Abstract
Background
Post-traumatic stress disorder (PTSD) is a serious psychological condition, which can develop both from physically experiencing and also from witnessing traumatic events. There is evidence that physical exercise can have a positive impact on the symptoms of PTSD. Relevant to this, in our previous pre-clinical work, beneficial effects of treadmill exercise were reported on PTSD-like behaviors in a social defeat paradigm, a rat model of direct physical trauma. However, the role of exercise on vicariously acquired PTSD-like phenotype was not examined.
Objective
In this study, we utilized a rodent PTSD model, which mimics both the physical as well as the witness experience of trauma, and examined the impact of moderate treadmill exercise in mitigating vicariously acquired PTSD-like behaviors in rats.
Methods
Our PTSD model is a modified social defeat paradigm, which involves aggressive encounters between a large Long-Evans male rat (resident) and a smaller Sprague-Dawley male rat (intruder), resulting in intruder social defeat. The cage mate of the intruder is positioned to witness intruder defeat. Rats were grouped as control (CON), social defeat (SD), exercise (EX), trauma witness (TW), and exercise prior to trauma witness (EX-TW). After acclimatization for 7 days, the exercised groups were subjected to a daily 30-min treadmill exercise regimen for 14 days. On day 21, the SD group was exposed for 7 days of social defeat, while the TW groups witnessed social defeat. On days 28–34, behavioral and cognitive tests including short-term (STM) and long-term (LTM) memory function, anxiety- and depression-like behaviors were conducted.
Results
TW and SD rats demonstrated the highest levels of anxiety- and depression-like behaviors, while EX-TW rats did not exhibit anxiety- and depression-like behaviors. TW and SD rats showed no impairments in STM. However, TW and SD rats showed impairments in LTM, and exercise rescued LTM impairments in EX-TW rats.
Conclusions
This study demonstrates that rats subjected to direct experience or witness of social defeat exhibited PTSD-like behaviors, while moderate treadmill exercise prevented trauma witness-induced behavioral impairments. These studies have important translational value suggesting that prior treadmill exercise might provide resilience to stressful stimuli and perhaps mitigate the witnessing effects of traumatic events.
Keywords: PTSD, Stress, Exercise, Behavior, Cognition
1. Introduction
Post-traumatic stress disorder (PTSD) is a maladaptive and common psychiatric disorder developed upon exposure to traumatic events. PTSD patients exhibit a broad range of symptoms including hyperarousal, intrusive memories, anxiety, depression and poor cognition [1]. Interestingly, PTSD can be triggered not only in people who personally experience traumatic events, but also in those who witness it [2]. For example, a child who repeatedly witnesses physical and emotional abuse of a parent or sibling can develop PTSD [3]. The Diagnostic and Statistical Manual of Mental Disorders has recognized that learning of or witnessing traumatic events experienced by friends or family can contribute to PTSD [4]. PTSD remains very difficult to treat. Currently, selective serotonin reuptake inhibitors (SSRIs) are the treatment of choice [5]. However, their use is controversial due to their frequently reported side effects and poor patient compliance [6]. Other non-pharmacological interventions, including physical exercise, are attractive side-effect free approaches. Low-to-moderate intensity exercise reportedly elevates mood, reduces anxiety [7] and acts as an overall stress-buffer [8]. It is also known to have a positive impact on the symptoms of depression and PTSD [7].
Relevant to this, in our previous pre-clinical work, the beneficial effect of moderate physical exercise was reported on PTSD-like behaviors using two separate stress paradigms, one was a social defeat paradigm [9], and the other was a single prolonged stress (SPS) model [10]. Both rat models were based on direct physical trauma. However, the role of physical exercise on vicariously acquired PTSD-like phenotype was not examined. Although animal models cannot accurately reveal the impact of traumatic events on psychiatric symptoms occurring in later life, rodent models are excellent tools that can provide useful insights. In this study, we utilized a rodent PTSD model which mimics both the physical as well as the witness experience of trauma and examined the impact of moderate treadmill exercise on mitigating vicariously acquired PTSD-like behaviors in rats. Rodent treadmill exercise apparatus is a well-suited method for subjecting rats to physical exercise [11]. Our studies presented herein offer important insights into the role of physical exercise in promoting resilience to trauma witness. This is particularly important and of high translational relevance for designing PTSD-related management and treatment strategies.
2. Methods
2.1 Animal Model
Upon arrival at the animal facility, rats were housed on a 12-hr light/dark cycle in a climate-controlled room with food and water provided ad libitum. Experiments with rats were conducted in accordance with the NIH guidelines using protocols approved by the University of Houston Animal Care and Use Committee. Male Sprague-Dawley rats (225–250 g) were used as controls or intruders, and male Long-Evans (LE) retired breeders (400–500 g) served as resident aggressors (Charles River, Wilmington, MA).
