakura M, et al. Characteristics and clinical significance of prostate cancers missed by initial transrectal 12-core biopsy. BJU international. 2012 Mar;109(5):665–71. doi: 10.1111/j.1464-410X.2011.10427.x. [DOI] [PubMed] [Google Scholar]
- 6.Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. The Journal of urology. 2011 Nov;186(5):1830–4. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simsir A, Kismali E, Mammadov R, et al. Is it possible to predict sepsis, the most serious complication in prostate biopsy? Urologia internationalis. 2010;84(4):395–9. doi: 10.1159/000296290. [DOI] [PubMed] [Google Scholar]
- 8.Koppie TM, Bianco FJ, Jr, Kuroiwa K, et al. The clinical features of anterior prostate cancers. BJU international. 2006 Dec;98(6):1167–71. doi: 10.1111/j.1464-410X.2006.06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moussa AS, Meshref A, Schoenfield L, et al. Importance of additional “extreme” anterior apical needle biopsies in the initial detection of prostate cancer. Urology. 2010 May;75(5):1034–9. doi: 10.1016/j.urology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Bott SR, Henderson A, Halls JE, et al. Extensive transperineal template biopsies of prostate: modified technique and results. Urology. 2006 Nov;68(5):1037–41. doi: 10.1016/j.urology.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 11.Taira AV, Merrick GS, Galbreath RW, et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate cancer and prostatic diseases. 2010 Mar;13(1):71–7. doi: 10.1038/pcan.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philip J, Dutta Roy S, Scally J, et al. Importance of TURP in diagnosing prostate cancer in men with multiple negative biopsies. The Prostate. 2005 Jul 1;64(2):200–2. doi: 10.1002/pros.20239. [DOI] [PubMed] [Google Scholar]
- 13.Ouzzane A, Puech P, Lemaitre L, et al. Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology. 2011 Dec;78(6):1356–62. doi: 10.1016/j.urology.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Hambrock T, Somford DM, Hoeks C, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. The Journal of urology. 2010 Feb;183(2):520–7. doi: 10.1016/j.juro.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. The Journal of urology. 2011 Oct;186(4):1281–5. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadaschik BA, Kuru TH, Tulea C, et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. The Journal of urology. 2011 Dec;186(6):2214–20. doi: 10.1016/j.juro.2011.07.102. [DOI] [PubMed] [Google Scholar]
- 17.Lawrentschuk N, Haider MA, Daljeet N, et al. ‘Prostatic evasive anterior tumours’: the role of magnetic resonance imaging. BJU international. 2010 May;105(9):1231–6. doi: 10.1111/j.1464-410X.2009.08938.x. [DOI] [PubMed] [Google Scholar]
- 18.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011 Feb;258(2):488–95. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology. 2010 Apr;255(1):89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel V, Merrick GS, Allen ZA, et al. The incidence of transition zone prostate cancer diagnosed by transperineal template-guided mapping biopsy: implications for treatment planning. Urology. 2011 May;77(5):1148–52. doi: 10.1016/j.urology.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 21.Falzarano SM, Zhou M, Hernandez AV, et al. Can saturation biopsy predict prostate cancer localization in radical prostatectomy specimens: a correlative study and implications for focal therapy. Urology. 2010 Sep;76(3):682–7. doi: 10.1016/j.urology.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 22.Dimmen M, Vlatkovic L, Hole KH, et al. Transperineal prostate biopsy detects significant cancer in patients with elevated prostate-specific antigen (PSA) levels and previous negative transrectal biopsies. BJU international. 2012 Jul;110(2 Pt 2):E69–75. doi: 10.1111/j.1464-410X.2011.10759.x. [DOI] [PubMed] [Google Scholar]
- 23.Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. The Journal of urology. 2010 Mar;183(3):963–8. doi: 10.1016/j.juro.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 24.Vargas HA, Akin O, Franiel T, et al. Normal central zone of the prostate and central zone involvement by prostate cancer: clinical and MR imaging implications. Radiology. 2012 Mar;262(3):894–902. doi: 10.1148/radiol.11110663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corcoran NM, Hovens CM, Hong MK, et al. Underestimation of Gleason score at prostate biopsy reflects sampling error in lower volume tumours. BJU international. 2012 Mar;109(5):660–4. doi: 10.1111/j.1464-410X.2011.10543.x. [DOI] [PubMed] [Google Scholar]
- 26.Dong F, Jones JS, Stephenson AJ, et al. Prostate cancer volume at biopsy predicts clinically significant upgrading. The Journal of urology. 2008 Mar;179(3):896–900. doi: 10.1016/j.juro.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 27.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. European urology. 2012 Jan;61(1):177–84. doi: 10.1016/j.eururo.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. The Journal of urology. 2011 Nov;186(5):1818–24. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krucker J, Xu S, Venkatesan A, et al. Clinical utility of real-time fusion guidance for biopsy and ablation. Journal of vascular and interventional radiology: JVIR. 2011 Apr;22(4):515–24. doi: 10.1016/j.jvir.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamey TA, Freiha FS, McNeal JE, et al. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer. 1993 Feb 1;71(3 Suppl):933–8. doi: 10.1002/1097-0142(19930201)71:3+<933::aid-cncr2820711408>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 31.Wolters T, Roobol MJ, van Leeuwen PJ, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. The Journal of urology. 2011 Jan;185(1):121–5. doi: 10.1016/j.juro.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 32.Baris Turkbey MD, Maria J, Merino MD, Gallardo Elma Carvajal, et al. Comparison of Endorectal coil and Non-endorectal coil T2W and DW MRI at 3T for Localizing Prostate Cancer: Correlation with Whole-mount Histopathology. Journal of Magnetic Resonance Imaging. 2013 doi: 10.1002/jmri.24317. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]


