Abstract
In organotypic hippocampal slice cultures (OHSC), the morphological and functional characteristics of both neurons and glial cells are well preserved. This model is suitable for addressing different research questions that involve studies on neuroprotection, electrophysiological experiments on neurons, neuronal networks or tumor invasion. The hippocampal architecture and neuronal activity in multisynaptic circuits are well conserved in OHSC, even though the slicing procedure itself initially lesions and leads to formation of a glial scar. The scar formation alters presumably the mechanical properties and diffusive behavior of small molecules, etc. Slices allow the monitoring of time dependent processes after brain injury without animal surgery, and studies on interactions between various brain-derived cell types, namely astrocytes, microglia and neurons under both physiological and pathological conditions. An ambivalent aspect of this model is the absence of blood flow and immune blood cells. During the progression of the neuronal injury, migrating immune cells from the blood play an important role. As those cells are missing in slices, the intrinsic processes in the culture may be observed without external interference. Moreover, in OHSC the composition of the medium-external environment is precisely controlled. A further advantage of this method is the lower number of sacrificed animals compared to standard preparations. Several OHSC can be obtained from one animal making simultaneous studies with multiple treatments in one animal possible. For these reasons, OHSC are well suited to analyze the effects of new protective therapeutics after tissue damage or during tumor invasion.
The protocol presented here describes a preparation method of OHSC that allows generating highly reproducible, well preserved slices that can be used for a variety of experimental research, like neuroprotection or tumor invasion studies.
Keywords: Neuroscience, Issue 126, Rat, mouse, slice cultures, brain slice cultures, organotypic hippocampal slice cultures, propidium iodide, invasiveness
Introduction
OHSC are a well-characterized in vitro model to study both physiological and pathological properties of neurons, astrocytes and microglia1. It is easy to control the extracellular environment and monitor the cellular and morphological changes after various stimuli. The organization of hippocampal neurons and their connections are well preserved after preparation2,3. Out of several advantages, OHSC allow monitoring of brain injury and tumor invasion without animal surgery. Six to eight OHSC can be obtained from a single rodent brain. OHSC therefore help to significantly reduce the number of animals and allow testing multiple drug concentrations, genetic manipulations or different lesion models in the same animal. In slice-based assays, experimental conditions can be precisely controlled. Additionally, time dependent development of pathological conditions like secondary damages can easily be monitored by time-lapse imaging.
In the given protocol, originally established by Stoppini et al.4, the preparation steps are described and important morphological landmarks for the selection of appropriate slices are highlighted. We recommend the preparation of postnatal day 7-9 rats or postnatal day 4-5 mice. In these periods, OHSC show a robust resistance to mechanical traumas and a high potential for reorganization of neuronal circuits. In contrast, preparations from embryonic or adult rats rapidly change their structure and lose their organotypic morphology during cultivation and are therefore less suitable for studying long-term processes in basic research5,6,7,8,9,10,11. Another critical point for the survival rate of OHSC is the thickness of the slice itself as the diffusion and thus nutrient supply are limited12,13,14.
Protocol
Animal experiments were performed in accordance with the Policy of Ethics and the Policy on the Use of Animals in Neuroscience Research as approved by the European Communities Council Directive 2010/63/EU of the European Parliament and of the Council of the European Union on the protection of animals used for scientific purposes.