The social defeat model [12], involving aggressive encounters between a large LE male rat (resident) and a smaller Sprague-Dawley male rat (intruder) is a well-recognized rodent model of PTSD [1, 13–16]. In this study, a modified version of the resident-intruder model was used [17]. Three Sprague-Dawley rats were housed together during the acclimatization period for 7 days. Later, one Sprague-Dawley rat, considered as an intruder and designated as socially defeated (SD), was introduced into the cage of the resident LE rat. The cage mates of the SD rat were placed in the Plexiglas enclosure surrounding the cage where both LE and SD rats were present (Fig. 1A). The cage mates were considered as the trauma witnessing (TW) rats. Introduction of the SD intruder rat into the cage of the resident LE rat results in social defeat of the intruder rat, indicated by the intruder surrendering, when attacked by the resident LE rat. After defeat, a perforated Plexiglas partition was placed in between the LE and SD rats to avoid injury to the intruder. This partition allowed visual, auditory, and olfactory interactions for the remainder of the 30-min session (Fig. 1A). The TW rat present outside the cage in the enclosure witnesses the social defeat of the SD rat. Two more bouts of social defeat were performed with 5-min separation, in order to reinforce the visual stress in the TW rat. After social defeat sessions, the SD and TW rats were housed together until the next day of social defeat protocol.
Fig 1.
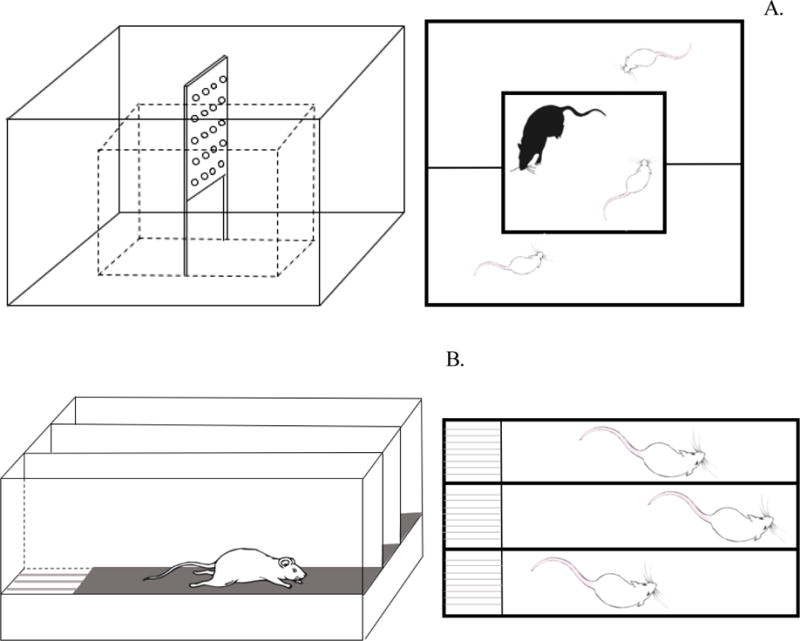
A schematic representation of the trauma witness apparatus comprising of a central enclosure where social defeat events occur and a surrounding area from the social defeat events are witnessed (A), treadmill exercise equipment comprising of three lanes of motorized running belts (B) and the experimental design depicting the experimental protocol (C). Group designations are as follows; Sprague-Dawley rat placed inside a novel cage and not subjected to social defeat (CON: control); Sprague-Dawley rat subjected to treadmill exercise (EX: exercise); Sprague-Dawley rat subjected to social defeat (SD: social defeat); Sprague-Dawley rat subjected to witness of social defeat (TW: trauma witness); Sprague-Dawley rat subjected to treadmill exercise prior to witness of social defeat (EX-TW: exercise-trauma witness).
2.2 Moderate Treadmill Exercise
Rats (n=70) were randomly selected into five groups (14 rats/group): control (CON), social defeat (SD), exercise (EX), trauma witness (TW), and exercise prior to trauma witness (EX-TW). The rats in the two exercise groups were subjected to treadmill exercise on a motorized rodent treadmill purchased from Columbus Instruments, Columbus, OH. The apparatus consisted of a 3-lane animal exerciser utilizing single belt construction with dividing walls suspended over the tread surface (Fig. 1B). The exercising belt is made with special material that facilitates the animals’ grip and is easy to clean. The overall dimensions of the treadmill are, 33 cm × 50.8 cm × 50.8 cm and each exercise lane dimension is 43.8 cm × 12 cm × 12.7 cm. All rats in the exercise groups were pre-trained on the treadmill in order for the animals to adapt to the apparatus. Overall, rats were able to run on the treadmill with minimal requirement for external stimuli or manual prodding. A set of three rats were placed on the treadmill in each session. The rats were subjected to a 30-min daily treadmill exercise protocol for a total of 2 weeks: at a speed of 10 m/min for the first week and a speed of 15 m/min for the second week [9]. The rats were given a rest period of 5-min after 15-min of exercise. All of the rats had free access to standard rodent chow and water during the entirety of the experiment. After each session, the treadmill was cleaned with 70% ethanol solution, wiped and air dried before the following session.
