Abstract
Endothelial cells (ECs) are the fundamental building blocks of the vascular architecture and mediate vascular growth and remodeling to ensure proper vessel development and homeostasis. However, studies on endothelial lineage hierarchy remain elusive due to the lack of tools to gain access as well as to directly evaluate their behavior in vivo. To address this shortcoming, a new tissue model to study angiogenesis using the mammary fat pad has been developed. The mammary gland develops mostly in the postnatal stages, including puberty and pregnancy, during which robust epithelium proliferation is accompanied by extensive vascular remodeling. Mammary fat pads provide space, matrix, and rich angiogenic stimuli from the growing mammary epithelium. Furthermore, mammary fat pads are located outside the peritoneal cavity, making them an easily accessible grafting site for assessing the angiogenic potential of exogenous cells. This work also describes an efficient tracing approach using fluorescent reporter mice to specifically label the targeted population of vascular endothelial stem cells (VESCs) in vivo. This lineage tracing method, coupled with subsequent tissue whole-mount microscopy, enable the direct visualization of targeted cells and their descendants, through which the proliferation capability can be quantified and the differentiation commitment can be fate-mapped. Using these methods, a population of bipotent protein C receptor (Procr) expressing VESCs has recently been identified in multiple vascular systems. Procr+ VESCs, giving rise to both new ECs and pericytes, actively contribute to angiogenesis during development, homeostasis, and injury repair. Overall, this manuscript describes a new mammary fat pad transplantation and in vivo lineage tracing techniques that can be used to evaluate the stem cell properties of VESCs.
Keywords: Developmental Biology, Issue 126, Vascular endothelial stem cells, endothelial hierarchy, angiogenesis, vascular regeneration, vascular remodeling, lineage tracing, mammary gland, fat pad transplantation
Introduction
During development and homeostasis, vascular growth and remodeling faithfully take place in accordance with organ growth and repair. Angiogenesis describes the generation of new vessels from pre-existing blood vessels and is deemed a major force mediating these dynamic vascular changes. Each blood vessel is inner-lined with a layer of endothelial cells (ECs), and they appear to be the foundation of vessel architecture. For a long time, the mechanism through which the EC pool is replenished during homeostasis remained unclear, and arguments were raised over whether vascular turnover is the result of mature EC proliferation or is the contribution of vascular stem/progenitor cell activities. Due to the lack of direct physiological evidence, the existence and cellular identity of vascular endothelial stem cells (VESCs) also remained controversial.
One of the most common approaches used to verify stem cell behavior is through the transplantation of putative stem cells into recipient mice. This method measures the stemness potential of candidate stem cells in vivo. Transplantation was first applied to the study of bone marrow stem cells1, which contributed to the establishment of the hierarchical characteristics of the hematopoietic system2. In the endothelial field, a basement membrane matrix (e.g., matrigel) plug inserted subcutaneously under the flank skin has been a standard in vivo angiogenesis assay used to address the vessel formation capabilities of transplanted ECs. Multiple experimental methods, including colony formation in 3D culture systems and transplantation, have suggested potential EC progenitor/VESC populations3,4,5,6. However, since ECs embedded in basement membrane matrix are relatively separate from the surrounding tissue, this does not provide the optimal niche environment required to fully explore the angiogenic potential of transplanted cells. As a result, vessels formed within the matrix plug are predominantly capillary-like and are functionally unmeasurable.
The mammary gland develops postnatally, with the most robust growth occurring during puberty and pregnancy. At the pubertal stage, the mammary epithelium undergoes rapid expansion, to occupy the whole mammary fat pad, accompanied by the efficient remodeling of the surrounding vascular structures. Thus, the mammary gland offers an excellent model for the study of angiogenesis. It provides space, matrix, and rich angiogenic stimuli from the growing mammary epithelium and therefore is an ideal grafting site for assessing the angiogenic potential of exogenous cells. In addition, the mammary fat pad allows the formed exogenous vessels to integrate with the host circulation system, enabling further functional evaluation and representing an advantage over subcutaneous transplantation.
Although in vitro culturing and transplantation assays are as effective way to investigate the regeneration properties of a cell population, it is known that such assays may stimulate plasticity as cells are taken away from their native habitats, and changes might be induced when cells are disconnected from their physiological surroundings7. Therefore, obtaining direct in vivo evidence of cell fate is the key approach to advancing the current understanding of the behavior of endothelial populations.
