Abstract
Objectives
To quantify the heterogeneity of the tumour apparent diffusion coefficient (ADC) using voxel-based analysis to differentiate malignancy from benign wall thickening of the urinary bladder.
Methods
Nineteen patients with histopathological findings of their cystectomy specimen were included. A data set of voxel-based ADC values was acquired for each patient’s lesion. Histogram analysis was performed on each data set to calculate uniformity (U) and entropy (E). The k-means clustering of the voxel-wised ADC data set was implemented to measure mean intra-cluster distance (MICD) and largest inter-cluster distance (LICD). Subsequently, U, E, MICD, and LICD for malignant tumours were compared with those for benign lesions using a two-sample t-test.
Results
Eleven patients had pathological confirmation of malignancy and eight with benign wall thickening. Histogram analysis showed that malignant tumours had a significantly higher degree of ADC heterogeneity with lower U (P = 0.016) and higher E (P = 0.005) than benign lesions. In agreement with these findings, k-means clustering of voxel-wise ADC indicated that bladder malignancy presented with significantly higher MICD (P < 0.001) and higher LICD (P = 0.002) than benign wall thickening.
Conclusions
The quantitative assessment of tumour diffusion heterogeneity using voxel-based ADC analysis has the potential to become a non-invasive tool to distinguish malignant from benign tissues of urinary bladder cancer.
Keywords: Bladder malignancy, Tumour heterogeneity, Apparent Diffusion Coefficient, Histogram analysis, K-means clustering
Introduction
One of the critical needs in bladder cancer is a non-invasive method to accurately differentiate a residual malignant tumour from benign wall thickening caused by inflammation and reactive changes after initial treatment (e.g. transurethral resection of the bladder tumour (TURBT) or neoadjuvant chemotherapy). Cystoscopy with cytology and biopsy remains the gold standard for bladder cancer detection. Bladder imaging including computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI) is an alternative and additional diagnostic tool. To date, the clinical need has remained unmet by these imaging modalities, and the bladder wall thickening continues to interfere with and cause inaccuracies in the diagnosis of bladder cancer [1–6].
Diffusion-weighted imaging (DWI) is an MRI method to quantify the diffusion of in vivo water using a parameter called apparent diffusion coefficient (ADC) [7, 8]. This quantitative method has been shown to be beneficial in distinguishing benign and malignant lesions in the urinary bladder [9]. However, this previous study obtained the measurement of mean ADC through a circular region of interest (ROI) on a lesion and did not take into account the heterogeneous distribution of voxel-wise ADC within an entire tumour volume.
Heterogeneity is an intrinsic characteristic of tumours, which makes tumour tissues distinctive from normal tissues. A number of previous studies have shown that the assessment of tumour heterogeneity is highly valuable for cancer diagnosis, prognosis, and treatment monitoring [10–12]. Histogram analysis is a widely used tool for heterogeneity quantification, particularly for MRI features, which are not always well visualised with the naked eye [11, 13]. Two quantities calculated by using this method are uniformity (U) and entropy (E). These two quantitative measurements characterise the uniformity and the irregularity of intra-tumour voxel-wise distribution [10]. Until now, there has been no reported study that applies this quantitative methodology to quantify the heterogeneity degree of MRI features in bladder tumours to solve unmet needs in the clinical care of bladder cancer.
The k-means clustering technique recently has been more recognised as a useful tool to analyse the inhomogeneous distribution of MRI features to assist in radiological diagnostics [14–18]. Srinivasan [18] concluded that voxel-wise ADC analysis with k-means clustering could better characterise and differentiate malignant from benign neck pathologies than whole-lesion mean ADC alone. In bladder cancer, it was also demonstrated that k-means clustering of voxel-wise pharmacokinetic parameters could characterise the spatial distribution of microcirculation characteristics in a tumour to facilitate early prediction of chemotherapeutic response [17]. Therefore, it is of clinical interest to investigate the diagnostic value of voxel-wise ADC analysis with k-means clustering in the characterisation of tumour diffusivity to differentiate malignant tumours from the benign wall thickening of the urinary bladder.
The aim of this prospective study is to quantitatively assess the heterogeneity of tumour ADC using voxel-wise analysis to differentiate malignant from benign urinary bladder tissue. While we use two well-known quantities (U and E) in the histogram analysis, we also propose the employment of two measurements in the k-means clustering analysis to characterise the ADC heterogeneity within malignancy versus the benign wall thickening.
