Abstract
Microalgae are considered to be an important and sustainable alternative to fish oil as a source for the polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Due to their health benefits, there is an increasing interest in the commercial application of these fatty acids (FA) to health and dietary products, and to aquaculture feeds. However, FA from microalgae are still expensive to produce compared to fish or plant oils. With only a few microalgal strains being cultivated on a large scale for commercial PUFA production, prospecting for new, robust and fast-growing strains with increased PUFA content is essential in order to reduce production costs. Microalgae from northern high latitudes, exposed to cold temperatures, may be especially promising candidates as previous studies have shown increasing unsaturation of FA in response to decreasing growth temperatures in different microalgae, most likely to maintain membrane fluidity and function. We have designed a screening pipeline, targeting a focused search and selection for marine microalgal strains from extreme North Atlantic locations with high robustness and biomass production, and increased levels of EPA and DHA. The pipeline includes a rational sampling plan, isolation and cultivation of clonal strains, followed by a batch growth experiment designed to obtain information on robustness, growth characteristics, and the FA content of selected isolates during both nutrient replete exponential cultivation and nutrient limited stationary cultivation. A number of clonal cultures (N = 149) have been established, and twenty of these strains have been screened for growth and FA content and composition. Among those strains, three showed growth rates ≥ 0.7 d− 1 at temperatures of 15 °C or below, and high amounts of EPA (> 3% DW), suggesting their potential as candidates for large scale production.
Keywords: Bioprospecting, Docosahexaenoic acid (DHA), Eicosapentaenoic acid (EPA), Microalgae, Northern high latitudes, Omega-3 fatty acids
Highlights
-
•
Fast growing microalgae with high EPA and DHA levels were prospected in North Atlantic waters.
-
•
A number of 149 clonal stock cultures were established, mostly represented by diatoms.
-
•
Three out of 20 strains showed fast growth together with high EPA content.
-
•
Highest EPA content of 4.6% of dry weight was found in an Arctic diatom.
-
•
Microalgae from northern high latitudes reveal potential for biotechnological applications.
1. Introduction
The omega-3 long-chained PUFAs (LC-PUFAs), eicosapentaenoic acid (EPA, 20:5) and docosahexaenoic acid (DHA, 22:6) have unique nutritional benefits for human health [1]. They appear to be essential in preventing cardiovascular disease, and most nutritional guidelines now include recommendations for increased intakes of these FA [2]. The current major source for these LC-PUFAs is marine fish oil. However, fish, like other animals, do not efficiently synthesize EPA and DHA themselves, but obtain and accumulate them via the marine food chain from PUFA-synthesizing microalgae [3], [4], [5], [6]. Because of their high EPA and DHA levels, oily wild fish are considered a health food, and their oil is in much demand for use in aquaculture feed as a source of these PUFAs. Approximately 70% of the globally-available fish oil is currently consumed by aquaculture feed production [7]. Additionally, other applications and markets for direct human consumption of EPA and DHA rich fish oil are developing rapidly. This increasing demand for fish oil, and its commercial production from wild fish stocks has led to economic, ethical and environmental concerns. Due to increasing competition in the global market, fish oil is increasingly being substituted with vegetable alternatives in modern aquafeeds [8]. However, as plant oils lack omega-3 LC-PUFAs [1], this leads to a decrease in the relative EPA and DHA levels in farmed fish, and the production of plant oils also uses valuable and increasingly limited farmland. Questions concerning the relatively high content of omega-6 FA in land-plant based oils and their potentially negative effects on human health have also been raised in the literature [9]. Thus, there is a great demand for new sources of omega-3 FA, and microalgae are currently regarded a promising alternative, as many species naturally produce high levels of EPA and DHA.
In recent decades, there have been increasing efforts to apply microalgae-based technologies for a more sustainable production of many different compounds used in the biofuel, pharmaceutical, functional food and aquafeed industries [10]. In addition to their nutritious biomass and high areal productivity, microalgae can be cultivated in seawater and on non-arable land, and do not compete for resources with conventional agriculture [11]. The lipids of microalgae are of particular commercial interest; triacylglycerides (TAG), which are storage lipids comprising mostly saturated and monounsaturated FA [12], are in much demand in the biofuel industry, whereas the health, food and feed sectors target the LC-PUFAs [7] that are typically present in the polar membrane lipids of microalgae [13]. However, the many advantages derived from using microalgae as feedstocks, contrast with their cost-inefficient large-scale production [14]. Today, the production of FA from microalgae continues to be expensive when compared with current fish oil sources [15] and farmland plant oils, due to the high energy requirements of water pumping, CO2 transfer, culture mixing, nutrient supply, biomass harvest and drying [16], [17].
An essential requirement for the successful improvement and progression of microalgae-based technologies is the discovery of new and robust strains that grow fast and produce naturally high levels of the desired compounds [10]. A number of different strategies have been implemented in bioprospecting and screening for new strains to optimize FA production for biofuels [18], [19], [20], [21], but so far, only a few studies have focused on the PUFA fraction [22], [23]. In this study, we developed a screening pipeline, targeting microalgae from different North Atlantic habitats, with high growth rates and increased concentrations of the high value products EPA and DHA. If found suitable for process upscaling, candidate strains could contribute significantly to the development of a more cost efficient large-scale production of microalgae.
2. Materials and methods
2.1. Sampling sites and times
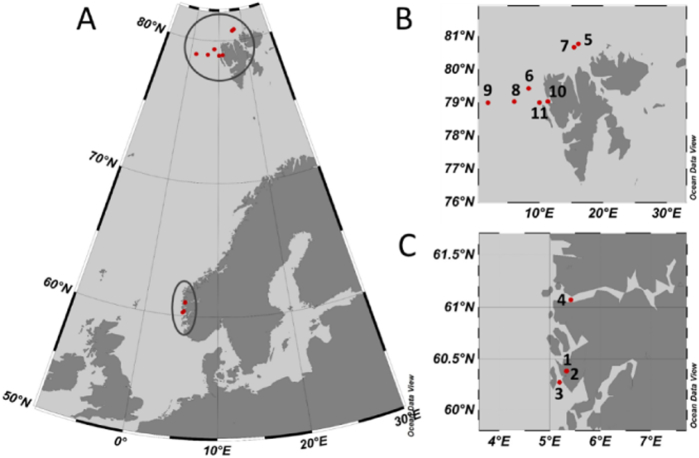
Microalgal samples were taken during 2014 at two different marine locations; the Atlantic waters around Spitsbergen and three fjord systems on the South-West coast of Norway (Fig. 1A). In the Arctic, samples were taken at a number of stations during four cruises in March, May, August, and November (Fig. 1B, sampling stations 5, 6, 7 and 8–11, respectively). The three fjord systems Store Lungegårdsvann, Puddefjorden and Raunefjorden (Fig. 1C, sampling stations 1, 2 and 3, respectively) were sampled four times during the year, in order to cover all four seasons. In addition to this, the re-isolation of natural samples obtained in 2012 from deep water masses at 1000 m in Sognefjorden (Fig. 1C, sampling station 4), was included. At all sampling sites, phytoplankton samples were taken by vertical net haul (mesh size 10 μm) at depths from 0 to 10 m in fjords and 0–50 m in Arctic waters, and Niskin water bottles were used for the deeper water layers. In the fjords, sediment and biofilm samples from the intertidal zone were also taken, with pre-sterilized equipment. Water and benthic samples were collected in 50 mL centrifuge tubes. The environmental samples were divided into two parts; one part was taken for single cell isolation directly, and the other part went through an enrichment phase, where 50% (v/v) Walne's medium [24], (prepared in 80% seawater [SW]), was added. The enrichments were initially incubated at 15 °C (fjord spring/summer samples) or 4–8 °C (fjord winter/spring samples and Arctic samples), and at a photon flux density (PFD) of 50 μmol photons m− 2 s− 1, until visible development of microalgal biomass, followed by single cell isolation.
Fig. 1.
Map illustrating the sampling sites (A) with seven different sampling stations to the north and west of Spitsbergen (B) and four different sampling stations at the Norwegian west coast (C). Names and coordinates of the sampling positions and sampling times can be found in Table 1.
2.2. Single cell isolation
Single cell sorting of environmental and enrichment samples was performed with a Becton Dickinson FACS Aria™ Cell sorter (BD Biosciences, San Jose, CA, USA) at a flowrate of 300 events s− 1. The software BD FACS DIVA (Version 8.0; BD Biosciences, San Jose, CA, USA) was used for data analysis. The selection criteria were set after initial analysis of fluorescence signals of a pure Phaeodactylum tricornutum culture and environmental samples. Cells were excited with a red laser at 633 nm, and the resulting scattered light and fluorescence emission were recorded by a forward scatter detector, and a FL-4 detector (661/16 nm), respectively (see Fig. 2 for an example of a two-dimensional dot plot of an environmental sample). Events with > 1000 arbitrary units of auto fluorescence from chlorophyll a (chl a) were set as selection criteria for cell sorting. All samples were pre-filtered (BD Falcon™ USA, 40 μm Nylon) before sorting. A 100 μm nozzle dispensed one drop containing a single cell into each well of 96-well plates, containing 150 μL solid or liquid growth medium (80% SW enriched with Walne's nutrients). After sorting, the plates with cells were incubated at 10 °C (Fjord winter/spring samples and Arctic samples) or 15 °C (Fjord spring/summer samples) at approximately 50 μmol photons m− 2 s− 1 until visible growth. In addition to this, cell isolation by serial dilution was performed with two enrichment samples from the Arctic by monthly transfer and dilution of cells growing in 3 mL sterile Walnes's medium.
