Abstract
Background and aim
α-complex protein-2 (αCP2) encoded by the poly (rC) binding protein 2(PCBP2) gene is responsible for the accumulation of type I collagen in fibrotic livers. In this study, we silenced the PCBP2 gene using a small interfering RNA (siRNA) to reverse alcohol-and cytokine-induced profibrogenic effects on hepatic stellate cells (HSCs).
Methods
Primary rat HSCs and the HSC-T6 cell line were used as fibrogenic models to mimic the initiation and perpetuation stages of fibrogenesis, respectively. We previously found that a PCBP2 siRNA, which efficiently silences expression of αCP2, reduces the stability of type I collagen mRNA. We investigated the effects of the PCBP2 siRNA on cell proliferation and migration. Expression of type I collagen in HSCs was analyzed by quantitative real-time PCR and western blotting. In addition, we evaluated the effects of the PCBP2 siRNA on apoptosis and the cell cycle.
Results
PCBP2 siRNA reversed multiple alcohol- and cytokine-induced profibrogenic effects on primary rat HSCs and HSC-T6 cells. The PCBP2 siRNA also reversed alcohol- and cytokine-induced accumulation of type I collagen as well as cell proliferation and migration. Moreover, the combination of LY2109761, a transforming growth factor-β1 inhibitor, and the PCBP2 siRNA exerted a synergistic inhibitive effect on the accumulation of type I collagen in HSCs.
Conclusions
Silencing of PCBP2 using siRNA could be a potential therapeutic strategy for alcoholic liver fibrosis.
Keywords: Hepatic stellate cells, Primary HSC, Myofibroblast, Liver fibrosis, Poly (rC) binding protein (PCBP)2, Transforming growth factor (TGF)-β, Platelet-derived growth factor (PDGF), Epidermal growth factor (EGF), LY2a09761, Migration
1. Introduction
Liver fibrosis/cirrhosis is a global health problem that leads to morbidity and mortality. Alcohol abuse is one of the most common causes of liver fibrosis/cirrhosis in western developed countries and accounts for more than 50% of cirrhosis cases [1, 2]. Alcoholic liver fibrosis is characterized by excessive accumulation of extracellular matrix (ECM) in the liver. While liver fibrosis is reversible and treatable in the initial stage, it becomes irreversible and untreatable in its advanced stages.
Hepatic stellate cells (HSCs) play a key role in fibrogenesis and are responsible for the production of the ECM. It is known that the transdifferentiation of quiescent HSCs to myofibroblast-like cells is a hallmark of liver fibrogenesis [3]. ECM secretion is significantly increased upon activation of HSCs. These conditions lead to liver fibrosis accompanied by enhanced HSC migration.
HSC activation is divided into two stages: initiation and perpetuation [4]. The initiation stage involves changes in gene expression and phenotypes. Sustained stimulation of HSCs over a long term results in the perpetuation stage that involves proliferation and fibrogenesis [5]. After activation, HSCs become more contractile, proinflammatory, and fibrogenic [6]. Additionally, after transdifferentiation, activated HSCs migrate to injury sites where they produce >50-fold higher levels of ECM in which type I collagen is the major component [7]. The excessive collagen accumulation in activated HSC is mainly due to the enhanced stability of type I collagen mRNA [8]. In our previous study [9], HSC-T6 cells exposed to alcohol for 48 h demonstrated enhanced αCP2 expression leading to the prolonged stability of type I collagen mRNA.
In a fibrotic microenvironment, various cytokines including transforming growth factor β1 (TGF-β1), platelet-derived growth factor (PDGF), and epidermal growth factor (EGF) play major roles in stimulating fibrosis and cirrhosis. These cytokines promote cell proliferation and migration as well as excessive collagen production. TGF-β1, one of the most potent profibrogenic cytokines, is involved in the initiation and maintenance of fibrogenesis. TGF-β1-stimulated activation of HSCs is reported to be the key fibrogenic response in liver fibrosis [3]. In addition, PDGF, an important cytokine, promotes collagen production upon HSC activation. PDGF and its receptor (PDGFR) are up-regulated after HSC activation and correlate with the degree of fibrosis. EGF plays a critical role in liver regeneration and HSC transdifferentiation. EGF and EGF receptor are highly overexpressed in fibrotic livers, resulting in an increased HSC migratory capacity and upregulated matrix metalloproteinase-2 activity [10, 11].
In the current study, primary rat HSCs and the HSC-T6 cell line were used as fibrogenic models to mimic the initiation and perpetuation stages of fibrogenesis, respectively. Furthermore, we explored the effects of a PCBP2 siRNA on liver fibrogenesis induced by alcohol and cytokines.
2. Materials and methods
2.1. Materials
Dulbecco’s modified Eagle’s medium (DMEM), Dulbecco’s phosphate-buffered saline (DPBS), penicillin, and streptomycin were purchased from Mediatech, Inc. (Manassas, VA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals Inc. (Lawrenceville, GA). Bovine serum albumin (BSA) was purchased from Sigma-Aldrich (St. Louis, MO). An iTaq Universal SYBR Green One-Step Kit was purchased from Bio-Rad (Hercules, Ca). PDGF and EGF was purchased from R&D Systems (Minneapolis, MN). TGF-β1 was purchased from Sino Biological Inc. (Beijing, China). PCBP2 siRNA and a scrambled siRNA (negative control siRNA) were obtained from Ambion Inc. (Austin, TX). Lipofectamine-iMAX and TRIzol reagent were obtained from Invitrogen (Carlsbad, CA). A Dead Cell Apoptosis Kit with Annexin V Alexa Fluor® 488 and Propidium Iodide was purchased from Thermo-Fisher Scientific (Pittsburgh, PA).
2.2.Cell culture
The rat HSC line HSC-T6 was kindly provided by Dr. Scott L. Friedman (Mount Sinai School of Medicine, New York, NY). Primary rat HSCs were isolated as described previously [12–14]. Cells were cultured as reported previously in DMEM supplemented with 10% FBS [15], penicillin (100 U/mL), and streptomycin (100 µg/mL) at 37°C in a humidified atmosphere containing 5% CO2. The medium was changed every other day. Cells were passaged at 80%–90% confluence. For alcohol stimulation experiments, HSCs were incubated with alcohol in a closed chamber as described previously [9, 16–18].
2.3. Isolation of primary rat HSCs
The animal use protocol was approved by the Institutional Animal Care and Use Committee of the University of Missouri (Kansas City, MO). Briefly, Sprague-Dawley rats (~250 g) were anesthetized with isoflurane. The abdomen was opened, and the portal vein was cannulated using polyethylene tubing. The rat liver was perfused with 2 mL of a heparin solution diluted at 20 U/mL to avoid blood clotting. Then, the rat liver was perfused with 200 mL Hank’s balanced salt solution at 15 mL/min, followed by 250 mL Hank’s balanced salt solution containing 0.05% type IV collagenase and 0.1% pronase at 10 mL/min. All solutions were maintained at 37°C. After the perfusion, the rat liver was removed and immersed in Hank’s balanced salt solution, and all other tissues were removed without damaging the liver. The liver was cut into small fragments, and the liver cells were released into the medium by mild agitation with forceps. The cell suspension was filtered through sterilized gauze and then centrifuged at 50 × g for 5 min. The supernatant was further centrifuged at 100 × g for 5 min. Then, the supernatant was collected and centrifuged at 500 × g for 10 min. HSCs were further separated using the density gradient centrifugation method. The cell pellet was dispersed in 10 mL of an 11.5% Nycodenz solution and slowly overlaid on 5 mL of a 17.5% Nycodenz solution. This gradient was covered by 2 mL PBS. The gradient was then centrifuged at 1450 × g for 17 min, and the top of the second layer contained the purified primary HSCs.
