Abstract
Currently available glucose test strip enzymes include glucose oxidase (GOD) and glucose dehydrogenase (GDH). In GDH-based glucometers, glucose oxidation can be catalysed by different cofactors: nicotinamide adenine dinucleotide (GDH-NAD), flavin adenine dinucleotide (GDH-FAD), pyrroloquinolinequinone (GDH-PQQ) and mutant GDH-PQQ. GOD-based and GDH-NAD-based glucometers are substrate-specific and do not react with sugars other than glucose. GDH-FAD reacts with xylose only in addition to glucose. GDH-PQQ is not glucose-specific; in addition to glucose, it reacts with different other sugars and produces falsely high values of capillary glucose in the presence of such substances. There are reports of several deaths associated with usage of GDH-PQQ-based test strips. A modified form of GDH-PQQ, the so-called mutant GDH-PQQ, is supposedly free from such interferences. In this article spuriously high glucose values due to maltose interference in a glucometer using the mutant GDH-PQQ chemistry are being reported.
Keywords: Emergency medicine, Diabetes, General practice / family medicine, Adult intensive care, Nursing
Background
With increasing prevalence of diabetes across the globe, point of care testing devices like glucometer have become an essential tool for successful management of patients with diabetes. Moreover, modern-day glucometers are affordable, small-sized and user-friendly, which have made them indispensable even in resource-restricted hospital care settings. Following blood application, the tests strips of the glucometers use different enzymes to selectively catalyse the oxidation of glucose; the product of the enzyme reaction then reacts with an intermediate and generates a signal that is detected either photometrically or amperometrically. Currently available glucose test strip enzymes include glucose oxidase (GOD) and glucose dehydrogenase (GDH). In GDH-based glucometers, glucose oxidation can be catalysed by different cofactors: nicotinamide adenine dinucleotide (GDH-NAD), flavin adenine dinucleotide (GDH-FAD), pyrroloquinolinequinone (GDH-PQQ) and mutant GDH-PQQ.
As GOD transfers its electrons to oxygen (being reduced into hydrogen peroxide), oxygen levels may have a negative impact on GOD-based glucometers; however, they exhibit higher substrate specificity and do not react with sugars other than glucose. The GDH-NAD-based systems do not exhibit interference by cross-reacting sugars, and GDH-FAD reacts with xylose only in addition to glucose. GDH-PQQ is not glucose-specific; in addition to glucose, it reacts with other sugars (maltose, galactose, cellobiose, lactose and xylose) and produces falsely high values of capillary glucose in the presence of such substances. A modified form of GDH-PQQ, the so-called mutant GDH-PQQ, is supposedly free from such interference.
Case presentation
A young man with Guillain-Barre syndrome developed sudden-onset severe respiratory distress during intravenous administration of human immunoglobulin (IVIG). The patient himself disconnected the transfusion tube from the intravenous cannula, resulting in soakage of his left hand with human immunoglobulin. Fingertip capillary blood glucose estimation from his left hand by Accu-Chek Performa (Roche Diagnostics, Mannheim, Germany) showed a value of 510 mg/dL. A repeat test from one of his fingers of the right hand using the same glucometer gave a value of 238 mg/dL. Venous samples were immediately sent to laboratory, and corresponding venous plasma glucose was 113 mg/dL and haemoglobin A1c was 5.5%. Apprehending a possible analytical error, we applied IVIG directly in three different glucometers: Accu-Chek Performa, which uses mutant GDH-PQQ; TRUEresult, which uses GDH-FAD; and AccuSure Soul, which uses GOD-based test strips. The reading in the first one was more than 600 mg/dL (displayed ‘HI’) (figure 1) and less than 20 mg/dL (displayed ‘Lo’) in the remaining two (figures 2 and 3). The maltose concentration in IVIG is 100 mg/mL (figure 4).
Figure 1.