Fig. 2.
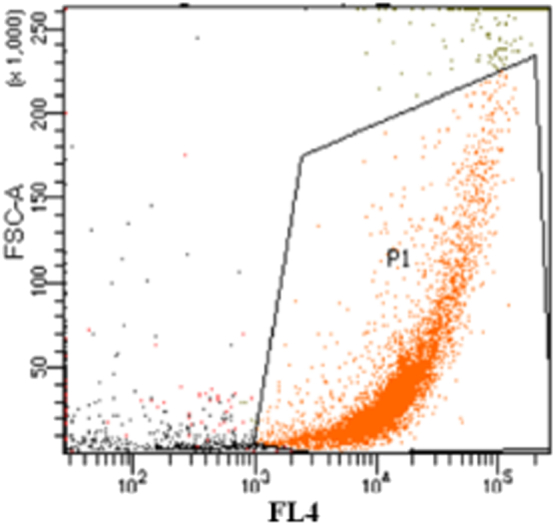
Single cell isolation using FACS Aria™ Cell sorter. Example of a two-dimensional dot plot combining forward scattering (FSC-A) and fluorescence emission by chlorophyll a (FL-4). Events with > 1000 arbitrary units of auto fluorescence from chlorophyll a was set as selection criteria for cell sorting.
2.3. Upscaling and culturing of clonal cultures
Proliferating strains from the 96-well plates and isolates from serial dilution were transferred to glass tubes with 3 mL sterile Walne's medium [24] prepared in 80% SW, and incubated under conditions as described in Section 2.2. The proliferating clonal strains were sustained as stock cultures by sub-culturing every month. Cultures were not axenic, but were maintained as sterilely as possible. For preliminary identification, and to monitor contamination by other microalgae, the cultures were observed frequently under the microscope. Isolates showing highest growth rates in the stock culture (by visual observation) were selected for the determination of their FA profile and growth rates under controlled conditions.
2.4. Growth and FA profiling
A 280 mL batch culture was grown for twenty selected isolates to investigate their growth rate and FA content and composition in both the exponential and the stationary phase. For the inoculum, biomass of each strain was upscaled to 100 mL, harvested by centrifugation (2264g, 5 min), washed twice with fresh medium, recultured in 280 mL fresh medium (Walne's medium [24] prepared in 80% SW) and transferred to a 300 mL glass cylinder (3.5 cm inner diameter). The twenty glass cylinders were placed into temperature-controlled water tanks to mirror temperatures used in the previous cultivation step (10 or 15 °C). Continuous illumination of 120–150 μmol photons m− 2 s− 1 (measured with a 4π quantum scalar irradiance sensor [QSL-100, Biospherical Instruments, San Diego, CA, USA], inside the empty glass cylinder) was provided by banks of six white fluorescent tubes (Philips MASTER, TL-D 90 Graphica, 58W/95) in the back of the water tanks, running perpendicular to the glass cylinders. To ensure mixing and carbon supply, 0.2 μm-filtered and 1% CO2-enriched air was bubbled through glass capillaries into the bottom of each 300 mL glass cylinder. The cultures were sampled every 24 h for optical density measurements at 750 nm (OD 750). The OD-based growth curves were used to determine a time point in the mid-exponential and the late stationary growth phase, for FA and biomass dry weight (DW) analyses. Due to variable growth rates, these sampling days were different for the individual strains. At the end of the experiment, 10 mL samples were taken for phylogenetic analysis of the isolates.
Optical density measurements were performed using a spectrophotometer (UV-1800, Shimadzu Corporation, Kyoto, Japan) at 750 nm wavelength. If the OD value exceeded 0.8, samples were diluted to give an attenuation between 0.2 and 0.8. The specific growth rate (μavg [d− 1]) was calculated for each culture by taking the average of the growth rates between two consecutive days (μx) during exponential phase. The growth rate between two consecutive days was calculated according to the changes in attenuation during 24 h with Eq. (1). Ntx and Ntx − 24 h are OD 750 at day x (tx) and the previous day x − 24 h (tx – 24 h), respectively. Exponential phase was determined individually for each strain based on the logarithmic shape of its growth curve. Dry weights were determined in triplicate, as described in Zhu & Lee (1997) [25], using GF/F (47 mm) Whatman® filters and 0.5 M ammonium formate as a washing buffer, and are expressed as weight of the dried biomass per volume. For FA analysis, quadruplicate 10 mL microalgal cultures were sampled into 10 mL glass tubes (PYREX), centrifuged for 6 min at 2264g, and the supernatant was discarded. Pellets were stored in nitrogen atmosphere to prevent oxidation at − 20 °C prior to analysis.
| (1) |
2.5. FA extraction and FAME analysis
Total lipids were extracted and derivatized to fatty acid methyl esters (FAME) by direct esterification, according to Meier et al. (2006) [26]. The sample pellet was dried in the 10 mL glass tubes by evaporating water under a nitrogen stream, and the internal standard (23:0 FAME, dissolved in isooctane) was added. The solvent was then evaporated, and 0.5 mL of methylation reagent (2 M HCl in methanol) was added to each tube. Tubes were flushed with nitrogen, sealed and incubated in an oven at 90 °C for 2 h. After cooling to room temperature, half of the methylation reagent was evaporated, and 0.5 mL water was added. The samples were thereafter extracted twice with 1 mL isooctane. Before analysis by gas chromatography (GC), the combined extracts were further diluted with isooctane in order to yield a final internal standard concentration of approximately 20 μg mL− 1. FAMEs were analysed by GC as described in Prestegard et al. (2015) [27]. To aid identification of the FAMEs, selected samples were analysed by gas chromatography–mass spectrometry (GC–MS) as described in Wasta & Mjøs (2013) [28]. In order to calculate the FA concentrations appropriately, the internal standard content should be approximately 30% of the most abundant FA in the sample. Thus, an initial analysis of the FA content and composition for each strain at both growth phases was necessary to determine the correct amount of internal standard to be added to each sample. Therefore, 36 μg internal standard (460 μg for some stationary phase samples with a higher biomass content) was added to every first replicate of the quadruplicate samples and samples were analysed as described above. After the initial test, the correct amount of internal standard was calculated for each sample, and added to the remaining triplicates.
2.6. Phylogenetic analysis of isolates selected for screening
Molecular methods were used to give taxonomic information of the isolates. DNA was isolated from the pellet of 10 mL algal culture (harvested at 1663g for 6 min) using E.Z.N.A. SP Plant Kit (Omega Bio-tek, Inc., Norcross, GA, USA). Microalgal DNA was amplified by PCR using the HotStarTaq DNA Polymerase Kit (QIAGEN, Valencia, CA, USA), according to the manufacturer's instructions. Amplification of a region of the 28S ribosomal RNA (rRNA) gene (for the large subunit [LSU] of eukaryotic cytoplasmic ribosomes) was performed using the primers D1R-F [29] and D3B-R [30]. The reaction conditions were as follows: An initial activation of the enzyme at 95 °C for 15 min, followed by 30 cycles of denaturation (94 °C, 1 min), annealing (56 °C, 1 min) and extension (72 °C, 1 min), and a final extension at 72 °C for 10 min. Before sequencing, the PCR product was purified with GenElute™ PCR Clean-Up Kit (Sigma-Aldrich, St. Louis, MO, USA) and quantified with Qubit ® dsDNA BR Assay Kit and Qubit® 2.0 (Invitrogen, Eugene, Oregon, USA). Bi-directional sequencing of the PCR products was performed using the PCR forward and reverse primers with the BigDye v.3.1 Kit (ThermoFisher Scientific, Watham, MA, USA) at the sequencing facility at the University of Bergen (http://www.uib.no/en/seqlab). Sequences were edited and aligned manually in BioEdit [31], and Blastn [32] was used to search for similarities with previously published diatoms in GenBank (www.ncbi.nlm.nih.gov/blast/Blast/cgi).
2.7. Statistics
The batch growth experiments were run with one biological replicate for each strain. One measurement replicate was used for OD measurements of cultures to monitor the growth phase, whereas triplicate samples were taken for DW and FA analyses. One sample per culture was taken for phylogenetic analysis of the strains. As the FA content and biomass DW were not analysed from the same sample, the standard deviation for FA content relative to the biomass DW was calculated with Eq. (2) with SD: standard deviation, FADW: fatty acid dry weight (mg), BMDW: biomass dry weight (g), FAc: fatty acid concentration (mg L− 1) and DWc: dry weight concentration (g L− 1).
| (2) |
Differences in the EPA and DHA content between exponential and stationary phases for the different strains was analysed by Student's t-test using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA), with p < 0.01 as a threshold for statistical significance.