2.4.Cell cycle analysis and apoptosis assay
The cell cycle analysis and apoptosis assay were performed as described in a previous study [19] with modifications. For the cell cycle assay, at 24 h after PCBP2 siRNA transfection, the cells were incubated with 50 ng/mL PDGF for an additional 12 h. The cells were collected and fixed with ice-cold 70% ethanol. They were then washed with DPBS and incubated with a propidium iodide/RNase staining buffer for 30 min. The cell cycle analysis was performed using a FACSCalibur flow cytometer (BD Biosciences). For the apoptosis assay, the cells were incubated with alcohol for 48 h or transfected with 50 nmol PCBP2 siRNA for 24 h and then harvested. All of these cells were treated according to the manufacturers’ protocols and analyzed using the FACSCalibur flow cytometer.
2.5. siRNA transfection
Cells were transfected using Lipofectamine-iMAX as described previously [9, 19, 20]. The cells were seeded in 24-well plates at a density of 10,000–30,000 cells/well at 12 h before the transfection. The transfection complex was prepared with 1.25 µL PCBP2 siRNA in 25 µL OPTI-MEM and 1.5 µL Lipofectamine-MAX in 25 µL OPTI-MEM by mixing two 25 µL solutions at room temperature for 5 min to form a complex. The cells were washed with DPBS and then incubated with 50 µL of the siRNA-Lipofectamine complex and 450 µL OPTI-MEM for a final concentration of 50 nM siRNA. After 6 h of incubation, another 500 µL of fresh DMEM was added, followed by incubation for 18 h before evaluation.
2.6. Real-time PCR
Total RNA was isolated from the cells using Direct-zol RNA Miniprep Plus (ZYMO research). Real-time RT-PCR was conducted to compare gene expression as reported previously [21]. The primers were as follows: PCBP2, forward primer 5′-ACCAATAGCACAGCTGCCAGTAGA-3′ and reverse primer 5’-AGTCTCCAACATGACCACGCAGAT-3′; type I collagen mRNA, forward primer 5′-TGGTCCCAAAGGTTCTCCTGGT-3′ and reverse primer 5’-TTAGGTCCAGGGAATCCCATCACA-3′; type I collagen pre-mRNA, forward primer 5′-CCAGCCGCAAAGAGTCTACATGTC-3′ and reverse primer 5′-TCACCTTCTCATCCCTCCTAA-3′. 18s rRNA, forward primer 5′-GTCTGTGATGCCCTTAGATG-3′ and reverse primer 5’-AGCTTATGACCCGCACTTAC-3′.
2.7. Cell proliferation assay
HSCs were seeded into a 96-well plate at a density of 7000 cells/well in complete DMEM at 12 h before transfection. They were transfected with 50 nmol PCBP2 siRNA in DMEM with 0.5% FBS and incubated for 6 h. Then, another 100 µL DMEM with 0.5% FBS was added, and the incubation was continued for another 18 h. PDGF was then added at 50 ng/mL in complete DMEM containing 10% FBS. After 12 h, MTT was added, followed by incubation for 2 h. Absorbance values were measured using a DTX880 multimode detector (Beckman Coulter, Indianapolis IN) [22, 23].
2.8. Migration assay
The migration assay was conducted as described previously [10] with modifications. Transwell chambers with 8 µm polycarbonate membranes (COSTAR, Corning, NY) were coated with 50 µg/mL matrigel on the top and 50 µg/mL type I collagen on the bottom. The wells were filled with DMEM containing 10% FBS with or without cytokines. HSCs were transfected with the PCBP2 siRNA for 24 h in DMEM with 0.5% FBS (serum starvation for 24 h) before migration. The transwell chambers were inserted into the well, loaded with 20,000 cells/chamber, and incubated for 4 h to allow migration through the membrane. The cells were fixed with 10% paraformaldehyde and stained with 0.05% crystal violet. All cells remaining on the upper side of the membrane were removed with a cotton swab. Images were obtained at 200× magnification, and the numbers of cells were counted.
2.9. Western blotting
RIPA buffer (150 mmol NaCl, 50 mmol Tris base, pH 8.0, 1 mmol EDTA, 1% Triton X-100, 0.1% SDS, 1 mmol PMSF, and 1 mmol Na3VO4) with a protease inhibitor cocktail (Roche, Indianapolis, IN) was used to lyse cells on ice for 5 min. The lysate was collected by centrifugation at 12,000 × g for 20 min at 4°C. The protein concentration of the supernatant was determined using a BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of protein were loaded into a 12% SDS- polyacrylamide gel for electrophoresis. The separated proteins were transferred to a polyvinylidene fluoride membrane. After blocking the membrane (5% nonfat dry milk solution), an anti-type I collagen antibody (Rockland Immunochemical Inc., Gilbertsville, PA) was added, followed by incubation for 2 h at room temperature. A horseradish peroxidase-conjugated secondary antibody and a chemiluminescence detection kit were used for detection. The membrane was re-probed with an anti-β-actin antibody (Rockland Immunochemical Inc., Gilbertsville, PA) as an internal control [3].
2.10. Statistical analysis
Data are expressed as the mean ± standard deviation (SD). Statistical analysis was performed by one-way analysis of variance. P<0.05 was considered to be significantly different.
3. Results
3.1. Silencing PCBP2 reverses the fibrogenic effects of alcohol and cytokines in primary HSCs
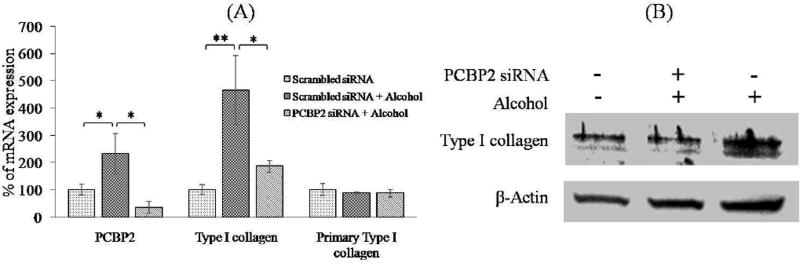
Primary HSCs were treated with 100 mmol alcohol for 48 h to induce profibrotic gene expression [9, 24]. Quantitative real-time PCR was performed to determine the mRNA levels of PCBP2 and type I collagen. As shown in Fig. 1A, incubation with 100 mmol alcohol for 48 h significantly increased the mRNA levels of PCBP2 and type I collagen by 2.30-fold and 4.65-fold, respectively. Compared with our previous study in HSC-T6 cells [9], the increase of PCBP2 mRNA was equivalent. However, the change of the type I collagen mRNA level was greater than that observed in our previous study (4.65-fold for primary HSCs compared with 2.7-fold for HSC-T6 cells). These results suggested that the primary HSCs were in a quiescent state and therefore more sensitive to alcohol stimulation compared with the HSC-T6 cell line. In addition, alcohol treatment had no significant effect on the expression of type I collagen primary mRNA in primary HSCs, which was not consistent with our previous report using HSC-T6 cells [9]. Primary mRNA is the primary transcript of its DNA. It proceeds into mature mRNA (also called mRNA) after splicing. We therefore believe that alcohol induced a profibrogenic effect in HSCs by upregulating the expression of αCP2 (PCBP2) that increases the stability of type I collagen mRNA at the post-transcriptional level [25].
