Abstract
N-arachidonoyl-l-serine (ARA-S) is an endogenous lipid, chemically related to the endocannabinoid, N-arachidonoyl ethanolamine (i.e., anandamide) and with similar physiologic and pathophysiologic functions. Reports indicate that ARA-S possesses vasoactive and neuroprotective properties resembling those of cannabinoids. However, in contrast to cannabinoids, ARA-S binds weakly to its known classical receptors, CB1 and CB2, and is therefore considered to be a ‘cannabinoid-like’ substance. The originally described ARA-S induced-endothelial-dependent vasorelaxation was not abrogated by CB1, CB2 receptor antagonists or TRPV1 competitive inhibitor. The present report demonstrates that ARA-S enhances the fluorescence staining of both cannabinoid receptors (CB1 and CB2) in human brain endothelial cells (HBEC). This reaction is specific since it was reduced by respective selective receptor antagonist (SR141716A and SR141728A). ARA-S alone or in the presence of ET-1 was shown to alter the cytoskeleton (actin). Both ARA-S stimulated phosphorylation of various kinases (MAPK, Akt, JNK and c-JUN) and alteration of cytoskeleton are mediated via CB1, CB2 and TRPV1 receptors. The findings also showed the involvement of Rho/Rock and PI3/Akt/NO pathways in the ARA-S-induced phosphorylation of kinases and actin reorganization in HBEC. All of the above mentioned ARA-S-induced effects were reduced by the treatment with LY294002 (inhibitor of PI3/Akt kinase), except MAPK kinase. In addition, MAPK, JNK, c-JUN phosphorylation were inhibited by H1152 (inhibitor of Rho/ROCK kinase), except Akt kinase. Furthermore, PI3/Akt pathway was inhibited by pretreatment with l-NAME (inhibitor of NOS). The findings suggest that ARA-S is a modulator of Rho kinase and may play a critical role in the regulation of its activity and subsequent effects on the cytoskeleton and its role in supporting essential cell functions like vasodilation, proliferation and movement.
Abbreviations: 2-AG, 2-Arachidonoylglycerol; ARA-S, N-arachidonoyl-l-serine; CB1 receptor, cannabinoid receptor 1; CB2 receptor, cannabinoid receptor 2; e-NOS, endothelial nitric oxide synthetase; Erk1/2, extracellular signal-regulated kinases 1and 2; ET-1, Endothelin 1; GPR55, G protein-coupled receptor 55; HBEC, Human brain endothelial cells; JNK, c-JUN N-terminal kinase; L-NAME, L-NG-Nitroarginine methyl ester; MAPK, Mitogen-activated protein kinases; NO, nitric oxide; PI3, Phosphatidylinositol-4,5-bisphosphate 3-kinase; ROCK, Rho-associated protein kinase; TPRV1, transient receptor potential vanilloid receptor 1
Keywords: Cannabinoid-like agent, N-arachidonoyl-L-serine, Signal transduction pathway, Cytoskeleton, Endothelin-1, Human brain endothelial cells
Highlights
-
•
The HBEC responses induced by ARA-S are mediated by CB1, CB2, and TRPV1 receptors.
-
•
ARA-S stimulated phosphorylation of various kinases (MAPK, Akt, JNK, and c-JUN).
-
•
ARA-S modulates Rho/ROCK and PI3/Akt/NO pathways and plays a role in regulating HBEC.
-
•
ARA-S induced phosphorylation of kinases coincides with cytoskeleton reorganization.
1. Introduction
The cerebromicrovascular endothelium derived from human brain (HBEC) plays a major role in the function of the blood brain barrier and contributes to vascular tone and blood flow. These cells are known to possess functional machinery to respond to endogenous and exogenous vasoactive substances as well as other factors [1]. HBEC also express CB1 and CB2 receptors that respond to endocannabinoids (e.g., 2-AG, anandamide) which induce Ca2+ influx and cytoskeleton (i.e., actin and vimentin) reorganization alone as well as in the presence of ET-1, a known potent vasoconstrictor [2], [3].
N-arachidonoyl-L-serine (ARA-S) is one of many endogenous lipids found in the brain. This agent is chemically related to the endocannabinoid N-arachidonoyl ethanolamide and was shown to have similar properties (i.e., vasoactive [4], pro-angiogenic [5], pro-neurogenic [6], and neuroprotective) and a similar physiologic role as those described for endocannabinoids [4]. However, the originally described ARA-S induced endothelial-dependent vasodilation observed in vivo in rat abdominal and mesenteric vessels was not abrogated by CB1, CB2 or TRPV1- antagonists [4]. Thus, ARA-S has been considered a ‘cannabinoid-like’ substance since, in contrast to the other cannabinoids, it binds weakly to the known classical receptors, namely CB1 and CB2 [4]. These studies also demonstrated that ARA-S stimulated phosphorylation of 44/42 MAPK kinase and Akt protein kinase. The mechanism by which ARA-S affects endothelial responses (i.e., angiogenesis, wound healing, inflammatory responses, etc.) is variable and involves different receptors. For example, some studies indicate that ARA-S effects are mediated by GPR55 receptors [5], [7] while other reports suggest that ARA-S mediated effects do not involve GPR55 [8]. Additional studies to clarify the precise mechanisms of ARA-S-induced responses will likely indicate differences dependent upon the model being studied.
In view of the above observations, it was of interest to evaluate if the cannabinoid-like substance, ARA-S, affected HBEC responses by examining its effects on cytoskeleton (actins) and signal transduction pathways.
