Abstract
Resting transmembrane potential (TMP) of primary human fibroblast cells was altered in predictable directions by subjecting cell cultures to specific monophasic and biphasic waveforms. Cells electrically stimulated with an anodal pulse resulted in hyperpolarization while a cathodal waveform depolarized the TMP to below that of non-paced control cells. The biphasic waveform, consisting of an anodal pulse followed immediately by an inverse symmetric cathodal pulse, also lessened the TMP similar to that of the cathodal pulse. The effect of short-term pacing on the TMP can last up to 4 h before the potentials equilibrate back to baseline. While subjecting the cells to this electrical field stimulation did not appear to damage the integrity of the cells, the three paced electrical stimulation waves inhibited expansion of the cultures when compared to non-paced control cells. With longer pacing treatments, elongation of the cells and electrotaxis towards the anodal polarity were observed. Pacing the fibroblasts also resulted in modest, yet very statistically significant (and likely underestimated) changes to cellular adenosine-5'-triphosphate (ATP) levels, and cells undergoing anodal and biphasic (anodal/cathodal) stimulation also exhibited altered mitochondrial morphology. These observations indicate an active role of electrical currents, especially with anodal content, in affecting cellular metabolism and function, and help explain accumulating evidence of cellular alterations and clinical outcomes in pacing of the heart and other tissues in general.
Keywords: Membrane Potential, Cellular Electrophysiology, Human fibroblast physiology, Anodal electrical stimulation, ATP production
Highlights
-
•
Cathodal currents impair cellular functions.
-
•
Anodal currents increase cardiac contractility and speed of conduction.
-
•
Anodal currents increase membrane potential which persists after stopping pacing.
-
•
Anodal currents produce ATP which is then used for the work of the cell.
-
•
The change result in stimulation of metabolism in immature fibroblasts.
1. Introduction
All live cells have a stable transmembrane potential (TMP) voltage differential across the cell membrane when the cell is at rest. This is the result of the accumulated ion concentrations within the cell compared to that outside the membrane. Electrically active cells, such as neurons, muscles, and pancreatic beta cells, are called excitable because they can produce an action potential due to a short-lived rapid depolarization of the TMP before returning to the higher resting state. These cells achieve this by expressing fast-acting voltage gated ion channels that allow the very rapid exchange of ions across the cellular membrane. While calcium and chloride ions make distinct contributions, it is particularly the sodium (Na+) and potassium (K+) which contribute most to this ion concentration potential.
Even cells that do not generate action potentials need to maintain a TMP in order to enable secondary active transport of metabolites. Non-excitable cells use slower membrane ion exchange pumps or transporters to move charges across the membrane, often in conjunction with the transport of a metabolite. Most of these transporters require a concentration gradient, a supply of energy, and are considerably slower than the fast-acting ion channels of the excitable cells.
Alterations to membrane potential are carefully modulated by ion channels within the cell membrane to maintain homeostasis. Subjecting cells to pacing waveforms alters the TMP since it results in a manipulation of these charged ions. We offer evidence that electrically altering the TMP can then have profound effects on cellular physiology in terms of both metabolism and function [1].
2. Materials and methods
2.1. Cells
Early passage human primary fibroblast cells (CRL-2703, ATCC; Manassas, VA) were subcultured in a 37 °C, high humidity incubator with 5% CO2 and maintained in Iscove's Modified Dulbecco's Medium (IMDM, Thermofisher Scientific; Waltham, MA) supplemented with 10% fetal bovine serum (FBS; ATCC), 25 mM HEPES (Thermofisher Scientific) and an antibiotic-antimycotic solution (ATCC). Cells were passaged at approximately 80% confluence and checked weekly or biweekly to be mycoplasma-free. Twenty-four hours prior to pacing, cells were seeded at a density of ~3.0×105 cells per cm2 for attachment in designated pacing chambers.