Genetic fate mapping (i.e., in vivo lineage tracing) is imperative for the identification of VESCs and for the investigation of their properties in the body system, as it can reveal in vivo stem cell behavior in its physiological context, and the actual stemness can be assessed. Lineage tracing provides direct evidence of the long-term persistence (i.e., self-renewal) of candidate VESCs and their ability to produce cell types for the tissue of origin (i.e., differentiation potency).
This protocol describes a novel mammary fat pad transplantation technique and a lineage tracing method to observe the vessel generation capability of VESCs. These techniques overcome shortcomings of currently available assays and provide a new way to optimally evaluate the stem cell properties of VESCs. These approaches are efficient tools that can be used to assess the behavior and vessel-forming properties of endothelial populations, as well as to determine vascular cell potency alteration within a pathological environment.
Protocol
Experimental procedures were approved by the Animal Care and Use Committee of Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences under the approved protocol SIBCB-NAF-15-002-S335-003.
1. Isolation of VESCs from the Mouse Mammary Gland
- Mammary gland harvest and tissue digestion.
- Use 8 week-old Actin-GFP mature virgin reporter mice, with weights range between 20 and 25 g, as tissue donors. Note: Cells originated from the donor can be easily tracked by GFP expression.
- Prepare tissue digestion base medium consisting of 5% fetal bovine serum (FBS), 1% pen/strep, and 25 mM HEPES in RPMI1640. Filter the mixture through a 0.22 µm filter to sterilize and pre-warm it to 37 °C in a water bath.
- Sacrifice the mice in a CO2 chamber. Dissect out the fourth pair of inguinal mammary glands by first making a vertical skin incision at the lower abdominal midline using a scalpel (or surgical scissors). Gently peel the skin to one side to expose the inguinal mammary gland using forceps. Obtain the fourth inguinal mammary glands from both sides and cut them into fine pieces in a 6 cm Petri dish using scissors.
- Measure the total weight of minced tissue and place it in a 50 mL tube. Note: Make sure the resultant tissue mince is smaller than 1 mm3 in size for each piece. A typical yield is approximately 5,000 VESCs per animal.
- Prepare digestion base medium in a 50-mL capped centrifuge tube at the volume of 10 mL per 1 g of tissue mince. Add collagenase Type 3 at the concentration of 300 units per 10 mL of digestion base medium to constitute the digestion buffer. Mix evenly and add it to the tissue mince.
- Horizontally place the 50-mL centrifuge tube on a rocking platform and set the speed to 100 rpm and the temperature to 37 °C. Agitate the mammary tissue for 2 h. Check and manually shake the tube every 20 min to ensure proper digestion. Note: The purpose of tissue digestion prior to FACS is to prepare a single-cell solution from solid tissue. Therefore, by the end of digestion, the content should be a thick solution without visible tissue pieces.
- Single-cell solution preparation.
- After digestion, top-up the 50 mL centrifuge tube with pre-warmed, sterile PBS and invert the tube to mix evenly. Centrifuge the tube at 200 x g for 5 min to obtain a cell pellet.
- Remove the supernatant and flick the bottom of the tube to loosen up the cell pellet. Add in 3 mL of red blood cell lysing buffer (see the Table of Materials) and incubate in a tissue culture hood at room temperature (RT) for 5 min to eliminate the red blood cells.
- Add 10 mL of sterile PBS and invert the tube to mix evenly. Centrifuge the tissue content at 200 x g for 5 min and discard the supernatant. Add in 3 mL of pre-warmed 0.05% trypsin and store the tube at 37 °C for 5 min to further digest the remaining tissue.
- Add in 3 mL of pre-warmed IMDM and then add 100 µL of DNase I solution (0.25 mg of DNase I diluted in 1 mL of PBS). Store the tube at 37 °C to break up the DNA filaments. Pass the tissue mixture through a 70-µm cell strainer to obtain the cell solution. Rinse the strainer twice with 2 mL of PBS + 5% FBS to neutralize the enzymatic digestion.
- Centrifuge the cell mixture at 200 x g for 5 min to pellet the cells. Discard the supernatant and resuspend the cell pellet in 1 mL of PBS + 5% FBS for antibody staining.
- FACS-based Procr+ VESC isolation.