Fig. 1. PCBP2 siRNA reverses alcohol-induced expression of type I collagen in primary HSCs.
Primary rat HSCs were transfected with 50 nmol PCBP2 siRNA or scrambled siRNA for 24 h and then treated with 100 mmol alcohol for 48 h. (A) Levels of PCBP2, type I collagen, and type I collagen primary mRNAs were quantified by real-time RT-PCR. (B) Protein expression of type I collagen was determined by western blotting. Results are presented as the mean ± SD (n=3). *P<0.05; **P<0.01.
Next, we treated primary HSCs with the PCBP2 siRNA followed by alcohol stimulation. Briefly, primary HSCs were transfected with 50 nmol PCBP2 siRNA for 24 h, and then the medium containing 100 mmol alcohol was replaced every 6 h for a total incubation time of 48 h. As shown in Fig. 1A, the PCBP2 siRNA remarkably knocked down the expression of PCBP2 (αCP2) in HSCs at the mRNA level. In our previous study, we demonstrated a similar silencing effect of the siNRA in HSC-T6 cells [9]. Real-time PCR demonstrated that, after alcohol simulation, the silencing effects on PCBP2 and type I collagen were up to 85% and 60%, respectively. The protein expression of type I collagen was silenced to a similar level as that prior to alcohol stimulation (Fig. 1B).
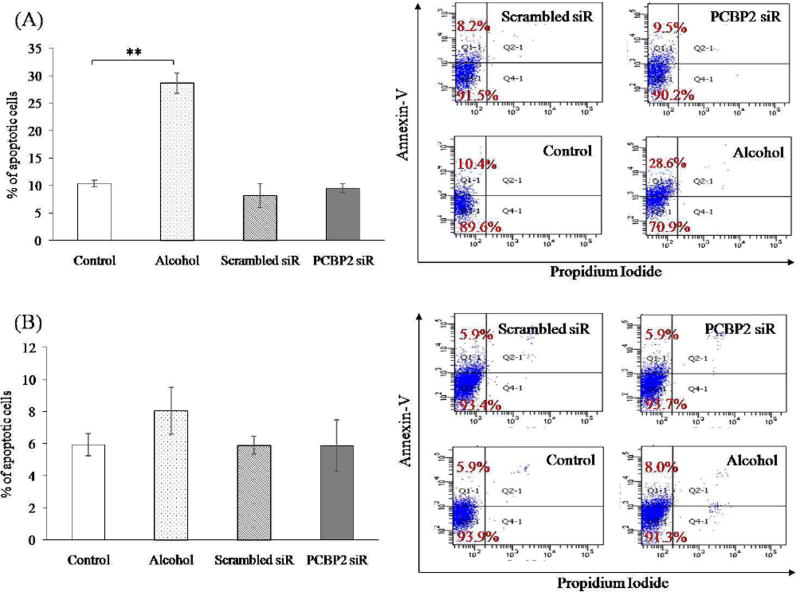
Flow cytometry was performed to examine apoptosis of HSCs after inhibition of PCBP2 using siRNA [19]. No significant difference in the numbers of annexin-V-positive apoptotic cells were observed in the PCBP2 siRNA-treated group compared with HSCs transfected with scrambled siRNA. As shown in Fig. 2, scrambled and PCBP2 siRNAs induced apoptosis by approximately 8.2% and 9.5%, respectively, in primary HSCs (Fig. 2A) and by 5.9% and 5.9% in HSC-T6 cells (Fig. 2B).
Fig. 2. Effect of PCBP2 siRNA and alcohol on apoptosis of primary rat HSCs (A) and HSC-T6 cells (B).
HSCs were treated with 50 nmol siRNA for 24 h or 100 mmol alcohol for 48 h. The cells were stained with Annexin-V-FITC and propidium iodide, and then analyzed by flow cytometry. Results are presented as the mean ± SD (n=3). *P<0.05; **P<0.01.
We also evaluated the apoptotic effect of alcohol stimulation on primary HSCs and HSC-T6 cells (Figure 2). The cells were incubated with DMEM containing 100 mmol alcohol for 48 h. The percentage of apoptosis in primary HSCs increased from 10.4% to 28.6%. However, 100 mmol alcohol did not induce a significant apoptotic effect in HSC-T6 cells (an increase from 5.9% to 8.0%).
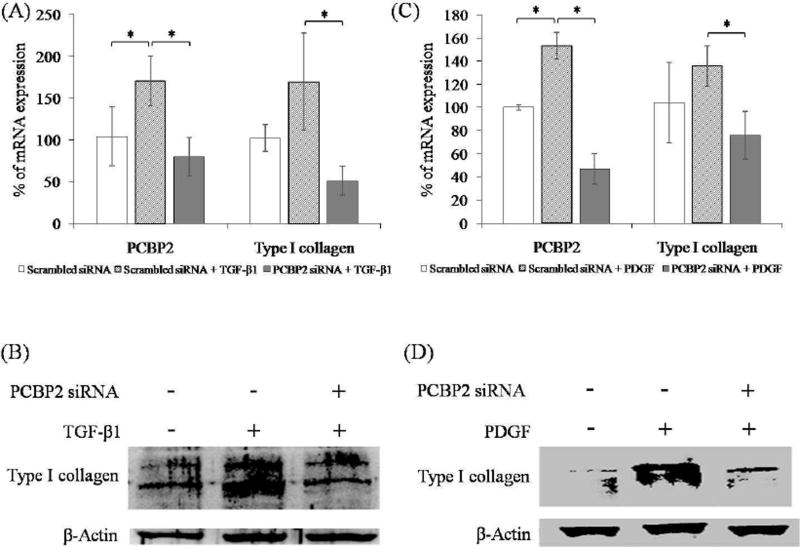
Previous studies have demonstrated that TGF-β1 and PDGF induce expression of type I collagen [26, 27]. In the current study, we transfected HSCs with PCBP2 siRNA three times over 6 days, and then incubated the cells with medium containing these cytokines. Primary HSCs were treated with TGF-β1 (12 ng/mL) or PDGF (10 ng/mL) for 24 h. The expression of PCBP2 and type I collagen were then determined at mRNA and protein levels. As shown in Figure 3A and C, both TGF-β1 and PDGF upregulated the mRNA levels of PCBP2 and type I collagen. Conversely, treatment of the cells with PCBP2 siRNA reversed the inductive effects of profibrotic cytokines TGF-β1 and PDGF on PCBP2 and type I collagen. Similar results were observed at the protein level of type I collagen (Fig. 3B and 3 D).
Fig. 3. PCBP2 siRNA reverses cytokine-induced expression of type I collagen in primary rat HSCs (A, B) and HSC-T6 (C, D) cells.
The cells were transfected with 50 nmol siRNA for 24 h and then treated with TGF-β1 or PDGF for 12 h. (A, C) mRNA levels of PCBP2 and type I collagen were quantified by real-time RT-PCR. (B, D) Protein expression of type I collagen was determined by western blotting. Results are presented as the mean ± SD (n=3). *P<0.05; **P<0.01.
