Abstract
A 26-year-old Japanese man was admitted to our unit with exacerbated paranoid schizophrenia. Prior to his admission, daily administration of olanzapine had been sufficient to maintain a partial remission of his schizophrenia, but due to an exacerbation of his delusions, he had then also been prescribed aripiprazole, which had been followed by no improvement in symptoms and a gradual further exacerbation of auditory delusions. Physical examinations, brain MRI and neurophysiological assessment were unremarkable. Blood analysis, however, revealed extremely low thyroid-stimulating hormone (TSH) and prolactin-releasing hormone (PRL) concentration. Interestingly, after aripiprazole discontinuation, he returned to partial remission with an increase in plasma TSH and PRL concentration.
Keywords: psychiatry, drugs: psychiatry, psychotic disorders (incl schizophrenia)
Background
There is still ongoing debate about the optimal approach for transitioning therapy to aripiprazole from other antipsychotics for schizophrenia. This is partly due to the fact that the mechanisms by which adding aripiprazole to olanzapine may exacerbate psychosis are not well understood. The patient had low thyroid-stimulating hormone (TSH) and prolactin-releasing hormone (PRL) concentration with exacerbated psychosis induced by addition of aripiprazole to olanzapine. We systematically measured all hormones such as TSH, PRL, adrenocorticotropic hormone (ACTH), luteinizing hormone (LH), follicle-stimulating hormone (FSH) and growth hormone (GH) in the anterior pituitary gland and found that only TSH and PRL were downregulated. After aripiprazole discontinuation, he returned to partial remission characterised by a reduction of auditory delusions and at the same time his plasma TSH and PRL concentration started to increase. This report describes the unique response of TSH and PRL in the anterior pituitary gland of a patient with schizophrenia whose chronic delusions were exacerbated when aripiprazole was added to olanzapine and discuss potential pathophysiological mechanisms.
Case presentation
A 26-year-old Japanese man was admitted to our unit on 23 September 2016 with exacerbated schizophrenia. He had previously developed gaze delusions and auditory hallucinations at 18 years of age and been diagnosed as having paranoid schizophrenia. He had been treated with conventional antipsychotic agents and supportive psychotherapy and had achieved partial remission of his symptoms. His medical history was unremarkable. Twenty milligrams of olanzapine daily had been sufficient to maintain a partial remission. Residual symptoms were paranoid ideas of influence, that is, ‘people are suffering torture from the gaze I am sending all over the place even through walls’ and a strong sense of guilt especially in the presence of others. He had had minimal, if any, negative symptoms. Due to an exacerbation of his delusions, from 20 June 2016, he had also been prescribed aripiprazole for 3 months, eventually being increased to 20 mg daily. The addition of aripiprazole had been followed by no improvement in symptoms and a gradual exacerbation of auditory delusions (neighbours are groaning with pain from his gaze and they are talking of killing him). The patient had been adherent to his medications. In response to his symptoms, he had begun repeatedly walking about in the room. There were no concomitant medications. Due to the deterioration of his symptoms (Brief Psychiatric Rating Scale (BPRS): 66 points), he was thus hospitalised in our unit for medical examination and drug adjustment.
Investigations
Blood analysis revealed extremely low TSH concentration (0.01 mIU/L, normal 0.35–4.00 mIU/L) and normal levels of free thyroxine (FT4) (1.0 ng/dL, normal 0.8–1.7 ng/dL) and free triiodothyronine (FT3) (2.7 pg/mL, normal 2.2–4.1 pg/mL) (table 1) before aripiprazole discontinuation. He had not been noted to have had any symptoms related to the thyroid gland in his medical history. The analysis also indicated low PRL concentration (2.3 ng/mL, normal 3.7–16.3 ng/mL) before aripiprazole discontinuation. Other laboratory investigation and neurological assessment findings were unremarkable. An MRI scan of the brain also did not reveal any remarkable signs such as atrophy or tumour.
Table 1.
Pituitary hormone secretion after aripiprazole discontinuation
| Weeks | TSH (mIU/L) |
Thyroglobulin (ng/mL) |
FT4 (ng/dL) |
FT3 (pg/mL) |
PRL (ng/mL) |
LH (mIU/mL) |
FSH (mIU/mL) |
ACTH (pg/mL) |
GH (ng/mL) |
| 0 | 0.01 | – | 1.30 | 3.60 | – | – | – | – | – |
| 1 | 0.04 | 65.50 | 1.00 | 2.70 | 2.30 | 4.60 | 4.00 | 42.10 | 0.03 |
| 2 | 5.03 | 70.10 | 1.00 | 2.80 | 5.80 | 4.50 | 3.70 | 35.60 | 0.36 |
| 3 | 7.07 | 38.50 | 1.20 | 3.40 | 12.60 | 4.80 | 3.00 | 57.40 | 0.13 |
| 4 | 5.20 | 30.50 | 1.10 | 3.30 | 21.50 | 4.70 | 2.00 | 73.40 | 0.28 |
| 5 | 5.47 | 25.10 | 1.20 | 3.20 | 24.00 | 4.40 | 2.00 | 24.30 | 0.15 |
| 6 | 5.92 | 23.00 | 1.10 | 3.20 | 28.00 | 5.00 | 2.10 | 47.20 | 0.17 |
Differential diagnosis
Primary hypothyroidism.