- Subject the cell mixture obtained from donor mammary tissue to antibody staining following published routine protocols8. Note: The following antibodies were used: anti-mouse blood lineage-FITC, anti-mouse CD31(PECAM1)-PECY7, anti-mouse CD105-APC (all used at a concentration of 1 µg/mL), anti-mouse CD201 (Procr)-biotin (used at a concentration of 2.5 µg/mL), and streptavidin-v450 (used at a concentration of 0.5 µg/mL).
- After antibody staining, top-up the cells with PBS + 5% FBS and then centrifuge at 200 g for 5 min. Discard the supernatant and resuspend the cells with 1 mL of PBS + 5% FBS. Filter through a 40-µm cell strainer. Place on ice until FACS sorting.
- Proceed to perform FACS-based sorting to isolate Procr+ VESCs.
- Analyze the unstained sample and each single-color control to determine the voltage for each color. Calculate the compensation parameters to avoid auto-fluorescence or background staining. Set up the template for proper gating and sorting for the desired cell populations.
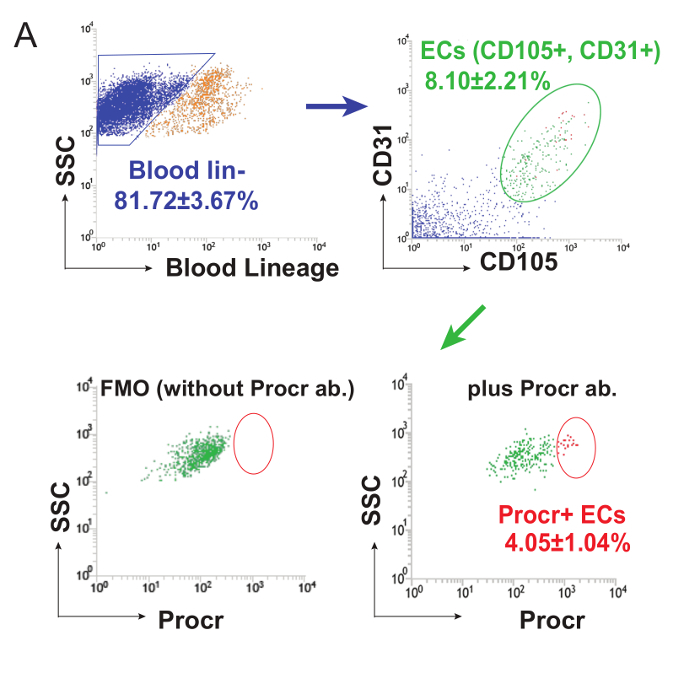
- To establish the hierarchy of FACS sorting, first eliminate debris from all events and then discard doublets or adhesive cell clusters by triggering with width. Gate-out blood lineage-cells and then use CD31+ and CD105+ to define the endothelial populations. Note: Procr+ cells were isolated from CD31+ CD105+ ECs compared to an FMO control. A representative FACS analysis plot is shown in Figure 1. A FACS-isolated VESC population should be suspended in IMDM + 50% FBS and stored on ice until further processing.
2. In Vivo VESC Vessel Formation Assay Using the Mammary Fat Pad
- Prepare primary ECs for transplantation.
- Top-up the FACS-sorted cells with 10 mL of PBS + 5% FBS and then centrifuge at 200 x g for 5 min to settle the cells down to the bottom.
- Aspirate the liquid carefully, count the cell number using a hemocytometer, and resuspend the cell pellet with basement membrane matrix mixture (consisting of 50% basement membrane matrix and PBS with 20% FBS, supplemented with 100 ng/mL rmVEGF and 60 U/mL heparin) to reach a working concentration of 15,000 cells/15 µL. Note: The basement membrane matrix mixture should always be stored on ice.
- Serially dilute the sorted and counted cells to reach the required input concentrations in a 1.5-mL microtube, making sure that the final injection volumes do not exceed 15 µL in total. Add 0.1% Trypan blue to each tube for better content visualization during transplantation. Pipette thoroughly to mix. Store the tubes on ice until use. Note: Successful vessel generation could be achieved with a minimum of 100 VESCs.
- Transplant primary ECs to the mammary fat pad.
- Disinfect the surgical instruments and benchtop. If using the same instruments on multiple animals, a bead sterilzer can be used for disinfection.
- Anesthetize 3-week-old BALB/c nude mice by using the anesthetic protocol recommended by your institution's veterinarian and IACUC. Lay each mouse in supine position, with its back on the operation table. Immobilize its limbs using adhesive tape.