3.2. PCBP2 siRNA reverses the proliferation effect of PDGF
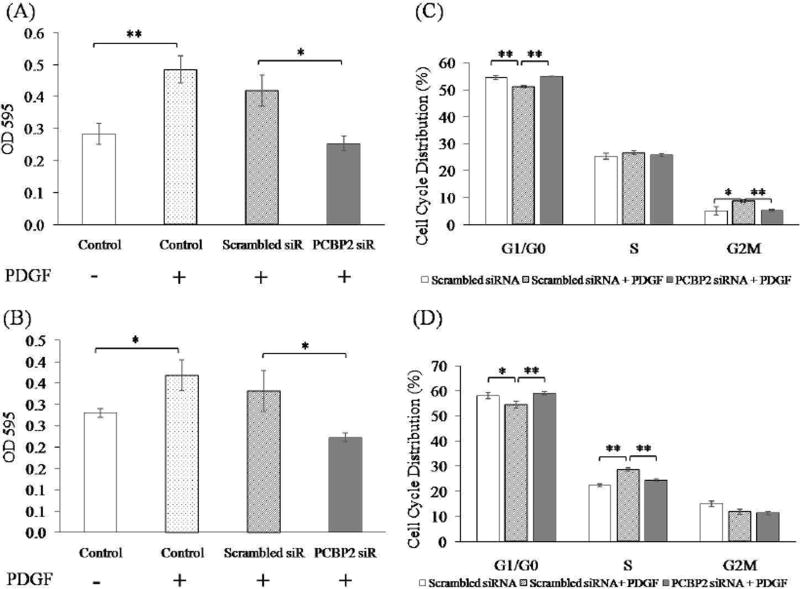
As a result of chronic liver injury, HSCs as the main ECM-producing cells activate, transdifferentiate into myofibroblast-like cells, and acquire proinflammatory properties. As reported previously [28], PDGF is upregulated during fibrogenesis and is the most potent cytokine for HSC proliferation. To evaluate the effect of the PCBP2 siRNA on PDGF-stimulated cell proliferation, both primary HSCs and HSC-T6 cells were transfected with 50 nmol PCBP2 siRNA in DMEM containing 0.5% FBS for 24 h and then incubated with PDGF for another 12 h. As shown in Fig. 4A, the proliferation of primary HSCs was induced by PDGF, and the stimulatory effect of PDGF was reversed by transfection of the PCBP2 siRNA. Similar results were observed in HSC-T6 cells (Fig. 4B). However, the stimulatory effect of PDGF on primary HSCs was more significant than that on HSC-T6 cells.
Fig. 4. PCBP2 siRNA reverses PDGF-induced cell proliferation of primary rat HSCs (A, C) and HSC-T6 cells (B, D).
The cells were transfected with 50 nmol siRNA for 24 h and then incubated with PDGF for 12 h. (A, B) Cell proliferation was measured by MTT assays. (C, D) Cell cycle analysis was performed by flow cytometry. Results are presented as the mean ± SD (n=3). *P<0.05; **P<0.01.
Cell cycle analysis was conducted to investigate the anti-proliferation mechanism of the PCBP2 siRNA. Primary HSCs treated with PDGF exhibited a decrease in G0/G1 phase and arrest at G2/M phase (Fig. 4C). Following transfection with the PCBP2 siRNA, the percentages of primary HSCs in G0/G1 and G2/M phases were restored to normal compared with cells without PDGF stimulation. Similar experiments were conducted in HSC-T6 cells (Fig. 4D). PDGF also decreased the proportion of cells in G1/G0 phase but arrested the cells in S phase rather than G2/M phase. Therefore, the PCBP2 siRNA restored the PDGF-induced cell distributions in G0/G1 and S phases.
3.3.PCBP2 siRNA inhibits the migration effect of cytokines and alcohol
HSCs reside in the space of Disse, the major storage site of vitamin A [29]. Following chronic injury of the liver, activated HSCs migrate and accumulate at the site of injury, where they produce large amounts of ECM and modulate the ECM degradation rate. Cytokines such as TGF-β1 [26], PDGF, and EGF have been previously reported to facilitate HSC migration [10, 28]. In the present study, we evaluated whether the PCBP2 siRNA inhibited the migration effect induced by cytokines and alcohol.
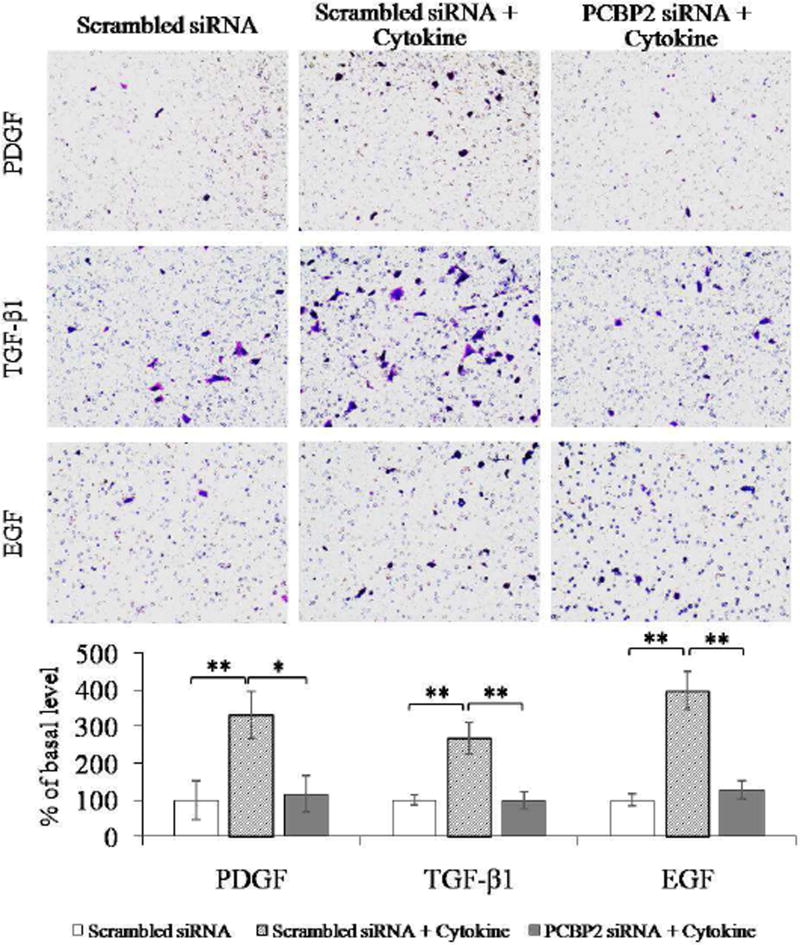
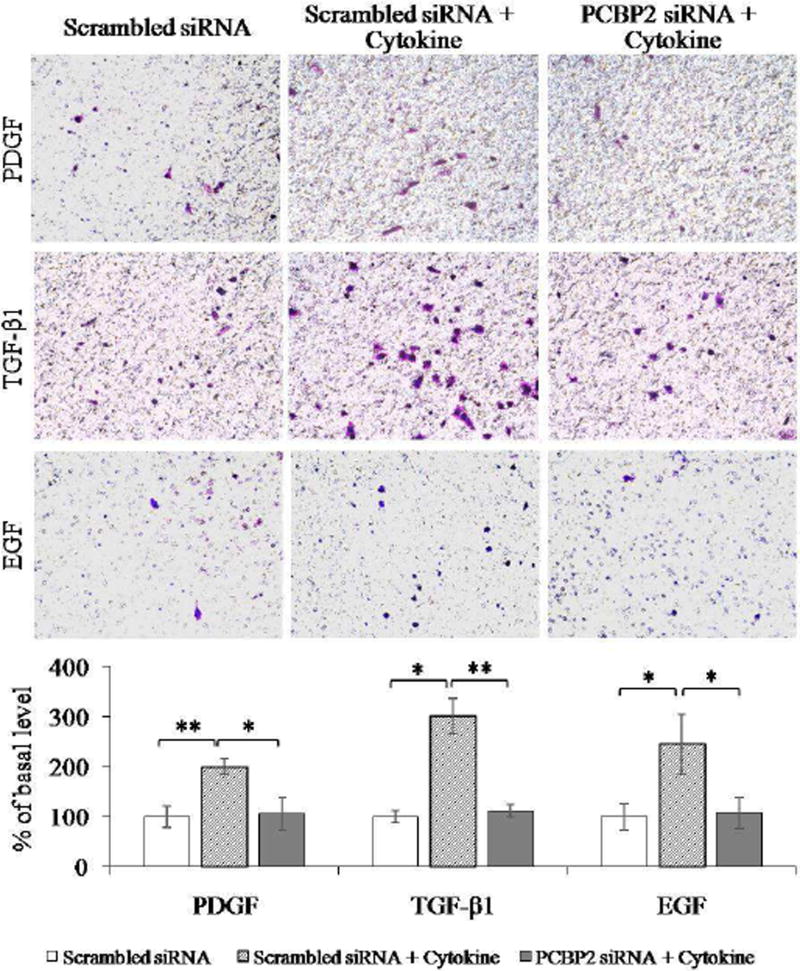
