Abstract
This is the case of a 53-year-old man with known coronary artery disease who underwent two exercise treadmill tests (ETT). The first test, which yielded an abnormal result, was undertaken shortly after he had drunk two cans of Red Bull, a popular energy drink (ED). A second ETT was undertaken 1 week later by the same team without EDs on board and the test result was normal. This case suggests that drinking EDs prior to an ETT could lead to a false positive result and should be discouraged prior to exercise testing.
Keywords: Arrhythmias, Ischaemic Heart Disease, Unwanted Effects / Adverse Reactions, Clinical Diagnostic Tests
Background
We believe that this is the first description of a false positive exercise treadmill test (ETT) resulting from energy drinks (EDs) taken prior to the test. The consumption of EDs has significantly increased over the last decade. The UK population consumed 600 million litres of EDs in 2014 compared with 370 million litres in 2008, with a market worth £1480 million, making up 4.1% of soft drinks consumption.1 These so-called ‘energy drinks’ are marketed as energy boosting and concentration enhancing as well as aiding athletic performance and weight loss. EDs are particularly popular with the adolescent and younger population. However, they are also being regularly drunk by people in older age groups, some of whom may have underlying coronary artery disease (CAD). Potential described effects have included cardiac arrhythmias, reduced coronary perfusion, increased myocardial workload, increased platelet aggregation, decreased endothelial function and even coronary artery vasospasm, which in turn may be risk factors for an acute myocardial infarction.2 3
Case presentation
A 53-year-old man presented to the hospital 9 hours after the onset of typical cardiac chest pain. The pain had lasted for 2 hours but he was pain free on arrival. His ECG showed partial right bundle branch block (QRS 113 ms) and no significant ST elevation. An echocardiogram was undertaken which showed preserved left ventricular function with mild concentric left ventricular hypertrophy (septum 1.1 cm, posterior wall 1.2 cm in diastole) and mild inferior hypokinesia. On admission, he was hypertensive at 175/105 mm Hg with a high-sensitivity troponin I at 349 (normal 0–59 ng/L), rising to 8636, 12 hours later. He underwent an angiogram with a view to intervention within 48 hours of his admission. This showed mild atheroma in an otherwise essentially normal left coronary tree. The right main coronary artery and posterolateral branch were free of disease but the posterior descending artery was occluded in its midportion. This was successfully wired and treated with a 2.75mm×23mm Xience drug-eluting stent. He was pain free throughout his admission and he was discharged the day following his procedure. His medications were aspirin 75 mg OD, clopidogrel 75 mg OD, atorvastatin 80 mg ON, ramipril 2.5 mg OD, bisoprolol 1.25 mg OD, amlodipine 5 mg OD and there were no concerns regarding adherence postdischarge.
As he was desperate to go on a skiing trip, 5 weeks post-myocardial infarction (MI), an ETT was organised 4 weeks after the index event to assess his functional state, look for ischaemia and provide appropriate advice. This yielded an abnormal result. The day after the first treadmill test, the patient informed the cardiology department that he had drunk two cans of Red Bull shortly before the test, thinking it would improve his performance. Accordingly, he was brought back 1 week later for repeat testing which showed a normal result.
Our centre's policy for ETT includes not eating in the hour preceding the test and discontinuation of rate limiting medications for 48 hours. The test results are detailed below.