2.3 General Body Parameters
Body weight was recorded on days 8 and 35. At the conclusion of the social defeat and trauma witnessing paradigm lasting for 7 days, depression- and anxiety-like behavior tests were conducted as published [11, 18]. Cognitive functions including short-term (STM) and long-term memory (LTM) tests using radial arm water maze (RAWM) paradigm were performed as published [19–21]. Rats were sacrificed 7 days after the conclusion of the social defeat protocol. The experimental design is summarized in Fig. 1C.
2.4 Anxiety-like Behavior Tests
First, light/dark test was conducted followed by elevated plus maze and open field tests as previously published [11, 18].
Light/dark (LD) exploration
The light/dark box consisted of a light and a dark compartment separated by a barrier with a single opening for passage from one compartment to the other. Total time spent in the lit area was recorded by an observer blinded to the treatment [11, 18].
Elevated plus maze (EPM)
A standard rat elevated plus maze with 43 cm arms extending from a 10 cm central area 90 cm above the floor was used (Med Associates Inc., St. Albans, VT). The rat was placed in the central area facing the open arms of the maze and each session lasted 5-min. The rat’s movements were tracked visually by an observer who was blinded to treatment, and the amount of time the rat spent in the open arms was determined [22].
Open field (OF) activity
Rats were placed in the center of the OF (60×40 cm) and left to explore the arena for 15-min. Total activity, ambulatory activity, and distance covered were determined using a Opto-Varimex Micro Activity Meter v2.00 system (Optomax, Columbus Instruments; OH) as previously published by our lab [11, 18].
2.5 Depression-like Behavior Test
Forced swim test (FST)
The FST is a test used for measuring depression-like behavior in rodents [23]. Rats were individually placed in a 24cm × 50cm cylinder filled with water (25°C) for 5-min. At some point after being placed in the water the rat assumes an immobile posture, marked by motionless floating and cessation of struggling. The total time spent immobile was recorded by an observer blinded to the treatment [24, 25].
2.6 Memory Function Test
Radial Arm Water Maze (RAWM)
The RAWM procedures were done as published [19–21]. The rats were subjected to the first set of six learning trials (trials # 1–6) followed by a 5-min rest period and then a second set of six learning trials (trials # 7–12). STM was assessed 30-min after the end of 12th trial. LTM was assessed 24-hr after the end of the STM.
2.7 Plasma Corticosterone
Rats were sacrificed 7 days after the conclusion of the social defeat protocol. The blood was collected, snap frozen, and stored at −80°C for further analysis. Corticosterone level in plasma was measured using an EIA based kit (No. 501320. Cayman Chemical, 1180 East Ellsworth Road. Ann Arbor, Michigan 48108, USA) [1].
2.8 Data Analysis
Data are expressed as mean ±SEM. Significance was determined by one-way ANOVA applying Tukey’s post-hoc test (GraphPad Software, Inc. San Diego, CA). A value of P< 0.05 was considered significant.
3. Results
3.1 General Body Parameters
Average body weight measured across the experimental period did not change among the groups. The weight gain for all groups are as follows: CON (37 gm, ± 3.82), EX (47 gm, ± 3.51), SD (42 gm, ± 1.20), TW (41 gm, ± 1.59), and EX-TW (51 gm, ± 5.03) (data not shown).
3.2 Analysis of Anxiety-like Behavior of Rats
In the LD test, SD rats spent 34.23 ± 6.05 seconds and TW rats spent 55.67 ± 7.80 seconds in the lit area of the LD apparatus when compared to control or exercise alone groups (CON: 141.20 ± 25.23 seconds, EX: 100.90 ± 10.91 seconds) (Fig. 2A), an indication of higher anxiety-like behavior [9–11, 18]. SD rats spent significantly less time in the lit area than CON (75.76%, p<0.05) or EX (66.08%, p<0.05) rats. Similarly, TW rats spent significantly less time in the lit area than CON (60.57%, p<0.05) or EX (44.83%, p<0.05) rats. Decreased time spent in the lit area of the LD apparatus during a 5-min session is indicative of high anxiety-like behavior [9–11, 18]. EX-TW rats spent 111.50 ± 9.24 seconds in the lit area of the LD apparatus when compared to TW or SD groups (TW: 55.67 ± 7.80 seconds, SD: 34.23 ± 6.05 seconds). The time spent in lit area by EX-TW rats was comparable to CON and EX groups (CON: 141.20 ± 25.23 seconds, EX: 100.90 ± 10.91 seconds). Furthermore, EX-TW rats spent significantly more time in the lit area than SD (69.30%, p<0.05) or TW (50.07%, p<0.05) rats, a behavior suggestive of reduced anxiety- like behavior [9–11, 18].
Fig 2. Examination of Anxiety-like Behavior.