- Dip a cotton swab into iodine-based antiseptic solution. Wipe the entire abdomen area of the mouse thoroughly to sterilize the skin surface before surgery.
- Hold and lift the abdomen skin of the mouse with forceps. Use a surgical scalpel to make a small, 1 cm vertical incision. Make two cuts about 0.5 - 1 cm in length, each towards the hind leg, to achieve an upside-down, Y-shaped incision.
- Use forceps and a cotton swab to gently separate the skin from the abdomen and pin the skin sideways to expose the inguinal mammary glands. Place the mouse under a stereoscope and set the magnification to 2.0X. Make sure to adjust the focus so that the mammary fat pad can be clearly seen and operated on.
- Place a 27G 1/2" needle on ice to pre-chill. Prime a 1-mL syringe with basement membrane matrix mixture in advance to remove possible air bubbles trapped in the needle-attaching region. Pipette out 15 µL of mixed solution onto the cap of the microcentrifuge tube and then aspirate the entire volume into the primed syringe.
- Hold the mammary fat pad in front of the inguinal lymph node, using fine tweezers, and lift it slightly for easy injection. Insert about 1/4 of the syringe needle gently into the fat pad.
- Release the tweezers from the fad pad to grip and stabilize the syringe needle. Inject the solution slowly to prevent liquid leak. Hold for a few seconds to make sure that the cell mixture is solidified to minimize the leakage when pulling out the needle.
- Close the skin flap with absorbable sutures. Suture the skin flap using size 5-0 non-reactive monofilament suture and close the wounds with a surgeon's-style knots, each knot approximately 0.3 cm apart. Alternatively, close the skin with wound staples.
- Place operated mice in a separate cage for recovery. Place a heating lamp over the cage to prevent the anesthetized mice from suffering from hypothermia. Place soft paper and cotton at the bottom of the cage to keep the mice warm post-surgery.
- Observe the mice carefully until all recipients are fully awake. For the next few days, apply post-surgical analgesics pre-emptively to all animals or as directed by the facility veterinarian. Apply antibiotics according to the approved animal protocol if wound infection occurs. If the surgical wound was closed with wound staples, remove the wound clips 10 days after surgery, when the wound has fully closed.
3. Whole-mount Tissue Preparation for Microscopic Observation
- Tissue harvest
- At 2 - 4 weeks post-implantation, anesthetize the recipient BALB/c nude mice with an intra-peritoneal injection of 2.5% anesthetizing agent at a dosage of 0.12 mL per 20 g of bodyweight, as in step 2.2.2. Note: Positive vessel growth can be detected 2 - 4 weeks after cell transplantation.
- Label the recipient systemic circulation with a tail-vein injection of isolectin-647 dye at a dosage of 50 µg/25 g of bodyweight, resuspended in 50 µL of sterile PBS. Allow a resting period of 5 - 10 min. Sacrifice the mice using CO2.
- Dissect out the area of mammary tissue where the cells were initially injected following step 2.2.4; use surgical scissors. Using a surgical blade, chop the tissue into roughly 3- to 4-mm3 cubes in a 6-cm Petri dish.
- Tissue digestion and preparation for antibody staining.
- Digest the tissue pieces in a 15 mL centrifuge tube filled with 10 mL of pre-warmed digestion base medium (step 1.1.2) supplemented with collagenase III (300 U per 10 mL).
- Place the 15 mL centrifuge tube horizontally on a rocking platform with the speed set to 100 rpm and the temperature set to 37 °C for approximately 1 h to loosen up the tissue structure and to remove excessive adipocyte tissue. Note: Adipocytes tend to produce auto-fluorescence which could result in a noisy fluorescent background. By the end of the digestion, the tissue pieces should have a soft, cloudy appearance. The optimal digestion period depends upon the size of chopped tissue pieces and should therefore be adjusted accordingly to obtain the best results. Since the tissue vasculature has already been labeled with fluorescence-tagged Isolectin, all following procedures should be carried out protected from light.
- Wash the tissue pieces with PBS at RT for 10 min. Fix the whole-mount tissue by carefully placing the pieces into a 6-cm Petri dish half-full with 4% PFA (pH 7.2 - 7.4) to preserve the fluorescence. Place the Petri dish on a rocking platform and gently agitate the tissue for 30 min at RT. Note: PFA is carcinogen and should be handled with caution.