Investigations
Treadmill test 1
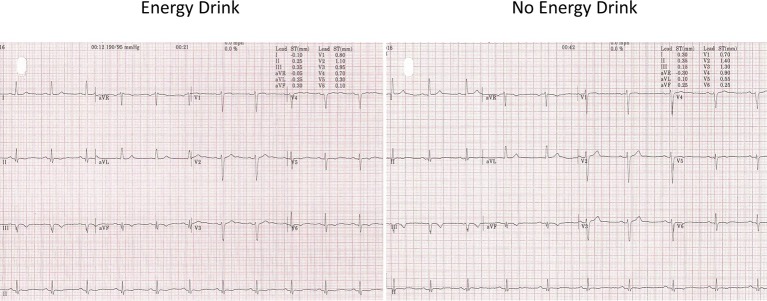
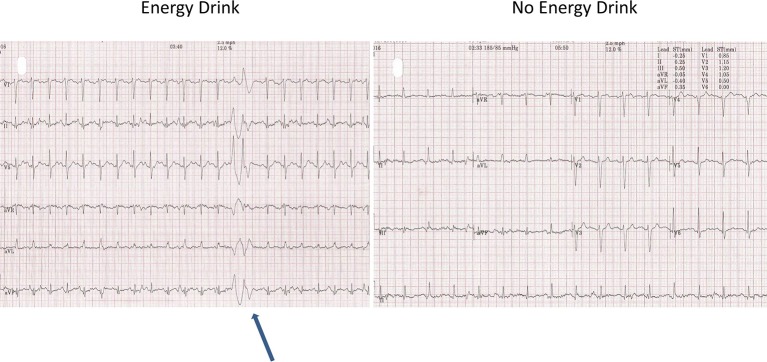
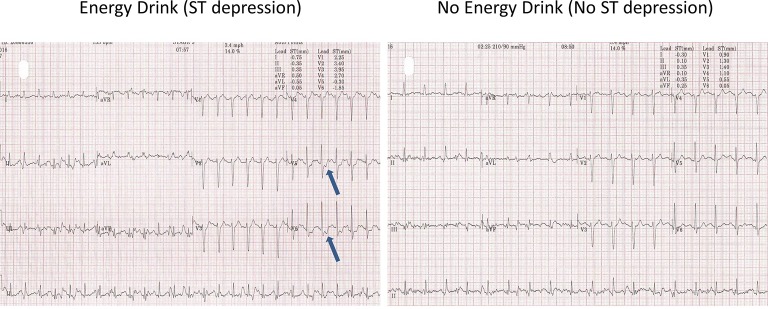
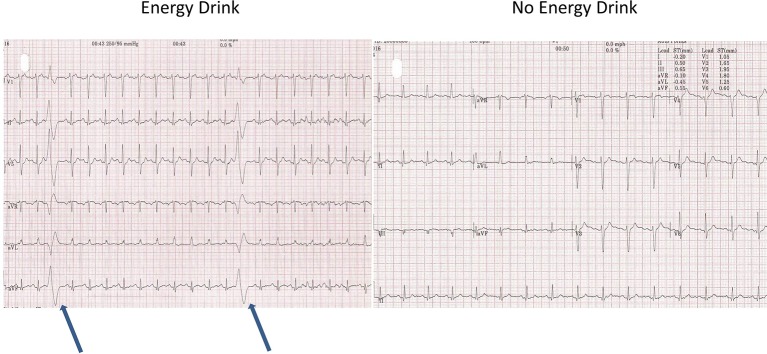
The resting blood pressure (BP) was high (190/95 mm Hg). The resting heart rate (HR) was 67 bpm and the resting ECG showed T-wave inversion in leads II, III, avF and V5-6 (figure 1, old ECG changes). During the test, the patient had difficulty in keeping coordinated on the treadmill. He denied any alcohol use. The patient managed 8.03 min on the Bruce protocol and achieved 10.40 Metabolic Equivalent of Tasks (METs) with a maximum HR of 153 bpm (91% of maximal age-predicted HR). The BP rose to a maximum of 250/100 and the test was stopped due to an exaggerated BP response. No chest pain was reported. During stage 1, premature ventricular contractions (PVCs) were seen. This was followed by further unifocal PVCs and a couplet in stage 2 (figure 2). During stage 2, upsloping ST depression was seen in leads V5-6. This progressed to 1.85 mm of horizontal ST depression during stage 3 in lead V6 and 0.5–1 mm of horizontal ST depression in lead V5 at maximum workload (figure 3). These ST changes resolved within 40 s of recovery. However, a minute into recovery, there was an increase in PVC frequency before subsequently settling (figure 4). The BP gradually returned to his baseline of 190/90 mm Hg.