Materials and methods
Patient cohort
This study was approved by the institutional review board (IRB) as a phase I clinical trial. All patients provided informed consent to be enrolled in the study. From May 2010 to May 2013, 19 patients [14 males, 5 females; age (mean ± standard deviation): 64 ± 9 years] who had a TURBT and were pathologically diagnosed with muscle-invasive bladder cancer were included in this data assessment. After TURBT, 17 patients underwent neoadjuvant chemotherapy (cisplatin-based, 2–3 weeks per cycle). The two other patients did not have chemotherapy because of their co-morbidity. Subsequently, the 19 patients (17 post-TURBT and post-chemotherapy and 2 post-TURBT) had an MRI prior to radical cystectomy. The pathological findings of the cystectomy specimen were correlated with the assessment of pre-operative MRI data.
MR imaging
Patient MRI examinations were performed on a 3-T multi-transmit system (Ingenia CX, Philips Healthcare) using a 32-channel phased-array surface coil. Anatomical T2-weighted MRI (T2W-MRI) was done before diffusion-weighted MRI (DWI).
T2W-MRI was performed with turbo spin echo sequence (TR/TE = 4264/80 ms; matrix = 468 × 380; in-plane FOV = 300 × 340; slice thickness = 3 mm; slice gap = 0.3 mm; number of slices = 40; number of signal average = 2; echo train length = 19). DWI was performed with a single-shot echo-planar imaging (ssh-EPI) sequence (TR/TE = 2017/59 ms; matrix = 100/140; in-plane FOV = 220/317; slice thickness = 3.0 mm; slice gap = 0.3 mm; number of slices = 25; number of signal average = 6; b-values = 0, 333, 667, 1000 s/mm2). Both scans were in the axial plane.
Voxel-wise ADC analysis
ADC maps were acquired after DWI data acquisition using Philips system software. For each patient, freehand regions of interest (ROIs) were placed on the whole tumour volume by a radiologist (Z.K.S) with 15 years of experience on ADC maps to obtain voxel-wise ADC values of the patient’s tumour. Pathological findings were used as the ground truth to ensure that ROIs were accurately placed on malignant tumours or benign thickenings. In addition, anatomical T2W images were used to help the radiologist localise the tumours.
Implementation of histogram analysis: The analysis was case-based and performed on Microsoft Excel (Version 2013) with the same bin size for all cases. Two quantities, uniformity (noted as U) and entropy (noted as E), were calculated as follows:
where pi is the probability of occurrence in the ith bin; N is the total number of bins and dependent on each data set.
The two quantities characterise the uniformity (U) and the irregularity (E) of voxel ADC values within a tumour. The more homogeneous voxel-wise ADC values are within the tumour, the higher U and the lower E are. For the detailed description and formula of U and E calculation, please refer to the appendix in a comprehensive review by Davnall [10]. Since U and E depend on the number of ADC values within each bin of a histogram, i.e. the number of voxels with similar ADC values, these quantities are unit-less.
Implementation of k-means clustering analysis: Previous studies on k-means clustering of MRI parameters showed that three clusters provided the best characterisation of cancer tissues [14, 17, 18]. Therefore, we selected k of 3 to perform k-means clustering of voxel-wise ADC values for each case. The case-based k-means clustering was executed on Microsoft Excel using a method described in [17] to determined three cluster centres. Subsequently, two widely used measurements were calculated for each case: (1) mean intra-cluster distance (MICD), which is the average of the distance from each data point (ADC value) to its centre cluster; (2) the largest inter-cluster distance (LICD), which is the largest distance among three cluster centres:
| (1) |
where N is the total number of data points in the data set, i ranges from 1 to 3 and is denoted for cluster i, ci is the centre of cluster i, Mi is the number of data points in cluster i, and xj is the jth data point of cluster i.
| (2) |
where ci and cj are the centres of cluster i and cluster j; i and j range from 1 to 3.
MICD and LICD are in the unit of ADC.
The calculated quantities U, E and MICD, and LICD for each case were then correlated to the pathological findings of the patient’s cystectomy specimen.
In addition, mean ADC values were also evaluated to compare their diagnostic value with that of the four quantities in the patient population of this study.
Statistical analysis
The values of uniformity, entropy, MICD, and LICD as well as mean ADC were summarised using descriptive statistics (i.e. mean and standard deviation) for patients with benign lesions or malignant tumours, respectively. Kolmogorov-Smirnov test has been used to check for normality. Those values were also compared between patients with benign lesions and patients with malignant tumours using a two-sample t-test. A commercial statistical package (SAS for Windows® Version 9.3; SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. P < 0.05 was considered statistically significant.