1. Preparation of Instruments and Culture Media
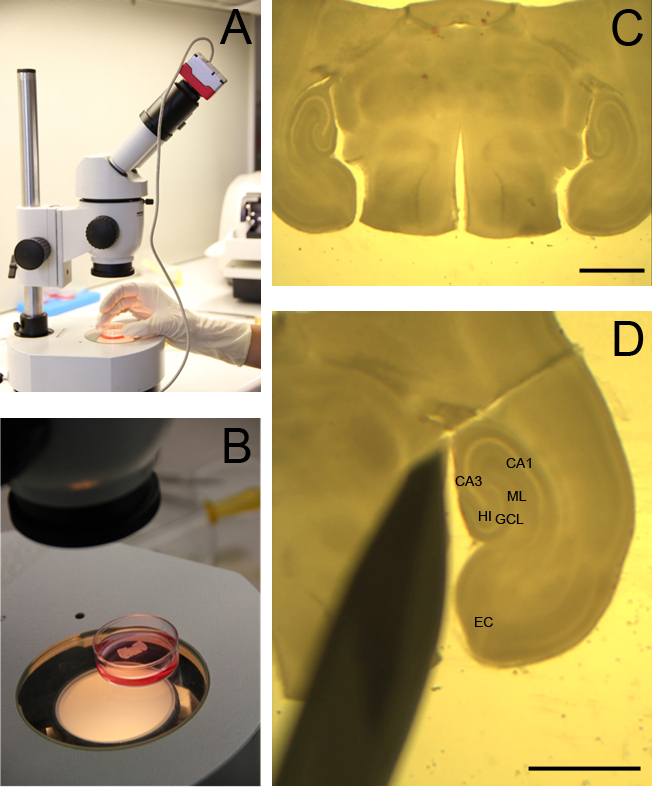
For preparation of OHSC use the following set of instruments: two small scissors, two curved tweezers, one tweezer with a fine tip, three blades (two of size 11, one of size 15), three scalpel holders, round filter papers (diameter: 35 mm), agar, one razor blade, one unmodified Pasteur pipette, and one modified Pasteur pipette without a tip. Sterilize all materials in an autoclave before usage (Figure 1).
Weigh 5 g agar and dissolve it in 100 mL distilled water. Sterilize the solution for 20 min at 121.7 °C at 210.8 kPa in an autoclave. Distribute the 3 mL liquid agar solution in 60-mm Petri dishes using a sterile glass pipette, allow it to solidify for 5 h, cover with plastic paraffin film to avoid contamination and store at 4 °C until further use. Agar blocks are needed to stabilize the brains during the slicing procedure.
- Media
- Make 200 mL of the preparation medium (pH 7.35) consisting of 198 mL minimal essential medium (MEM) and 2 mL L-glutamine solution (final concentration 2 mM). Prepare the solution on the day of media preparation and store it at 4 °C.
- Prepare 100 mL of the culture medium consisting of 49 mL MEM, 25 mL Hanks' balanced salt solutions (HBSS), 25 mL (v/v) normal horse serum (NHS), 1 mL L-glutamine solution (final concentration 2 mM), 100 µg insulin, 120 mg glucose, 10 mg streptomycin, 10,000 U penicillin, and 800 µg vitamin C as reported previously. Warm the medium (37 °C), adjust the pH value to pH 7.4 and sterile filter (0.2 µm pore size). Repeat the procedure (warming up, pH adjustment, etc.) every second day before changing the medium. Use the medium at the most for one week when stored at 4 °C.
Fill one 35-mm Petri dish with preparation medium to store the brain. Place two empty Petri dishes for collecting the tissue on a cooling pack in the working area.

2. Preparation and Slicing with a Vibratome
Use brains from 7-9 day old rats or 4-5 day old mice for the OHSC preparation according to Stoppini et al.4 After decapitation of animals, remove the skin from the skull with scissors.
Introduce the blade of a fine scissor into the foramen magnum and open the skull by cutting along the caudal (back) rostral (front) axis. Make two cuts perpendicular to the first one, so that the scissors point towards the left and right ear, respectively ("T-incision").
Open the skull carefully with fine forceps, paying attention not to injure the brain. Use a scalpel (blade size 11) to cut the most rostral part of the frontal pole and the cerebellum.
By means of a spatula, remove the brain and place it carefully in the Petri dish filled with preparation medium (Figure 2). Position the brain on the specimen holder and fix it with medical cyanoacrylate glue. Use the pieces of agar to assure mechanical stabilization.
Dissect the tissue horizontally in 350 µm thick OHSC using a sliding vibratome.
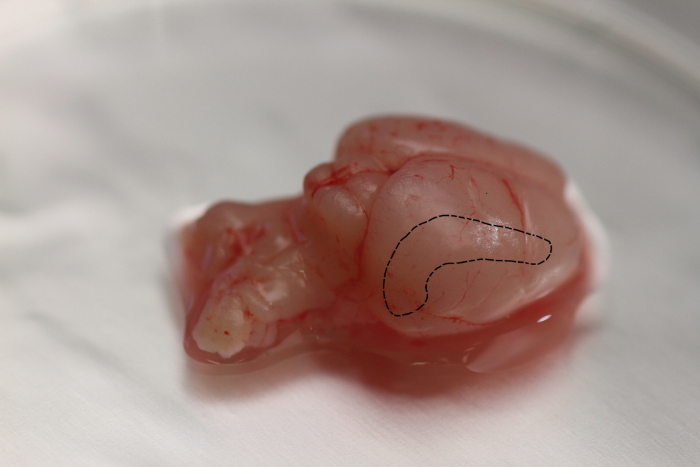
Evaluate the slices optically using a binocular microscope. Discard OHSC of low quality immediately. It is important to take only those slices with intact cytoarchitecture isolated from the middle part of the hippocampus (see Figures 3 and 4) between the dorsal and the ventral hippocampus.