Figure 1.
Resting ECG.
Figure 2.
Stage 2 couplet.
Figure 3.
Stage 3 ST depression after energy drink.
Figure 4.
Premature ventricular contractions in recovery.
Treadmill test 2
No changes in medications were made in between the tests. The same doctor and technician supervised the test. On this occasion, the resting BP and HR were 164/80 and 64 bpm, respectively. The patient managed 10.23 min on the BRUCE protocol and achieved 12.30 METS. The heart rate rose to 126 bpm (75% of age-predicted maximal HR) with an appropriate maximum BP response (220/100 mm Hg). On this occasion, no PVCs or ST changes were seen. No symptoms were reported and the patient had excellent coordination during the entire test.
Outcome and follow-up
The patient went on a skiing holiday following the second normal test without any complications and no adverse cardiovascular effects have occurred from initial discharge to the second exercise test. At 12-month follow-up, he was doing extremely well, has been on a second skiing holiday and is free of exertional chest pain.
Discussion
European guidelines only suggest exercise testing post-MI in patients with incompletely revascularised multivessel disease.4 They also go on to state that no generalisable recommendations can be made regarding the delay to resumption of activities but that decisions should be individualised based on left ventricular function, completeness of revascularisation and rhythm control.4 The American scientific statement is more detailed in this respect supporting the use of ETT in patients with CAD to predict cardiovascular events, all-cause death and to evaluate physical capacity, effort tolerance and exercise-related symptoms.5
The rationale for organising an ETT here prior to the skiing holiday is borne from the concern around high altitude and cold weather coupled with the planned strenuous physical activity.6–8 Acute hypoxia,9 physical activity, dehydration and cold weather all cause sympathetic activation at altitude resulting in vasoconstriction, a rise in heart rate and blood pressure, hence increasing cardiac output.10
This case highlights the ECG and haemodynamic changes that can occur on treadmill testing in a patient with known CAD after consumption of EDs. These abnormalities disappeared during the second ETT, with no EDs on board. Differences seen after ingestion of two EDs included impaired coordination on the treadmill, higher resting and peak BP, higher resting and peak HR, more arrhythmias (PVCs and couplets seen only when EDs were on board) and significant ST depression during exercise. Thus, when EDs were on board, the ETT was classified as ECG positive for ischaemia, but was reclassified as completely normal when no EDs were on board.
Other adverse cardiovascular events and findings have been described with EDs including increased platelet aggregation, decreased endothelial function, reduced coronary artery blood flow (worse in underlying CAD and hypoxic conditions), increased cardiac strain by increasing heart rate and blood pressure and possibly coronary artery vasospasm. There has also been the suggestion that they can trigger acute coronary syndromes, QT prolongation and cardiac arrest.2 3 11–19
Some of these changes are due to the high caffeine content in EDs. Namdar et al15 in a study of 30 subjects, 15 with CAD, with a mean age of 58, concluded that caffeine impairs the exercise-induced hyperaemic myocardial blood flow response in patients with CAD to a greater degree than in age-matched controls. Using positron emission tomography scanning to measure myocardial blood flow, they found that 50 min after oral caffeine intake, the latter did not affect resting coronary blood flow. However, exercise-induced myocardial blood flow was reduced by 14% in controls, 18% in patients with CAD in their non-affected coronary arteries and by 25% in patients with CAD in arteries that had stenotic segments. Caffeine may cause this by triggering catecholamine release and by directly inhibiting adenosine-mediated vasodilatation, with intrinsic adenosine production linked to the hyperaemic response to exercise.15 Namdar et al16 have also found that myocardial blood flow is reduced during hypoxia with up to a 39% reduction in flow reserve.