3. Results and discussion
A screening-pipeline, including five different steps (Fig. 3) was developed for bioprospecting new, robust and fast growing microalgal strains from extreme North Atlantic habitats, with increased levels of the omega-3 fatty acids EPA and DHA, and thus with potential for future biotechnological applications. Selected habitats were sampled during different seasons of the year (step 1), and single cell isolation (step 2), upscaling and culturing of proliferating strains (step 3) led to the establishment of stock cultures. In a subsequent batch growth experiment (step 4), strains with high growth rates (≥ 0.7 d− 1) and EPA or DHA levels of minimum 3% of biomass DW in either the exponential or the stationary phase were targeted. The selected benchmark levels represent an upper average level that is found for species that are already being applied in aquaculture and mariculture, e.g. Nannochloropsis sp. (Eustigmatophyceae) with a typical EPA content between 2.1 and 3.8% DW [33], [34] and P. tricornutum (Bacillariophyceae) with values between 2.6 and 3.1% DW [4], [35], [36]. Isolates that were robust (to the mechanical shear forces from bubbling) and also fulfilled the desired requirements, were selected to be investigated in more detail (step 5).
Fig. 3.
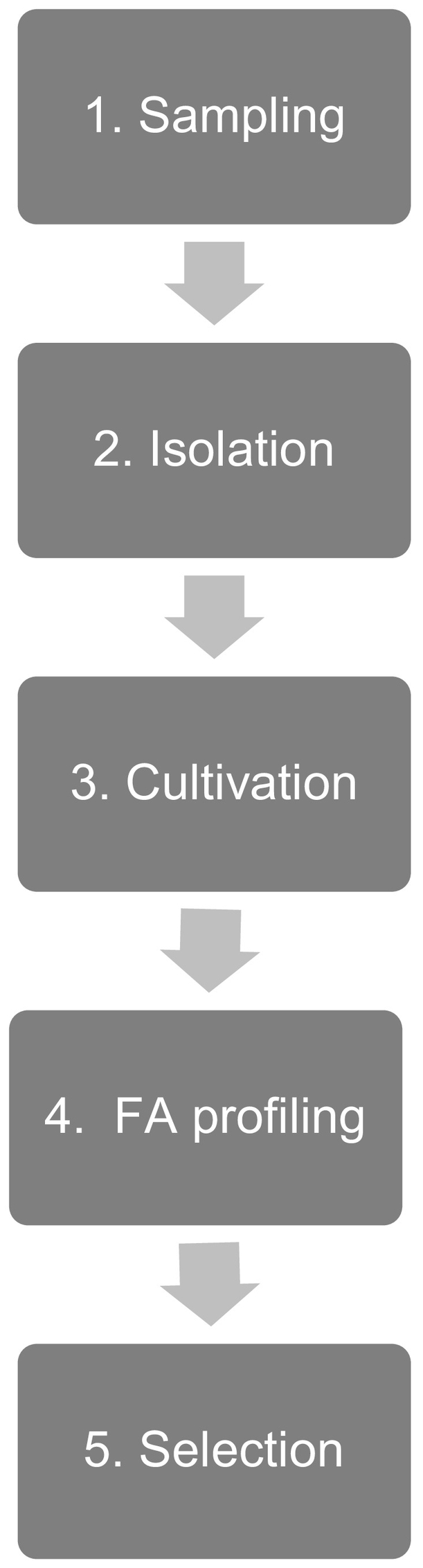
Screening pipeline including five different steps.
3.1. Sampling site
Microalgal samples were obtained from two marine locations, the Atlantic waters around Spitsbergen and four fjord systems on the South-West coast of Norway (Fig. 1A). The fjord waters represent a wide range of environmental conditions, from the brackish water of the land-locked fjords Store Lungegårdsvann and Puddefjorden, with their frequent and strong fluctuations of environmental conditions (Salinity 11–32, temperature − 0.5–20 °C), to the saline waters of the open coastal fjord Raunefjorden, and the world's deepest fjord (Sognefjorden) with annual variations in temperature and salinity in the surface layer of between 4 and 16 °C and 26–33, respectively. The photoperiod in Western Norway varies between 19:5 (light:dark) around mid-summer and 6:18 around winter solstice. Hence, microalgae isolated from these environments were exposed to large seasonal variations and fluctuations in salinity, temperature and irradiance, and were thus hypothesized to be robust and fast growing [37], and able to tolerate fluctuations in growth conditions which routinely occur in commercial production ponds. In contrast, the Arctic waters around Spitsbergen are characterized by lower variations in temperatures (− 1.8–5.8 °C) and salinities (33.0–35.1) [38], but greater variations in photoperiod, typically ranging from 16:9 (light:dark) in early March to 24:0 after 25th of May, and complete darkness in winter. Microalgae found in these environments are expected to be promising candidates for EPA or DHA production, as the low temperature levels in the Arctic are assumed to cause increasing unsaturation of the FA in microalgae, most likely to maintain membrane fluidity and function [39].
3.2. Isolation and cultivation of clonal cultures
Altogether, 75 samples were sorted on 96-well plates (liquid and solid media) resulting in approximately 7200 individual cells (Table 1). In total, 147 clonal strains (strain designation “M”), constituting 2% of the sorted cells, were established successfully as clonal cultures under the conditions used. A higher fraction of clonal strains was established from Arctic samples (4.6% of sorted cells; M21–M24, M30–M116) compared with fjord samples (1.1% of the sorted cells; M01–M05, M08–M20, M25–M29, M117–M149) among which the least were from Raunefjorden (0.2% of sorted cells). In the fjord systems, isolates were established from spring and summer samples (March, May and August), but not from samples taken in November, possibly due to very low phytoplankton abundance or fragility of strains. In the Arctic, strains were established from samples taken in May, August and from two stations sampled in November. Additionally, two Arctic strains were isolated by serial dilution of an enrichment culture (m06 and m07) and designated with “m” in order to distinguish them from the clonal isolates, as serial dilution is less accurate when it comes to establishment of cultures from one single cell. Microscopic observations of the isolates allowed for a preliminary morphological characterization of the stock cultures. All ninety-three isolates (Table 1) derived from the Arctic were identified as diatoms. Isolates from the fjords (56) were identified as diatoms (89.3%), Chlorophytes (8.9%) and Cyanobacteria (1.8%).
Table 1.
Results of the different steps in the screening pipeline. Strains designated with “M” are clonal strains obtained from single cell isolation by cell sorting flow cytometry. Strains designated with “m” were isolated by serial dilution.
| Stat. no. | 1. Sampling 2014 |
2. Isolation |
3. Cultivation |
4. FA profiling |
5. Final selection | ||||
|---|---|---|---|---|---|---|---|---|---|
| Site | Coordinates | Month | No. of 96-well plates | No. of strains in culture | Strain designation | No. of clonal strains | Strain designation | ||
| Norwegian fjord waters | 54 | 56 | 10 | 2 | |||||
| 1 | Store Lungegårdsvann | N 60° 22.93733′ E 05° 20.17962′ | March | 4 | – | – | – | – | |
| May | 2 | 2 | M08, M09 | – | – | ||||
| Aug. | 7 | 35 | M26, M27, M117–M149 | 2 | M26, M27 | M26 | |||
| Nov. | 2 | – | – | – | – | ||||
| 2 | Puddefjorden | N 60° 22.86558′ E 05° 19.52838′ | March | 5 | 6 | M02–M05, M28, M29 | 3 | M04, M28, M29 | M28 |
| May | 2 | 6 | M11–M16 | – | – | ||||
| Aug. | 2 | – | – | – | – | ||||
| Nov. | 2 | – | – | – | – | ||||
| 3 | Raunefjorden | N 60° 16.265′ E 05° 11.456′ | March | 2 | – | – | – | – | |
| May | 18 | 1 | M10 | – | – | ||||
| Aug. | 5 | 5 | M01, M17–M20 | 4 | M17,M18,M19,M20 | ||||
| Nov. | 2 | – | – | – | – | ||||
| 4 | Sognefjorden | N 61° 02.467′ E 05° 24.962′ | 2012 Aug. | 1 | 1 | M25 | 1 | M25 | |
| Arctic | 21 | 93 | 10 | 1 | |||||
| 5 | N 80° 45.80′ E 16° 07.20′ | March | 2 | – | – | – | – | ||
| 6 | N 79° 25.14′ E 08° 18.84′ | May | 1 | 3 | M21, M22, m06 | 2 | M21, m06 | M21 | |
| 7 | N 80° 39.72′ E 15° 26.55′ | Aug. | 4 | 3 | M23, M24, m07 | 2 | M23, m07 | ||
| 8 | N79° 01.46′ E 06° 02.98′ | Nov. | 4 | – | – | – | – | ||
| 9 | N 78° 59.33′ E 01° 54.94′ | Nov. | 2 | – | – | – | – | ||
| 10 | N 79° 01.64′ E 11° 19.49′ | Nov. | 4 | 7 | M30–M36 | – | |||
| 11 | N 78° 59.66′ E 10° 00.17′ | Nov. | 4 | 80 | M37–M116 | 6 | M41, M44, M46, M58, M62, M65 | ||
| Total no. | 75 | 149 | 20 | 3 | |||||
Diatoms were the main target in our screening pipeline, as they are known to be extremely robust and often comprise a high content of PUFAs like EPA [40]. They are one of the most important primary producers in marine environments, such as Arctic and temperate waters, and may constitute 25% of global primary production [41]. However, even though diatoms were the most highly represented group among the 149 isolated cultures, it was surprising that the diversity among the isolates was so low. Other microalgal groups, such as dinoflagellates [42] and prymnesiophytes (especially Phaeocyctis pouchetii) [43], frequently dominate the Atlantic and Arctic waters, but could not be isolated here. The mesh size of the net haul (10 μm) and the physical stress experienced by the cells during single cell isolation by the flow cytometry, are probable reasons for having not collected smaller algae and fragile strains, respectively. Using traditional isolation techniques like a micropipette, agar plating and serial dilutions could minimize cell damage during isolation. However, this was considered an important selection step, as robust strains (which could handle moderate mechanical stress) were preferred. Different types of medium and variation in the growth conditions might also have increased the diversity among the isolates. The conditions were selected so, as to provide a gentle transition of the sampled algae from their natural habitat to the laboratory conditions. Compared to the strains isolated from the fjords, which are expected to be highly tolerant to a rapidly changing environment, microalgae isolated from the Arctic could be more challenging candidates in terms of their decreased flexibility and tolerance to changing conditions, especially with regards to temperature. Culture conditions of 10 °C were higher than the ambient temperature Arctic strains experience in their natural habitat, but were deliberately chosen in order to acclimate the strains to slightly higher temperatures. For consideration for outdoor production, strains need to grow well at higher temperatures, as cooling is one of the main cost factors in large-scale algal production. The application of different isolation methods, media types, and growth conditions might have led to a higher diversity among our isolates, but would have been time- and cost-prohibitive. Thus, the potential risk of loss should be evaluated from a cost/benefit perspective in future bioprospecting studies.