First, we treated HSCs with serial concentrations of TGF-β1, PDGF, and EGF to determine the optimal concentration that induces the highest degree of HSC migration (data not shown). Primary HSCs (Fig. 5) and HSC-T6 cells (Fig. 6) were transfected with the PCBP2 siRNA, followed by incubation with the cytokines for 4 h. Scrambled siRNA was used as a negative control. As illustrated in Fig. 5, after stimulation with the cytokines, the number of migrated primary HSCs was increased by approximately 3.3-fold (PDGF), 2.7-fold (TGF-β1), and 4-fold (EGF). In contrast, transfection of the cells with the PCBP2 siRNA reversed the stimulatory effects of these cytokines. Similar results were observed in HSC-T6 cells (Fig. 6). The number of migrated HSC-T6 cells was increased by 2-fold (PDGF), 3-fold (TGF-β1), and 2.5-fold (EGF) after cytokine stimulation. Transfection with the PCBP2 siRNA also reversed the stimulatory effect of cytokines in HSC-T6 cells.
Fig. 5. PCBP2 siRNA inhibits cytokine-induced migration of primary rat HSCs.
After transfection with 50 nmol PCBP2 siRNA for 24 h, primary rat HSCs were seeded onto transwell chambers and incubated for 4 h to allow migration through the membrane. The migrated cells were stained, and images were obtained at 200× magnification. Six random microscopic fields were counted in each transwell. Cells treated with scrambled siRNA were used as the negative control. Results are presented as the mean ± SD (n=3). *P<0.05; **P<0.01.
Fig. 6. PCBP2 siRNA inhibits cytokine-induced migration of HSC-T6 cells.
After transfection with 50 nmol PCBP2 siRNA for 24 h, HSC-T6 cells were seeded onto transwell chambers and incubated for 4 h to allow migration through the membrane. The migrated cells were stained, and images were obtained at 200× magnification. Six random microscopic fields were counted in each transwell. Cells treated with scrambled siRNA were used as the negative control. Results are presented as the mean ± SD (n=3). *P<0.05; **P<0.01.
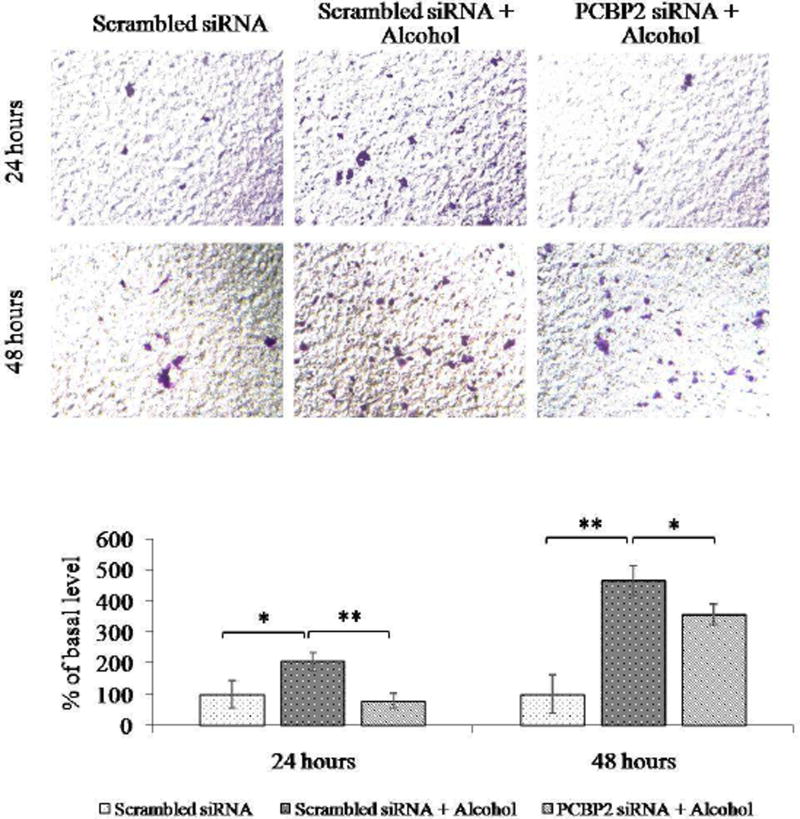
Next, we evaluated the stimulatory effect of alcohol on the migration of primary HSCs. As shown in Fig. 7, incubation with alcohol for 24 h increased the number of migrated cells by approximately 2-fold, whereas the PCBP2 siRNA was able to completely reverse the stimulatory effect on migration. In contrast, alcohol exhibited a more significant effect (4.65-fold increase) on cell migration when the incubation time was extended to 48 h. Treatment of the cells with the PCBP2 siRNA attenuated, but could not completely reverse, the stimulatory effect of alcohol on migration. This result could be due to the transient silencing effect of the PCBP2 siRNA during the longer alcohol stimulation period.
Fig. 7. PCBP2 siRNA inhibits alcohol-induced migration of primary rat HSCs.
Primary rat HSCs were transfected with 50 nmol PCBP2 siRNA for 24 h and then incubated with 100 mmol alcohol for 24 or 48 h. The cells were then seeded onto transwell chambers and incubated for 4 h to allow migration through the membrane. The migrated cells were stained, and images were obtained at 200× magnification. Six random microscopic fields were counted in each transwell. Cells treated with scrambled siRNA were used as the negative control. Results are presented as the mean ± SD (n=3). *P<0.05; **P<0.01.