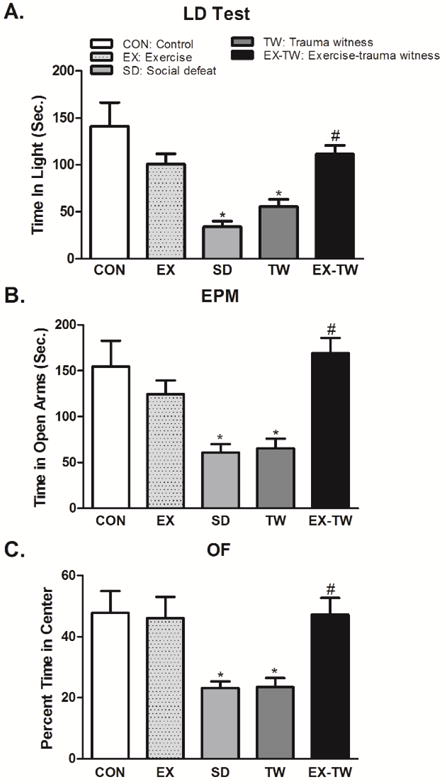
Total time spent in the lit compartment in light/dark (LD) test (A) and in the open arms in the elevated plus maze (EPM) test (B) as well as time spent in the center of the open arena in the open field (OF) test (C) was used to measure anxiety-like behavior. Group designations: rats not subjected to social defeat (CON: control); rats subjected to treadmill exercise (EX: exercise); rats subjected to social defeat (SD: social defeat); rats subjected to witness of social defeat (TW: trauma witness); rats subjected to treadmill exercise prior to application of trauma witness procedure (EX-TW: exercise-trauma witness). (*) indicates significantly different from CON, P<0.05; (#) indicates significantly different from TW, P<0.05. Bars represent means ± SEM, n =10–14 rats/group.
In the EPM test, SD rats spent 60.50 ± 9.43 seconds and TW rats spent 65.36 ± 10.35 seconds in the open arms of the EPM apparatus when compared to control or exercise alone groups (CON: 154.60 ± 27.99 seconds, EX: 124.50 ± 14.91 seconds) (Fig. 2B), an indication of higher anxiety-like behavior [9, 10]. SD rats spent significantly less time in the open arms than CON (60.87%, p<0.05) or EX (51.41%, p<0.05) rats. Similarly, TW rats spent significantly less time in the open arms than CON (57.72%, p<0.05) or EX (47.50%, p<0.05) rats. Increased time spent in the closed arms during a 5-min session is indicative of high anxiety-like behavior [9, 10]. EX-TW rats spent 169.00 ± 16.81 seconds in the open arms when compared to TW or SD groups (TW: 65.36 ± 10.35 seconds, SD: 60.50 ± 9.43 seconds). The time spent in open arms by EX-TW rats was comparable to CON and EX groups (CON: 154.60 ± 27.99 seconds, EX: 124.50 ± 14.91 seconds). Furthermore, EX-TW rats spent significantly more time in open arms than SD (64.20%, p<0.05) or TW (61.33%, p<0.05) rats, a behavior suggestive of reduced anxiety- like behavior [9, 10].
In the OF test, SD rats spent significantly less time in the center of the OF arena than CON (51.46%, p<0.05) or EX (49.76%, p<0.05) rats (Fig. 2C). Similarly, TW rats spent significantly less time in the center than CON (50.89%, p<0.05) or EX (49.18%, p<0.05) rats. Less percent time spent in the center of the open arena of the OF apparatus is considered as an indication of anxiety-like behavior [9–11, 18]. EX-TW rats spent significantly greater percent time in the center of the OF arena than SD (50.96%, p<0.05) or TW (50.39%, p<0.05) rats. In fact, percent time spent by EX-TW rats (47.23%) was comparable to CON (47.71%), and EX (46.10%) groups.
In summary, in three distinct tests assessing anxiety-like behavior, both SD and TW rats exhibited anxiety-like behavior. The exposure to moderate treadmill exercise prior to trauma witness stress mitigated the TW-induced anxiety-like behavior in rats.
3.3 Analysis of Depression-like Behavior of Rats
In the FST, SD rats spent 39.25 ± 4.85 seconds and TW rats spent 39.50 ± 8.08 seconds being more immobile when compared to control or exercise alone groups (CON: 15.50 ± 3.91 seconds, EX: 14.64 ± 2.49 seconds) (Fig. 3), a sign of increased depression-like behavior [17, 26]. EX-TW rats spent less time, 18.21 ± 2.69 seconds, in the immobile position when compared to TW or SD groups (TW: 39.50 ± 8.08 seconds, SD: 39.25 ± 4.85 seconds). The time spent immobile by EX-TW rats was comparable to CON and EX groups (CON: 15.50 ± 3.91 seconds, EX: 14.64 ± 2.49 seconds). Furthermore, EX-TW rats spent significantly less time immobile than SD (53.61%, p<0.05) or TW (53.90%, p<0.05) rats, a behavior suggestive of a reduced despair-like behavior [17, 26]. In the FST, assessing depression-like behavior, both SD and TW rats presented high depression-like behavior. This TW-induced depression-like behavior was reduced by moderate treadmill exercise prior to the TW-induced stress.