3.3. Batch experiment—strain identification and growth rates
Initially, twenty isolates that (by visual observation) grew most rapidly in stock cultures were selected for further examination of growth rate and total FA (TFA), EPA and DHA contents during both the exponential and the stationary phase. Therefore, ten Fjord isolates (M04, M17–M20, M25–M29, 15 °C) and ten Arctic isolates (m06, m07, M21, M23, M41, M44, M46, M58, M62, M65, 10 °C), were grown in a controlled batch experiment at continuous irradiance (120–150 μmol photons m− 2 s− 1), constant temperature (10 or 15 °C) and bubbled with 1% CO2-enriched air.
In total eighteen isolates (9 Fjord and 9 Arctic) grew at the defined conditions. Sequencing of parts of the LSU rRNA gene revealed that 15 isolates belonged to the class Bacillariophyceae and one isolate (M04) to the Chlorophyceae. Two isolates (M19 and M27) could not be identified by molecular methods, but were recognized as type Chlorophyceae and Bacillariophyceae by microscopic examination, respectively. Eight of the Arctic strains were identified as Thalassiosira hispida (m06, m07, M41, M44, M46, M58, M62, M65) and one as Attheya septentrionalis (M21). The Fjord strains showed higher diversity with five different species: the green algae Micractinium sp. (M04) and the diatoms Nitzschia laevi (M18), Arcocellulus cornucervis (M20), Nanofrustulum shiloi (M25) and P. tricornutum (M26, M28 and M29) (see Table 2 for details).
Table 2.
Phylogenetic classification of the eighteen strains examined in the batch experiment by sequencing a region of the LSU. Asterisks indicate isolates which could not be characterized by molecular methods and were identified at the class level by microscopy only. Strains designated with “M” are clonal strains obtained from single cell isolation by cell sorting flow cytometry. Strains designated with “m” were isolated by serial dilution. ND: not detected.
| Origin | Strain | Temp °C | Closest species (GenBank) | Class | Length (bp) | Coverage (%) | Similarity (%) |
|---|---|---|---|---|---|---|---|
| Fjord | M04 | 15 | Micractinium sp. (HE861877.1) | Chlorophyceae | 1021 | 83 | 88 |
| M18 | 15 | Nitzschia laevis (AF417673.1) | Bacillariophyceae | 767 | 98 | 100 | |
| M19 | 15 | ND | Chlorophyceae⁎ | ND | |||
| M20 | 15 | Arcocellulus cornucervis (JQ995445.1) | Bacillariophyceae | 863 | 90 | 96 | |
| M25 | 15 | Nanofrustulum shiloi (AB430640.1) | Bacillariophyceae | 765 | 67 | 99 | |
| M26 | 15 | Phaeodactylum tricornutum (EF553458.1) | Bacillariophyceae | 830 | 100 | 99 | |
| M27 | 15 | ND | Bacillariophyceae⁎ | ND | |||
| M28 | 15 | Phaeodactylum tricornutum (EF553458.1) | Bacillariophyceae | 832 | 100 | 99 | |
| M29 | 15 | Phaeodactylum tricornutum (EF553458.1) | Bacillariophyceae | 842 | 100 | 99 | |
| Arctic | m06 | 10 | Thalassiosira hispida (JQ995464.1) | Bacillariophyceae | 865 | 87 | 100 |
| m07 | 10 | Thalassiosira hispida (JQ995464.1) | Bacillariophyceae | 863 | 88 | 100 | |
| M21 | 10 | Attheya septentrionalis (GQ219678.1) | Bacillariophyceae | 896 | 96 | 99 | |
| M41 | 10 | Thalassiosira hispida (JQ995464.1) | Bacillariophyceae | 545 | 100 | 100 | |
| M44 | 10 | Thalassiosira hispida (JQ995464.1) | Bacillariophyceae | 882 | 86 | 100 | |
| M46 | 10 | Thalassiosira hispida (JQ995464.1) | Bacillariophyceae | 863 | 88 | 99 | |
| M58 | 10 | Thalassiosira hispida (JQ995464.1) | Bacillariophyceae | 870 | 87 | 100 | |
| M62 | 10 | Thalassiosira hispida (JQ995464.1) | Bacillariophyceae | 874 | 87 | 100 | |
| M65 | 10 | Thalassiosira hispida (JQ995464.1) | Bacillariophyceae | 871 | 87 | 99 |
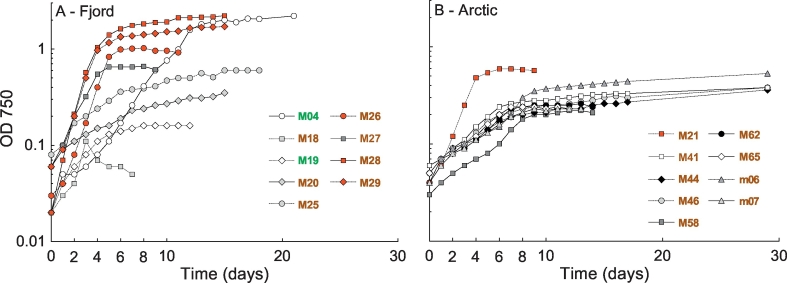
Growth curves and growth rates differed between the eighteen strains (Fig. 4). Fjord strains (grown at 15 °C) generally grew faster and reached stationary phase earlier than the Arctic strains (grown at 10 °C). Except for strain M21 (A. septentrionalis), the Arctic strains (T. hispida) had low average growth rates (μavg) of between 0.2 and 0.3 d− 1. Four strains, the three P. tricornutum strains (Fjord) and A. septentrionalis (Arctic), reached the targeted average growth rate of ≥ 0.7 d− 1. Strain M28 had the overall highest growth rate of 1.0 d− 1, followed by M29 (0.8 d− 1) and M26 and M21, both with 0.7 d− 1 (Table 3).
Fig. 4.
Growth curves based on optical density measurements (n = 1) at 750 nm of 18 isolates in a batch growth experiment (one biological replicate each) for Fjord isolates (A) and Arctic isolates (B). Each strain was sampled for dry weight and fatty acid analysis during both, the exponential growth phase (day 2 for M27, M28 and M29, day 3 for M21, day 4 for M25 and M26, day 5 for M18 and day 7 for M04, M19, M20, m06, m07, M41, M44, M46, M58, M62 and M65) and the stationary growth phase (last point of the respective growth curve). Red colored growth curves indicate an average growth rate in exponential phase with ≥ 0.7 d− 1. Green strain designation highlight the Chlorophyceae and brown the Bacillariophyceae. Strains designated with “M” are clonal strains obtained from single cell isolation by cell sorting flow cytometry. Strains designated with “m” were isolated by serial dilution. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Table 3.