2. Materials and methods
2.1. Chemicals
Arachidonoyl-L-serine (ARA-S) was obtained from Cayman Chemical Co., Ann Arbor, MI. Endothelin-1 was obtained from Sigma (Saint Louis, MO). Alexa Fluor Phalloidin 635 was purchased from Molecular Probes (Eugene, OR). N G-nitro-L-arginine methyl ester (L-NAME), an inhibitor of nitric oxide synthase (eNOS) and H1152 ((S)-(+)-2-Methyl-1-[(4-methyl-5-isoquinolinyl)sulfonyl]-hexahydro-1H-1,4-diazepine dihydrochloride), an inhibitor of Rho/ROCK kinase, were obtained from Calbiochem (La Jolla, CA). SR141716A, a selective CB1 receptor antagonist, and SR141728A, a selective CB2 receptor antagonist, were provided by the Research Triangle Institute, Research Triangle Park, NC. Capsazepine, a transient receptor potential vanilloid receptor (TRPV-1) antagonist, was obtained from Enzo Life Sciences International, Inc., Plymouth, PA. LY 2940002 (2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride), an inhibitor of PI3/Akt kinase, was obtained from EMD Chemical, Inc., Gibbstown, NJ.
2.2. Cell culture and treatment
The technique used for preparing HBEC has been previously described [9]. Briefly, the cultured microvascular endothelium were dissociated from microvessels of human brain surgically removed for the treatment of idiopathic epilepsy. For this study, HBEC (from two cell lines [each derived separately from gray matter and white matter from different brains], passages 7-15) were grown to confluence on 1% gelatin-coated 75 cm2 flasks at 37° C in humidified atmosphere of 5% CO2/air. The purity of the endothelial cell cultures was >98%, as determined by positive immunostaining for von Willebrand (factor VIII)-related antigen and incorporation of acetylated low density lipoprotein, as well as negative staining for glial cell (GFAP, galactocerebroside, and ED-2), muscle cell (α-actin), and pericyte (tropomyosin) markers.
L-NAME and H1152 were dissolved in H2O. ARA-S, SR141716A, SR141728A, LY294002, and capsazepine were prepared in 50% ethanol. The final ethanol concentration in HBEC culture was a negligible 0.0005% and no ethanol vehicle was added to the control. Cultured HBEC were incubated with different signal transduction pathway inhibitors or receptor antagonists alone or prior to stimulation with ARA-S. Specifically, cells were incubated with: 1) 20 μM LY2940022, for 15 min; 2) 10 μM H1152, for 15 min; 3) 100 μM L-NAME (eNOS), for 40 min; or 4) 10 μM capsazepine, for 30 min. HBEC cultures were incubated with SR141716A or SR141728A for 30 min as previously described [2]. Dose-dependent studies indicated that 1 μM of SR141716A or SR141728A produced the maximum inhibitory effect on Ca2+ uptake and cytoskeleton rearrangements induced by endocannabinoids [2]. The cells were subsequently exposed to 50 μM ARA-S for 15 min followed by ET-1 (20 nM) for 1 min; the ARA-S concentration was determined by dose-dependent pilot studies evaluating the effect on Ca2+ uptake (to be similar to that of the cannabinoid effect).
2.3. Western blot analysis
All antibodies used for determination of protein levels were obtained from Cell Signaling Technologies (Danvers, MA). HBEC grown on 60 mm petri dishes were washed with HBSS and the feeding medium was withdrawn and replaced with serum-free medium (M199) overnight. After treatment with test agents, the cells were lysed in a solution containing 150 mM NaCl, 50 mM Tris–HCl, 0.25% sodium deoxycholate 1 mM EGTA, 1 mM NaF, 1 mM Na3VO4, and a cocktail of proteinase inhibitors (Calbiochem, La Jolla, CA). Protein assays and Western blot analyses were determined as described previously [3]. Briefly, proteins were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were blocked in 5% nonfat milk in PBS at room temperature for 1 h and incubated with antibodies against phosphorylated Akt (ser473), phosphorylated MAPK (Erk1/2), phosphorylated c-JUN, or phosphorylated JNK at 4 °C overnight. The next day, the membranes were washed 3 times with a 0.1% Tween 20 in PBS washing buffer and incubated with HRP-conjugated secondary antibody for 45 min at room temperature. The membranes were washed 3 times in washing buffer then developed in chemiluminescence reagent (SuperSignal West Femto, Thermo Scientific, Rockford, IL) and imaged in a Fuji Image analyzer (LAS-3000, Fujifilm Medical Systems U.S.A., Inc. Stamford, CT) to detect the HRP-antibodies complex. Band density was measured using the software MultiGage (version 2). To detect total (phosphorylated and non-phosphorylated) proteins, the membranes were incubated in a stripping buffer (Thermo Scientific) for 15 min to remove the immune complex and re-probed with total Akt, MAPK, c-JUN, and JNK antibodies.
2.4. Immunofluorescence staining and confocal microscopy
HBEC were grown on gelatin-coated chambered culture slides (Thermo Scientific) for 48 h before treatment with test agents. For staining of CB1 and CB2 receptors, cells were washed 3 times with PBS and then fixed in 4% paraformaldehyde for 10 min and permeabilized with 0.2% TritonX-100 in PBS for 15 min. After blocking with 1% bovine serum albumin (BSA) in PBS for 1 h, CB1 and CB2 expression was probed by incubating the samples in goat anti-CB1 (sc-10066, Santa Cruz Biotechnology Inc.; Dallas, TX) or rabbit anti-CB2 (sc-10073, Santa Cruz) for 1 h at 1:200 ratio in BSA. After 3 washes with PBS, the cells were treated with Alexa Fluor 488-conjugated anti-goat or anti-rabbit antibody to detect CB1 and CB2 expression, respectively.