Separate the hippocampal region and the entorhinal cortex using a scalpel (round blade size 15; Figure 3). The perforant pathway and entorhinal cortex must be preserved.
Six to eight OHSC are obtained from each brain. Transfer 2-3 slices into one cell culture insert (pore size 0.4 µm) and place it in one well of a 6-well culture dish containing 1 mL culture medium per well.
Incubate the 6-well dishes at 35 °C in a fully humidified atmosphere with 5% (v/v) CO2 and change the cell culture medium every second day. NOTE: Conduct your experiments at 6 day in vitro (div). Inflammatory reactions associated with the slicing procedure disappear by day 6. At this stage, microglial cells show a ramified morphology again and synaptic connections have matured.
3. Evaluation of Tissue Quality
- Fixation, labeling and visualization of degenerating neurons in OHSC
- After performing the experiments, incubate the OHSC with 5 µg/mL propidium iodide (PI) for 2 h prior to fixation, in order to stain the nuclei of degenerating neurons. CAUTION: PI is a suspected carcinogen, always wear personal protective equipment (PPE) such as gloves.
- Wash the OHSC with 0.1 M phosphate buffer (pH 7.4) and fix them with a 4% (v/v) solution of paraformaldehyde (PFA) for 24 h. CAUTION: PFA is a toxic and suspected carcinogen. Work under fume hood and wear PPE.
- Wash the OHSC in inserts with 1 mL PBS and use a rigger brush to separate slices from the membrane.
- Label the OHSC with IB4 dye in a 24-well plate (Figure 5).
- Conduction of tumor invasion experiments using fluorescently labeled single cells
- 24 h prior to the start of an experiment, label the tumor cells by using the fluorescent dye Carboxyfluorescein diacetate succinimidyl ester (CFDA SE).
- Detach and count the tumor cells using a Neubauer chamber.
- Resuspend the cells in medium, such that 10 µL of suspension contains the desired cell number (normally 50,000 or 100,000 cells).
- Apply 10 µL of the cell suspension onto the slice culture and allow the cells to invade for 3 days.
- At the end of the experiment, fix the slice cultures using 4% (v/v) PFA for 24 h, and label the co-cultures with PI for another 24 h to visualize the cytoarchitecture. CAUTION: PFA is a toxic and suspected carcinogen. Work under fume hood and wear PPE.
- Mount the co-cultures onto a cover slip for further analysis using the mounting media.
4. Evaluation of OHSC Experiments
Analyze the OHSC with a confocal laser scanning microscope (CLSM). For detection of PI labeled, degenerating neurons or the PI labeled cytoarchitecture use monochromatic light of the wavelength λ = 543 nm and an emission band pass filter for wavelengths λ = 585-615 nm. For CFDA labeled tumor cells or IB4 microglia, use an excitation wavelength of λ = 488 nm. For both experimental types, record a z-stack with 2 µm thick optical slices and used for evaluation.
Representative Results
Neuroprotection studies: To determine neuronal damage, the number of PI positive nuclei and IB4 positive microglia in every third optical section of the granule cell layer (GCL) of the dentate gyrus (DG) was counted. For tumor invasion experiments, the maximal intensity z-projection of the stack was used for calculating the area covered by tumor cells, as a measure of invasion and to visualize different invasion patterns (Figure 6).