Summary of batch experimental data. Average and standard deviation (SD) of growth rates between two consecutive days during exponential phase based on changes in optical density (OD750, n = 1), sampling day, and average dry weight (DW) and fatty acid (FA) data with SD (n = 3), of the 20 isolates for both the exponential and the stationary phase. Average values and SD are from measurement replicates from one biological replicate. Strains designated with “M” are clonal strains obtained from single cell isolation by cell sorting flow cytometry. Strains designated with “m” were isolated by serial dilution.
| No. | Growth rate |
Exponential phase |
Stationary phase |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | DW |
TFA |
EPA |
DHA |
Day | DW |
TFA |
EPA |
DHA |
||||||||
| μavg d− 1 | g L− 1 | mg L− 1 | % DW | % DW | % TFA | % DW | % TFA | g L− 1 | mg L− 1 | % DW | % DW | % TFA | % DW | % TFA | |||
| M04 | 0.4 ± 0.1 | 7 | 0.12 ± 0.02 | 12.8 ± 0.26 | 10.3 ± 1.55 | 0.0 | 0.0 | 0.0 | 0.0 | 21 | 0.83 ± 0.02 | 278.3 ± 6.80 | 33.5 ± 1.12 | 0.0 | 0.0 | 0.0 | 0.0 |
| M17 | No growth | ||||||||||||||||
| M18 | 0.5 ± 0.3 | 5 | 0.12 ± 0.01 | 15.3 ± 0.18 | 12.9 ± 0.77 | 2.00 ± 0.16 | 15.5 ± 0.75 | 0.62 ± 0.04 | 4.85 ± 0.08 | 7 | 0.18 ± 0.02 | 15.7 ± 0.44 | 8.9 ± 1.17 | 1.30 ± 0.20 | 14.6 ± 0.84 | 0.59 ± 0.08 | 6.60 ± 0.18 |
| M19 | 0.4 ± 0.1 | 7 | 0.09 ± 0.01 | 4.6 ± 0.03 | 5.0 ± 0.49 | 0.0 | 0.0 | 0.0 | 0 | 12 | 0.09 ± 0.00 | 4.5 ± 0.13 | 5.0 ± 0.22 | 0.0 | 0.0 | 0.0 | 0.0 |
| M20 | 0.2 ± 0.1 | 7 | 0.07 ± 0.01 | 8.03 ± 0.05 | 11.6 ± 1.43 | 1.72 ± 0.21 | 14.8 ± 0.20 | 0.26 ± 0.03 | 2.26 ± 0.05 | 15 | 0.13 ± 0.00 | 14.2 ± 0.40 | 11.3 ± 0.33 | 1.36 ± 0.04 | 12.0 ± 0.30 | 0.23 ± 0.01 | 2.02 ± 0.06 |
| M25 | 0.3 ± 0.1 | 4 | 0.12 ± 0.01 | 14.5 ± 0.92 | 11.9 ± 1.24 | 0.98 ± 0.14 | 8.22 ± 0.43 | 0.0 | 0.0 | 18 | 0.40 ± 0.01 | 102.9 ± 4.00 | 25.8 ± 1.20 | 1.28 ± 0.03 | 4.98 ± 0.18 | 0.0 | 0.0 |
| M26 | 0.7 ± 0.2 | 4 | 0.15 ± 0.01 | 19.4 ± 0.42 | 12.9 ± 1.01 | 3.10 ± 0.25 | 24.1 ± 0.08 | 0.49 ± 0.04 | 3.84 ± 0.02 | 11 | 0.44 ± 0.02 | 112.2 ± 0.62 | 25.5 ± 1.35 | 3.40 ± 0.18 | 13.3 ± 0.01 | 0.36 ± 0.02 | 1.41 ± 0.00 |
| M27 | 0.5 ± 0.1 | 2 | 0.08 ± 0.01 | 14.5 ± 0.17 | 18.4 ± 2.08 | 1.40 ± 0.16 | 7.61 ± 0.07 | 0.0 | 0.0 | 9 | 0.23 ± 0.01 | 68.0 ± 0.92 | 29.3 ± 1.07 | 2.41 ± 0.10 | 8.23 ± 0.06 | 0.0 | 0.0 |
| M28 | 1.0 ± 0.3 | 2 | 0.08 ± 0.01 | 10.2 ± 0.69 | 13.2 ± 1.42 | 2.66 ± 0.29 | 20.3 ± 0.08 | 0.38 ± 0.04 | 2.91 ± 0.10 | 15 | 0.90 ± 0.02 | 385.7 ± 7.48 | 42.9 ± 1.13 | 3.14 ± 0.07 | 7.33 ± 0.13 | 0.25 ± 0.01 | 0.58 ± 0.02 |
| M29 | 0.8 ± 0.1 | 2 | 0.09 ± 0.01 | 9.7 ± 0.11 | 11.4 ± 1.37 | 2.21 ± 0.26 | 19.4 ± 0.05 | 0.33 ± 0.04 | 2.89 ± 0.05 | 15 | 0.85 ± 0.01 | 192.3 ± 17.22 | 22.6 ± 2.04 | 2.30 ± 0.13 | 10.2 ± 0.33 | 0.24 ± 0.01 | 1.06 ± 0.04 |
| m06 | 0.2 ± 0.2 | 7 | 0.10 ± 0.01 | 11.6 ± 0.08 | 12.2 ± 1.22 | 2.70 ± 0.27 | 22.2 ± 0.16 | 0.47 ± 0.05 | 3.87 ± 0.05 | 29 | 0.27 ± 0.01 | 87.3 ± 0.54 | 32.9 ± 1.09 | 4.63 ± 0.15 | 14.1 ± 0.07 | 0.61 ± 0.01 | 1.86 ± 0.02 |
| m07 | 0.2 ± 0.1 | 7 | 0.11 ± 0.01 | 12.0 ± 0.17 | 11.0 ± 0.82 | 2.55 ± 0.19 | 23.0 ± 0.11 | 0.47 ± 0.04 | 4.27 ± 0.01 | 13 | 0.12 ± 0.01 | 41.8 ± 0.40 | 34.2 ± 2.04 | 3.62 ± 0.22 | 10.6 ± 0.02 | 0.47 ± 0.03 | 1.37 ± 0.01 |
| M21 | 0.7 ± 0.0 | 3 | 0.09 ± 0.01 | 11.0 ± 0.31 | 11.8 ± 1.60 | 3.00 ± 0.40 | 25.4 ± 0.22 | 0.56 ± 0.08 | 4.78 ± 0.08 | 9 | 0.20 ± 0.00 | 37.5 ± 0.19 | 19.0 ± 0.29 | 4.58 ± 0.07 | 24.1 ± 0.15 | 0.60 ± 0.01 | 3.17 ± 0.04 |
| M23 | No growth | ||||||||||||||||
| M41 | 0.2 ± 0.0 | 7 | 0.16 ± 0.02 | 32.1 ± 0.47 | 20.2 ± 1.93 | 3.09 ± 0.30 | 15.3 ± 0.15 | 0.56 ± 0.05 | 2.78 ± 0.04 | 29 | 0.25 ± 0.01 | 75.6 ± 0.79 | 29.8 ± 1.10 | 3.65 ± 0.13 | 12.3 ± 0.11 | 0.56 ± 0.02 | 1.88 ± 0.02 |
| M44 | 0.2 ± 0.1 | 7 | 0.12 ± 0.01 | 23.3 ± 0.63 | 20.2 ± 2.10 | 2.78 ± 0.28 | 13.7 ± 0.10 | 0.52 ± 0.05 | 2.59 ± 0.01 | 29 | 0.25 ± 0.01 | 77.2 ± 0.50 | 28.7 ± 0.91 | 2.44 ± 0.08 | 8.52 ± 0.05 | 0.28 ± 0.01 | 0.96 ± 0.01 |
| M46 | 0.2 ± 0.1 | 7 | 0.16 ± 0.0 | 38.8 ± 0.34 | 24.1 ± 0.71 | 2.81 ± 0.09 | 11.7 ± 0.09 | 0.49 ± 0.02 | 2.04 ± 0.03 | 29 | 0.29 ± 0.03 | 96.0 ± 0.60 | 31.0 ± 3.38 | 2.74 ± 0.30 | 8.84 ± 0.03 | 0.27 ± 0.03 | 0.88 ± 0.01 |
| M58 | 0.3 ± 0.0 | 7 | 0.10 ± 0.01 | 7.4 ± 0.09 | 7.7 ± 0.53 | 1.86 ± 0.13 | 24.3 ± 0.14 | 0.45 ± 0.03 | 5.94 ± 0.06 | 14 | 0.14 ± 0.01 | 47.3 ± 2.44 | 33.2 ± 3.90 | 3.24 ± 0.38 | 9.74 ± 0.13 | 0.37 ± 0.04 | 1.12 ± 0.02 |
| M62 | 0.2 ± 0.1 | 7 | 0.15 ± 0.02 | 23.0 ± 0.38 | 15.5 ± 1.91 | 2.75 ± 0.34 | 17.7 ± 0.19 | 0.55 ± 0.07 | 3.52 ± 0.06 | 14 | 0.18 ± 0.02 | 62.9 ± 3.86 | 35.7 ± 5.19 | 4.10 ± 0.56 | 11.5 ± 0.24 | 0.47 ± 0.06 | 1.33 ± 0.05 |
| M65 | 0.3 ± 0.1 | 7 | 0.14 ± 0.04 | 28.2 ± 0.28 | 20.8 ± 5.46 | 3.22 ± 0.85 | 15.5 ± 0.19 | 0.60 ± 0.16 | 2.90 ± 0.06 | 12 | 0.17 ± 0.01 | 63.3 ± 0.24 | 38.1 ± 1.18 | 3.77 ± 0.12 | 9.89 ± 0.08 | 0.45 ± 0.01 | 1.19 ± 0.00 |
3.4. Batch experiment—DW and TFA content
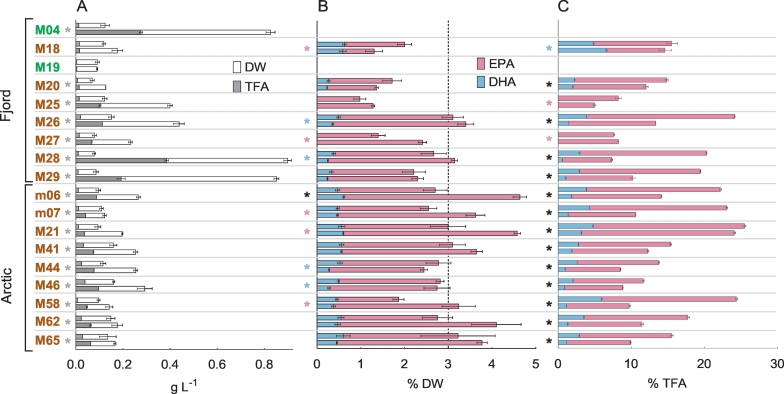
The eighteen strains growing in the batch experiment were sampled for DW and FA analysis in the exponential and the stationary growth phase, with sampling days being chosen individually for each strain (Table 3). Large differences were observed in both DW and TFA content between strains, and between growth phases (Fig. 5A). Interestingly, the two P. tricornutum strains M28 and M29, and the green algae strain M04 reached much higher DW in stationary phase compared to the others, with DW of 0.90, 0.85 and 0.83 g L− 1, respectively. This response could be due to silicate becoming the limiting nutrient for most of the diatom strains. As green algae do not require silicate to grow and P. tricornutum requires only modest amounts [44], other nutrients such as nitrate or phosphate may have been limiting for them at a later stage, thus allowing them to grow to greater cell densities. To verify this, the nutrient composition during the growth experiment would need to be measured. However, the DW of the third P. tricornutum strain, M26, was lower in stationary phase (0.44 g L− 1) compared to strains M28 and M29. One explanation could be that strain M26 had not accumulated as much storage compound, as it was sampled earlier in the stationary phase (day 11) than M28 and M29 (sampled on day 15), resulting in a comparatively lower DW.