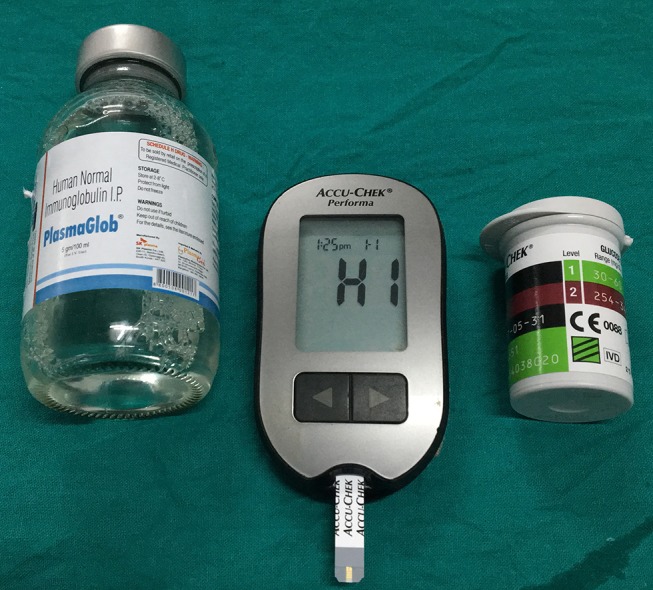
Accu-Chek Performa displaying HI (value over 600 mg/dL). The green box with three black bars over the strip box suggests that it uses mutant glucose dehydrogenase-pyrroloquinolinequinone and the strips are not subject to clinically relevant maltose interference.
Figure 2.

Lo reading in TRUEresult (which uses glucose dehydrogenase-flavin adenine dinucleotide) denoting a glucose value of less than 20 mg/dL.
Figure 3.
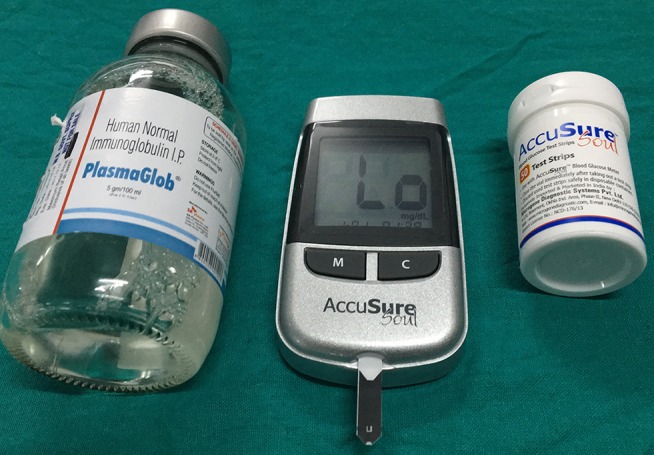
AccuSure Soul, which uses glucose oxidase-based test strips, displays Lo suggesting glucose value of less than 20 mg/dL.
Figure 4.
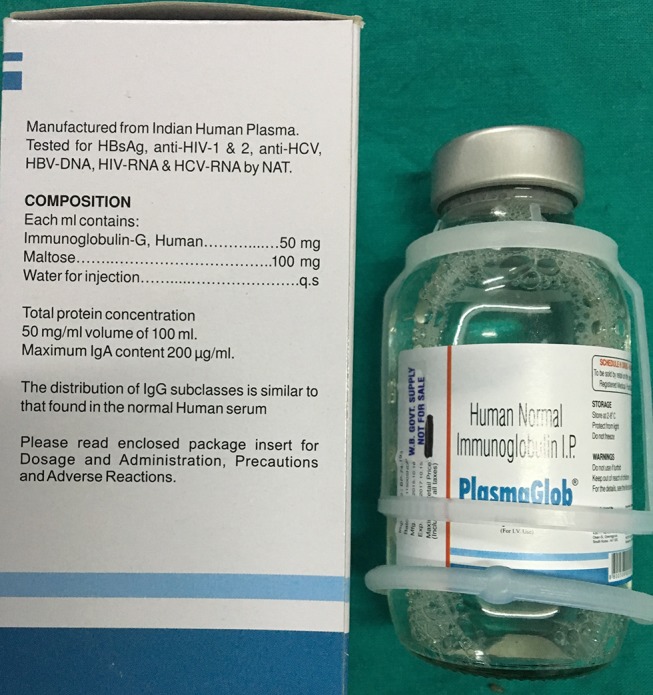
Intravenous immunoglobulin having a maltose content of 100 mg/mL.