- Remove the remaining PFA and wash the fixed tissue three times with whole-mount wash buffer (0.1% tween in PBS), with a 10-min interval between each wash.
- Whole-mount tissue antibody staining.
- Dilute the primary antibody in antibody staining buffer in a 2 mL centrifuge tube. Incubate the tissue with primary antibody overnight at 4 °C. Note: Rat anti-CD31 (concentration: 10 µg/mL) or rat anti-VE-Cadherin (CD144/Cdh5; concentration: 2.5 µg/mL) were used as the primary antibodies. Both CD31 and VE-Cadherin are cell surface markers used to recognize endothelium. 5x antibody staining buffer is prepared with 500 mM maleic acid, pH 7.5; 750 mM NaCl; and 0.5% (v/v) tween dissolve in PBS. Only add in an appropriate serum volume (5%) when preparing fresh 1x working solution9.
- Remove the antibody staining buffer and wash the whole-mount tissue three times with wash buffer, with a 10-min interval. Incubate the tissue with secondary antibody (see the Table of Materials) plus DAPI at RT for 4 h on a rocking platform or overnight at 4 °C. Note: Use DAPI (4',6-diamidino-2-phenylindole) at a final concentration of 5 µg/mL to visualize the nuclei.
- Wash the whole-mount tissue with wash buffer 3 times for 10 min each. Incubate the tissue pieces in 80% glycerol or 80% sucrose overnight to dehydrate the tissue for better preservation.
- Place the individual tissue pieces onto a glass slide and remove the excessive dehydration buffer. Cover the tissue with mounting medium, place a coverslip, and gently squeeze the tissue to set it.
- Perform confocal microscopic image acquisition. For whole-mount mammary glands, acquire images using a 40X with N.A. = 1.3 oil objective. Acquire tile scans of Z-stacks at an optical section resolution of 1,024 x 1,024 and a layer separation of 1.0 - 1.2 µm. Note: To capture the correct fluorescence light signal, the filter beam splitter should be set to triple dichroic (TD) 488/553/638, with the gain setting at PMT Gain 600-800. The following excitation/emission sources should be used: mGFP, with excitation/emission maxima of 488/509 nm; mTomato with excitation/emission maxima of 532/588 nm; Alexa-647, with excitation/emission maxima of 594/665; and DAPI, with excitation/emission maxima of 350/470.
4. Lineage Tracing Using a Genetically Modified Mouse Model
- Detect Procr+ VESCs using the mouse model.
- Use the ProcrCreERT2-IRES-tdTomato/+ (referred to as ProcrCreERT2) knock-in mouse strain. Note: In this mouse model, a CreERT2-IRES-tdTomato cassette10 is inserted after the first ATG codon of Procr11.
- Cross ProcrCreERT2 mice with the Rosa26mTtomatomGFP (referred to as R26mTmG) reporter strain to trace the fate of endogenous Procr+ ECs in vivo11. Identify all labeled endogenous Procr+ ECs and their descendent using GFP expression.
- Administer tamoxifen solution11 (10 mg of tamoxifen dissolved in 1 mL of peanut oil + 10% ethanol to make up a stock solution of 10 mg/mL) intraperitoneally at a dose of 4 mg/25 g bodyweight during the pubertal stage (5 weeks old) to track the developmental fate of Procr+ ECs.
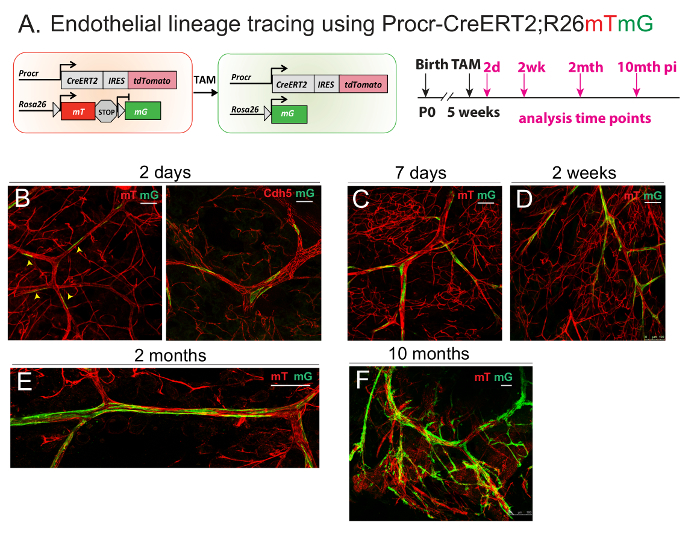
- Analyze the clone-forming efficiency of labeled cells by whole-mount immunostaining following the preparation steps outlined in step 3. Obtain mammary fat pads from treated mice both after short-term (2 days) and extended time periods (up to 10 months). See Figure 3.