Caffeine has been proposed as a potential trigger for acute myocardial infarction.2 In a retrospective case-cross-over design study, Baylin et al2 found that in 503 incident cases of non-fatal MIs, occasional coffee drinkers had a 4.1-fold relative risk of suffering their MI within 1 hour of a cup of coffee. This was less common in more frequent coffee drinkers. Similarly, patients with three or more cardiac risk factors had a 2.1-fold relative risk of suffering their MI within 1 hour of a cup of coffee.2
Although caffeine is likely to be the major player in EDs, their physiological and pathological effects on the heart are unlikely to be limited to caffeine alone. Thus, while a normal can of Red Bull contains 80 mg of caffeine, equivalent to a cup of coffee, it also contains 1000 mg of taurine and 600 mg of glucuronolactone which may in turn also have cardiovascular effects.19 Glucuronolactone, for example, has been shown to increase blood pressure.18 Another additive found in a number of EDs is Guarana, which contains caffeine hence augmenting the caffeine-related effects.3
Although no direct causal link between ED and the documented ETT changes has been demonstrated in this report, the timing of the ED consumption prior to the first ETT and the resolution of the ETT changes on subsequent testing without ED on board lead us to consider that the observed changes could be secondary to ED consumption. Although a higher peak heart rate and peak blood pressure was seen during ETT1, which could confound the findings, and might explain some of the differences between the two tests, this is unlikely to explain all the differences. For example, PVCs and couplets were only seen in ETT1, starting in stage I and II, respectively, at heart rates and blood pressures that were reached in ETT2 where no PVCs or couplets were seen. Furthermore, upsloping ST depression was seen towards the end of stage 1 in ETT1, again at heart rates and blood pressures seen in ETT2 where no such ST depression was seen. By the time horizontal ST depression was seen in ETT1, it was at heart rates and blood pressures that were higher than those achieved during ETT2, and thus it is unclear whether these haemodynamic changes caused the ST depression, on a background of hypertensive heart disease, rather than having EDs on board. However, these changes in ETT1 persisted in recovery for almost 40 s leading us to believe that the EDs may have been contributory here.
Causes of false positive ETTs include female gender, hyperventilation, lead placement, structural heart disease, valvular dysfunction, digitalis therapy, abnormal baseline ECG and electrolyte disturbances.5 Recent ingestion of alcohol and caffeine can also exacerbate arrhythmias5 but it would be difficult to attribute all the effects observed in this case to caffeine alone.
This case report highlights the need for clear instructions to patients prior to ETT to avoid not only caffeine, but energy drinks as well. Consumption of these prior to an ETT might alter the test result and hence affect patient advice as well as further management. In our opinion, EDs should be held-off for at least 24 hours, if not 48 hours, prior to an ETT. It appears that, in this case, after 7 days, there w no residual systemic effects.
Learning points.
Energy drinks could potentially have haemodynamic, proarrhythmic and myocardial effects in individuals with coronary artery disease.
Energy drinks can affect the outcome of exercise tests due to the exaggerated blood pressure and heart rate response.
Energy drinks can alter the result and interpretation of exercise tests in the form of ischaemia mimics on the ECG.
There might be a need for clear instructions to patients prior to exercise treadmill test to avoid caffeine and energy drinks for at least 24 hours, if not 48 hours prior to testing.
Footnotes
Contributors: TRC and MAA have devised and written the article. GG has supervised the submission and made alterations to the final manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.BSDA. British Soft Drinks Association Annual Report 2015. British Soft Drinks Association Annual Report. 2015. http://www.britishsoftdrinks.com/write/MediaUploads/Publications/BSDA_Annual_Report_2015.pdf.