Results
Pathological findings
According to the cystectomy pathological reports, 8 out of 19 patients were found to have chronic inflammation or reactive changes after the initial treatments (TURBT only or both TURBT and neoadjuvant chemotherapy) and were negative for malignancy. Of the eight patients, one patient was not treated with neoadjuvant chemotherapy (as described in the methods) and had no tumour in the cystectomy specimen.
Pathological examination found the presence of carcinoma in the other 11 cases. One patient was only treated with TURBT and did not receive neoadjuvant chemotherapy. In three patients, malignancy locations were identified continuously in multiple sites (left, right, anterior, and posterior) of the bladder wall. Three were found with malignancy on the anterior or dome aspects of the bladder. Three other patients had a malignant tumour in the trigone area. Malignancy was found in the anterior wall in one case and in the left wall in the other.
Correlation of malignancy with heterogeneity degree
Table 1 lists the mean value and standard deviation for each quantity in the groups of malignant and benign cases.
Table 1.
The mean value and standard deviation of each quantity for the malignant and benign groups
| Quantity | Malignant (Mean ± Standard Deviation) |
Benign (Mean ± Standard Deviation) |
P value |
|---|---|---|---|
| U | 0.07 ± 0.01 | 0.09 ± 0.02 | 0.016 |
| E | 418 ± 0.18 | 3.81 ± 0.31 | 0.005 |
| MICD+ | 162.3 ± 16.4 | 122.6 ± 22.0 | <0.001 |
| LICD+ | 948.3 ± 120.0 | 732.0 ± 144.3 | 0.002 |
The units is (×10−9 mm2/s)
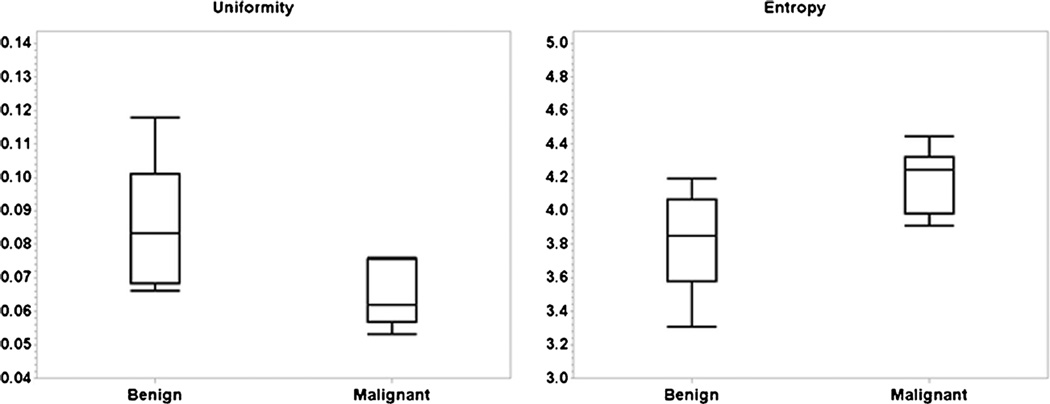
Compared to benign bladder wall thickening, malignant tumours had a significantly lower U (P = 0.016) and higher E (P = 0.005). Figure 1 shows the box plots that demonstrate the differences of U and E in malignant versus benign lesions. Correlation with U implied that the ADC values were more uniform from voxel to voxel in benign lesions. Similarly, correlation with E indicated that voxel-based ADC values were more irregular in malignancies. These two correlations were in agreement and both suggested that malignant tumours had a significantly higher degree of heterogeneity in voxel-based ADC values, i.e. a higher level of heterogeneity in micro-cellularity.
Fig. 1.