To examine cytoskeletal organization, cells grown in monolayers were washed with PBS, fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.1% TritonX-100 in PBS for 5 min, and then incubated in BSA for 20 min. To detect F-actin, cells were incubated in Alexa Fluor-635 phalloidin for 20 min. All stained cells were washed in PBS prior to mounting with anti-fade mounting medium (Vectashield, Vector Laboratories; Burlingame, CA) containing 4′,6-diamidino-2-phenylindole (DAPI) to detect nuclear DNA.
Immunofluorescent staining was detected with a confocal microscope (Zeiss, LSM 510; Oberkochen, Germany). The cells were imaged using standard filter sets and laser lines to acquire double labeled images. DAPI, CB1/CB2 or actin fluorescence were excited with 405 nm, 488 nm, and 633 nm laser lines, respectively. The images were captured using LSM software. Z-stacks were acquired from the base of the cells (at cover slip) to beyond the top of the cells to encompass all the cells in the region of interest (ROI).
Fluorescence intensity of actin fibers was estimated using the software Image J (National Institutes of Health). ROI of equal size were defined within the cytoplasmic region of phalloidin-stained non-overlapping cells. Fluorescence intensity was estimated using an average of 33 ROIs per treatment group.
2.5. Statistical analysis
Statistical analysis of fluorescence and phosphorylation data was performed using Prism 5 (GraphPad Software Inc., San Diego, CA), and are presented as the mean±S.E.M. Statistical significance was determined by one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparisons test (P<0.05).
3. Results
3.1. General observation
Overall, all the tested responses of HBEC to ARA-S showed some variability. The degree of stimulation was independent of age, passage and whether the HBEC were derived from gray or white matter. However, some changes in level of stimulation were found to correlate with the length of storage of ARA-S preparations (i.e., fresh ARA-S was more potent than older agent).
3.2. Effects of ARA-S on the CB1 or CB2 cannabinoid receptors
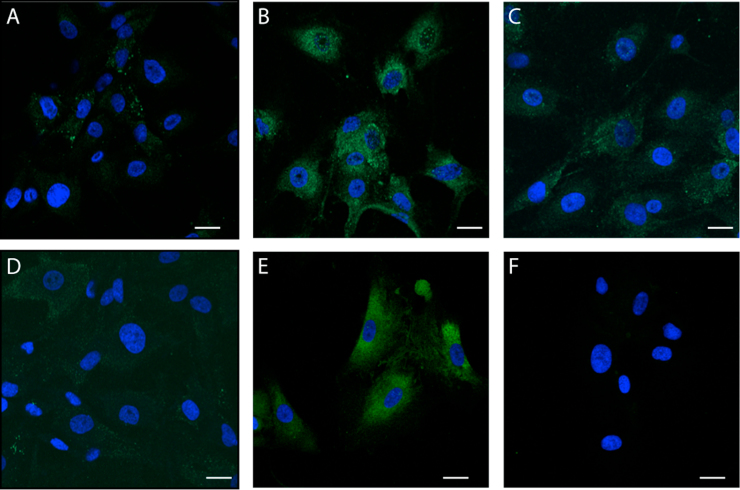
The fluorescence staining of both CB1 and CB2 receptors in the cytoplasm and nuclei of HBEC were increased by ARA-S treatment (see Fig. 1). The expression of these receptors was manifested by granular fluorescence uniformly dispersed in the cytoplasm but more condensed in the perinuclear areas. In contrast, the nuclear expression of the fluorescence was punctuated; the granules being more separated and distinct from each other as compared to their arrangement in the cytoplasm (Fig. 1B, E). The relative level of fluorescence induced by ARA-S was reduced or abolished by treatment with CB1 and CB2 selective antagonists (Fig. 1C, F).
Fig. 1.
Effect of ARA-S on CB1 (A-C) and CB2 (D-F) receptor expression. Cultured HBEC were untreated (A, D) or exposed to 50 μM ARA-S for 15 min alone (B, E) or pre-treated with selective antagonists for CB-1 [1 μM SR141716A (SR16)] (C) or CB-2 [1 μM SR141728A (SR28)] (F) receptors. Confocal analysis of immunofluorescent intensity of HBEC labeled with Alexa-488-conjugated antibodies against CB1 (B, C) and CB2 (E, F) receptors. ARA-S stimulated CB1 (B) and CB2 (E) receptors manifested by granular fluorescence uniformly dispersed in the cytoplasm but more condensed in the perinuclear areas. Scale bar=10 µm.