- 2.Baylin A, Hernandez-Diaz S, Kabagambe EK, et al. . Transient exposure to coffee as a trigger of a first nonfatal myocardial infarction. Epidemiology 2006;17:506–11. 10.1097/01.ede.0000229444.55718.96 [DOI] [PubMed] [Google Scholar]
- 3.Sanaei-Zadeh H. With which mechanism the overuse of energy drinks may induce acute myocardial ischemia? Cardiovasc Toxicol 2012;12:273–4. 10.1007/s12012-012-9160-4 [DOI] [PubMed] [Google Scholar]
- 4.Steg PG, James SK, Atar D, et al. . ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–619. 10.1093/eurheartj/ehs215 [DOI] [PubMed] [Google Scholar]
- 5.Fletcher GF, Ades PA, Kligfield P, et al. . Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013;128:873–934. 10.1161/CIR.0b013e31829b5b44 [DOI] [PubMed] [Google Scholar]
- 6.Mieske K, Flaherty G, O'Brien T. Journeys to high altitude--risks and recommendations for travelers with preexisting medical conditions. J Travel Med 2010;17:48–62. 10.1111/j.1708-8305.2009.00369.x [DOI] [PubMed] [Google Scholar]
- 7.Schmid JP, Noveanu M, Gaillet R, et al. . Safety and exercise tolerance of acute high altitude exposure (3454 m) among patients with coronary artery disease. Heart 2006;92:921–5. 10.1136/hrt.2005.072520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West JB, Schoene RB, Milledge JS. Pre-existing medical conditions at altitude High altitude medicine and physiology. 4th Ed London: Hodder Arnold, 2000:337–48. [Google Scholar]
- 9.Hainsworth R, Drinkhill MJ, Rivera-Chira M. The autonomic nervous system at high altitude. Clin Auton Res 2007;17:13–19. 10.1007/s10286-006-0395-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bärtsch P, Gibbs JS. Effect of altitude on the heart and the lungs. Circulation 2007;116:2191–202. 10.1161/CIRCULATIONAHA.106.650796 [DOI] [PubMed] [Google Scholar]
- 11.Ali F, Rehman H, Babayan Z, et al. . Energy drinks and their adverse health effects: A systematic review of the current evidence. Postgrad Med 2015;127:308–22. 10.1080/00325481.2015.1001712 [DOI] [PubMed] [Google Scholar]
- 12.Grasser EK, Yepuri G, Dulloo AG, et al. . Cardio- and cerebrovascular responses to the energy drink Red Bull in young adults: a randomized cross-over study. Eur J Nutr 2014;53:1561–71. 10.1007/s00394-014-0661-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozik TM, Shah S, Bhattacharyya M, et al. . Cardiovascular responses to energy drinks in a healthy population: the C-energy study. Am J Emerg Med 2016;34:1205–9. 10.1016/j.ajem.2016.02.068 [DOI] [PubMed] [Google Scholar]
- 14.Grasser EK, Dulloo AG, Montani JP. Cardiovascular and cerebrovascular effects in response to red bull consumption combined with mental stress. Am J Cardiol 2015;115:183–9. 10.1016/j.amjcard.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 15.Namdar M, Schepis T, Koepfli P, et al. . Caffeine impairs myocardial blood flow response to physical exercise in patients with coronary artery disease as well as in age-matched controls. PLoS One 2009;4:e5665 10.1371/journal.pone.0005665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namdar M, Koepfli P, Grathwohl R, et al. . Caffeine decreases exercise-induced myocardial flow reserve. J Am Coll Cardiol 2006;47:405–10. 10.1016/j.jacc.2005.08.064 [DOI] [PubMed] [Google Scholar]
- 17.Wilson RE, Kado HS, Samson R, et al. . A case of caffeine-induced coronary artery vasospasm of a 17-year-old male. Cardiovasc Toxicol 2012;12:175–9. 10.1007/s12012-011-9152-9 [DOI] [PubMed] [Google Scholar]
- 18.Worthley MI, Prabhu A, De Sciscio P, et al. . Detrimental effects of energy drink consumption on platelet and endothelial function. Am J Med 2010;123:184–7. 10.1016/j.amjmed.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 19.Red Bull Caffeine Content. Secondary Red Bull Caffeine Content. 2016. http://energydrink-uk.redbull.com/red-bull-caffeine-content.