Fig. 5.
Superimposed dry weight (DW) and total fatty acid (TFA), EPA and DHA content of eighteen strains in the exponential (upper bar) and the stationary growth phase (lower bar) during the batch experiment. Data are average values with standard deviation (n = 3) of measurement replicates from one biological replicate. A: Average DW and TFA concentration. B: Average EPA and DHA amount relative to DW. C: Average EPA and DHA amount relative to TFA. The dotted vertical line marks the particular benchmark level for EPA and DHA. Strains identified as Chlorophyceae are displayed in green and strains belonging to the class Bacillariophyceae in brown. Asterisks in front of the bars indicate significant difference between the exponential and the stationary phase (t-test, p < 0.01), in grey for TFA (relative to DW), red for EPA, blue for DHA and black for both EPA and DHA. Strains designated with “M” are clonal strains obtained from single cell isolation by cell sorting flow cytometry. Strains designated with “m” were isolated by serial dilution. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
The TFA content relative to the biomass DW (w/w) varied between 5.0 (M19) and 24.1% (M46) in exponential phase, and between 5.0 (M19) and 42.9% (M28) in stationary phase (Table 3). All strains (except for M18 and M19) showed a significant increase in their TFA content relative to the DW from the exponential to the stationary phase (p < 0.01), indicating TAG accumulation (Fig. 5A). The sampling time point in exponential phase (day 7) was unfortunately chosen slightly too late for some T. hispida strains, as most had already entered early stationary phase except for strains M58, m06 and m07, as can be seen in Fig. 4. This was also reflected in the TFA content, which was much lower in exponential samples for strains M58, m06 and m07 (7.7, 12.2, and 11.0% DW, respectively), than for the others with TFA between 15.5 and 24.1% DW. However, the TFA content increased further in stationary phase (between 28.7 and 38.1% DW) in all T. hispida strains. The three P. tricornutum strains M26, M28 and M29 had a very similar TFA content in the exponential phase (between 11.4 and 13.2% DW), but showed differences in the stationary phase. M28 had with 42.9% DW a much higher TFA content compared to M26 (25.5%) and M29 (22.6%). As mentioned above, M26 was sampled earlier in stationary phase (day 11), thus its lower TFA content is probably due to lower level of TAG accumulation. However, M29 was sampled on the same day as M28 (day 15) and also reached a similar DW, and thus seems to differ in its physiological response to nutrient starvation.
3.5. Batch experiment—EPA and DHA content
EPA and DHA content in the exponential and the stationary phase of the eighteen strains grown in the batch experiment are presented in percentage (w/w) relative to biomass DW and to TFA (Fig. 5B, and C, respectively). Both EPA and DHA were present in all Arctic strains investigated, but not in all strains isolated from the fjords. EPA was not detected in the two chlorophytes M04 and M19. The absence (or only insignificant amounts) of EPA has been reported for some green algae previously, while high levels of EPA have been found in other green algae species [45]. All sixteen diatom strains possessed EPA, but in different concentrations. The EPA content relative to the biomass DW ranged between 1.0 (M25) and 3.2% (M65) in exponential, and between 1.3 (M25) and 4.6% (m06) in stationary phase. Strain M18 showed a significant decrease, whereas the five strains M27, m06, m07, M21 and M58 revealed a significant increase in their DW-based EPA content from exponential to stationary phase (p < 0.01). In contrast to this, the EPA content in percentage of TFA decreased significantly in all strains, except in M18 and M27 (p < 0.01), from exponential to stationary phase, possibly caused by accumulation of TAG. TAGs mostly comprise the saturated and monounsaturated FA and therefore TAG accumulation causes the relative amount of PUFAs like EPA to decrease in stationary phase [13]. Values varied between 7.6 (M27) and 25.4% TFA (M21) in exponential phase and between 5.0 (M25) and 24.1% TFA (M21) in stationary phase. Nine strains (7 Arctic, 2 fjord), comprising 50% of the isolates that grew in the experiment, obtained the targeted EPA content of 3% DW in either exponential or stationary phase or both.
DHA was found in fourteen isolates, and was not detected in the chlorophytes M04 and M19, and the two diatoms M25 and M27. The DHA content relative to DW was low, and varied between 0.26 (M20) and 0.62% (M18) in exponential, and 0.23 (M20) and 0.61% (m06) in stationary phase, and remained on the same level or decreased (M26, M28, M44, M46) from exponential to stationary phase. DHA content relative to the TFA content ranged between 2.0 (M46) and 5.9% (M58), and between 0.6 (M28) and 6.6% (M18) in exponential and stationary phase, respectively. A significant increase in M18 and a decrease in all other strains from exponential to stationary phase was observed. None of the strains reached the targeted DHA content relative to the DW.
Eight T. hispida strains and three P. tricornutum strains were investigated in the batch experiment. Interestingly, both the different T. hispida strains and the P. tricornutum strains showed differences in their growth, and TFA and EPA content that could only be explained to a certain degree by the different sampling times. This emphasizes that variations can be observed at the strain level, and it is therefore advisable to test multiple strains of specific species. This has also been reported before [20].
3.6. Batch experiment—relative TFA composition
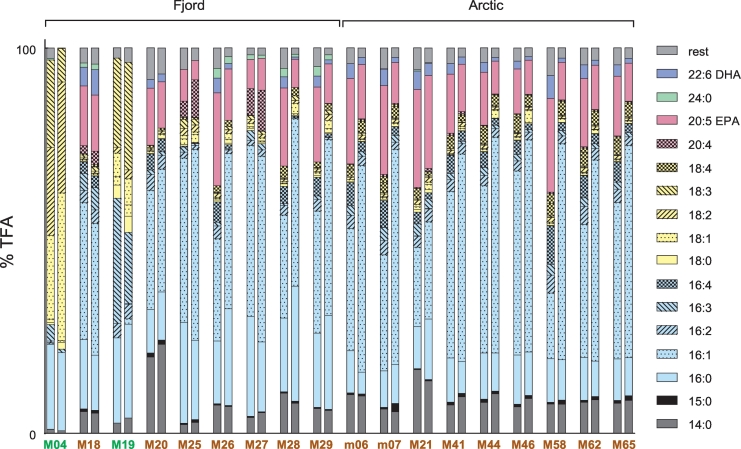
The relative TFA composition of microalgae is often used as a taxonomic indicator, as different microalgal classes are characterized by a specific FA profile [46]. Fig. 6 shows an overview on the FA composition as a percentage of TFA for the eighteen strains that grew in the batch experiment (FA with > 2% TFA in at least one strain are listed). The two chlorophytes, M04 and M19, clearly differed from the diatom strains. They had no, or only negligible amounts of FA with a carbon chain length higher than C18, besides having C18 mono- and polyunsaturated FA as their major FA. All diatoms showed a similar overall picture of the FA composition, with C16 FA representing the most abundant, followed by LC-PUFAs (C > 20) and myristic acid (14:0), and low levels of C18 derivatives. Similar results were found by Zhukova & Aizdaicher (1995) [46], with a preference of palmitoleic acid (16:1) over palmitic acid (16:0), and high levels of myristic acid and EPA, together with an insignificant amount of C18 FA for diatoms. However, some differences among the diatom species were observed in our study. Only strains M18, M21, M25 and M27 possessed arachidonic acid (20:4) in amounts higher than 2% TFA, whereas only trace amounts were detected for all other diatoms. M18, M27 and the three P. tricornutum strains (M26, M28 and M29) were the only diatoms with lignoceric acid (24:0), and DHA was not detected for two diatoms (M25 and M27). In the future, when additional FA data from more strains are available, a cluster analysis of the FA composition data could reveal, if and to what degree the FA profile reflects the phylogenetic classification of the microalgae.
