Description
A 64-year-old male was diagnosed with clinical stage IV lung adenocarcinoma. Biopsy was negative for both epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement. We administered five lines of chemotherapy. The disease continued to progress (figure 1A), and the patient received antiprogrammed death(PD)-1 antibody (nivolumab, scheduled at 3 mg/kg once every 2 weeks). After 2 months, he received supportive care due to progression (figure 1B). After 1 month, CT demonstrated a decrease in the size of the lung cancer and metastases (figure 1C). We concluded that this was an atypical response, or pseudoprogression, caused by the immunotherapeutic agent. After 2 months, nivolumab was reinitiated due to progression (figure 2A). After 1 month, the cancer increased in size (figure 2B), and the histopathology of the specimen obtained from the left cervical lymph nodes showed tumour cells and a mild to moderate inflammatory cell infiltrate (figure 3). His serum CA19-9 levels had increased from 56.9 U/mL to 2210.0 U/mL, over 4 months. He expired 5 months later.
Figure 1.
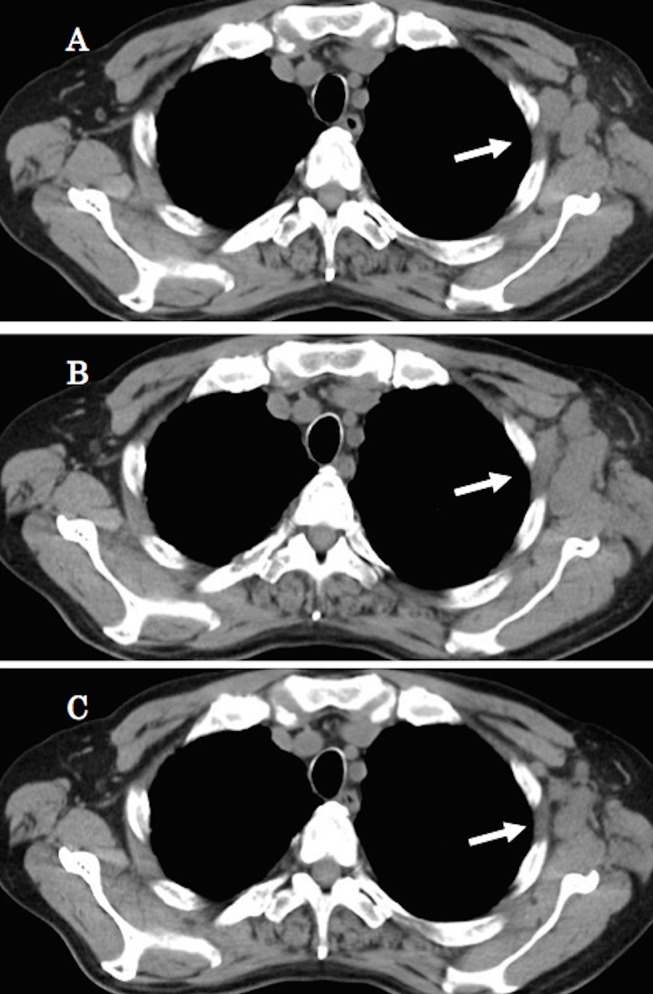
After five lines of chemotherapy for lung adenocarcinoma, CT revealed the enlarged lymph node in the left axilla (A). Eight weeks later, after six cycles of nivolumab treatment, CT revealed that it became larger (B). After 1 month, CT demonstrated a decrease in the size of the lung cancer and metastases (C).
Figure 2.