3.4. Combinational treatment with PCBP2 siRNA and LY2109761
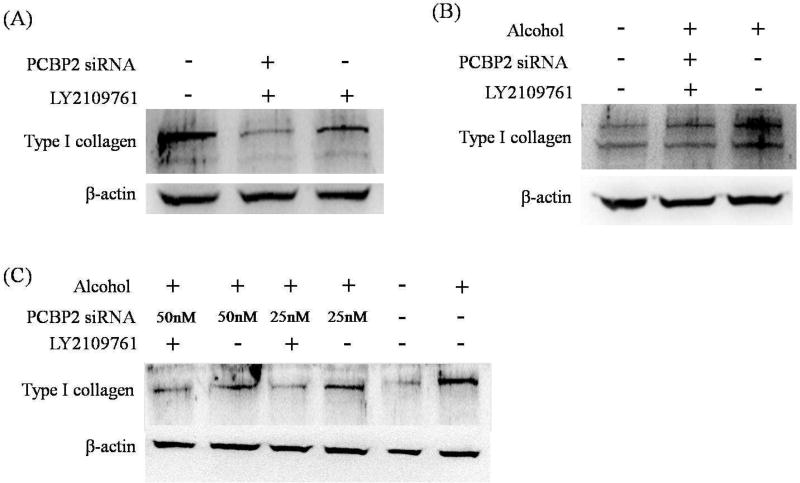
LY2109761 is a small molecule inhibitor of TGF-β receptor type I/II kinases and widely used for the treatment of various diseases [30, 31]. It has be used to treat pulmonary fibrosis [32] and human hypertrophic scar fibroblasts [33] because of its inhibitory effect on the expression of type I collagen. In this study, we treated HSCs with a combination of the PCBP2 siRNA and LY2109761 and then monitored the expression of type I collagen by western blotting (Figure 8A). HSC-T6 cells are activated HSCs and accordingly express high levels of type I collagen. While LY2109761 alone significantly reduced the expression of type I collagen, the combination of LY2109761 and PCBP2 siRNA had a much stronger inhibitory effect on type I collagen. The protein expression of type I collagen in HSC-T6 cells was reduced to near negligible levels by the combinational treatment.
Fig. 8. Combinational treatment with PCBP2 siRNA and LY2109761.
(A) HSC-T6 cells were transfected with 50 nmol PCBP2 siRNA three times on days 1, 3, and 5. LY2109761 (10 µmol) was added on day 3, and alcohol (100 mmol) was added on day 6. The cells were harvested for western blot analysis of type I collagen on day 7. (B) Primary rat HSCs were transfected with 50 nmol PCBP2 siRNA three times on days 1, 3, and 5. LY2109761 (10 µmol) was added on day 3, and alcohol (100 mmol) was added on day 6. The cells were harvested for western blot analysis of type I collagen on day 7. (C) Primary rat HSCs were transfected with 25 or 50 nmol PCBP2 siRNA for 24 h, followed by incubation with 100 mmol alcohol for another 48 h. LY2109761 (10 µmol) was added to the medium during the siRNA transfection and alcohol incubation periods. Protein expression of type I collagen was evaluated by western blotting.
We next treated primary HSCs (quiescent) with the same combination of PCBP2 siRNA and LY2109761 before subsequent alcohol stimulation (Fig. 8B). Compared with HSC-T6 cells, primary HSCs are quiescent and therefore express low levels of type I collagen. Incubation with alcohol significantly induced the expression of type I collagen, and the combinational treatment reversed the expression level of type I collagen.
Next, we evaluated whether single transfection of PCBP2 siRNA can also reverse the alcohol-induced expression of type I collagen in primary HSCs. As shown in Figure 8C, PCBP2 siRNA alone exhibited a dose-dependent inhibitory effect on the alcohol-induced expression of type I collagen. The combination of LY2109761 with PCBP2 siRNA further reduced the expression of type I collagen to a negligible level.
4. Discussion
In our previous study, we demonstrated that a synthetic PCBP2 siRNA has a potent silencing effect on the expression of αCP2 that stabilizes type I collagen α1 mRNA in activated HSCs [9]. As an activated HSC line, HSC-T6 cells reflect a fibroblast-like phenotype that proliferates rapidly in culture [34]. HSC-T6 cells express cytoskeletal proteins, such as desmin, alpha-smooth muscle actin, glial acidic fibrillary protein, and vimentin, which are typical markers of activated stellate cells. In the current study, primary HSCs isolated from normal rats were stimulated with alcohol and cytokines. This model was used to mimic the activation of quiescent HSCs in the initiation stage of fibrogenesis. We also used HSC-T6 cells as a model of the perpetuation stage of fibrogenesis.
HSCs are the main contributor to ECM accumulation in the process of alcoholic liver fibrosis. HSCs transdifferentiate into active fibroblasts after continuous exposure to alcohol or other chronic injuries [3]. An increase in cytokine release has been reported to occur during the chronic wound-healing process [10, 35–38]. The activated fibroblasts migrate to injury sites where they demonstrate enhanced proliferation and collagen production. In our previous study, we found overexpression of αCP2 and collagen type I in HSC-T6 cells after exposure to alcohol, and the PCBP2 siRNA reverses the collagen accumulation in HSC-T6 cells [9].
In the current study, we first isolated primary HSCs from rat liver and then treated the cells with alcohol. As shown in Fig. 1, the mRNA levels of PCBP2 and type I collagen were increased significantly. In addition, the type I collagen mRNA level was more significantly increased in primary HSCs than in HSC-T6 cells. We infer that the primary HSCs isolated from a healthy rat were quiescent and therefore more sensitive to the alcohol stimulation. We also demonstrated that treatment of primary HSCs with the PCBP2 siRNA significantly suppressed the alcohol-induced mRNA levels of PCBP2 and type I collagen as well as the protein level of type I collagen (Fig. 1 and 8).
It is known that activated HSCs produce more type I collagen upon initiation of fibrogenesis and upregulate PDGF and TGF-β1 secretion in the fibrotic environment. In our study, as shown in Figure 3A, type I collagen expression was increased by PDGF and TGF-β1 stimuli. Treatment with the PCBP2 siRNA significantly decreased the expression of type I collagen at mRNA and protein levels. αCP2, which is encoded by the PCBP2 gene, binds to the 3′ end of type I collagen mRNA and increases its stability. This stability leads to the accumulation of a large amount of collagen in the fibrotic liver [9, 39]. Here, we showed that treatment of alcohol- and cytokine-stimulated primary rat HSCs with the PCBP2 siRNA significantly inhibited the collagen accumulation.
PDGF is a cytokine with the strongest mitogenic stimulatory effects on HSC proliferation. PDGF and its receptors are highly expressed in cirrhotic liver tissue [40]. As Lin et al. reported, primary rat HSCs stimulated with PDGF for 24 h show a dose-dependent increase in cell proliferation [23]. Adachi et al. [41] reported that PDGF activates NAD(P)H oxidase in HSCs, leading to the generation of reactive oxygen species, which further activates p38 mitogen-activated protein kinase (MAPK) and induces the proliferation of HSCs. Similarly, we found a significant proliferation effect on both primary HSCs and HSC-T6 cells exerted by PDGF stimulation (Fig. 4A, 4B). This result is also in accordance with a report showing that microRNA-214 (mir-214) suppresses glioma proliferation by targeting PCBP2 [42]. Mir-214 binds to the 3′-untranslated region of PCBP2 mRNA, which leads to the degradation of PCBP2 mRNA. As a result, mir-214 inhibits the proliferation and growth of glioma cells. In contrast, the restoration of PCBP2 dramatically reverses these tumor-suppressive effects. PCBP2 has been also reported to regulate astrocyte proliferation after spinal cord injury [43] and facilitate the progression of esophageal squamous cell carcinoma by regulating cellular proliferation and apoptosis [44]. In this study, HSC cells were transfected with PCBP2 siRNA and then treated with PDGF. The PCBP2 siRNA remarkably blocked the PDGF-stimulated proliferation of HSCs.