2.2. Electrodes and pacing chambers
Pacing chambers were T-25 cell culture flasks, 6-well culture plates, and 3-D printed plastic forms with electrodes attached with similar spacing. Non-reactive carbon rods (4.0 mm diameter, Frey Scientific; Appleton, WI) or platinum wires (0.5 mm diameter, WPI; Sarasota, FL) were inserted 4 cm apart. There were no apparent variations of results in experiments based on the material composition of the electrodes. The chambers were tilted so that the seeded cells attached and were localized close to the active electrode, defined as the polarity of the defined waveform when measured on an oscilloscope. The other electrode served as the ‘non-active’ reference electrode.
2.3. Pacing treatment
An external cardiac pacer (PACE Medical, Inc.; Waltham, MA) set at 5 V (V) magnitude and 1.8 ms (mSec) corresponding to an output of 10 mA (mA) at a rate of 1.7 Hz was used as the input signal to a Slave Stimulator (Model 71006, Rivertek Medical Systems; Minneapolis, MN). This circuit produced the actual monophasic anodal and cathodal, and biphasic (anodal followed by cathodal) pulsed square waveforms used for stimulation. The monophasic waveforms selected for these experiments were ±5.0 Vx1.8 ms. The biphasic waveform was defined as a +2.5 V×0.9 ms anodal pulse immediately followed by a −2.5 V×0.9 ms cathodal pulse. Selected waveforms were verified on the electrodes before each experiment using digital oscilloscopes with probes placed within the electrolyte near the active electrode. All pacing treatments were continuous for times stated with the cells incubated in the conditions described. Electrolyte for cells paced less than 24 h was the defined media; cells paced 24 h or longer included 5% FBS.
2.4. Transmembrane potential assay
After pacing treatment, cells were suspended in phenol red-free IMDM with 2% FBS and 25 nM DiOC6(3) and allowed to equilibrate to room temperature prior to cytometric analysis. The cationic potentiometric carbocyanine dye, 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3), Thermofisher Scientific) was used to measure TMP on a flow cytometer (Beckton Dickenson Cantos, Japan) with BD FACSDiva software (Ver. 8.0). The positively charged dye is taken up by the cell in proportion to the negative charge on the membrane. Using 488 nm excitation, a minimum of 25 k resulting cellular mean florescence events on the FITC channel (MFCH) were averaged for each treatment. The TMP thus recorded is directly related to the MFCH on a linear histogram. MFCH from non-paced cells treated with valinomycin (30 nM, Sigma-Aldrich; St. Louis, MO) defined the hyperpolarization setpoint (−90 mV, arbitrary); and gramicidin (100 nM, Sigma-Aldrich) the depolarization setpoint (0 mV, arbitrary). Controls were included on each experiment.
2.5. Proliferation assay
Cells were checked for respiration using 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT Assay; Thermofisher Scientific) following manufacturer's protocol. Immediately after pacing, the electrolyte was discarded and replaced with whole phenol red-free IMDM with 1.2 nM MTT. After incubation, all but 25% of the media was discarded and 50% (v/v) dimethyl sulfoxide (DMSO, Sigma-Aldrich) was added to the remaining media to dissolve the resulting formazan crystals. Optical density for the resulting DMSO solutions was read at 540 nm. Cell cytotoxicity was estimated by comparison to absorbance levels generated by non-paced cells of similar number and treatments.
2.6. Trypan blue vital staining
To assess cellular death, cells paced for 3 h were subjected to Trypan Blue (0.1% Thermofisher) exclusion staining assay. The monolayer was gently rocked for 15 min in the cell culture incubator then washed in PBS for digital imaging under 10x magnification. The monolayer was then lysed with 1% sodium dodecyl sulfate (SDS) and the cleared lysate also read for optical density at 590 nm.
2.7. Electrotaxis
Prior to pacing, cells were directly passaged onto a 0.5% gelatin substratum with appropriate whole media, and incubated overnight. They were then paced for 96 h with the electrolyte changed daily. Digital imaging with 10x magnification was used to capture the results near the electrodes.