Differences in U (left) and E (right) between malignant vs. benign tissues
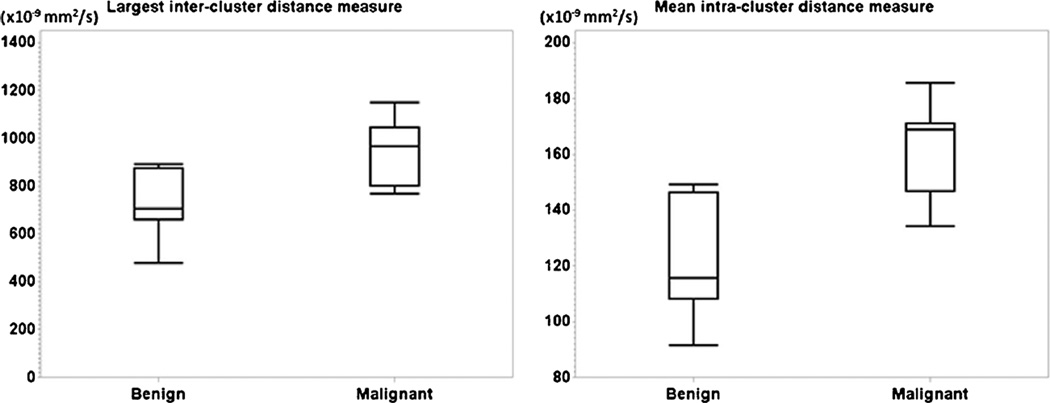
The analysis with k-means clustering gave the result that malignant tumours had significantly higher MICD (P < 0.001) and LICD (P = 0.002) than benign lesions. Figure 2 shows the box plots of the MICD and LICD in the malignant and benign groups. The difference in MICD demonstrated that voxel-wise ADC values of benign lesions were significantly more compact in a cluster than those of malignant tumours. The LICD difference showed that the three clusters of voxel-wise ADC values in a malignancy were more spread out from each other. These results also demonstrated that voxel-wise ADC distribution was more heterogeneous in malignant tissues.
Fig. 2.
Differences in LICD (left) and MICD (right) between malignant vs. benign tissues
The two different analysis approaches both showed that malignancies had higher level of ADC heterogeneity than benign bladder wall thickening. The statistical analysis indicated that the quantities measured by k-means clustering were more robust in differentiating malignant from benign lesions.
Meanwhile, no significant differences (all P > 0.1) in mean ADC value and in the size as well as the number of voxels were found between malignant and benign lesions.
Visualisation of heterogeneity
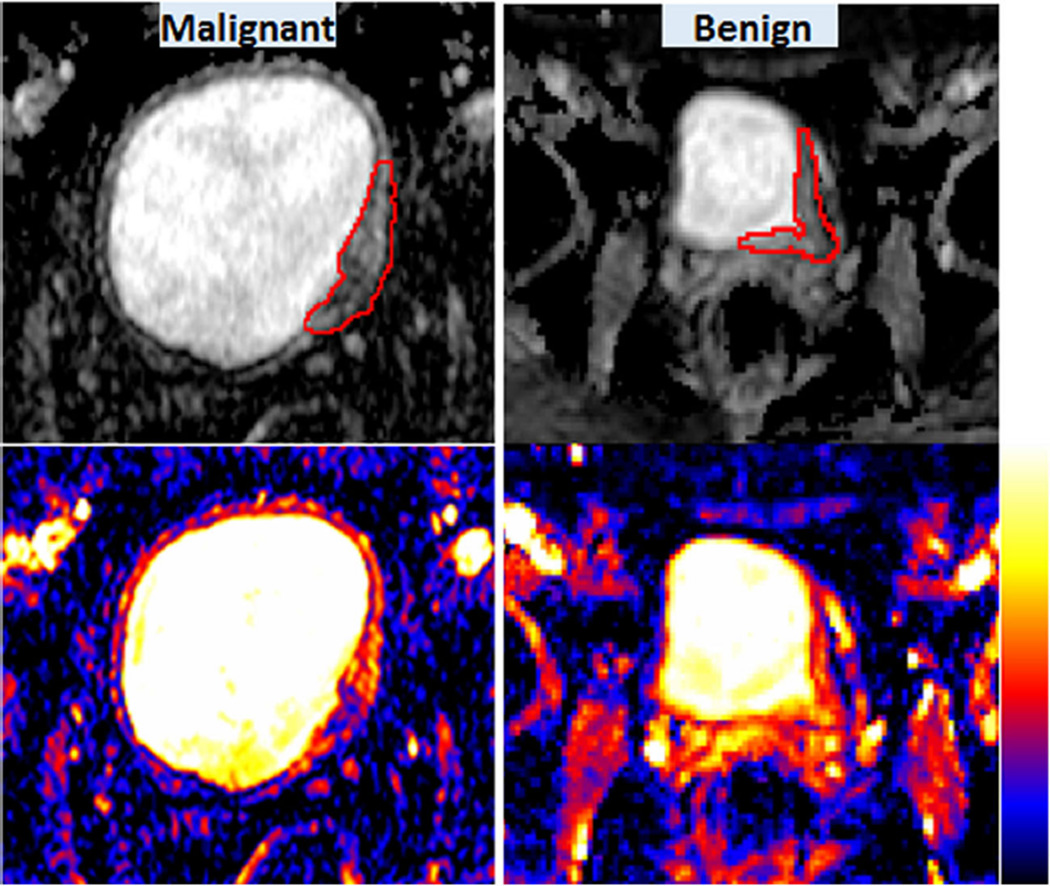
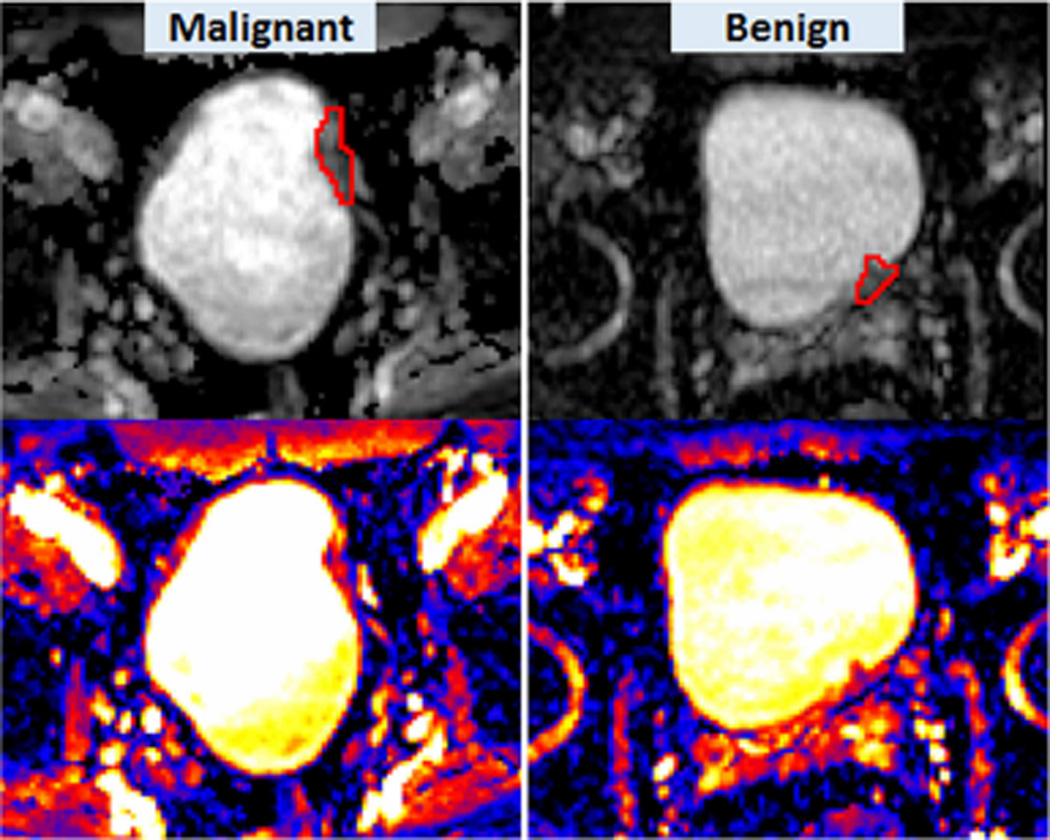