Representative Results
Mammary VESCs Isolation:
To isolate the endothelial population for the consequent transplantation assay, mature (8 week-old), virgin mouse mammary glands were harvested as donor material. Endothelial cells were isolated using an antibody-based cell sorting technique. Representative plots of the FACS analysis of the VESC population in 8-week-old C57BL/6 mammary gland ECs are shown in Figure 1 (Figure adapted from Yu et al.8, with permission).
Mammary Fat Pad Transplantation:
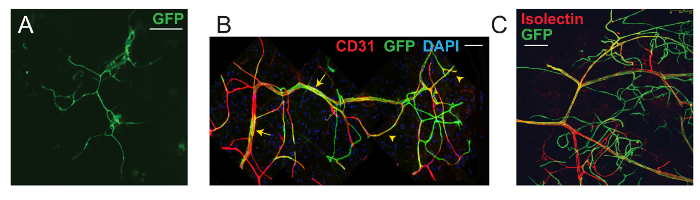
The mammary fat pad provides an ideal site for the study of angiogenesis. After FACS-based isolation, Procr+ ECs were mixed with 0.5% basement membrane matrix and 0.1% Trypan blue indicator and were then transplanted to the empty fat pad of 3-week-old SCID recipients. During puberty, the mammary fat pad creates an angiogenic environment, promoting the remodeling of the vasculature in alignment with mammary epithelium expansion. As a result, transplanted Procr+ ECs (GFP+) incorporated into the large vessels of the host (Figure 2B, arrows) and also formed secondary and tertiary GFP+ vessel branches (Figure 2B, arrowheads). Although transplanted Procr+ ECs can also generate vessels in a matrix plug assay (inserted under the flank skin), the formed vessels only appear capillary-like (Figure 2A; figure adapted from Yu et al.8, with permission).
Functionality of the Vessels Formed by Procr+ VESCs:
To investigate whether the GFP+ vessels in mammary fat pads are part of the functional circulatory vasculature, isolectin was administered by intravenous injection prior to harvest. The GFP+ vessels were isolectin+, indicating that the formed vessels are luminized and connected with the host vasculature (Figure 2B; figure adapted from Yu et al.8, with permission).
Mammary VESC Lineage Tracing Using the ProcrCreERT2; Rosa26mTmGMouse Model:
ProcrCreERT2; Rosa26mTmG mice were used for the fate tracing of Procr+ VESCs in vivo (Figure 3A). To investigate how Procr+ VESCs contribute to the robust angiogenesis and vascular remodeling during mammary gland pubertal development, ProcrCreERT2; Rosa26mTmG were administered with tamoxifen to induce GFP expression in the Procr+ endothelial population. The mammary fat pads of drug-treated ProcrCreERT2; Rosa26mTmG mice were harvested both short-term (2 days post-injection; Figure 3B) and long-term (up to 10 months post-injection; Figure 3C-F; figure adapted from Yu et al.8, with permission). The whole-mount preparation was carried out to visualize the tracing outcome. The vascular endothelium identity can be validated by staining with endothelial surface marker VE-Cadherin (Cdh5; Figure 3B, right image).
Figure 1: Analysis of VESCs in Mouse Mammary Gland. FACS analysis of Procr expression in 8-week-old C57BL/6 mammary gland ECs. 8 week-old virgin C67BL/6 female mice were used for mammary gland collection. After cell samples were prepared, unstained sample and each single-color control were analyzed to determine the voltage for each color. The compensation parameters were calculated to avoid auto-fluorescence or background staining. A template was established for proper gating and to sort for the desired cell populations. To establish the hierarchy of FACS sorting, debris from all events was eliminated and then doublets or adhesive cell clusters were discarded. Lineage- cells were gated out, and CD31 and CD105 were used to define the endothelial populations. Procr+ cells were isolated from CD31+ CD105+ ECs compared to FMO control. This figure has been modified from Yu et al.8, with permission. Please click here to view a larger version of this figure.