2.8. ATP assay
After pacing treatments, cell pellets were suspended in PBS supplemented with a protease inhibitor cocktail (Sigma-Aldrich) and flash frozen in liquid nitrogen. After sonication, protein concentrations of lysates were determined by Bradford assay (Sigma-Aldrich) and samples normalized to the lowest concentration value. Lysates were deproteinized with trichloroacetic acid precipitation and subjected to ATP assay (ABCam; Cambridge, MA) utilizing the phosphorylation of glycerol to produce a colorimetric product quantitated by absorbance at 570 nm.
2.9. Mitochondrial imaging
Cells were attached to poly-l-lysine treated cover coverslips and paced for 1 h or 24 h with non-paced controls. Cells were incubated and stained with 100 nM MitoTracker Red CMXRos (Thermofisher Scientific) before being fixed with 3.7% paraformaldehyde (Sigma-Aldrich). Fluorescence imaging (ex. 579 nm/em. 599 nm) was captured on a Zeiss AxioObserver Z1, 63X oil, confocal microscope using ZEN Blue Pro 2.0 program to produce the images.
2.10. Statistical analyses
Statistical analyses were performed on GraphPad Prism (ver. 4.0, GraphPad Software Inc.; La Jolla, CA). Results are given as means±Standard Error of the Mean (SEM). ANOVA's were performed with Bonferroni's or Dunnett's Multiple Comparison Post Testing. P-values were considered significant if p<0.05 compared to the similar non-paced control values. The respective symbols * indicate p<0.05, ** p<0.01, and *** p<0.001 in comparison to the unpaced cells.
3. Results
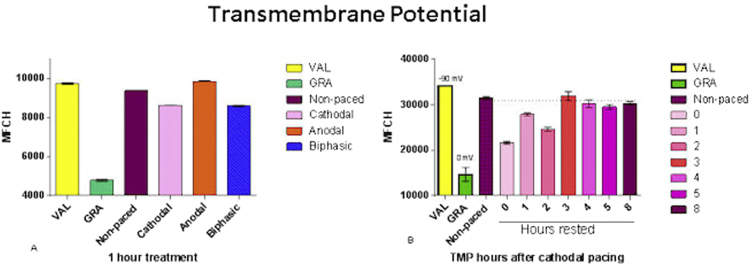
Paced cells showed altered global TMPs based on the polarity of the active electrode. The TMP of the non-paced cells was determined to be −83.2 mV±0.5 mV. Anodal pacing resulted in hyperpolarization with TMP increasing to −91.3 mV±1.0 mV. Cathodal paced cells exhibited an expected hypopolarization with TMP of −69.2 mV±0.3 mV. The symmetric biphasic waveform resulted in a TMP of −69.0 mV±0.5 mV similar to that of the cathodal paced cells. In the case of the cathodal cells, after removing the stimulus, multiple chambers were rested in an incubator and TMP was measured hourly. Non-paced cells were used to establish the TMP baseline, and assays were compared to the 0 h time point. The cells remained in a depolarized state for several hours before equilibrating back to near the resting baseline. Pacing for longer periods of time did not appear to affect the magnitude of the altered potential however did produce elongation of the cells and migration towards the anodal polarity (Fig. 1).
Fig. 1.
Transmembrane potential measured with a cationic fluorescent dye after one hour of each treatment in panel A. Valinomycin caused hyperpolarization and gramicidin depolarization. Anodal pacing produced increased potential and cathodal and biphasic pacing showed less potential than unpaced cells. In panel B, the reduction of polarization caused by cathodal pacing required several hours to return to the baseline unpaced state.
A Trypan blue exclusion assay showed no staining in any of the cells as noted by both absorption fluorescence and phase contrast microscopy, indicating no dead cells or cells with ruptured membranes. All the electrically stimulated cultures showed inhibition of expansion of the cultures when compared to non-paced control cells by MTT data and duplicate hemocytometer counts although anodal and biphasic paced cells exhibited less inhibition of growth than the cathodal pulse (anodal vs non-paced 92.9%; cathodal vs non-paced 70.0%; biphasic vs non-paced 80.2%) (Fig. 2).