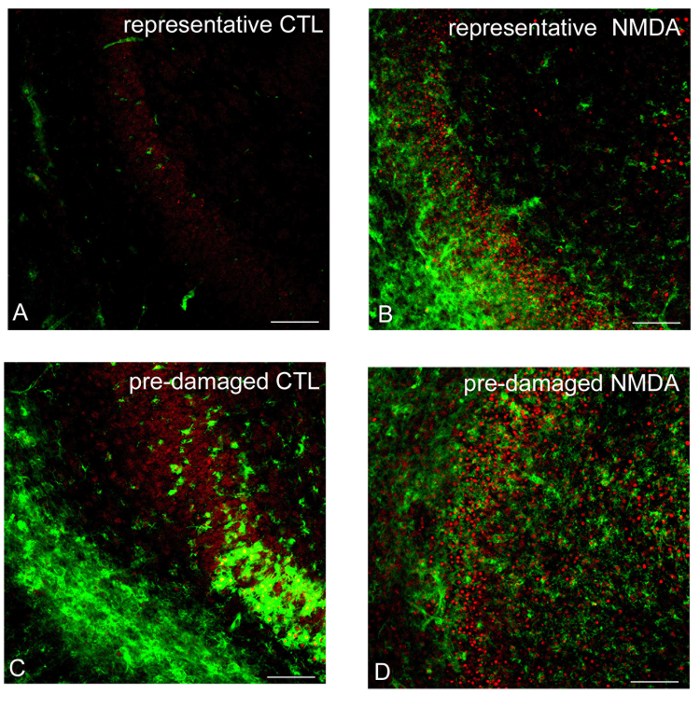
In the untreated control OHSC (CTL), neurons remained well-preserved. In the GCL of the DG almost no PI positive neuronal nuclei (Figure 5A) were observed. In the molecular layer, the majority of microglial cells were ramified. In the GCL of the DG, only very few IB4 positive microglial cells (Figure 5A) were detected. After incubation with 50 µM N-methyl-D-aspartic acid (NMDA) a strong increase in the number of PI positive neuronal nuclei (Figure 5B) and IB4 positive microglial cells (Figure 5B) was detected when compared to control OHSC. Pre-damaged OHSC of lower quality can be identified by higher numbers of amoeboid microglia and PI positive cells in the DG (Figure 5C) under control conditions. Treating pre-damaged slices with NMDA led to destruction of DG cytoarchitecture and a very high number of PI positive death cells spreading to the hilum and CA3 region (Figure 5D).
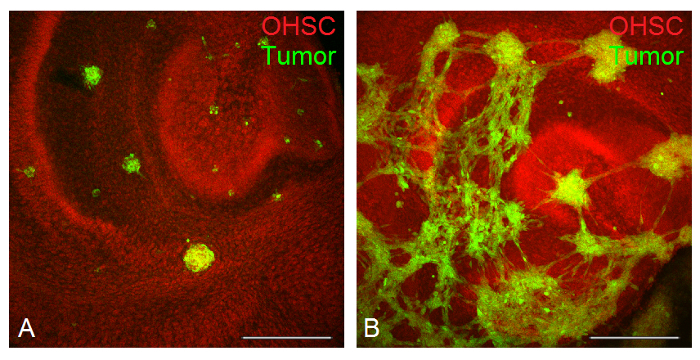
Tumor invasion studies: Slices were treated with 50,000 cells of the two glioblastoma cell lines U138 (Figure 6A) and LN229 (Figure 6B) for three days. In both cases the cytoarchitecture of the slice cultures remained intact and the cornu ammonis and the DG were preserved. Furthermore, both cell lines showed distinct invasion behavior. While U138 cells formed round, clearly distinct tumors (Figure 6A), LN229 cells built a tumor network within single tumor spheroids and were often hard to distinguish. When looking at the amount of tumor mass in slice cultures, a higher invasiveness for LN229 cells was found when compared with U138 cells.
FIGURE 1: Dissection tools and materials. For preparation of slice cultures, a suitable set of dissection tools and materials is required as shown. The set includes three scalpels with small exchangeable blades, one spatula, two fine scissors, three forceps, one normal Pasteur pipette, one Pasteur pipette without tip, agar, Petri dishes, filter paper, a cooling pack, preparation medium, and medical cyanoacrylate glue. The dissection tools must be autoclaved before usage and placed in a plastic package to keep it in a sterile atmosphere. Please click here to view a larger version of this figure.
FIGURE 2: Location of the hippocampal formation in the rat brain. The hippocampal formation, shown by the black dotted line is a bilateral structure enclosed by the cerebral cortex and the thalamus. The hippocampus bulges into the temporal horn of the lateral ventricle. The hippocampus is bilaminar, consisting of the cornu ammonis (hippocampus proper) and the dentate gyrus (or fascia dentate), with one lamina rolled up inside the other. Please click here to view a larger version of this figure.