The grey-scaled or colour-coded ADC maps could visually show the heterogeneity of ADC within tumour tissues (Figs. 3 and 4). On ADC maps, it was seen that ADCs varied voxel by voxel within a lesion. However, the difference in the degree of heterogeneity was not always visually seen without quantitative assessment. Figure 3 illustrates an example in which the difference in the degree of ADC heterogeneity between a malignant tumour and a benign lesion could be visualised using ADC maps. The malignancy shown on Fig. 3 consisted of voxels in a spectrum of red (higher ADC) to blue (lower ADC) while the benign lesion was mainly composed of red voxels. From this observation, it can be concluded that voxel-wise ADCs were more heterogeneous in the malignancy. Figure 4 illustrates another example in which the degree of heterogeneity was visually seen to be similar between malignant and benign lesions and only the quantification using the four parameters U, E and MICD, LICD could show the difference in the degree of heterogeneity.
Fig. 3.
ADC heterogeneity in a malignancy (left) vs. a benign lesion (right). Grey scaled ADC maps are shown in the upper row, colour-coded ADC maps in the lower row. Colour scale is shown next to colour ADC maps. Red contours depict the ROIs placed on the lesions. Malignant: U = 0.053; E = 4.44; MICD = 185.5 (×10−9 mm2/s); LICD = 1150.0 (× 10−9 mm2/s). Benign: U = 0.078; E = 3.98; MICD = 144.4 (× 10−9 mm2/s); LICD = 749.0 (× 10−9 mm2/s)
Fig. 4.