3.3. ARA-S activated various components of signal transduction pathways
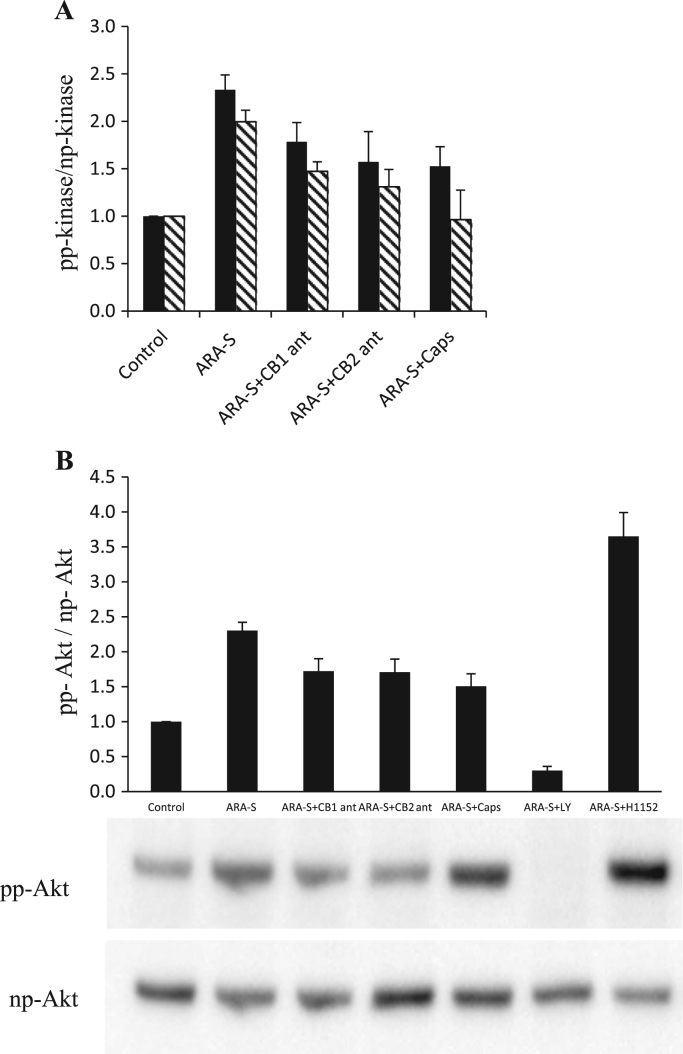
After exposure of HBEC to ARA-S, the MAPK phosphorylation was increased 2–2.5 fold above the control (Fig. 2A and Table 1A) and the stimulation of c-JUN was 1.5 fold above the control after exposure of HBEC to ARA-S (Table 1A). The Akt phosphorylation was also increased 2–2.5 fold above the control (Fig. 2B and Table 1A). All the above findings regarding kinase phosphorylation were reduced by pretreatment with CB1-, CB2- receptor antagonists and competitive inhibitor of TRPV-1; no significant differences were seen between CB1, CB2 and TRPV-1 inhibitory effects) (Fig. 2 and Table 1A). However, c-JUN was least affected by CB1-antagonist (Table 1A). Nevertheless, all the studied kinases, except for MAPK, were almost completely blocked by LY294002, an inhibitor of PI3/Akt kinase (Table 1B).
Fig. 2.
(A) Effect of inhibitors on ARA-S -stimulated JNK and MAPK. Bars (means±SE of pp/np ratio) indicate semi-quantitative analysis of effect of inhibitors on Western blot analysis of ARA-S -stimulated JNK (hatched) and MAPK (solid black). HBEC were untreated or pre-treated with ARA-S+indicated receptor antagonists as described in the Materials and Methods section. (B) Effect of inhibitors on ARA-S-stimulated Akt phosphorylation. Bars (means±SE of pp/np ratio) indicate quantitative analysis of effect of inhibitors on Western blot analysis of effect of inhibitors on ARA-S-stimulated Akt phosphorylation. Shown are representative Western blots of at least 7 independent experiments.
Table 1.
Characterization of the effects of (A) the antagonists of the CB1, CB2 and vanilloid receptors and (B) inhibitor of Rho kinase and PI3 on phosphorylation of protein kinases in ARA-S-stimulated HBEC.
| (A) | |||||
|---|---|---|---|---|---|
| Control | ARA-S | ARA-S+CB1antagonist | ARA-S+CB2 antagonist | ARA-S+vanilloid antagonist | |
| Akt | 1 | 2.30±0.12 | 1.72±0.18 | 1.71±0.19 | 1.51±0.18 |
| MAPK | 1 | 2.33±0.16 | 1.78±0.20 | 1.57±0.32 | 1.53±0.21 |
| c-JUN | 1 | 1.49±0.07 | 1.22±0.12 | 0.97±0.09 | 0.72±0.18 |
| JNK | 1 | 2.00±0.12 | 1.47±0.10 | 1.31±0.18 | 0.96±0.31 |
| (B) | |||||
|---|---|---|---|---|---|
| Control | ARA-S | ARA-S+LY294002 | ARA-S+H1152 | ARA-S+LY294002+ H1152 | |
| Akt | 1 | 2.32±0.15 | 0.30±1.00 | 3.73±0.35 | 0.57±0.36 |
| MAPK | 1 | 2.28±0.19 | 1.73±0.19 | 1.10±0.10 | 0.26±0.05 |
| c-JUN | 1 | 1.58±0.10 | 0.69±0.14 | 1.19±0.10 | 1.25±0.02 |
| JNK | 1 | 2.02±0.17 | 1.10±0.10 | 0.95±0.13 | 0.57±0.13 |
Data indicates density analysis of Western blots. Values (ratio relative to control value) were analyzed using ANOVA followed by Bonferroni pairwise comparisons. (A) Treatment with the CB1, CB2 or vanilloid receptor antagonists causes a statistically significant reduction in phosphorylation of all four kinases relative to the ARA-S- stimulated value (p<0.05). (B) ARA-S phosphorylated value vs. ARA-S with LY294002, H1152, or LY294002+H1152 were statistically different for Akt, MAPK, c-JUN, and JNK (p<0.05 in all cases). LY294002 and H1152 inhibit ARA-S-stimulated phosphorylation of MAPK, c-JUN and JNK. LY294002 also inhibits the phosphorylation of Akt, while H1152 enhances the ARA-S-induced phosphorylation of Akt.