Figure 2: VESC Mammary Fat Pad Transplantation Assay. (A) A vessel formed from Procr+ ECs within a basement membrane matrix plug inserted subcutaneously under the flank skin. (B) Confocal image of a recipient mammary fat pad, indicating the integration and contribution of transplanted Procr+ ECs (GFP+) to host mammary vasculature. ECs were counterstained with CD31. (C) Intravenous injection in recipient mice with isolectin showed that the outgrowths formed luminized vessels. Scale bar, 50 µm. Images were acquired using a 40X N.A. 1.3 oil objective. Tile scans of Z-stacks were acquired at an optical section resolution of 1,024 x 1,024, and each layer was 1.0 - 1.2 µm apart. To capture the correct fluorescence light signal, the filter beam splitter was set to TD 488/553/638, with the gain setting at PMT Gain 600 - 800. The following excitation/emission sources were used: mGFP, with excitation/emission maxima of 488/509 nm; mTomato, with excitation/emission maxima of 532/588 nm; Alexa-647, with excitation/emission maxima of 594/665; and DAPI, with excitation/emission maxima of 350/470. This figure has been modified from Yu et al.8, with permission. Please click here to view a larger version of this figure.
Figure 3: Lineage Tracing of Procr+ ECs Demonstrates their Contribution to EC Clonal Expansion During Development. (A) Illustration of the lineage tracing strategy using ProcrCreERT2; Rosa26mTmG line (Left panel). Experimental setup used in the short-term (2 days) and long-term (7 days to 10 months), as indicated (Right panel). (B-F) Whole-mount confocal imaging of mammary vasculature after different lengths of tracing periods, indicating the initial location of labeled Procr+ ECs and their contribution towards mammary vasculature at various tracing stages. Scale bar = 50 µm. This figure has been modified from Yu et al.8, with permission. Please click here to view a larger version of this figure.
Discussion
Angiogenesis assays represent a good experimental approach to study vascular dynamics. Mouse retinal vasculature, which develops postnatally, has proven to be an attractive model to study angiogenesis12. Despite it being relatively accessible, actual manipulation within the retina vascular bed is rather difficult. So far, the best-described in vivo transplantation has been the plug assay, which encloses cells inside a basement membrane matrix mass and surgically implants/injects subcutaneously at the flank region of a recipient mouse. This angiogenesis assay is effective at testing the regenerative potential and vessel-forming ability of embedded cells. However, since the location of the implanted matrix plug creates a comparatively isolated environment for the cells within, it does not provide an optimal physiological niche. Recently, a new lung angiogenesis model has also been developed by applying a fibrin patch onto the surface site of the mouse lung13. However, the manipulates pose yet another risk, because this technique operates invasively inside the mouse chest cavity, thus make it difficult to use in a more generalized assay setting.
In the angiogenesis assay described here, a small volume of cell mixture is transplanted inside the fat pad of the mammary gland, which provides space, matrix, and rich angiogenic stimuli during puberty. This allows the generated vessels to comply and integrate into remodeling host vasculature, enabling further functional evaluation, as well as representing an advantage over the subcutaneous plug assay. Also, the mammary fat pad is located outsiade the peritoneal cavity, making it a very accessible site. Operation on the mammary fat pad introduces limited distress to the recipient animal, avoiding the invasive surgical procedures involved in retinal and lung-based assays.
Blood vasculature within an organ often intertwines between tissue components to maximize the vascular coverage so that the provision of blood oxygen and material transfer can be optimized. For that reason, the conventional sectioning of the tissue frequently intercepts the tubular structure of its vessels. Analysis on vascular properties is best carried out when the vessels can be preserved and remain intact. The whole-mount preparation of a tissue can largely preserve the entirety of the vascular architecture within. This method allows not only the observation of the authentic spatial distribution of the blood vessel within an organ, but also the relationship with other tissue components.