Fig. 2.
In panel A, manual cell counts after 24 h of pacing showed inhibition of cell culture expansion with cathodal and biphasic waveforms, significant at p values less than 0.01 level. In panel B, MTT assay showed less cellular staining with any of the waveforms compared to non-paced at p values less than 0.01 (anodal and biphasic) and p value less than 0.05 level with cathodal.
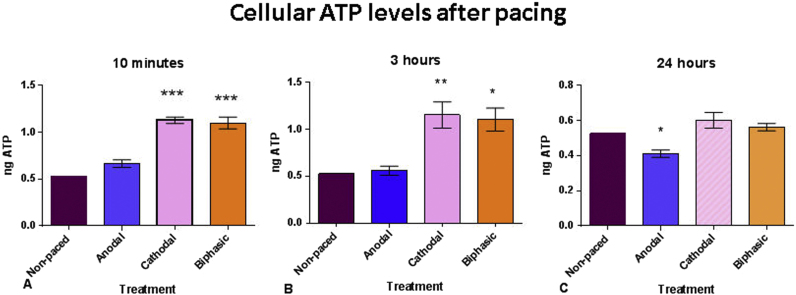
ATP production, as another surrogate of metabolic activity, was measured in mono- and biphasic paced cells and in non-paced cells after 10 min, 3 h, and 24 h treatments using a colorimetric assay utilizing phosphorylation of glycerol to produce a colorimetric product quantitated by optical density at 570 nm. Cathodal and biphasic stimulated cells showed statistically significant increased ATP levels after 10 min of treatment while the ATP level in the anodal paced cells was similar to that of the non-paced controls (non-paced 0.53 ng ATP vs anodal 0.66 ng; vs cathodal 1.12 ng; vs biphasic 1.09 ng). After 3 h of treatment, the increased available ATP was still significantly higher than non-paced cells. At 24 h treatments, ATP levels in the cathodal and biphasic paced cells returned to non-paced control levels however, levels in the anodal paced cells were now significantly less than all the others (non-paced 0.53 ng ATP vs anodal 0.41 ng; vs cathodal 0.60 ng; vs biphasic 0.56 ng) (Fig. 3).
Fig. 3.
Cellular ATP levels measured colorimetrically after ten minutes, three hours, and 24 h of pacing in panels A, B, and C respectively. In panel A, ATP levels were increased by cathodal and biphasic pacing at p values less than 0.001, in panel B increased at p values less than 0.01 and 0.05 respectively, and in panel C decreased by anodal pacing at a p value less than 0.05.
Together, the previously noted MTT and these ATP results suggest alterations in mitochondrial function when cells are influenced with pulsed electrical fields. These mitochondrial images at 24 h are A=non-paced, B=Anodal paced, and C=Cathodal paced, and D=Biphasic paced. The size bars indicate 20 µm of length. The arrows indicate mitochondria with increased bright staining and roundness indicating increased mitochondrial membrane potential in the case of anodal and biphasic (anodal/cathodal) stimulation (Fig. 4).
Fig. 4.
Mitochondrial membrane potential measured with fluorescent dye after 24 h of treatment in panel A- unpaced, panel B- anodal, panel C- cathodal, and panel D- biphasic. White arrows in the anodal and biphasic paced cells show increased mitochondrial membrane potential. The white horizontal size bars indicate 20 µm of length.