ADC heterogeneity in a malignancy (left) vs. a benign lesion (right). Shown here are grey-scaled ADC maps in the upper row, colour-coded ADC maps in the lower row. Red contours illustrate the ROIs placed on the lesions. Malignant: U = 0.062; E = 4.25; MICD = 159.4(× 10−9 mm2/s); LICD = 986.5 (× 10−9 mm2/s). Benign: U = 0.088; E = 3.72; MICD = 112.3 (× 10−9 mm2/s); LICD = 664.5 (× 10−9 mm2/s)
Discussion
As tumoral tissue is heterogeneous with respect to a variety of micro-environmental characteristics including the micro-vasculature and micro-cellularity, it is of high clinical importance to establish surrogate markers of the heterogeneity in these characteristics. In this study, we employed two widely used measurements (U and E) in histogram analysis and introduced two quantities (MICD and ULICD) in the k-means clustering method to quantify the heterogeneity in micro-cellularity within a bladder tumour. Our data have shown that all four quantities could become a potential biomarker for distinguishing a bladder malignancy from the benign wall thickening. MICD calculated from k-means clustering provided the best characterisation with a significant difference of P < 0.001 between malignant and benign groups.
After initial treatments including TURBT and neoadjuvant chemotherapy, the bladder has reactive changes with concomitant acute, chronic, and granulomatous inflammation. These changes cause bladder wall thickening that can be misdiagnosed as residual or recurrent cancer. This may interfere with the diagnosis using cystoscopy [4], CT [2], or MRI [6, 19]. Thus, there is a critical need to distinguish the treatment-induced benign thickening from bladder malignancy. A previous study showed that 3-T dynamic contrast-enhanced MRI (DCE-MRI) can be helpful in visually localising malignant tumours within the bladder wall thickening [20]. Our study provided a different perspective on characterising bladder malignancy, which is not always appreciated by the naked eye. As illustrated in the results, the difference in the level of heterogeneity, which is difficult to perceive visually, can be demonstrated by using a quantitative readout. The utility of the four measurements (U, E from the histogram, and MICD, LICD from k-means clustering) could provide radiologists with critical additional information for the cancer diagnosis.
A number of studies have applied quantitative measurement of ADC in the diagnosis of bladder cancer [21–27]. These studies showed that there was correlation of tumour ADC with the histological grade [27], stage [25], and invasive and proliferative potential [24] of the bladder tumour. However, these studies only acquire simple statistics of tumour ADC such as the mean of the whole tumour volume or only part of the tumour. These assessments neglect the heterogeneous distribution of ADC among voxels of the whole tumour. The study by Daggulli [21] assessed the difference in mean ADC between benign and malignant lesions in 45 bladder patients and did not find statistical significance in mean ADC in this patient population, which is in agreement with the findings in our study. A recent study by Suo [28] applied histogram analysis to differentiate bladder cancer from benign lesions and assess tumour stage and found significant differences in skewness and kurtosis. Although the study demonstrated the value of histogram analysis, the findings were not consistent because they obtained two different ADC values associated with two different b-values. In our study, curve fitting of signal intensities with four different b-values was used to obtain only one ADC value for each voxel. Our voxel-based analyses using histogram and k-means clustering were found to have statistical significance in all quantities between malignant and benign lesions.
The four different quantities characterise different aspects of the inhomogeneous distribution of ADC values among voxels within the entire tumour. While U and E are widely used to describe the uniformity and irregularity, which reflect the heterogeneity, of data points within the data set, no studies have used the two k-means clustering quantities for heterogeneity description. However, it is well known that MICD shows the compactness of data points within clusters and that LICD demonstrates the separation among clusters. Therefore, we have proposed using these two quantities in the assessment of heterogeneity and demonstrated that the findings with MICD and LICD were in agreement with the results on U and E differences to consistently show that malignant tissues had a significantly higher degree of heterogeneity than benign lesions.
A limitation in this study is that the number of patients is not large. However, in this phase I study, we have demonstrated the potential of the methodology and the need for further investment in the research to enhance its statistical significance. With a larger patient population, further statistical analysis including ROC curve analysis can be done to demonstrate the accuracy of using the proposed quantities as a biomarker in the identification of bladder malignancy.
In conclusion, this study has shown that non-invasive quantification of ADC heterogeneity using histogram analysis and k-means clustering is a valuable assessment to characterise malignancy in the urinary bladder. The four quantities acquired from these voxel-based analyses have the potential to be a biomarker to differentiate malignancy from benign bladder wall thickening induced by TURBT or neoadjuvant chemotherapy.