Fig 3. Examination of Depression-like Behavior.
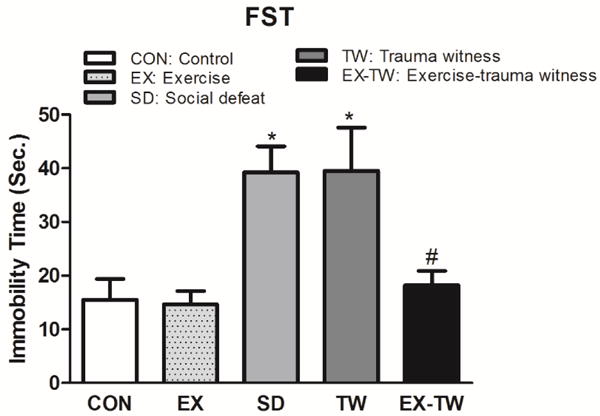
Total immobility time in the forced swim test (FST) was used to examine depression-like behavior. Group designations: rats not subjected to social defeat (CON: control); rats subjected to treadmill exercise (EX: exercise); rats subjected to social defeat (SD: social defeat); rats subjected to witness of social defeat (TW: trauma witness); rats subjected to treadmill exercise prior to application of trauma witness procedure (EX-TW: exercise-trauma witness). (*) indicates significantly different from CON, P<0.05; (#) indicates significantly different from TW, P<0.05. Bars represent means ± SEM, n =10–14 rats/group.
3.4 Analysis of Memory Deficits of Rats
STM was not significantly impaired in SD and TW rats compared to CON or EX groups in the RAWM apparatus. All groups including CON, EX, SD, TW and EX-TW rats made comparable errors in the STM (Fig. 4A) but not in LTM (Fig. 4B) tests. Errors made in the STM: CON (0.71 ± 0.32), EX (1.93 ± 0.49), SD (0.94 ± 0.30), TW (1.86 ± 0.53) and EX-TW (2.5 ± 0.63). Errors made in the LTM: CON (1.46 ± 0.45), EX (0.92 ± 0.34), SD (4.25 ± 0.65), TW (4.67 ± 0.61) and EX-TW (2.46 ± 0.67). EX-TW rats made significantly less errors than TW (47.40%, p<0.05) rats. In the RAWM test, assessing memory function, both SD and TW rats exhibited high LTM impairment. Moderate treadmill exercise, prior to trauma witness stress, mitigated the TW-induced memory impairments.
Fig 4. Examination of Memory Function.
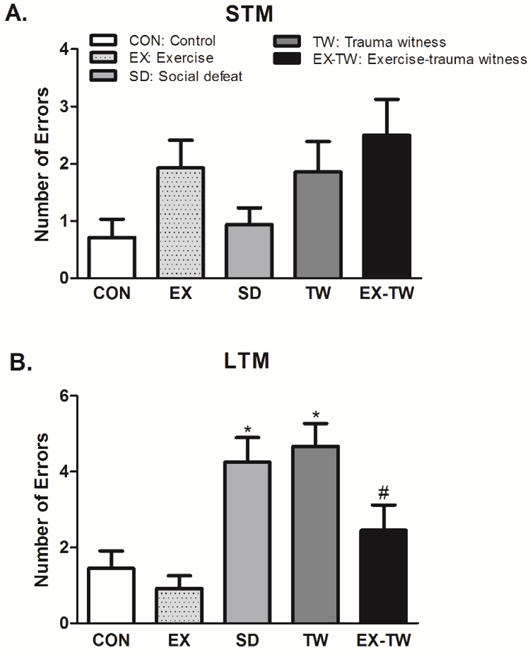
Number of errors made in the short-term memory (STM) test (A) and long-term memory (LTM) test (B) examined learning and memory function using radial arm water maze (RAWM) test. Group designations: rats not subjected to social defeat (CON: control); rats subjected to treadmill exercise (EX: exercise); rats subjected to social defeat (SD: social defeat); rats subjected to witness of social defeat (TW: trauma witness); rats subjected to treadmill exercise prior to application of trauma witness procedure (EX-TW: exercise-trauma witness). (*) indicates significantly different from CON, P<0.05; (#) indicates significantly different from TW, P<0.05. Bars represent means ± SEM, n =10–14 rats/group.