Experiments evaluating the effects of H1152, the inhibitor of Rho/ROCK pathway, demonstrated that it abrogated the stimulatory effect on ARA-S-induced phosphorylation by MAPK, JNK and c-JUN (Table 1B). Interestingly, in contrast to these observations, H1152 treatment actually increased the level of ARA-S-induced phosphorylation of Akt (>3-fold increase; Fig. 2B and Table 1B). This effect of ARA-S was also abrogated by LY294002 (Table 1A). In addition, the ARA-S stimulated phosphorylation of Akt was reduced (50%) with L-NAME (not shown). None of the antagonists or inhibitors exhibited any effect on kinase phosphorylation in the absence of ARA-S.
3.4. Effect of ARA-S and ET-1 on cytoskeleton (actin)
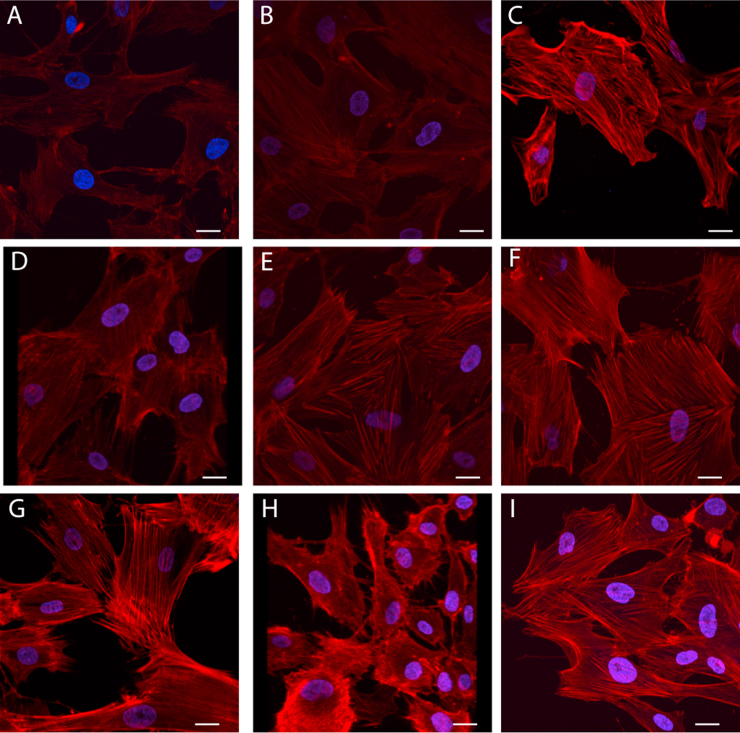
The treatment of HBEC with ARA-S led to morphological changes in the cytoskeleton with filaments and bundles becoming less distinct as compared to those in the controls (Fig. 3A and B); the semi-quantitative fluorescence intensity was not significantly different than controls (Table 2). Studies regarding cytoskeletal actin expression showed that treatment with ET-1 resulted in markedly thicker actin filaments and bundles with a considerably higher level of fluorescence as compared to normal controls (Fig. 3A and C). This ET-1 induced-actin rearrangement was altered by pretreatment with ARA-S; it led to rarefaction and decreased thickness as well as reduced fluorescence intensity (Fig. 3D and Table 2).
Fig. 3.
Effect of ARA-S and ET-1 on Cytoskeleton (Actin). Cultured HBEC were untreated (A) or exposed to 50 μM ARA-S alone (B), 20 nM ET-1 alone (C), or both ARAS and ET-1 (D). HBEC treated with both ARA-S and ET-1 were also pre-treated with selective antagonists for CB-1 (E) or CB-2 (F) receptors or LY, inhibitor of Akt kinase (G) H1152, inhibitor of rho kinase (H) or L-NAME, nitric oxide inhibitor (I). Scale bar=10 µm.
Table 2.
Semi-quantitative analysis of fluorescence intensity of actin filaments labeled with Alexa-635 phalloidin.
| Treatment | Absolute fluorescence intensity (arbitrary unit) | Percent change in intensity from ARA-S (%) | P-value for comparison relative to ARA-S | Percent change in intensity from ET-1 (%) | P-value for comparison relative to ET-1 |
|---|---|---|---|---|---|
| Control | 15.34 | 7.4 | NS | −39.5 | P<0.0001 |
| ARA-S | 14.29 | 0.0 | – | −43.6 | P<0.0001 |
| ET-1 | 25.34 | 77.3 | P<0.0001 | 0.0 | – |
| ARAS+ET-1 | 17.28 | 20.9 | P<0.001 | −31.8 | P<0.0001 |
| ARA-S+ET-1+CB1 antagonist | 16.27 | 13.9 | NS | −35.8 | P<0.0001 |
| ARA-S+ET-1+CB2 antagonist | 18.43 | 29.0 | P<0.0001 | −27.3 | P<0.0001 |
| ARA-S+ET+LY294002 | 18.86 | 32.0 | P<0.0001 | −25.6 | P<0.0001 |
| ARA-S+ET-1+ H1152 | 22.14 | 54.9 | P<0.0001 | −12.6 | NS |
| ARA-S+ET+L-NAME | 19.95 | 39.6 | P<0.0001 | −21.3 | P<0.0001 |
Fluorescence intensities were compared to the ARA-S or ET-1 values using an ANOVA followed by a Bonferroni test for multiple comparisons.