Treatment
In case the exacerbation of the patient’s paranoid schizophrenia was due to aripiprazole, administration of the drug was abruptly discontinued and only 20 mg olanzapine continued to be administered daily to the patient.
Outcome and follow-up
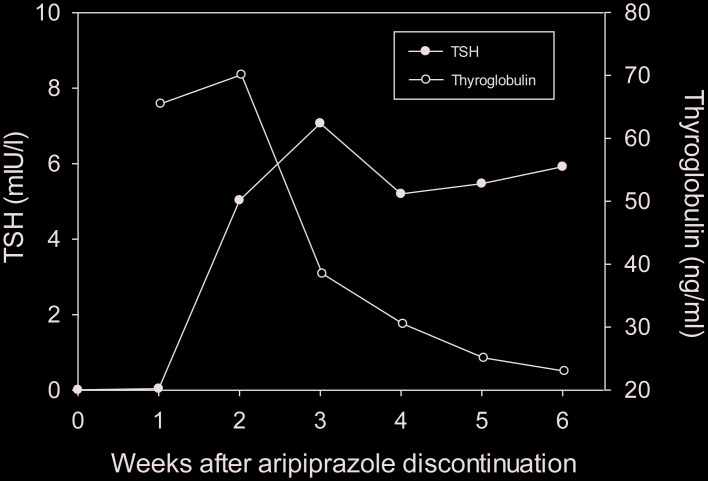
His psychotic symptoms subsided 3 weeks after the discontinuation of aripiprazole and he returned to partial remission characterised by delusions that did not influence his behaviour significantly (BPRS: 44 points). At the same time, his plasma TSH concentration increased to 5.20 mIU/L (figure 1). Blood analysis also indicated that TSH and PRL in the anterior pituitary gland had increased and thyroglobulin in the thyroid had decreased after aripiprazole discontinuation but other hormones such as ACTH, LH, FSH and GH in the anterior pituitary gland were not affected (table 1). Aripiprazole discontinuation seemed to restart TSH secretion in the pituitary gland, leading to free T4 production using thyroglobulin in the thyroid follicular cells (figure 1).
Figure 1.
Plasma TSH and thyroglobulin concentration after aripiprazole discontinuation. TSH, thyroid-stimulating hormone.
Discussion
No clear answer has been proposed to the question of why adding aripiprazole to olanzapine may exacerbate psychosis. The advantages and disadvantages of aripiprazole as a combination therapy with other antipsychotics have not been fully clarified.1 Currently, aripiprazole is the only available dopamine 2 (D2) receptor partial agonist with antipsychotic properties and has stronger affinity to dopamine D2 receptors than olanzapine. One speculation is that displacing olanzapine with aripiprazole may lead to greater partial D2 receptor agonist effect than its antagonist effect, resulting in exacerbation of psychotic symptoms through stimulation of mesolimbic dopamine D2 receptors, whose number may have increased due to long exposure to olanzapine prior to commencement of aripiprazole administration.2 The relatively long period of 3 weeks after aripiprazole discontinuation until reduction of psychosis in the patient may also reflect aripiprazole’s long elimination half life in the present case.
Another speculation is that aripiprazole may directly exacerbate the patient’s psychotic symptoms by leading to dysfunction of the anterior pituitary gland, where TSH and PRL are secreted, or the hypothalamus, where thyroid-releasing hormone (TRH) stimulates TSH and PRL secretions in the anterior pituitary gland at the upper level.
From our findings, we can make the following two hypotheses. First, our data suggest that aripiprazole more strongly affects D2 receptors in TSH and PRL cells in the anterior pituitary gland and causes them to downregulate their secretion, which influences an unknown brain mechanism to exacerbate psychosis in patients with schizophrenia. This is consistent with previous reports, in which low PRL was observed in patients with schizophrenia whose psychotic symptoms were exacerbated by aripiprazole administration, although these studies did not report TSH level, another hormone in the anterior pituitary gland.3 4 Interestingly, a recent study reported that the D2 dopamine receptor is expressed in the majority of the cell populations of the normal human pituitary gland, and particularly, in the different corticotroph cell populations localised in the anterior lobe and the intermediate zone of the pituitary gland.5 The present data also suggest that aripiprazole more strongly affects D2 receptors in TSH and PRL cells than D2 receptors in ACTH, LH, FSH and GH cells in the anterior pituitary gland.