HSC migration is one of the major features in liver fibrosis. In addition to alcohol, several cytokines were used in this study to mimic the fibrotic environment in the liver. As Yang et al. reported [10], the stimulation of HSCs with PDGF, TGF-β1, or EGF enhanced the migratory capacity of HSCs. Furthermore, TGF-β1 has been reported to facilitate the migration of HSCs in a modified Boyden Chamber model via both chemotactic and haptotactic mechanisms [45]. In addition, PDGF promotes the migration of murine HSC line GRX [46]. Alcohol has also been reported to promote migration and invasion of triple-negative breast cancer cells through activation of p38 MAPK and c-Jun N-terminal kinase [47]. In this study, PDGF, TGF-β1, EGF, and alcohol were used to exert a migration effect on HSCs. We found that treatment with PCBP2 siRNA attenuated or reversed the HSC migration induced by alcohol, PDGF, TGF-β1, and EGF, which is consistent with the study of Lin et al. who found that knocking down PCBP2 inhibits glioma cell migration and invasion via Rho GDP dissociation inhibitor alpha [48].
In addition, we demonstrated that the combination of PCBP2 siRNA and LY2109761 had a potent silencing effect on collagen expression (Fig. 8). Both HSC-T6 cells and primary HSCs were used to evaluate the combinational treatment. LY2109761, a TGFβ receptor type I/II kinase inhibitor, has been used to treat pulmonary fibrosis and human hypertrophic scar fibroblasts. It has been reported that LY2109761 strongly inhibits pulmonary fibrosis [32]. It was also found that LY2109761 reduces the expression of pSMAD1 and pSMAD2, and attenuates the gene expression involved in canonical and noncanonical TGF-β signaling. These data indicate that LY2109761 has an antifibrogenic effect through inhibition of proinflammatory, proangiogenic and profibrotic signals. Furthermore, Wang et al. reported that LY2109761 suppresses the mRNA levels of type I collagen and TGF-β1 by approximately 86% and 85% in hypertrophic scar-derived fibroblasts, respectively [33]. TGF-β1 enhances collagen expression through its cognate receptors to Smad proteins, which increases the transcription of their target genes including procollagen I and procollagen III [4]. Here, we used LY2109761 combined with PCBP2 siRNA to suppress collagen synthesis and inhibit collagen stability, respectively, which produced a strongly synergistic silencing effect on the accumulation of type I collagen.
In conclusion, rat primary HSCs and the HSC-T6 cell line were used to model the initiation and perpetuation stages of liver fibrosis. We observed that the expression of αCP2 and type I collagen was significantly enhanced in primary HSCs and HSC-T6 cells that were exposed to alcohol or fibrogenic cytokines. Notably, we demonstrated that PCBP2 siRNA inhibited overexpression of PCBP2 and type I collagen. Moreover, PCBP2 siRNA reversed the cell proliferation and migration induced by alcohol and fibrotic cytokines. This study suggests that the PCBP2 siRNA can be used alone or in combination with LY2109761 to reverse alcoholic liver fibrosis.
Acknowledgments
This work was supported by an award (aR0aAA02a5a0) from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 2.Cheng K, Mahato RI. Gene modulation for treating liver fibrosis. Crit Rev Ther Drug Carrier Syst. 2007;24:93–146. doi: 10.1615/critrevtherdrugcarriersyst.v24.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng K, Yang N, Mahato RI. TGF-beta1 gene silencing for treating liver fibrosis. Mol Pharm. 2009;6:772–779. doi: 10.1021/mp9000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 6.Bonis PA, Friedman SL, Kaplan MM. Is liver fibrosis reversible? N Engl J Med. 2001;344:452–454. doi: 10.1056/NEJM200102083440610. [DOI] [PubMed] [Google Scholar]
- 7.Lindquist JN, Marzluff WF, Stefanovic B. Fibrogenesis. III. Posttranscriptional regulation of type I collagen. Am J Physiol Gastrointest Liver Physiol. 2000;279:G471–G476. doi: 10.1152/ajpgi.2000.279.3.G471. [DOI] [PubMed] [Google Scholar]
- 8.Lindquist JN, Parsons CJ, Stefanovic B, et al. Regulation of alpha1(I) collagen messenger RNA decay by interactions with alphaCP at the 3'-untranslated region. J Biol Chem. 2004;279:23822–23829. doi: 10.1074/jbc.M314060200. [DOI] [PubMed] [Google Scholar]
- 9.Shukla RS, Qin B, Wan YJ, et al. PCBP2 siRNA reverses the alcohol-induced profibrogenic effects in hepatic stellate cells. Pharm Res. 2011;28:3058–3068. doi: 10.1007/s11095-011-0475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C, Zeisberg M, Mosterman B, et al. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 11.Fujii T, Fuchs BC, Yamada S, et al. Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 2010;10:79. doi: 10.1186/1471-230X-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng K, Ye Z, Guntaka RV, et al. Biodistribution and hepatic uptake of triplex-forming oligonucleotides against type alpha1(I) collagen gene promoter in normal and fibrotic rats. Mol Pharm. 2005;2:206–217. doi: 10.1021/mp050012x. [DOI] [PubMed] [Google Scholar]
- 13.Ye Z, Cheng K, Guntaka RV, et al. Receptor-mediated hepatic uptake of M6P-BSA-conjugated triplex-forming oligonucleotides in rats. Bioconjug Chem. 2006;17:823–830. doi: 10.1021/bc060006z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng K, Ye Z, Guntaka RV, et al. Enhanced hepatic uptake and bioactivity of type alpha1(I) collagen gene promoter-specific triplex-forming oligonucleotides after conjugation with cholesterol. J Pharmacol Exp Ther. 2006;317:797–805. doi: 10.1124/jpet.105.100347. [DOI] [PubMed] [Google Scholar]
- 15.Jin W, Qin B, Chen Z, et al. Discovery of PSMA-specific peptide ligands for targeted drug delivery. Int J Pharm. 2016;513:138–147. doi: 10.1016/j.ijpharm.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 16.Kato J, Sato Y, Inui N, et al. Ethanol induces transforming growth factor-alpha expression in hepatocytes, leading to stimulation of collagen synthesis by hepatic stellate cells. Alcohol Clin Exp Res. 2003;27:58S–63S. doi: 10.1097/01.ALC.0000078614.44983.97. [DOI] [PubMed] [Google Scholar]
- 17.Nieto N, Greenwel P, Friedman SL, et al. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem. 2000;275:20136–20145. doi: 10.1074/jbc.M001422200. [DOI] [PubMed] [Google Scholar]
- 18.Anania FA, Womack L, Jiang M, et al. Aldehydes potentiate alpha(2)(I) collagen gene activity by JNK in hepatic stellate cells. Free Radic Biol Med. 2001;30:846–857. doi: 10.1016/s0891-5849(01)00470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin B, Cheng K. Silencing of the IKKε gene by siRNA inhibits invasiveness and growth of breast cancer cells. Breast Cancer Res. 2010;12:R74. doi: 10.1186/bcr2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tai W, Qin B, Cheng K. Inhibition of breast cancer cell growth and invasiveness by dual silencing of HER-2 and VEGF. Mol Pharm. 2010;7:543–556. doi: 10.1021/mp9002514. [DOI] [PubMed] [Google Scholar]