These effects of ARA-S on ET-1 treated cells were altered by the pretreatment with either CB1- or CB2- receptor antagonists (Fig. 3E and F, respectively). The reduction of ET-1- induced increase in fluorescence by ARA-S was not prevented by pretreatment with CB1-receptor antagonist. However, the structural appearance of the cytoskeleton was similar to control. Treatment with CB2-receptor antagonist had a similar effect on the structural appearance of the cytoskeleton but also reversed the inhibitory effect of ARA-S on ET-1-induced fluorescence. Pretreatment with LY294002 prevented the ARA-S effects on ET-1-induced cytoskeletal effects (Fig. 3G). However, pretreatment with H1152 not only increased fluorescence but also completely disrupted the appearance of the cytoskeleton observed in cells either untreated or treated with ET-1 alone or ET-1 with ARA-S (Fig. 3H). The pretreatment of the HBEC with L-NAME has a similar effect on the cytoskeleton as that observed with LY294002 (thickened actin filaments) and reversed the ARA-S-induced inhibitory effect on ET-1-stimulated cytoskeletal rearrangements (Fig. 3I).
4. Discussion
The results of this study demonstrated the involvement of ARA-S in the protein phosphorylation of a number of signal transduction pathways (e.g. MAPK, Akt, JNK, and c-JUN) that are often associated with stress, most of these factors are part of the signal transduction pathway involved in cytoskeleton actin assembly. It is noteworthy that the effects of ARA-S alone, or in combination with ET-1, were prevented by treatment with CB1, CB2 receptor antagonists, TRPV-1 competitive inhibitor or L-NAME (an inhibitor of eNOS). The reduction of ARA-S induced-increase of cannabinoid receptors (fluorescence) by specific antagonists suggests the CB1 and CB2 mediation of ARA-S-induced effects. The implicated involvement of cannabinoid receptors in these events was further explored and the precise location of these receptors, whether present on the surface or internalized inside of the membranes, was not determined in the present study. However, the internalization of the receptors does not necessarily rule out the possibility of involvement in the activation of the signal transduction pathways since this has been previously reported [10].
These findings are in contrast to the reported weak binding of ARA-S to classical cannabinoid (CB1 and CB2) receptors and the described ineffectiveness of the cannabinoid receptor antagonists to affect ARA-S-induced vascular relaxation and angiogenesis [4], [5]. Nonetheless, neuroprotection induced by ARA-S was shown to be mediated by CB2 receptors [8], [11] and pro-neurogenic activation by ARA-S was found to be mediated by both CB1 and CB2 receptors [6]. Such observations could be due to different vascular and/or cellular derivations, species, and cell types. The discrepancy between functional effects and physical binding associated with a given receptor (i.e., CB2) may depend upon the structural orientation of a ligand to the receptor binding site [11]. Although the unstimulated presence of CB1 and CB2 receptors in the cytoplasm and nuclei of cerebromicrovascular endothelial cells were described previously [3], the present report is the first demonstration of the effect of ARA-S on endothelial cytoplasm and nuclear receptors suggesting that not only cytoplasmic but also nuclear cannabinoid reactivity in HBEC.
The observed responsiveness of nuclear receptors to a lipid agent such as ARA-S is in agreement with the findings regarding effects of other lipid-like substances and growth factors on nuclear receptors [12]. The specific role of lipid activated nuclear receptors has been extensively studied but is still not fully understood [13]. They are characterized as transcriptional factors activated by soluble lipid therefore acting as membrane-permeable ligands regulating the expression of target genes involved in diverse physiological and pathological responses. Many studies suggest that fatty acids and their derivatives (eicosanoids, phospholipids, sphingolipids), including some cannabinoid-like molecules, bind and activate nuclear receptors [12], [14]. Thus, it seems likely that ARA-S acts as a cannabinoid-like lipid substance that may bind and activate these receptors. Future studies may clarify additional aspects of their processes namely the precise location (i.e., outer or inner surface of membranes) and activation of specific signal transduction pathways as well as metabolic pathways [14].
The reorganization of the cytoskeleton induced by ARA-S alone or in combination with ET-1-stimulation was shown to be mediated by CB1, CB2, and TRPV-1 receptors, since observed effects were prevented by their respective receptor antagonists or inhibitors (Fig. 3 and Table 2). In addition, effects on cytoskeleton also involved Rho/ROCK and PI3/Akt/NO pathways, since the observed alterations were affected by selective inhibitors of these factors. In support of these conclusions, our preliminary studies were concerned with the effects of ARA-S on Rho/ROCK kinase and PI3/Akt kinase activities. In these studies our observations indicated that ARA-S reduced the activity (30%) of Rho/ROCK kinase in control and ET-1 stimulated cells; ET-1 was evaluated because it is a known stimulator of Rho/ROCK kinase. On the other hand, the same treatment with ARA-S increased (50%) Akt kinase activity. Our studies are consistent with the findings that cannabinoid ligands (stable analogues of anandamide or CB-1 ligands) reduced Rho/ROCK kinase, stimulated PI3/Akt kinase, reorganized cytoskeleton (actin) and decreased cell migration [15], [16], [17]. These findings are also in agreement with reports that Rho kinase inhibition leads to rapid activation of PI3/Akt pathway [18].
We previously reported that vasoactive substances such as NO, 2-AG and ET-1 affected the HBEC cytoskeleton (actin and vimentin) [1], [3]. It is presently not clear whether the prevention of ET-1-induced effects on the cytoskeleton reorganization by ARA-S was directly or indirectly the consequence of GTPase inhibition. Rho-GTPase has been extensively studied and functions as a cellular cytoskeleton actin stabilizer. In its activated state, RhoA regulates cellular structure by controlling the dynamic interactions of microfilaments and microtubulin. This event occurs via binding and activating specific downstream effector proteins such as Rho associated coil/coiled kinase (i.e., ROCKI and ROCK II) [19]. Following ROCK activation this molecule becomes a regulator, especially of actin cytoskeleton remodeling, F-actin stabilization, actin-network assembly, and actin-membrane linkage [20], [21], [22].