Fig. 6.
Relative fatty acid composition of the 18 isolates for both the exponential (first bar) and the stationary (second bar) phase during the batch experiment. Data are average values (n = 3) of measurement replicates from one biological replicate. FA with amounts < 2% of total fatty acids (TFA) in all strains are summarized in rest. Strains identified as Chlorophyceae are displayed in green and strains belonging to the class Bacillariophyceae in brown. Strains designated with “M” are clonal strains obtained from single cell isolation by cell sorting flow cytometry. Strains designated with “m” were isolated by serial dilution. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Most strains showed variations in the relative FA abundance between the exponential and the stationary phase, with palmitoleic acid (16:1) content predominantly increasing in stationary phase, and PUFAs such as EPA and DHA accounting for a higher fraction of TFA in the exponential phase for most, but not all strains. This correlates favorably with the aforementioned typical pattern of TAG accumulation, as TAGs comprise mostly saturated and monounsaturated FA, whereas PUFAs are typically present in membrane lipids [13]. However, in some microalgae, TAGs can be another, but much less common PUFA source [12], [47]. TAG accumulation together with an incorporation of EPA and DHA into the TAGs have been observed, for example, in Thalassiosira pseudonana and Pavlova lutheri (Prymnesiophyceae) in the stationary growth phase [48].
3.7. Selection of candidate strains
Based on our findings and selection criteria for growth rate (≥ 0.7 d− 1) and EPA/DHA content (≥ 3% DW), the three strains M21 (A. septentrionalis) and M26 and M28 (P. tricornutum) were selected, and recommended for further characterization with respect to upscaling and future utilization in microalgae-based technologies. Strain M21 seemed especially interesting and promising, as it had a high growth rate of 0.7 d− 1 at low temperatures (10 °C) and a high EPA content relative to biomass DW, which increased in stationary phase to 4.6%. Similar, but slightly lower growth rates (0.6 d− 1) at 8.5 °C have been reported for another Attheya species, A. longicornis, isolated from northern Norwegian coastal waters [49]. Additional investigations might reveal if it is possible to increase the EPA levels further, suggesting sampling later in the stationary phase and varying growth conditions. As we did not discriminate between polar and non-polar lipids in this study, such analyses might provide information about which lipid fraction the EPA accumulates in, during the stationary phase. High growth rates and different EPA levels have been reported for P. tricornutum strains previously; Jiang & Gao (2004) [35] and Patil et al. (2006) [4] found an EPA content of 2.6 and 2.8% DW respectively, which is in a similar range as our findings. The two P. tricornutum strains selected in our study differed in their EPA content, with M26 reaching higher amounts (3.1% DW) in exponential phase than M28 (2.7% DW), but at the same time having a lower growth rate. Thus, further experiments will be needed to show which combination will result in a higher EPA productivity.
The selected strains are adapted to, and have so far been tested at only one growth condition, but in future upscaling and outdoor large-scale productions, growth conditions may differ from those used in our experiments. In this case higher media nutrient concentrations are necessary in order to reach higher biomass densities, and temperatures and irradiance will vary, both being important factors that regulate the relative content of FA and the absolute quantity of EPA [33]. Therefore, the influence of different growth factors (e.g. irradiance, temperature and nutrient content) on the growth rate and the EPA content, and thus EPA productivity, needs to be investigated in future studies.
3.8. Evaluation of the pipeline
The growth and FA profiling of the isolates was determined to be the rate-limiting step of our pipeline. Despite the experiment design being one of simplicity and speed, considerable time was needed in order to evaluate the FA content and composition for each isolate, as no rapid methods currently exist without conducting FA extraction and GC analysis. Furthermore, the fact that the strains were sampled at different time points during their exponential and stationary growth phase makes empirical comparisons difficult. However, the growth characteristics of the individual isolates were very different, and therefore appropriate sampling time points had to be estimated during the ongoing experiment.
Nevertheless, the first screening results obtained in this study are promising, and by continuing to investigate more isolates, additional information can be surmised from our data. Besides discovering new candidate strains with potential to be used in biotechnological applications, statistical analysis of a subsequent larger-scale dataset could reveal which of the factors (sampling location, time of the year, microalgae species or culture conditions) have the most beneficial effect on the bioprospecting success. This would help greatly improve future sampling strategies and also give a much more detailed insight into the ecophysiological properties of the microalgae.
4. Conclusion
A screening pipeline was developed in order to find new and promising North Atlantic microalgal strains to be used in biotechnological application targeting the production of the omega-3-fatty acids EPA and DHA. One hundred and forty nine different isolates (comprising mostly diatoms) were established as stock cultures, and twenty of these were investigated in terms of their growth rates and EPA/DHA content. Arctic strains generally had a higher EPA content than fjord strains, but lower growth rates. Promising data were found for three strains represented by the diatoms Phaeodactylum tricornutum (fjord) and Attheya septentrionalis (Artic), which showed high growth rates (≥ 0.7 d− 1), together with an increased EPA content (> 3% DW), suggesting their potential use in microalgae-based technologies and EPA production. Similar findings have been described for P. tricornutum previously, and this species has widely been used in aquaculture [4], [35], [36]. Yet, to our knowledge, this is the first report on a high EPA content for A. septentrionalis; with an EPA content of 4.6% DW in stationary phase, this value is higher than those typically reported from industrially-applied microalgae (Nannochloropsis sp. 2.1–3.8%, P. tricornutum 2.6–3.1%). However, as those EPA values are generally derived from exponentially-grown algae, the EPA production potential of A. septentrionalis must be further evaluated, as growth rates in the stationary phase are low. This study highlights the value of establishing a screening pipeline with a targeted focus on productive microalgae strains from northern high latitude waters.
Acknowledgments
Acknowledgements
This work was supported by EU MIRACLES project and has received funding from the European Union’s Seventh Framework Programme for research; technological development and demonstration under grant agreement No. 613588. We thank Maria Lund Paulsen at Marine Microbiology, University of Bergen for sampling and Research Project MicroPolar (number 807785, Norwegian Research Council) for including us for sampling. We would also like to acknowledge the staff at the Flow Cytometry Core Facility, Department of Clinical Science, University of Bergen for performing the flow cytometry/cell sorting analyses. Many thanks to Bryan Wilson at Marine Microbiology, University of Bergen for revising and improving the language quality of this paper.