Key Points.
Heterogeneity is an intrinsic characteristic of tumoral tissue.
Non-invasive quantification of tumour heterogeneity can provide adjunctive information to improve cancer diagnosis accuracy.
Histogram analysis and k-means clustering can quantify tumour diffusion heterogeneity.
The quantification helps differentiate malignant from benign urinary bladder tissue.
Acknowledgments
The scientific guarantor of this publication is Dr. Michael V. Knopp at The Ohio State University. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. The DCE-MRI data of the patient cohort were reported in Investigative Radiology and the Journal of Magnetic Resonance Imaging. Methodology: prospective, diagnostic or prognostic study, performed at one institution.
References
- 1.Bhatt J, Cowan N, Protheroe A, Crew J. Recent advances in urinary bladder cancer detection. Expert Rev Anticancer Ther. 2012;12:929–939. doi: 10.1586/era.12.73. [DOI] [PubMed] [Google Scholar]
- 2.Sadow CA, Silverman SG, O'Leary MP, Signorovitch JE. Bladder cancer detection with CT urography in an Academic Medical Center. Radiology. 2008;249:195–202. doi: 10.1148/radiol.2491071860. [DOI] [PubMed] [Google Scholar]
- 3.Setty BN, Holalkere NS, Sahani DV, Uppot RN, Harisinghani M, Blake MA. State-of-the-art cross-sectional imaging in bladder cancer. Curr Probl Diagn Radiol. 2007;36:83–96. doi: 10.1067/j.cpradiol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Shariat SF, Karam JA, Lotan Y, Karakiewizc PI. Critical evaluation of urinary markers for bladder cancer detection and monitoring. Rev Urol. 2008;10:120–135. [PMC free article] [PubMed] [Google Scholar]
- 5.Tatsugami K, Kuroiwa K, Kamoto T, Nishiyama H, Watanabe J, Ishikawa S, et al. Evaluation of narrow-band imaging as a complementary method for the detection of bladder cancer. J Endourol / Endourol Soc. 2010;24:1807–1811. doi: 10.1089/end.2010.0055. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura K, Fujiyama C, Nakashima K, Satoh Y, Tokuda Y, Uozumi J. The effects of neoadjuvant chemotherapy and chemo-radiation therapy on MRI staging in invasive bladder cancer: comparative study based on the pathological examination of whole layer bladder wall. Int Urol Nephrol. 2009;41:869–875. doi: 10.1007/s11255-009-9566-5. [DOI] [PubMed] [Google Scholar]
- 7.Lambregts DM, Lahaye MJ, Heijnen LA, Martens MH, Maas M, Beets GL, Beets-Tan RG. MRI and diffusion-weighted MRI to diagnose a local tumour regrowth during long-term follow-up of rectal cancer patients treated with organ preservation after chemoradiotherapy. Eur Radiol. 2016;26(7):2118–2125. doi: 10.1007/s00330-015-4062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner M, Ronot M, Doblas S, Giraudeau C, Van Beers B, Belghiti J, et al. Assessment of the residual tumour of colorectal liver metastases after chemotherapy: diffusion-weighted MR magnetic resonance imaging in the peripheral and entire tumour. Eur Radiol. 2016;26:206–215. doi: 10.1007/s00330-015-3800-6. [DOI] [PubMed] [Google Scholar]
- 9.Avcu S, Koseoglu MN, Ceylan K, Bulut MD, Unal O. The value of diffusion-weighted MRI in the diagnosis of malignant and benign urinary bladder lesions. Br J Radiol. 2011;84:875–882. doi: 10.1259/bjr/30591350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3:573–589. doi: 10.1007/s13244-012-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Just N. Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer. 2014;111:2205–2213. doi: 10.1038/bjc.2014.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alic L, Niessen WJ, Veenland JF. Quantification of heterogeneity as a biomarker in tumor imaging: a systematic review. Plos One. 2014;9:e110300. doi: 10.1371/journal.pone.0110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerome NP, Miyazaki K, Collins DJ, Orton MR, d’Arcy JA, Wallace T, et al. Repeatability of derived parameters from histograms following non-Gaussian diffusion modelling of diffusion-weighted imaging in a paediatric oncological cohort. Eur Radiol. 2016 doi: 10.1007/s00330-016-4318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen EK, Kristensen GB, Lyng H, Malinen E. Pharmacokinetic analysis and k-means clustering of DCEMR images for radiotherapy outcome prediction of advanced cervical cancers. Acta Oncol. 2011;50:859–865. doi: 10.3109/0284186X.2011.578586. [DOI] [PubMed] [Google Scholar]