The reduction of RhoA activity induced by ARA-S was never previously studied even though many ARA-S stimulated events or processes (i.e., vasorelaxation, angiogenesis, ischemia, and trauma) were demonstrated to be modulated by PI3/Akt pathway [4]. The ARA-S reorganization of actin fibers observed in normal and ET-1-stimulated HBEC as shown in the present paper is most likely due to its effect on RhoA activity since inhibition of RhoA/Rock activity has been known to affect the changes in cytoskeleton [19], [20].
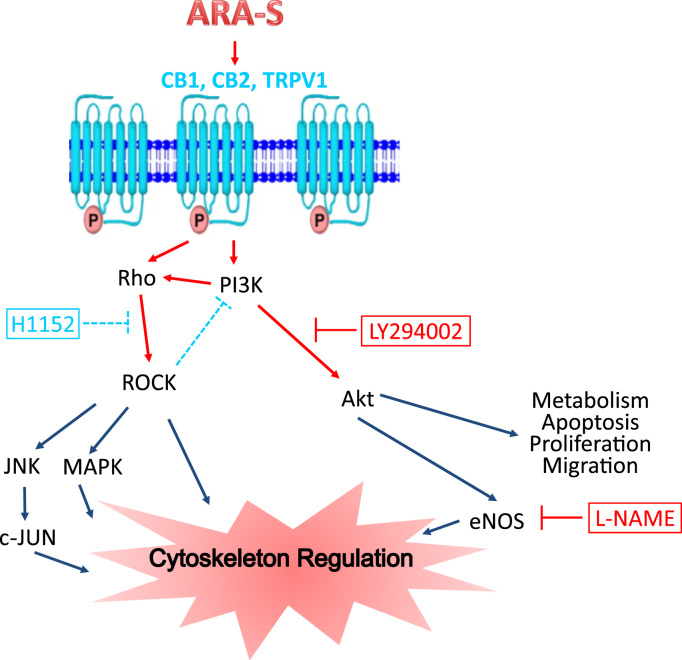
Previous reports indicate that the inhibition of Rho kinase affects the PI3/Akt signal transduction pathway which, in turn, controls many biological processes (i.e., cell migration, proliferation, cytoskeleton reorganization) [18] that are stimulated in many physiological and pathological conditions (Fig. 4). Many studies have also demonstrated that Rho/ROCK activation or reduction of its activity plays a major role in these processes [17], [23]. Indeed, our results (as shown in Fig. 3) are in agreement with reported inhibitory effects of cannabinoid ligands on the cellular cytoskeleton reorganization induced by ET-1. Furthermore, the pretreatment of the ARA-S induced reorganization of HBEC cytoskeleton with specific inhibitors indicates that this event is mediated by PI3/Akt induced NO signal transduction pathway (Fig. 4). Taken together, the findings suggest that ARA-S acts as a modulator of Rho/ROCK kinases which are functionally active in HBEC. Thus the properties of ARA-S, including potential roles in vasorelaxation, may have therapeutic value for treatment of vascular diseases.
Fig. 4.
Postulated signal transduction pathways of ARA-S modulation of cytoskeleton via CB1, CB2, TRPV1 receptors. Possible mechanisms of receptor-mediated activation involve different routes including both Rho/ROCK kinase and PI3/Akt kinase pathways resulting in cytoskeletal alterations. Inhibition of Rho/ROCK kinase by H1152 decreases ROCK and ROCK-mediated inhibition of PI3K. Inhibition of PI3/Akt kinase by LY294002 completely inhibits Akt formation and effects on cytoskeleton. Akt effects on cytoskeleton are also inhibited by treatment with L-NAME, inhibitor of e-NOS activity.
Disclaimer
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. I am an employee of the U.S. Government and this work was prepared as part of my official duties. Title 17 USC. $ 105 provides that “Copyright protection under this title is not available for any work of the United States Government.’ Title 17 USC. $101 defines a U.S. Government work as a work prepared by an employee of the U.S. Government as part of that person's official duties.
Acknowledgments
We thank Professor Raphael Mechoulam for providing N-arachidonoyl-L-serine in the pilot study, Professor Esther Shohami for helpful discussion, and Dr. Francoise Arnaud for helping in editing the manuscript. Funding received from Congressionally Directed Medical Research Programs’ Psychological Health and Traumatic Brain Injury (PH/TBI) Research Program USAMRMC 14147001, BUMED 603115HP.3730.001. A1269.
Footnotes
The results of this study were partly presented at the 2014 Society for Neuroscience Meeting.
Transparency data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.09.002.
Appendix A. Transparency document
Supplementary material
.