Second, our data also suggest that aripiprazole affects D2 receptors in TRH cells in the hypothalamus6 and causes them to downregulate TRH secretions, which leads to a decrease in TSH and PRL secretions at the lower level of the anterior pituitary gland. The hypothesis can well explain why secretions of only TRH and PRL and not other hormones such as ACTH, LH, FSH and GH are downregulated in the anterior pituitary gland (table 1), since TRH is known to control only TSH and PRL secretions in the anterior pituitary gland.7 8 As is known from the TRH load test, the time lag between TRH secretion in the hypothalamus and TSH/PRL secretions in the pituitary gland is less than 1 hour. Therefore, the onset of TSH/PRL secretion recovery in the pituitary gland 2 weeks after aripiprazole discontinuation seems to have been induced immediately after recovery of TRH secretion in the hypothalamus. An unknown brain mechanism related to TRH secretion in the hypothalamus may exacerbate psychosis in patients with schizophrenia.
That may partly explain why we encounter many aripiprazole-treated patients who recovered from some schizophrenia symptoms even with low-level PRL secretions. Only once TRH secretion in the hypothalamus starts to be downregulated by aripiprazole administration, exacerbated psychosis may happen through an unknown mechanism, resulting in low TSH and PRL secretions at the same time in the pituitary gland induced by low TRH secretion in the hypothalamus. Therefore, TSH might be a better objective biomarker to determine whether aripiprazole is an appropriate treatment for the patient or whether it should be stopped to avoid exacerbating the patient’s psychotic symptoms.
Through this report, we hope to increase awareness of the unique response of TSH and PRL in the anterior pituitary gland, as seen in a patient with schizophrenia whose chronic delusions were exacerbated when aripiprazole was added to olanzapine and discuss potential pathophysiological mechanisms. In this patient, aripiprazole downregulated TSH and PRL secretion levels while discontinuation of aripiprazole treatment resulted in the smooth recovery of TSH and PRL secretions and a reduction in severity of the patient’s symptoms. Low plasma TSH and PRL levels can be used as simple biological markers to assess the side effects of aripiprazole in schizophrenia patients. Further basic and clinical studies are required to confirm the present findings.
Patient’s perspective.
We had a difficulty in asking our patient for writing the patient’s perspective in English because he is Japanese and does not write English sentences himself.
Learning points.
Low plasma thyroid-stimulating hormone (TSH) and prolactin-releasing hormone (PRL) levels can be used as biological markers to assess the side effects of aripiprazole in patients with schizophrenia.
Only TSH and PRL secretions in the anterior pituitary gland were downregulated when aripiprazole was additionally prescribed to olanzapine to treat exacerbation of delusions in the patient.
Thyroid-releasing hormone secretion in the hypothalamus, which specifically controls TSH and PRL secretions in the anterior pituitary gland, may be the key to understanding the mechanism of how aripiprazole exacerbates the symptoms of schizophrenia.
Footnotes
Contributors: All authors cared for the patient and wrote the report, which was reviewed by them.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chan J, Sweeting M. Review: Combination therapy with non-clozapine atypical antipsychotic medication: a review of current evidence. J Psychopharmacol 2007;21:657–64. 10.1177/0269881106071334 [DOI] [PubMed] [Google Scholar]
- 2.Gurevich EV, Bordelon Y, Shapiro RM, et al. Mesolimbic dopamine D3 receptors and use of antipsychotics in patients with schizophrenia. A postmortem study. Arch gen psychiatry 1997;54:225–32. 10.1001/archpsyc.1997.01830150047009 [DOI] [PubMed] [Google Scholar]
- 3.Sogawa R, Shimomura Y, Minami C, et al. Aripiprazole-associated hypoprolactinemia in the clinical setting. J Clin Psychopharmacol 2016;36:385–7. 10.1097/JCP.0000000000000527 [DOI] [PubMed] [Google Scholar]
- 4.Propst AJ, Jarvis GE, Margolese HC. Aripiprazole-induced hypoprolactinemia in an adult male with first-episode psychosis. Clin schizophr relat psychoses 2016;9:173–6. 10.3371/CSRP.PRJA.022015 [DOI] [PubMed] [Google Scholar]
- 5.Pivonello R, Waaijers M, Kros JM, et al. Dopamine D2 receptor expression in the corticotroph cells of the human normal pituitary gland. Endocrine 2017;57 10.1007/s12020-016-1107-2 [DOI] [PubMed] [Google Scholar]
- 6.Lewis BM, Dieguez C, Lewis MD, et al. Dopamine stimulates release of thyrotrophin-releasing hormone from perfused intact rat hypothalamus via hypothalamic D2-receptors. J Endocrinol 1987;115:419–24. 10.1677/joe.0.1150419 [DOI] [PubMed] [Google Scholar]
- 7.Bowers CY, Friesen HG, Folkers K. Further evidence that TRH is also a physiological regulator of PRL secretion in man. Biochem Biophys Res Commun 1973;51:512–21. 10.1016/0006-291X(73)91344-2 [DOI] [PubMed] [Google Scholar]
- 8.Anderson MS, Bowers CY, Kastin AJ, et al. Synthetic thyrotropin-releasing hormone. A potent stimulator of thyrotropin secretion in man. N Engl J Med 1971;285:1279–83. 10.1056/NEJM197112022852302 [DOI] [PubMed] [Google Scholar]