FIGURE 3: Visualization of rat OHSC. A whole brain slice containing OHSC is visualized by a binocular to assess the tissue quality. Slices are kept in a Petri dish filled with cooled preparation medium (A, B). Magnification of the brain slice. The intact hippocampus (HC) and entorhinal cortex are visible on the left and right side of the image (C). OHSC is separated from the rest of the brain. The granule cell layer (GCL) of the DG, the cornu ammonis subfields CA1 and CA3, the hilus region (HI), the entorhinal cortex (EC) and the molecular layer (ML) are clearly distinguishable (D). C, D: scale bar = 4 mm.
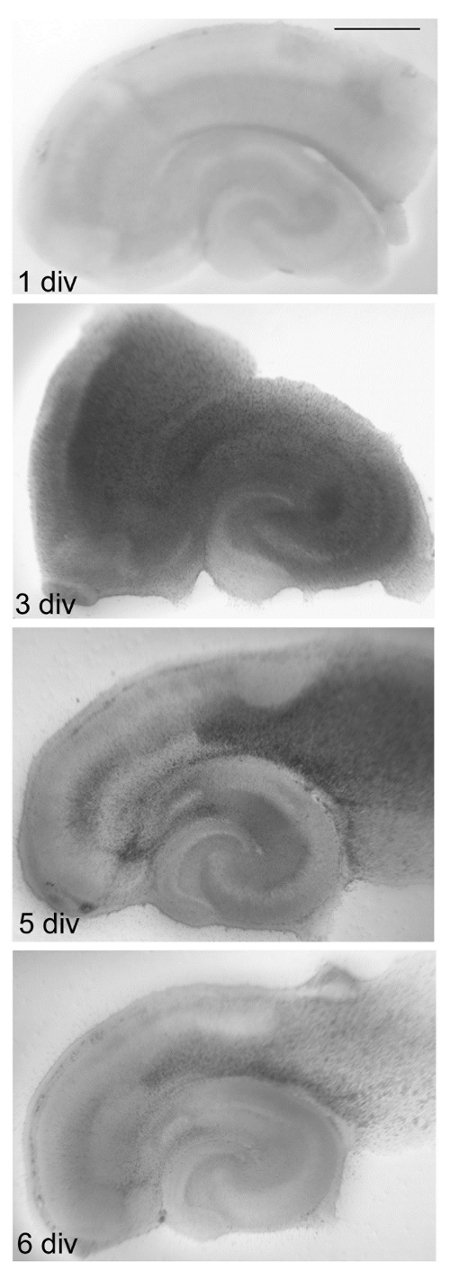
FIGURE 4: Time dependent changes in OHSC. Directly after the preparation, microglia and astrocytes begin to form a glial scar. From 1-3 days in vitro (div) gliosis and edema formation are observed. The stratification of hippocampal formation and DG is no longer visible. At the cellular level, OHSC on 5 div display a reduction in the number of activated cells. At 6 div, almost no edema is present, the slice is thinner, and the areas included in the intrinsic neuronal circuits have survived. OHSC can now be used for the experiments. Scale bar = 1 mm. Please click here to view a larger version of this figure.
FIGURE 5: Evaluation of tissue quality by CLSM. As revealed by CLSM, a good neuronal preservation is observed in the non-lesioned control OHSC (CTL). Virtually no PI positive neuronal nuclei (red) and only a few IB4 positive microglial cells (green) are found in the granule cell layer (GCL) of the DG (A). In contrast to non-lesioned OHSC, a strong increase in the number of PI and IB4 positive cells is visible in the GCL of NMDA-lesioned OHSC (B). Please note the morphology of the DG, activated microglial cells and a large number of PI positive nuclei in pre-damaged OHSC (C, D). Bars = 50 µM. Please click here to view a larger version of this figure.
FIGURE 6: Visualization of tumor invasion by CLSM. PI (in red) applied after the fixation labels the cytoarchitecture of the OHSC, while CFDA (in green) illustrates the tumor cells invading the slice. The invasion process of U138 cells is visualized after three days of invasion time. Single tumor spheroids are clearly distinguishable (A). In contrast, the glioblastoma cell line LN229 formed a tumor network (B). Bars = 400 µm. Please click here to view a larger version of this figure.