3.5 Plasma Corticosterone
Corticosterone level in plasma was measured using an EIA based kit [1]. Serum corticosterone levels were significantly increased in SD (80% from CON, 74.3% from EX p<0.05) and in TW rats (79.1% from CON, 73.1% from EX p<0.05) when compared to CON or EX rats. The corticosterone levels were significantly decreased in EX-TW group (45.7% from SD, 43.3% from TW, p<0.05) when compared to SD and TW rats. Serum corticosterone levels were 14 ng/ml in CON rats; 18 ng/ml in EX rats; 70 ng/ml in SD rats, 67 ng/ml in TW rats and 38 ng/ml in EX-TW rats (Fig. 5). In the corticosterone assay, assessing systemic stress levels, both SD and TW rats showed high systemic stress levels. The exposure to moderate treadmill exercise prior to the trauma witness stress, reduced the systemic stress levels in rats.
Fig 5. Plasma Corticosterone (CORT).
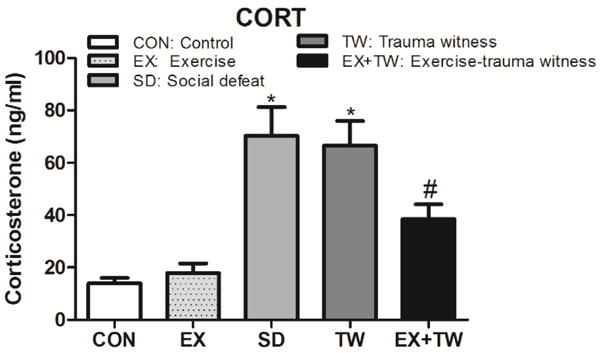
Plasma corticosterone was measured in rat plasma using a kit based ELISA assay. Group designations: rats not subjected to social defeat (CON: control); rats subjected to treadmill exercise (EX: exercise); rats subjected to social defeat (SD: social defeat); rats subjected to witness of social defeat (TW: trauma witness); rats subjected to treadmill exercise prior to application of trauma witness procedure (EX-TW: exercise-trauma witness). (*) indicates significantly different from CON, P<0.05; (#) indicates significantly different from TW, P<0.05. Bars represent means ± SEM, n =10–14 rats/group.
4. Discussion
Our animal model has previously shown anxiety-like and depression-like behavior following social defeat-mediated trauma directly (SD) or indirectly (TW) [1, 17]. The results of this study corroborate with our previous findings as SD and TW rats had increases in anxiety-like behavior evaluated through the LD, EPM and OF behavior tests. Anxiety-like behavior is indicated through the reduced time spent, by SD and TW rats, in the light compartment of the LD apparatus, reduced time spent in the open arms of the EPM apparatus, and lastly, reduced percent time spent in the central area of the OF arena. In contrast, the anxiety-like behavior of EX-TW rats were comparable to the CON rats, which suggests that moderate treadmill exercise prior to application of indirect trauma is protective on anxiety-like behavior. Perhaps, moderate treadmill exercise provided TW rats with the ability to manage and mitigate the anxious phenotype of the TW-induced stress.
Previous studies have shown that moderate treadmill exercise results in prevention of anxiety-like behavior in other models of stress including direct social defeat [9], SPS model [10] and sleep-deprivation [18]. While some studies have reported anxiolytic effect of exercise on rats and humans [27, 28], there are studies that have observed the opposite effect or no change in anxious behavior phenotype [18, 29, 30]. Some possible explanations for the discrepancy in the results may be the different exercise protocols used, the type of anxiety test performed, and the parameters measured. Similar to the anxiety-like behavior, SD and TW rats demonstrated significant depression-like behavior when compared to the CON or EX rats. Whereas, the EX-TW rats did not exhibit depression-like behavior. These results suggest that moderate treadmill exercise prevented the occurrence of depression-like behavior despite application of indirect social defeat-mediated trauma. These results are interesting considering that depression is associated with low levels of physical activity [31] and physical exercise is known to elicit mood elevating effects [32, 33]. The anti-depressant mimicking effects of treadmill exercise are perhaps due to the release of beta-endorphin, catecholamine, and/or serotonin [34–37]. Beta-endorphins can create a “feel good” effect, and previous studies have shown that exercise-induced increases in beta-endorphin levels may occur via three different proposed mechanisms: analgesic response, pH balances, and/or metabolic regulation [34, 35, 38]. Catecholamine release can also be beneficial in stressful events because it can lead to an adaptation to the stress [39]. Lastly, exercise-induced increases in serotonin levels can enhance neurogenesis, which may be valuable to protect against depression-like behavior [40]. Prior moderate treadmill exercise most likely released high amounts of beta-endorphin, catecholamine, and/or serotonin levels in EX-TW rats, which contributed to lessening or overcoming of the negative effects of trauma witnessing stress.