- 21.Mahato R, Qin B, Cheng K. Blocking IKKα expression inhibits prostate cancer invasiveness. Pharm Res. 2011 Jun;28:1357–1369. doi: 10.1007/s11095-010-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borkham-Kamphorst E, Herrmann J, Stoll D, et al. Dominant-negative soluble PDGF-beta receptor inhibits hepatic stellate cell activation and attenuates liver fibrosis. Lab Invest. 2004;84:766–777. doi: 10.1038/labinvest.3700094. [DOI] [PubMed] [Google Scholar]
- 23.Lin J, Chen A. Activation of peroxisome proliferator-activated receptor-gamma by curcumin blocks the signaling pathways for PDGF and EGF in hepatic stellate cells. Lab Invest. 2008;88:529–540. doi: 10.1038/labinvest.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontana L, Jerez D, Rojas-Valencia L, et al. Ethanol induces the expression of alpha 1(I) procollagen mRNA in a co-culture system containing a liver stellate cell-line and freshly isolated hepatocytes. Biochim Biophys Acta. 1997;1362:135–144. doi: 10.1016/s0925-4439(97)00056-2. [DOI] [PubMed] [Google Scholar]
- 25.Lindquist JN, Stefanovic B, Brenner DA. Regulation of collagen alpha1(I) expression in hepatic stellate cells. J Gastroenterol. 2000;35(Suppl 12):80–83. [PubMed] [Google Scholar]
- 26.Pignatelli J, Tumbarello DA, Schmidt RP, et al. Hic-5 promotes invadopodia formation and invasion during TGF-β-induced epithelial-mesenchymal transition. J Cell Biol. 2012;197:421–437. doi: 10.1083/jcb.201108143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin X, Kong LN, Huang C, et al. Hesperetin derivative-7 inhibits PDGF-BB-induced hepatic stellate cell activation and proliferation by targeting Wnt/β-catenin pathway. Int Immunopharmacol. 2015;25:311–320. doi: 10.1016/j.intimp.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Roderfeld M, Weiskirchen R, Atanasova S, et al. Altered factor VII activating protease expression in murine hepatic fibrosis and its influence on hepatic stellate cells. Liver Int. 2009;29:686–691. doi: 10.1111/j.1478-3231.2008.01897.x. [DOI] [PubMed] [Google Scholar]
- 29.Bataller R1, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Kleber S, Röhrich M, et al. Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011;71:7155–7167. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 31.Mazzocca A, Fransvea E, Lavezzari G, et al. Inhibition of transforming growth factor beta receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology. 2009;50:1140–1151. doi: 10.1002/hep.23118. [DOI] [PubMed] [Google Scholar]
- 32.Flechsig P, Dadrich M, Bickelhaupt S, et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-β and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res. 2012;18:3616–3627. doi: 10.1158/1078-0432.CCR-11-2855. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Gao Z, Wu X, et al. Inhibitory effect of TGF-β peptide antagonist on the fibrotic phenotype of human hypertrophic scar fibroblasts. Pharm Biol. 2016;54:1189–1197. doi: 10.3109/13880209.2015.1059862. [DOI] [PubMed] [Google Scholar]
- 34.Vogel S, Piantedosi R, Frank J, et al. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882–893. [PubMed] [Google Scholar]
- 35.Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J Gastroenterol Hepatol. 1999;14:618–633. doi: 10.1046/j.1440-1746.1999.01928.x. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Liu X, Gao B, et al. New Approaches for studying alcoholic liver disease. Curr Pathobiol Rep. 2014;2:171–183. doi: 10.1007/s40139-014-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H, Wang J, Fan JH, et al. Ilexgenin A exerts anti-inflammation and anti-angiogenesis effects through inhibition of STAT3 and PI3K pathways and exhibits synergistic effects with Sorafenib on hepatoma growth. Toxicol Appl Pharmacol. 2017;315:90–101. doi: 10.1016/j.taap.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Zhao J, Liu Y, et al. A novel pentacyclic triterpenoid, Ilexgenin A, shows reduction of atherosclerosis inapolipoprotein E deficient mice. Int Immunopharmacol. 2016;40:115–124. doi: 10.1016/j.intimp.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Lindquist JN, Kauschke SG, Stefanovic B, et al. Characterization of the interaction between alphaCP(2) and the 3'-untranslated region of collagen alpha1(I) mRNA. Nucleic Acids Res. 2000;28:4306–4316. doi: 10.1093/nar/28.21.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinzani M, Milani S, Herbst H, et al. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am J Pathol. 1996;148:785–800. [PMC free article] [PubMed] [Google Scholar]
- 41.Adachi T, Togashi H, Suzuki A, et al. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology. 2005;41:1272–1281. doi: 10.1002/hep.20719. [DOI] [PubMed] [Google Scholar]
- 42.Tang SL, Gao YL, Chen XB. MicroRNA-214 targets PCBP2 to suppress the proliferation and growth of glioma cells. Int J Clin Exp Pathol. 2015;8:12571–12576. [PMC free article] [PubMed] [Google Scholar]
- 43.Mao X, Liu J, Chen C, et al. PCBP2 modulates neural apoptosis and astrocyte proliferation after spinal cord injury. Neurochem Res. 2016;41:2401–2414. doi: 10.1007/s11064-016-1953-6. [DOI] [PubMed] [Google Scholar]
- 44.Ye J, Zhou G, Zhang Z, et al. Poly (C)-binding protein 2 (PCBP2) promotes the progression of esophageal squamous cell carcinoma (ESCC) through regulating cellular proliferation and apoptosis. Pathol Res Pract. 2016;212:717–725. doi: 10.1016/j.prp.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Li L, Wang JY, Yang CQ, et al. Effect of RhoA on transforming growth factor β1-induced rat hepatic stellate cell migration. Liver Int. 2012;32:1093–1102. doi: 10.1111/j.1478-3231.2012.02809.x. [DOI] [PubMed] [Google Scholar]
- 46.Bitencourt S, Mesquita F, Basso B, et al. Capsaicin modulates proliferation, migration, and activation of hepatic stellate cells. Cell Biochem Biophys. 2014;68:387–396. doi: 10.1007/s12013-013-9719-0. [DOI] [PubMed] [Google Scholar]
- 47.Zhao M, Howard EW, Parris AB, et al. Alcohol promotes migration and invasion of triple-negative breast cancer cells through activation of p38 MAPK and JNK. Mol Carcinog. 2017;56:849–862. doi: 10.1002/mc.22538. [DOI] [PubMed] [Google Scholar]
- 48.Lin X, Yang B, Liu W, et al. Interplay between PCBP2 and miRNA modulates ARHGDIA expression and function in glioma migration and invasion. Oncotarget. 2016;7:19483–19498. doi: 10.18632/oncotarget.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]