References
- 1.Miller M.R., Nichols P.D., Carter C.G. n-3 oil sources for use in aquaculture—alternatives to the unsustainable harvest of wild fish. Nutr. Res. Rev. 2008;21:85–96. doi: 10.1017/S0954422408102414. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D., Wu J.H.Y. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 3.Venegas-Calerón M., Sayanova O., Napier J.A. An alternative to fish oils: metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog. Lipid Res. 2010;49:108–119. doi: 10.1016/j.plipres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Patil V., Källqvist T., Olsen E., Vogt G., Gislerød H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac. Int. 2006;15:1–9. [Google Scholar]
- 5.Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006;101:87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- 6.Rubio-Rodríguez N., Beltrán S., Jaime I., de Diego S.M., Sanz M.T., Carballido J.R. Production of omega-3 polyunsaturated fatty acid concentrates: a review. Innovative Food Sci. Emerg. Technol. 2010;11:1–12. [Google Scholar]
- 7.Chauton M.S., Reitan K.I., Norsker N.H., Tveterås R., Kleivdal H.T. A techno-economic analysis of industrial production of marine microalgae as a source of EPA and DHA-rich raw material for aquafeed: research challenges and possibilities. Aquaculture. 2015;436:95–103. [Google Scholar]
- 8.Norambuena F., Lewis M., Hamid N.K.A., Hermon K., Donald J.A., Turchini G.M. Fish oil replacement in current aquaculture feed: is cholesterol a hidden treasure for fish nutrition? PLoS One. 2013;8 doi: 10.1371/journal.pone.0081705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simopoulos A.P. The omega-6/omega-3 fatty acid ratio: health implications. Oilseeds Fats Crop. Lipids. 2010;17:267–275. [Google Scholar]
- 10.Barclay W., Apt K. 2013. Strategies for bioprospecting microalgae for potential commercial applications; pp. 69–79. (Handb. Microalgal Cult. Appl. Phycol. Biotechnol.). [Google Scholar]
- 11.Draaisma R.B., Wijffels R.H., Slegers P.M.E., Brentner L.B., Roy A., Barbosa M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013;24:169–177. doi: 10.1016/j.copbio.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Sharma K.K., Schuhmann H., Schenk P.M. High lipid induction in microalgae for biodiesel production. Energies. 2012;5:1532–1553. [Google Scholar]
- 13.Olofsson M., Lamela T., Nilsson E., Bergé J.P., del Pino V., Uronen P., Legrand C. Seasonal variation of lipids and fatty acids of the microalgae Nannochloropsis oculata grown in outdoor large-scale photobioreactors. Energies. 2012;5:1577–1592. [Google Scholar]
- 14.Breuer G., Lamers P.P., Martens D.E., Draaisma R.B., Wijffels R.H. The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour. Technol. 2012;124:217–226. doi: 10.1016/j.biortech.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Harwood J.L., Guschina I.A. The versatility of algae and their lipid metabolism. Biochimie. 2009;91:679–684. doi: 10.1016/j.biochi.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Rodolfi L., Chini Zittelli G., Bassi N., Padovani G., Biondi N., Bonini G., Tredici M.R. Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009;102:100–112. doi: 10.1002/bit.22033. [DOI] [PubMed] [Google Scholar]
- 17.Jegathese S.J.P., Farid M. Microalgae as a renewable source of energy: a niche opportunity. J. Renew. Energy. 2014;2014 [Google Scholar]
- 18.Lim D.K.Y., Garg S., Timmins M., Zhang E.S.B., Thomas-Hall S.R., Schuhmann H., Li Y., Schenk P.M. Isolation and evaluation of oil-producing microalgae from subtropical coastal and brackish waters. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araujo G.S., Matos L.J.B.L., Gonçalves L.R.B., Fernandes F.A.N., Farias W.R.L. Bioprospecting for oil producing microalgal strains: evaluation of oil and biomass production for ten microalgal strains. Bioresour. Technol. 2011;102:5248–5250. doi: 10.1016/j.biortech.2011.01.089. [DOI] [PubMed] [Google Scholar]
- 20.Slocombe S.P., Zhang Q., Ross M., Anderson A., Thomas N.J., Lapresa Á., Rad-Menéndez C., Campbell C.N., Black K.D., Stanley M.S., Day J.G. Unlocking nature's treasure-chest: screening for oleaginous algae. Sci Rep. 2015;5:9844. doi: 10.1038/srep09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee K., Eisterhold M.L., Rindi F., Palanisami S., Nam P.K. Isolation and screening of microalgae from natural habitats in the midwestern United States of America for biomass and biodiesel sources. J. Nat. Sci. Biol. Med. 2014;5:333–339. doi: 10.4103/0976-9668.136178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C.-Y., Chou H.-N. Screening of red algae filaments as a potential alternative source of eicosapentaenoic acid. Mar. Biotechnol. 2002;4:189–192. doi: 10.1007/s10126-002-0002-4. [DOI] [PubMed] [Google Scholar]
- 23.Yongmanitchai W., Ward O.P. Screening of algae for potential alternative sources of eicosapentarnoic acid. Phytochemistry. 1991;30:2963–2967. [Google Scholar]
- 24.Walne P.R. Vol. 26. 1970. Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria and Mytilus; pp. 1–62. (Fish. Invest. Lond. Ser. 2). [Google Scholar]
- 25.Zhu C.J., Lee Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997;9:189–194. [Google Scholar]
- 26.Meier S., Mjøs S.A., Joensen H., Grahl-Nielsen O. Validation of a one-step extraction/methylation method for determination of fatty acids and cholesterol in marine tissues. J. Chromatogr. A. 2006;1104:291–298. doi: 10.1016/j.chroma.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 27.Prestegard S.K., Erga S.R., Steinrücken P., Mjøs S.A., Knutsen G., Rohloff J. Specific metabolites in a Phaeodactylum tricornutum strain isolated from western Norwegian fjord water. Mar. Drugs. 2015;14:9. doi: 10.3390/md14010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasta Z., Mjøs S.A. A database of chromatographic properties and mass spectra of fatty acid methyl esters from omega-3 products. J. Chromatogr. A. 2013;1299:94–102. doi: 10.1016/j.chroma.2013.05.056. [DOI] [PubMed] [Google Scholar]
- 29.Scholin C.A., Herzog M., Sogin M., Anderson D.M. Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J. Phycol. 1994;30:999–1011. [Google Scholar]
- 30.Nunn G.B., Theisen B.F., Christensen B., Arctander P. Simplicity-correlated size growth of the nuclear 28S ribosomal RNA D3 expansion segment in the crustacean order Isopoda. J. Mol. Evol. 1996;42:211–223. doi: 10.1007/BF02198847. [DOI] [PubMed] [Google Scholar]
- 31.Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 32.Zhang Z., Schwartz S., Wagner L., Miller W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- 33.Sukenik A., Zmora O., Carmeli Y. Biochemical quality of marine unicellular algae with special emphasis on lipid composition. II. Nannochloropsis sp. Aquaculture. 1991;117:313–326. [Google Scholar]
- 34.Hu H., Gao K. Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol. Lett. 2006;28:987–992. doi: 10.1007/s10529-006-9026-6. [DOI] [PubMed] [Google Scholar]
- 35.Jiang H., Gao K. Effects of lowering temperature during culture on the production of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum (Bacillariophyceae) J. Phycol. 2004;40:651–654. [Google Scholar]
- 36.Sánchez S., Martínez M.E., Espinola F. Biomass production and biochemical variability of the marine microalga Isochrysis galbana in relation to culture medium. Biochem. Eng. J. 2000;6:13–18. doi: 10.1016/s1369-703x(00)00071-1. [DOI] [PubMed] [Google Scholar]
- 37.Duong V.T., Li Y., Nowak E., Schenk P.M. Microalgae isolation and selection for prospective biodiesel production. Energies. 2012;5:1835–1849. [Google Scholar]
- 38.Owrid G., Socal G., Civitarese G., Luchetta A., Wiktor J., Nöthig E.-M., Andreassen I., Schauer U., Strass V. Spatial variability of phytoplankton, nutrients and new production estimates in the waters around Svalbard. Polar Res. 2000;19:155–171. [Google Scholar]
- 39.Boelen P., van Dijk R., Sinninghe Damsté J.S., Rijpstra W.I.C., Buma A.G.J. On the potential application of polar and temperate marine microalgae for EPA and DHA production. AMB Express. 2013;3:26. doi: 10.1186/2191-0855-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hildebrand M., Davis A.K., Smith S.R., Traller J.C., Abbriano R. The place of diatoms in the biofuels industry. Biofuels. 2012;3:221–240. [Google Scholar]
- 41.Buchan A., LeCleir G.R., Gulvik C.A., González J.M. Master recyclers: features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 2014;12:686–698. doi: 10.1038/nrmicro3326. [DOI] [PubMed] [Google Scholar]
- 42.Okolodkov Y.B., Dodge J.D. Biodiversity and biogeography of planktonic dinoflagellates in the Arctic Ocean. J. Exp. Mar. Biol. Ecol. 1996;202:19–27. [Google Scholar]
- 43.Degerlund M., Eilertsen H.C. Main species characteristics of phytoplankton spring blooms in NE Atlantic and Arctic waters (68-80° N) Estuar. Coasts. 2010;33:242–269. [Google Scholar]
- 44.Riedel G.F., Nelson D.M. Silicon uptake by algae with no known Si requirement. II. Strong pH dependence of uptake kinetic parameters in Phaeodactylum tricornutum (Bacillariophyceae) J. Phycol. 1985;21:168–171. [Google Scholar]
- 45.Lang I., Hodac L., Friedl T., Feussner I. Fatty acid profiles and their distribution patterns in microalgae: a comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011;11:124. doi: 10.1186/1471-2229-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhukova N.V., Aizdaicher N.A. Fatty acid composition of 15 species of marine microalgae. Phytochemistry. 1995;39:351–356. [Google Scholar]
- 47.Mühlroth A., Li K., Røkke G., Winge P., Olsen Y., Hohmann-Marriott M.F., Vadstein O., Bones A.M. Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of chromista. Mar. Drugs. 2013;11:4662–4697. doi: 10.3390/md11114662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tonon T., Harvey D., Larson T.R., Graham I.A. Long chain polyunsaturated fatty acid production and partitioning to triacylglycerols in four microalgae. Phytochemistry. 2002;61:15–24. doi: 10.1016/s0031-9422(02)00201-7. [DOI] [PubMed] [Google Scholar]
- 49.Huseby S., Degerlund M., Eriksen G.K., Ingebrigtsen R.A., Eilertsen H.C., Hansen E. Chemical diversity as a function of temperature in six northern diatom species. Mar. Drugs. 2013;11:4232–4245. doi: 10.3390/md11114232. [DOI] [PMC free article] [PubMed] [Google Scholar]