The homeostasis of a stem-cell-containing tissue is sustained by balancing the generation and loss of cell mass. Stem cells within the specific inhabiting organ are often sheltered and in close contact with the surrounding tissue environment, referred to as the stem cell niche. Therefore, studies analyzing adult stem cell tissue should preferably be performed in an intact physiological context. Transplantation assays could provide a means of assessing the cell potential, while the lineage-tracing technique enables the exploration of true cell fate within the native habitat. In recent years, in vivo lineage tracing has become a powerful technique for the experimental evaluation of stemness properties. This strategy has been used to identify tissue residue stem cells and their offspring in multiple tissues, including the intestine10,14, hair follicles15, stomach16, and pancreas17. The lineage tracing method largely relies on the genetic marking of stem cells and is initiated in defined populations. Therefore, it is difficult to exclude the possibility that actual stemness is found in an even smaller subpopulation within the labeled cells. It is therefore imperative to map the cell-of-origin of traced clones with precision and to quantitatively measure the tracing efficiencies over time. In that way, the self-renewal capacity of the labeled population can be apprehended.
The described angiogenesis assay can be modified for specific study purposes: it can be used not only to test the vessel generation ability of endothelial populations of interest, but also to evaluate the effect of angiogenic factors on localized vessel remodeling. However, since the mammary gland undergoes dynamic morphological changes during different developmental stages (e.g., puberty, estrus cycles, and pregnancy) one should take into consideration the effect of systemic changes, such as hormonal level fluctuation, during result interpretation. The critical steps in this assay include primary endothelial cell isolation. To optimally obtain viable cells for transplantation, procedures for FACS isolation should be carried out gently. Caution should be taken to avoid harsh handling during cell preparations, such as cell pellet loosening and suspending. Another critical step is the cell mixture injection into the fat pat. During the fat pad injection, it is important to ensure that the cell mixture is precisely deposited inside the fat pad. This can be tricky if the needle insertion is too deep/shallow or if the overall volume exceeds the recommended dosage.
This protocol provides a series of experimental methods that assisted in the identification of an endothelial stem cell population. Through these techniques, it was possible to evaluate stem cell properties through transplantation, as well as to track and observe their behavior under physiological processes in vivo. These approaches are efficient tools that can be applied to multiple disciplines, including studies of endothelial populations in tumor environments and of alterations in their cell potency. In particular, the fat pad transplantation and whole-mount preparation are reproducible and powerful methods that may aid in future efforts to investigate different aspects of vascular biology.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31530045 and 31371500 to Y.A.Z., 31401245 to Q.C.Y.), the Ministry of Science and Technology of China (2014CB964800), The Chinese Academy of Sciences (XDB19000000 to Y.A.Z.), and the Chinese Society of Cell Biology (Early Career Fellowship to Q.C.Y.).
References
- Thomas ED, Lochte HL, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- Chao MP, Seita J, Weissman IL. Establishment of a normal hematopoietic and leukemia stem cell hierarchy. Cold Spring Harb Symp Quant Biol. 2008;73:439–449. doi: 10.1101/sqb.2008.73.031. [DOI] [PubMed] [Google Scholar]
- Bompais H, et al. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood. 2004;103(7):2577–2584. doi: 10.1182/blood-2003-08-2770. [DOI] [PubMed] [Google Scholar]
- Fang S, Wei J, Pentinmikko N, Leinonen H, Salven P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 2012;10(10):e1001407. doi: 10.1371/journal.pbio.1001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito H, Kidoya H, Sakimoto S, Wakabayashi T, Takakura N. Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels. EMBO J. 2012;31(4):842–855. doi: 10.1038/emboj.2011.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder MC, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, Clevers H. Tracking adult stem cells. Embo Rep. 2011;12(2):113–122. doi: 10.1038/embor.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu QC, Song W, Wang D, Zeng YA. Identification of blood vascular endothelial stem cells by the expression of protein C receptor. Cell Res. 2016;26(10):1079–1098. doi: 10.1038/cr.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios AC, Fu NY, Lindeman GJ, Visvader JE. In situ identification of bipotent stem cells in the mammary gland. Nature. 2014;506:322–327. doi: 10.1038/nature12948. [DOI] [PubMed] [Google Scholar]
- Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Wang DS, et al. Identification of multipotent mammary stemcells by protein C receptor expression. Nature. 2015;517:81–U201. doi: 10.1038/nature13851. [DOI] [PubMed] [Google Scholar]
- Stahl A, et al. The Mouse Retina as an Angiogenesis Model. Invest Ophth Vis Sci. 2010;51(6):2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T, Mammoto A. Implantation of Fibrin Gel on Mouse Lung to Study Lung-specific Angiogenesis. J. Vis. Exp. 2014. p. e52012. [DOI] [PMC free article] [PubMed]
- Snippert HJ, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143(1):134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Barker N, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6(1):25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Solar M, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17(6):849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]