Discussion
The present protocol describes the preparation of OHSC. This model allows testing of intrinsic capabilities and reactions of brain tissue after the application of physiological and pathological stimuli. Besides analyses of electrophysiological parameters, OHSC can be lesioned and the effects of damage on all cell types can be determined. Treatment with different substances and the detailed description of lesioning processes or healing in the absence of macrophages and lymphocytes is possible.
The most critical steps for a successful preparation and culturing are to: 1) work under sterile conditions, 2) prepare the media correctly, considering temperature and pH, and 3) develop the necessary manual dexterity during handling of OHSC.
Characteristics of the OHSC
Because of the unaltered morphology of neuronal cells and their in vivo-like organization, the slices are called organotypic. The OHSC consist of the stratum pyramidale and stratum granulosum, composed of the pyramidal cornu ammonis (CA1, CA2, CA3) and DG granular cells. Parts of the hippocampus are highly ordered and the afferent fiber projections terminate in distinct layers in a non-overlapping way. The efferences between entorhinal cortex (the perforant pathway), DG, CA3 and CA1 neurons remain intact after the preparation and behave functionally similar to those in vivo. As previously shown, development of dendritic trees, synapse formation and network activities of the granular cells in OHSC are comparable to acute slices obtained from time matched controls14,15,16.
Oligodendrocytes and astrocytes have been studied in OHSC by both light and electron microscopy. Oligodendrocytes display an organotypic spatial distribution, including different morphological subtypes17,18. Also, the axons become myelinated during the first two weeks in vitro. Some authors postulated that the layer specific localization of astrocytes is absent in slice cultures and believe that the astrocytes do not reach a full maturation in vitro. This aspect cannot be fully answered, since specific markers for all activation steps of astrocytes are still missing1,19,20.
In the first 3 days after the preparation, microglial cells respond with migration and proliferation and accumulate at the slice surface where they form the glial scar together with astrocytes. All cells seem to be in a highly-activated state. However, in the inner layers of OHSC and between 3 and 6 div, microglial cells change their amoeboid morphology towards a ramified form, characterized by small cellular bodies and long branching fine cytoplasmic protrusions extended in all directions21,22.
Similar expression and distribution of glutamate receptors have been reported in adult tissue and OHSC2,23. The frequency of synaptic currents, the miniature synaptic currents and the frequency of GABAergic currents in OHSC (7, 14 and 21 div) are not significantly different compared to acute preparations on postnatal days 14 or 15 respectively. In contrast, the frequency of glutamatergic signals is higher in OHSC than in acute slices15.
Comparison of different preparation methods
The preparation of embryonic and early postnatal tissue is quite easy, because of the good cell survival rates in vitro. It should be considered that at this early stage, the neuronal cells are still migratory and active leading to a loss of organotypic organization of slices1. After 7-9 postnatal days, some synaptic connections in rats are established. During the next 2-3 weeks in vitro the synapses mature13. One of the advantages of the presented model is its resistance to mechanical trauma during the preparation and its high plasticity due to the age of the animals (7-9 days)1,14,24. It must be mentioned that the slices are highly sensitive to mechanical manipulations by instruments. Even the wrong angle of slicing may cause incline cutting of neuronal fibers responsible for anterograde or retrograde neuronal demise. The preparation of slice cultures from adult animals for long-term purposes is challenging and still not established. Such OHSC show a massive degeneration shortly after the preparation presumably due to the loss of plasticity1. Nevertheless, there is a strong need for a preparation method allowing for work with adult slices.
For several preparation methods, the freshly excised tissue is sliced to a thickness of 300-400 µm, using a tissue chopper to cut the isolated hippocampus. This quick method may frequently result in widespread tissue damage. Critical factors for the quality of OHSC are their final thickness and the time they are kept in vitro. The best results with the highest survival rates have been achieved for 300-400 µm thick slices. In thicker slices the upper layers degenerate during the culture time due to diffusion limitations1. Depending on the scientific questions asked, OHSC are used either directly after preparation or after several days in culture. Therefore, over the years several cultivation techniques were established, like the Roller-Tube technique1,13 or static slice techniques4.
Out of different preparation methods we are convinced that using the interface technique together with a vibratome is the most precise and least damaging procedure for preparing OHSC to date. This method is further improved by using porous membranes. OHSC keep their 3D structure, stay morphologically and functionally intact for months, and can visually be evaluated during the cultivation period25.