4. Discussion
There is increasing evidence that there are histologic changes in the myocardium and impaired contractility [2], and unfavorable morbidity and mortality outcomes in patients paced with the standard monophasic cathodal waveform [3], [4]. Previous work in the literature has shown improved acute hemodynamics in a number of species paced with anodal and biphasic (anodal/cathodal) stimulation [5], [6], [7], [8]. In Sheep with induced myocardial infarctions paced for three months with a biphasic (anodal/cathodal) waveform, statistically significant reduction of left ventricular volumes and increased percent fractional shortening has been noted [9]. In addition, deliberate acute pacing of the heart with an anodal waveform in Humans has shown improved hemodynamics [10].
Clearly we show that ATP levels are increased by electrical stimulation, and in fact may be drastically underestimated due to influences of the creatine phosphokinase buffering system. As we are dealing both with glycolysis and the Kreb's cycle, the absolute amounts measured can vary both by its buffering when being synthesized and rate-limiting when needed. The amounts of reaction products produced can vary by the number of viable cells or by the amount of NADH/NADPH present in those cells.
Our findings suggest possible underlying mechanisms which may play a role in producing these observations. We conclude that anodal pacing, or a waveform including an anodal element, clearly alters mitochondrial morphology and function, however it is not clear whether this relates to dysfunction or might actually produce beneficial effects in the healing of areas of tissue damage. Specific pacing waveforms increase transmembrane potential and these effects last several hours after stopping pacing, cause migration of cells towards the anodal polarity, and appear not to cause cellular damage.
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2016.09.004.
Appendix A. Transparency document
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Hille B. Sinauer Associates; Massachusetts: 2001. Ion channels of excitable Membranes Introductory Chapter; pp. 1–22. [Google Scholar]
- 2.K.G. Yamazaki F.J. Villarreal Ventricular pacing-induced loss of contractile function and development of epicardial inflammation Am J. Physiol. Heart Circ. Physiol. 2011 H1282 H1290. [DOI] [PMC free article] [PubMed]
- 3.Smit M.D., Van Dessel P.F.H.M., Nieuwland W., Weisfeld A.C.P., Tan E.S., Anthonio R.L., Van Erven L., Van Veldhuisen D.J., Van Gelder I.C. Right ventricular pacing and risk of heart failure in implantable defibrillator patients. Heart Rhythm. 2006;3:1397–1403. doi: 10.1016/j.hrthm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A.D., Rizo-Patron C., Hallstrom A.P., O’Neill G.P., Royhbart S., Martins J.B., Roelke M., Steinberg J.S., Greene H.L., the DAVID Investigators Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm. 2005;2:830–834. doi: 10.1016/j.hrthm.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Hummel J.D., Davis J.H., Mower M.M. Augmentation of cardiac output by anodal pacing. Circulation. 1994;90(4, part2) :I-69. [Google Scholar]
- 6.Thakor N.V., Ranjan R., Rajasekhar S., Mower M.M. Effect of varying pacing shapes on propagation and hemodynamics in the rabbit heart. Am. J. Cardiol. 1997;79(6A):36–43. doi: 10.1016/s0002-9149(97)00120-3. [DOI] [PubMed] [Google Scholar]
- 7.Tondato F., Rougee L., Ostrander G.K., Ungs M., Mower M.M. Effects of biphasic pacing in large animal species. J. Investig. Med. 2006;54(1):S302. [Google Scholar]
- 8.Thakral A., Stein L., Shenai M., Grammatikov B., Thakor N., Mower M. Effects of varying the pacing waveform on mechanical performance in the rabbit heart. Pace. 1999;22:750. [Google Scholar]
- 9.Mower M.M., Hepp D., Hall R. Comparison of chronic biphasic pacing versus cathodal pacing of the right ventricle on left ventricular function in sheep after myocardial infarction. Ann. Noninvasive Electrocardiol. 2011;16(2):111–116. doi: 10.1111/j.1542-474X.2011.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd M.S., Heeke S., Lerakis S., Langberg J.J. Reverse polarity pacing: the hemodynamic benefit of anodal currents at lead tips for cardiac resynchronization therapy. Cardiovasc. Electrophysiol. 2007;18(11):1167–1171. doi: 10.1111/j.1540-8167.2007.00943.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material