References
- 1.Chen Y., McCarron R.M., Bembry J., Ruetzler C., Azzam N., Lenz F.A., Spatz M. Nitric oxide modulates endothelin 1-induced Ca2+ mobilization and cytoskeletal F-actin filaments in human cerebromicrovascular endothelial cells. J. Cereb. Blood Flow. Metab. 1999;19:133–138. doi: 10.1097/00004647-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y., McCarron R.M., Ohara Y., Bembry J., Azzam N., Lenz F.A., Shohami E., Mechoulam R., Spatz M. Human brain capillary endothelium: 2-arachidonoglycerol (endocannabinoid) interacts with endothelin-1. Circ. Res. 2000;87:323–327. doi: 10.1161/01.res.87.4.323. [DOI] [PubMed] [Google Scholar]
- 3.Golech S.A., McCarron R.M., Chen Y., Bembry J., Lenz F., Mechoulam R., Shohami E., Spatz M. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res. Mol. Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Milman G., Maor Y., Abu-Lafi S., Horowitz M., Gallily R., Batkai S., Mo F.M., Offertaler L., Pacher P., Kunos G., Mechoulam R. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc. Natl. Acad. Sci. USA. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X., Maor Y., Wang J.F., Kunos G., Groopman J.E. Endocannabinoid-like N-arachidonoyl serine is a novel pro-angiogenic mediator. Br. J. Pharmacol. 2010;160:1583–1594. doi: 10.1111/j.1476-5381.2010.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen-Yeshurun A., Willner D., Trembovler V., Alexandrovich A., Mechoulam R., Shohami E., Leker R.R. N-arachidonoyl-L-serine (AraS) possesses proneurogenic properties in vitro and in vivo after traumatic brain injury. J. Cereb. Blood Flow. Metab. 2013;33:1242–1250. doi: 10.1038/jcbfm.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nevalainen T., Irving A.J. GPR55, a lysophosphatidylinositol receptor with cannabinoid sensitivity? Curr. Top. Med. Chem. 2010;10:799–813. doi: 10.2174/156802610791164229. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Yeshurun A., Trembovler V., Alexandrovich A., Ryberg E., Greasley P.J., Mechoulam R., Shohami E., Leker R.R. N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J. Cereb. Blood Flow. Metab. 2011;31:1768–1777. doi: 10.1038/jcbfm.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spatz M., Kawai N., Merkel N., Bembry J., McCarron R.M. Functional properties of cultured endothelial cells derived from large microvessels of human brain. Am. J. Physiol. 1997;272:C231–239. doi: 10.1152/ajpcell.1997.272.1.C231. [DOI] [PubMed] [Google Scholar]
- 10.Stornaiuolo M., Bruno A., Botta L., La Regina G., Cosconati S., Silvestri R., Marinelli L., Novellino E. Endogenous vs exogenous allosteric modulators in GPCRs: a dispute for shuttling CB1 among different membrane microenvironments. Sci. Rep. 2015;5:15453. doi: 10.1038/srep15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smoum R., Baraghithy S., Chourasia M., Breuer A., Mussai N., Attar-Namdar M., Kogan N.M., Raphael B., Bolognini D., Cascio M.G., Marini P., Pertwee R.G., Shurki A., Mechoulam R., Bab I. CB2 cannabinoid receptor agonist enantiomers HU-433 and HU-308: an inverse relationship between binding affinity and biological potency. Proc. Natl. Acad. Sci. USA. 2015;112:8774–8779. doi: 10.1073/pnas.1503395112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryant D.M., Stow J.L. Nuclear translocation of cell-surface receptors: lessons from fibroblast growth factor. Traffic. 2005;6:947–954. doi: 10.1111/j.1600-0854.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 13.Kiss M., Czimmerer Z., Nagy L. The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: from physiology to pathology. J. Allergy Clin. Immunol. 2013;132:264–286. doi: 10.1016/j.jaci.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 14.Burris T.P., Solt L.A., Wang Y., Crumbley C., Banerjee S., Griffett K., Lundasen T., Hughes T., Kojetin D.J. Nuclear receptors and their selective pharmacologic modulators. Pharmacol. Rev. 2013;65:710–778. doi: 10.1124/pr.112.006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laezza C., Pisanti S., Malfitano A.M., Bifulco M. The anandamide analog, Met-F-AEA, controls human breast cancer cell migration via the RHOA/RHO kinase signaling pathway. Endocr. Relat. Cancer. 2008;15:965–974. doi: 10.1677/ERC-08-0030. [DOI] [PubMed] [Google Scholar]
- 16.Nithipatikom K., Gomez-Granados A.D., Tang A.T., Pfeiffer A.W., Williams C.L., Campbell W.B. Cannabinoid receptor type 1 (CB1) activation inhibits small GTPase RhoA activity and regulates motility of prostate carcinoma cells. Endocrinology. 2012;153:29–41. doi: 10.1210/en.2011-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z., Ren J.H., Li Z.Y., Nong L., Wu G. Fasudil inhibits lung carcinoma-conditioned endothelial cell viability and migration. Oncol. Rep. 2012;27:1561–1566. doi: 10.3892/or.2012.1686. [DOI] [PubMed] [Google Scholar]
- 18.Wolfrum S., Dendorfer A., Rikitake Y., Stalker T.J., Gong Y., Scalia R., Dominiak P., Liao J.K. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler. Thromb. Vasc. Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amano M., Nakayama M., Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amin E., Dubey B.N., Zhang S.C., Gremer L., Dvorsky R., Moll J.M., Taha M.S., Nagel-Steger L., Piekorz R.P., Somlyo A.V., Ahmadian M.R. Rho-kinase: regulation, (dys)function, and inhibition. Biol. Chem. 2013;394:1399–1410. doi: 10.1515/hsz-2013-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Zheng X.R., Riddick N., Bryden M., Baur W., Zhang X., Surks H.K. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ. Res. 2009;104:531–540. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loirand G., Guerin P., Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ. Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 23.Chen M., Liu A., Ouyang Y., Huang Y., Chao X., Pi R. Fasudil and its analogs: a new powerful weapon in the long war against central nervous system disorders? Expert Opin. Investig. Drugs. 2013;22:537–550. doi: 10.1517/13543784.2013.778242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material