- 15.Docquier PL, Paul L, Menten R, Cartiaux O, Francq B, Banse X. Measurement of bone cyst fluid volume using k-means clustering. Magn Reson Imaging. 2009;27:1430–1439. doi: 10.1016/j.mri.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Gray C, MacGillivray TJ, Eeley C, Stephens NA, Beggs I, Fearon KC, et al. Magnetic resonance imaging with k-means clustering objectively measures whole muscle volume compartments in sarcopenia/cancer cachexia. Clin Nutr. 2011;30:106–111. doi: 10.1016/j.clnu.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen HT, Jia G, Shah ZK, Pohar K, Mortazavi A, Zynger DL, Wei L, Yang X, Clark D, Knopp MV. Prediction of chemotherapeutic response in bladder cancer using K-means clustering of dynamic contrast-enhanced (DCE)-MRI pharmacokinetic parameters. J Magn Reson Imaging. 2015;41:1374–1382. doi: 10.1002/jmri.24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasan A, Galban CJ, Johnson TD, Chenevert TL, Ross BD, Mukherji SK. Utility of the k-means clustering algorithm in differentiating apparent diffusion coefficient values of benign and malignant neck pathologies. AJNR Am J Neuroradiol. 2010;31:736–740. doi: 10.3174/ajnr.A1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abou-El-Ghar ME, El-Assmy A, Refaie HF, El-Diasty T. Bladder cancer: diagnosis with diffusion-weighted MR imaging in patients with gross hematuria. Radiology. 2009;251:415–421. doi: 10.1148/radiol.2503080723. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen HT, Pohar KS, Jia G, Shah ZK, Mortazavi A, Zynger DL, et al. Improving bladder cancer imaging using 3-T functional dynamic contrast-enhanced magnetic resonance imaging. Investig Radiol. 2014;49:390–395. doi: 10.1097/RLI.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daggulli M, Onur MR, Firdolas F, Onur R, Kocakoc E, Orhan I. Role of diffusion MRI and apparent diffusion coefficient measurement in the diagnosis, staging and pathological classification of bladder tumors. Urol Int. 2011;87:346–352. doi: 10.1159/000330925. [DOI] [PubMed] [Google Scholar]
- 22.El-Assmy A, Abou-El-Ghar ME, Mosbah A, El-Nahas AR, Refaie HF, Hekal IA, et al. Bladder tumour staging: comparison of diffusion- and T2-weighted MR imaging. Eur Radiol. 2009;19:1575–1581. doi: 10.1007/s00330-009-1340-7. [DOI] [PubMed] [Google Scholar]
- 23.Kilickesmez O, Cimilli T, Inci E, Kayhan A, Bayramoglu S, Tasdelen N, et al. Diffusion-weighted MRI of urinary bladder and prostate cancers. Diagn Interv Radiol. 2009;15:104–110. [PubMed] [Google Scholar]
- 24.Kobayashi S, Koga F, Kajino K, Yoshita S, Ishii C, Tanaka H, et al. Apparent diffusion coefficient value reflects invasive and proliferative potential of bladder cancer. J Magn Reson Imaging: JMRI. 2014;39:172–178. doi: 10.1002/jmri.24148. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi S, Koga F, Yoshida S, Masuda H, Ishii C, Tanaka H, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol. 2011;21:2178–2186. doi: 10.1007/s00330-011-2174-7. [DOI] [PubMed] [Google Scholar]
- 26.Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, Katsuoka Y. Diffusion-weighted MR imaging for urinary bladder carcinoma: initial results. Eur Radiol. 2007;17:201–204. doi: 10.1007/s00330-006-0281-7. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T, et al. Urinary bladder cancer: diffusion-weighted MR imaging–accuracy for diagnosing T stage and estimating histologic grade. Radiology. 2009;251:112–121. doi: 10.1148/radiol.2511080873. [DOI] [PubMed] [Google Scholar]
- 28.Suo ST, Chen XX, Fan Y, Wu LM, Yao QY, Cao MQ, et al. Histogram analysis of apparent diffusion coefficient at 3.0 T in urinary bladder lesions: correlation with pathologic findings. Acad Radiol. 2014;21:1027–1034. doi: 10.1016/j.acra.2014.03.004. [DOI] [PubMed] [Google Scholar]