Discussion
Maltose-containing agents commonly used in clinical practice are icodextrin-containing peritoneal dialysis solution, IVIG, Rho (D) immunoglobulin, Abatacept (used in rheumatoid arthritis), Adept Solution (used for reduction of postsurgical adhesions) and tositumomab (used in non-Hodgkin’s lymphoma). There are reports of several deaths associated with usage of GDH-PQQ-based test strips in which the most commonly implicated agents were icodextrin and IVIG.1 The deaths are attributed to GDH-PQQ-related falsely elevated glucose readings in the presence of maltose, resulting in insulin overdose. The Food and Drug Administration recommended that these metres should preferably not be used in hospital settings, and following this public health notification they have largely been replaced by glucometers using mutant GDH-PQQ.2 Mutant GDH-PQQ-based glucometers exhibit a striking specificity for glucose and have been claimed to be free from maltose interference. Despite the claim that such glucometers do not cross-react with sugars other than glucose, falsely high glucose values due to high galactose in a proven case of galactosaemia have been reported.3
Contamination of the left hand with extremely high concentration of maltose in this man resulted in grossly elevated capillary glucose values when measured initially by Accu-Chek Performa glucometer. The high capillary glucose on his right hand was likely due to cross-contamination of the right hand with maltose-rich IVIG.
Metabolism of intravenously administered maltose depends on the receiver’s health status and on the infusion rate, total infusion time and amount of the infused maltose. A number of studies have been conducted with intravenous infusion of maltose in subjects with normal renal function and in patients of end-stage renal disease on peritoneal dialysis with icodextrin-containing solutions to assess the possible maltose concentrations in plasma. The authors of those studies concluded that the expected maximum plasma concentration of maltose in those individuals was 10.5 mmol/L. Accu-Chek Performa test strips perform reasonably well and demonstrate suitable accuracy when tested with solutions having similar maltose concentration.4 However, extremely high concentration of maltose present in IVIG infusion (100 g/L or 292 mmol/L) is expected to show erroneous results with these test strips.
Maltose concentrations in blood following infusion of the maltose-containing agents that are used in day-to-day clinical practice are not elevated to a degree that can alter the metre readings, and the specificity of glucometers using mutant GDH-PQQ-based strips is still sufficient in most ‘not-that-extraordinary’ clinical situations.
The package insert of Accu-Chek Performa test strips states that galactose concentration of >15 mg/dL, triglyceride concentration of >1800 mg/dL and ascorbic acid concentration of >3 mg/dL will result in overestimation of blood glucose result by the metre. Nothing has been mentioned about possible maltose interference. To the best of our knowledge, this is the first report of spuriously high glucose values due to maltose interference in a glucometer using the mutant GDH-PQQ chemistry.
Learning points.
Caregivers should be made aware of the potential pitfalls of the mutant GDH-PQQ-based glucometers, and given the widespread use of different maltose-containing agents healthcare providers across a range of specialties need to be familiar with this issue.
All such glucometers should carry prominent warning information regarding possible clinically relevant maltose interference, and the package inserts should carry the clinically relevant maltose concentration above which possible interference becomes a serious consideration.
The Food and Drug Administration should ask the manufacturers to look into this particular issue in properly designed clinical experimental studies involving adequate number of patients.
Footnotes
Contributors: PPC and SP were involved in managing the patient, literature search and preparing the manuscript. RB and SC were involved in literature search, preparing the manuscript and providing intellectual inputs.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Frias JP, Lim CG, Ellison JM, et al. . Review of adverse events associated with false glucose readings measured by GDH-PQQ-based glucose test strips in the presence of interfering sugars. Diabetes Care 2010;33:728–9. 10.2337/dc09-1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration. FDA Public Health Notification: Potentially Fatal Errors with GDH-PQQ* Glucose Monitoring Technology. August 13, 2009. https://wayback.archiveit.org/7993/20170111190502/http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm176992.htm(accessed May 2017).
- 3.Mathew V, Ramakrishnan A, Srinivasan R, et al. . Erroneous glucose recordings while using mutant variant of quinoprotein glucose dehydrogenase glucometer in a child with galactosemia. Indian J Endocrinol Metab 2013;17:289–91. 10.4103/2230-8210.119616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkes JL, Slatin SL, Pardo S, et al. . A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care 2000;23:1143–8. 10.2337/diacare.23.8.1143 [DOI] [PubMed] [Google Scholar]