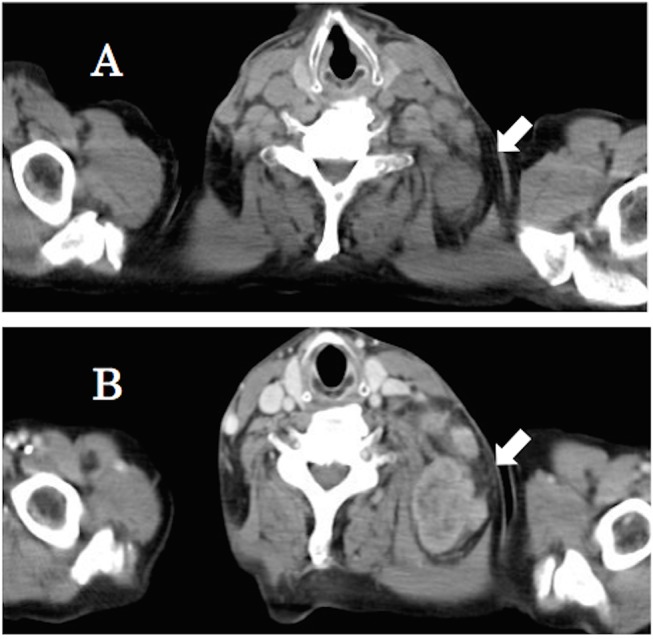
Nivolumab was reinitiated due to progression (A). After 1 month, CT revealed the lung cancer and metastases, especially the left cervical lymph nodes, became larger (B).
Figure 3.
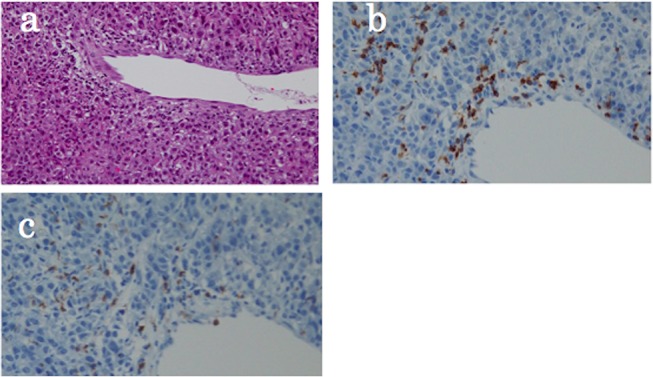
The histopathology of the specimen obtained from these nodes, illustrating tumour cells and a mild to moderate inflammatory cell infiltrate (a; H&E; ×200), containing cytotoxic T lymphocytes (b, c: CD3 and CD8, respectively; ×400).
Nivolumab is a fully humanised monoclonal antibody against a PD-1, which is a key immune-checkpoint receptor expressed by activated T lymphocytes.1 It has been known to cause pseudoprogression. It may be misinterpreted as disease progression and may lead to erroneous treatment decisions.2 3 The presence of a dense inflammatory cell infiltrate may be useful to distinguish pseudoprogression from progression.3 It is unclear when and how to assess it by the histopathology of the biopsy specimen. Our experience has led us to carefully assess the therapeutic benefit of nivolumab.
Learning points.
Nivolumab is a fully humanised monoclonal antibody against a programmed death (PD)-1, which is a key immune-checkpoint receptor expressed by activated T lymphocytes.
Nivolumab has been known to cause pseudoprogression. It may be misinterpreted as disease progression and may lead to erroneous treatment decisions.
It is important to carefully assess the therapeutic benefit of nivolumab.
Footnotes
Contributors: YK is the guarantor of the content of the manuscript. TI, YK and HT have made substantial contributions to the concept and design, or acquisition of data or analysis and interpretation of data; have drafted the submitted article or revised it critically for important intellectual content; have provided final approval of the version to be published; and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Concept and design: TI, YK and HT. Acquisition of data: TI, YK and HT. Analysis/interpretation of data: TI, YK and HT. Approval of manuscript: TI, YK and HT.
Competing interests: None declared.
Patient consent: Consent obtained from guardian.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gandini S, Massi D, Mandalà M. PD-L1 expression in Cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2016;100:88–98. 10.1016/j.critrevonc.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 2.Chiou VL, Burotto M. Pseudoprogression and Immune-Related response in solid tumors. J Clin Oncol 2015;33:3541–3. 10.1200/JCO.2015.61.6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Giacomo AM, Danielli R, Guidoboni M, et al. . Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 2009;58:1297–306. 10.1007/s00262-008-0642-y [DOI] [PMC free article] [PubMed] [Google Scholar]