The third component examined in this study was learning and memory function. Actually, beneficial effects of physical exercise in improving cognitive function in humans as well as in experimental animals are well studied [41–44]. Impairment of learning and memory function also has been demonstrated in various animal models of stress [45]. In agreement with our previous study [17], here, we did not observe any STM impairment in SD or in TW rats but LTM was impaired. Interestingly, TW-induced LTM impairment was prevented with prior moderate treadmill exercise as EX-TW rats did not show LTM impairment. Our postulation is that social defeat and traumatic stress increases alertness and causes hyperactivity in rats, as a result they are able to locate the platform quickly without making many errors in STM. However, this state of hyperactivity is over once the rats get a 24-hr rest period following completion of STM test. The actual phenotype in the form of LTM impairment surfaces later once the initial hyperactivity phase is passed.
Finally, elevated stress levels were indicated by increased plasma corticosterone levels in SD and TW rats which was markedly reduced in EX-TW rats. This is in agreement with our previously published work in which high corticosterone levels, induced by direct social defeat, were mitigated with exercise intervention in rats [9]. This data suggests there is a protective effect of moderate treadmill exercise on direct trauma [9] and also on indirect traumatic stress.
5. Conclusion
In previous studies, the beneficial role of moderate treadmill exercise, following direct physical trauma, was examined in two different stress paradigms [9, 10]. In order to further examine the beneficial properties of moderate treadmill exercise, this study focused on measuring its effect on vicariously acquired PTSD-like phenotype using a rodent animal model. The results revealed that moderate treadmill exercise prevents development of vicarious trauma-induced anxiety- and depression-like behavior as well as long-term memory impairment perhaps by modulating corticosterone levels.
Highlights.
Witnessing stressful events causes behavioral deficits in rats
Exercise induces resilience against stressful stimuli in rats
Exercise has preventive effects against negative behavioral deficits
Exercise is beneficial for both vicarious and direct trauma experiences
Acknowledgments
Funding for this research was provided by an NIH grant awarded to Samina Salim (2R15MH093918-02).
Abbreviations
- PTSD
post-traumatic stress disorder
- SD
social defeat
- TW
trauma witness
- Ex
exercise
- LD
light/dark test
- EPM
elevated plus maze test
- STM
short-term memory
- LTM
long-term memory
- FST
forced swim test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Interest
The authors report no conflict of interest.
References
- 1.Patki G, et al. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013 doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimering R, et al. Posttraumatic stress disorder in disaster relief workers following direct and indirect trauma exposure to ground zero. Journal of Traumatic Stress. 2006;19(4):553–557. doi: 10.1002/jts.20143. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann P. The development of posttraumatic stress disorder (PTSD) in a sample of child witnesses to mother assault. Journal of Family Violence. 1997;12(3):241–257. [Google Scholar]
- 4.American Psychiatric, A., A. American Psychiatric, and D.S.M.T. Force. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- 5.Schoenfeld FB, Marmar CR, Neylan TC. Current concepts in pharmacotherapy for posttraumatic stress disorder. Psychiatr Serv. 2004;55(5):519–31. doi: 10.1176/appi.ps.55.5.519. [DOI] [PubMed] [Google Scholar]
- 6.Penn E, Tracy DK. The drugs don’t work? antidepressants and the current and future pharmacological management of depression. Ther Adv Psychopharmacol. 2012;2(5):179–88. doi: 10.1177/2045125312445469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shamus E, Cohen G. Depressed, Low Self-Esteem: What Can Exercise Do For You? The Internet Journal of Allied Health Sciences and Practice. 2009;7(2) [Google Scholar]
- 8.Tsatsoulis A, Fountoulakis S. The protective role of exercise on stress system dysregulation and comorbidities. Ann N Y Acad Sci. 2006;1083:196–213. doi: 10.1196/annals.1367.020. [DOI] [PubMed] [Google Scholar]
- 9.Patki G, et al. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiol Behav. 2014;130:135–44. doi: 10.1016/j.physbeh.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patki G, et al. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav. 2014;130:47–53. doi: 10.1016/j.physbeh.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salim S, et al. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behavioural Brain Research. 2010;208(2):545–552. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60(3):253–9. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- 13.Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43(1):158–65. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 14.Bhatnagar S, et al. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18(1):13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- 15.Wood SK, et al. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151(4):1795–805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood SK, et al. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berl) 2012;222(2):325–36. doi: 10.1007/s00213-012-2648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patki G, Solanki N, Salim S. Witnessing traumatic events causes severe behavioral impairments in rats. Int J Neuropsychopharmacol. 2014;17(12):2017–29. doi: 10.1017/S1461145714000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vollert C, et al. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224(2):233–40. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Alhaider IA, et al. Caffeine prevents sleep loss-induced deficits in long-term potentiation and related signaling molecules in the dentate gyrus. Eur J Neurosci. 2010;31(8):1368–76. doi: 10.1111/j.1460-9568.2010.07175.x. [DOI] [PubMed] [Google Scholar]
