Description
Primary sclerosing cholangitis (PSC) is an idiopathic and insidious cholestatic liver disease characterised by persistent inflammation of the biliary tree, leading to fibrosis and stricturing.1 Often associated with inflammatory bowel disease, pathogenesis may be related to activated intestinal T-cells expressing the integrin cell surface receptor α4β7. These T-cells are recruited to the liver parenchyma resulting in persistent biliary inflammation.1 Patients typically remain asymptomatic until a diagnosis is attained by detection of elevated liver enzymes.2 No effective medical management exists, and the progressive fibrotic nature of the disease usually results in the necessity of liver transplantation.
We present a case of a male in his 40s with a 30-year history of ulcerative colitis and newly diagnosed PSC, who demonstrated an improvement in inflammation and stricturing of the biliary tree with vedolizumab (figure 1). On routine follow-up our patient was found to have an elevated alkaline phosphatase of 225 IU/L (alanine transaminase (ALT) 121 IU/L; aspartate transaminase (AST) 69 IU/L). Magnetic resonance cholangiopancreatography (MRCP) revealed stricturing and narrowing of the left and right intrahepatic ducts, findings consistent with PSC. A trial of ursodiol did not improve his clinical course. Treatment with vedolizumab was initiated with a standard loading dose of 300 mg at 0, 2 and 6 weeks followed by every 8 weeks thereafter. Thirteen months following induction of treatment, a follow-up MRCP demonstrated improvement in stricturing, and alkaline phosphatase was 127 IU/L (ALT 64 IU/L; AST 42 IU/L). A humanised monoclonal antibody, vedolizumab specifically recognised α4β7 heterodimer and selectively blocking gut lymphocyte trafficking.3 This inhibition of lymphocytic homing may aid in slowing or reverse the detrimental fibrotic nature of PSC.
Figure 1.
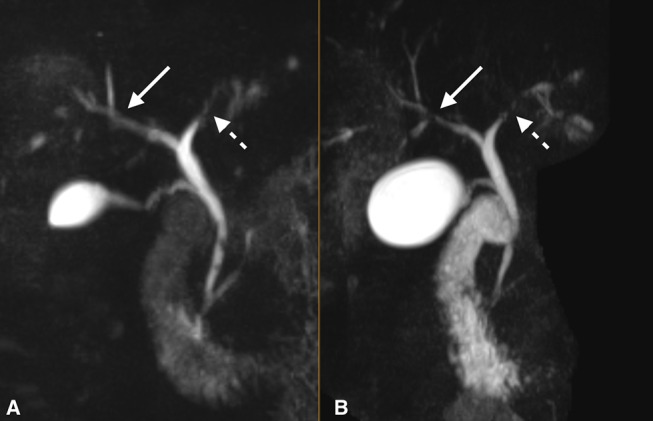
T2-weighted three-dimensional maximum intensity projection coronal images with fat saturation from prior to treatment induction (B) and 13 months following induction (A) demonstrate improvement in the strictures of the intrahepatic biliary tree, particularly the right hepatic duct and its segmental branches (solid arrows). The left hepatic duct also shows decreased irregular luminal narrowing (dashed arrows) following vedolizumab therapy. Both images are shown at equal levels as they are centred at the hepatic hilum with.
Learning points.
In primary sclerosing cholangitis, it is believed that intestinal T-cells express the cell surface integrin α4β7 and are recruited to the hepatic parenchyma. Once in the hepatic tissue, these T-cells will bind to their ligand, mucosal addressin cell adhesion molecule 1. This binding of T-cell to its ligand has been associated with continued inflammation and subsequent fibrosis.
Treatment with vedolizumab, a monoclonal antibody that selectively blocks gut lymphocyte trafficking, may improve or slow progression of inflammation and fibrosis of the biliary tree.
Patients may be asymptomatic when initially diagnosed with primary sclerosing cholangitis (PSC). However, if symptoms do occur they are predominantly abdominal pain, pruritus, and fatigue. Standard loading dose of vedolizumab of 300 mg at 0, 2 and 6 weeks followed by every 8 weeks over a period of 1 year, improved the associated PSC symptoms of pruritus and abdominal pain in our patient.
Footnotes
Contributors: DW, JG, LB and SG contributed to this project as a team; portions were written and edited by each member of the team multiple times. SG, the attending physician, gave the final approval to this work.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med 2016;375:1161–70. 10.1056/NEJMra1506330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan GG, Laupland KB, Butzner D, et al. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol 2007;102:1042–9. 10.1111/j.1572-0241.2007.01103.x [DOI] [PubMed] [Google Scholar]
- 3.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. 10.1056/NEJMoa1215734 [DOI] [PubMed] [Google Scholar]