- 20.Alhaider IA, et al. Chronic caffeine treatment prevents sleep deprivation-induced impairment of cognitive function and synaptic plasticity. Sleep. 2010;33(4):437–44. doi: 10.1093/sleep/33.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aleisa AM, et al. Acute nicotine treatment prevents REM sleep deprivation-induced learning and memory impairment in rat. Hippocampus. 2011;21(8):899–909. doi: 10.1002/hipo.20806. [DOI] [PubMed] [Google Scholar]
- 22.Bert B, et al. Fischer 344 and wistar rats differ in anxiety and habituation but not in water maze performance. Neurobiol Learn Mem. 2002;78(1):11–22. doi: 10.1006/nlme.2001.4040. [DOI] [PubMed] [Google Scholar]
- 23.Can A, et al. The mouse forced swim test. J Vis Exp. 2012;(59):e3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cougle JR, Resnick H, Kilpatrick DG. Does prior exposure to interpersonal violence increase risk of PTSD following subsequent exposure? Behav Res Ther. 2009;47(12):1012–7. doi: 10.1016/j.brat.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck CT. Secondary traumatic stress in nurses: a systematic review. Arch Psychiatr Nurs. 2011;25(1):1–10. doi: 10.1016/j.apnu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Patki G, et al. Witnessing traumatic events and post-traumatic stress disorder: Insights from an animal model. Neurosci Lett. 2015;600:28–32. doi: 10.1016/j.neulet.2015.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strohle A, et al. The acute antipanic activity of aerobic exercise. Am J Psychiatry. 2005;162(12):2376–8. doi: 10.1176/appi.ajp.162.12.2376. [DOI] [PubMed] [Google Scholar]
- 28.Strohle A, et al. The acute antipanic and anxiolytic activity of aerobic exercise in patients with panic disorder and healthy control subjects. J Psychiatr Res. 2009;43(12):1013–7. doi: 10.1016/j.jpsychires.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Dishman RK, et al. Increased open field locomotion and decreased striatal GABAA binding after activity wheel running. Physiol Behav. 1996;60(3):699–705. doi: 10.1016/0031-9384(96)00102-3. [DOI] [PubMed] [Google Scholar]
- 30.Fulk LJ, et al. Chronic physical exercise reduces anxiety-like behavior in rats. Int J Sports Med. 2004;25(1):78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- 31.Smith P, Blumenthal J. Exercise and physical activity in the prevention and treatment of depression. Handbook of physical activity and mental health. 2013:145–216. [Google Scholar]
- 32.Rosenbaum S, et al. Exercise augmentation compared to usual care for Post Traumatic Stress Disorder: A Randomised Controlled Trial (The REAP study: Randomised Exercise Augmentation for PTSD) Bmc Psychiatry. 2011;11 doi: 10.1186/1471-244X-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lepore SJ. Expressive writing moderates the relation between intrusive thoughts and depressive symptoms. Journal of Personality and Social Psychology. 1997;73(5):1030–1037. doi: 10.1037//0022-3514.73.5.1030. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz L, Kindermann W. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 1992;13(1):25–36. doi: 10.2165/00007256-199213010-00003. [DOI] [PubMed] [Google Scholar]
- 35.Hamner MB, Hitri A. Plasma beta-endorphin levels in post-traumatic stress disorder: a preliminary report on response to exercise-induced stress. J Neuropsychiatry Clin Neurosci. 1992;4(1):59–63. doi: 10.1176/jnp.4.1.59. [DOI] [PubMed] [Google Scholar]
- 36.Zouhal H, et al. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008;38(5):401–23. doi: 10.2165/00007256-200838050-00004. [DOI] [PubMed] [Google Scholar]
- 37.Dey S, Singh RH, Dey PK. Exercise training: significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiol Behav. 1992;52(6):1095–9. doi: 10.1016/0031-9384(92)90465-e. [DOI] [PubMed] [Google Scholar]
- 38.Goldfarb AH, Jamurtas AZ. Beta-endorphin response to exercise. An update Sports Med. 1997;24(1):8–16. doi: 10.2165/00007256-199724010-00002. [DOI] [PubMed] [Google Scholar]
- 39.Stone EA, McCarty R. Adaptation to stress: tyrosine hydroxylase activity and catecholamine release. Neurosci Biobehav Rev. 1983;7(1):29–34. doi: 10.1016/0149-7634(83)90005-2. [DOI] [PubMed] [Google Scholar]
- 40.Klempin F, et al. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J Neurosci. 2013;33(19):8270–5. doi: 10.1523/JNEUROSCI.5855-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ang ET, Gomez-Pinilla F. Potential therapeutic effects of exercise to the brain. Curr Med Chem. 2007;14(24):2564–71. doi: 10.2174/092986707782023280. [DOI] [PubMed] [Google Scholar]
- 42.Berchtold NC, et al. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–61. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 43.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 44.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Klenerova V, et al. Impaired passive avoidance acquisition in Sprague-Dawley and Lewis rats after restraint and cold stress. Behav Brain Res. 2002;136(1):21–9. doi: 10.1016/s0166-4328(02)00093-1. [DOI] [PubMed] [Google Scholar]