Applications
OHSC can be prepared from both wild type and transgenic animals14,26. They survive for months and provide options for long-term experiments12,13. OHSC are used to address physiological, pharmacological, morphological and developmental questions14,27 under highly standardized conditions such as chronic application of neurotherapeutics or stem cell therapy26,27,28. The investigation of developmental aspects of neuronal connectivity or processes related to synaptic transmission, synaptic plasticity, effects of chronic epilepsy of dendritic spines of pyramidal cells, or neurogenesis all represent additional fields29,30,31,32.
Glutamate, the primary excitatory neurotransmitter, and ionotropic glutamate receptors play a central role in excitotoxicity. Excitotoxic lesions occur during neurological diseases like stroke, Alzheimer disease, HIV neurotoxicity, traumatic brain injury and repair mechanisms14,24. Application of glutamate receptor agonists NMDA, α-amino-3-hydroxy-5-methy-4-isoxazolepropionic acid (AMPA), or kainic acid to OHSC mimics selective and region specific neuronal cell death26,28,33,34. Furthermore, glial cell response to neuronal injury e.g., microglial migration and phagocytosis in OHSC have previously been analyzed3,35. The influence of microglia during the lesion can be predicted by pharmacological depletion of these cells by the bisphosphonate clodronate36.
A further important application arises mimicking in vivo conditions for investigations of tumor invasion using OHSC. Spheroids formed by glioma cell lines were capable of invading the slice cultures, a phenomenon that was species overlapping37. The diffuse infiltration pattern of the cell lines used resembled the observed pattern in vivo37. In addition, primary glioblastoma spheroids implanted into OHSC kept their invasion potential and stem cell character over several days in vitro38. Thus, the model allows the observation of invasion patterns and interactions between neuronal tissue and tumor cells that correspond to those observed in vivo37,38. In comparison to collagen or gel based models (e.g.)39, the use of OHSC seems more beneficial for analyses on metastasis and tumor invasion in the brain. The physiological milieu, including neuronal and glial cell types and extracellular matrix, remains intact.
Furthermore, OHSC allow studying direct interactions between single tumor cells and brain tissue under controlled conditions. For example, microglial cells were found to enhance the capabilities of tumor cells to enter the brain by forming guiding structures for invasion35.
In addition, the effect of transient and permanent cellular alterations on invasion processes can be analyzed. A stable expression of metastasis associated colon cancer gene 1 (MACC1) in glioma cells was associated with an increased proliferation and subsequent invasion40. The transient alteration of cell stiffness in glioblastoma cell lines was directly correlated with the invasive properties of the cell lines41. OHSC and tumor cell co-cultures can thus potentially be used for drug testing, reproduction of clinical observations, and to model and image tumor invasion under highly controlled and standardized conditions.
After studies in primary cell cultures, OHSC are the logical next choice for performing experiments in a more complex system to approximate the in vivo conditions. Another field of interest in brain slices is the screening of potential therapeutic drugs before testing in vivo. Brain slices allow monitoring and mimicking of injuries under observer control and without animal surgery. Up to 8 OHSC can be obtained and the number of necessary animals is significantly reduced. It is possible to obtain results in the same animal under similar settings but with different or multiple conditions. Thus, OHSC is a powerful tool to observe amongst others morphological, cellular, molecular changes during the lesion period, development of secondary damage and tumor spreading.
Disclosures
The authors have nothing to disclose
Acknowledgments
The authors would like to thank Christine Auste for her support with the video recording and Chalid Ghadban for his excellent technical assistance. Urszula Grabiec was supported by the Roux Programm FKZ 29/18.
References
- Gähwiler B. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20(10):471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Bahr BA. Long-Term Hippocampal Slices: A Model System for Investigating Synaptic Mechanisms and Pathologic Processes. J Neurosci Res. 1995;42(3):294–305. doi: 10.1002/jnr.490420303. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Eyo U, Fuller L, Hass J, Kurpius D. Imaging microglia in brain slices and slice cultures. Cold Spring Harbor Protoc. 2013;2013(12):1142–1148. doi: 10.1101/pdb.prot079483. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. Journal of Neurosci Methods. 1991;37(2):173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Kleinberger-Doron N, Schramm M. Culture of mature hippocampus slices for 4 days in a newly developed medium: preservation of transmitter release and leucine incorporation into protein. Brain Res. 1990;533(2):239–247. doi: 10.1016/0006-8993(90)91345-h. [DOI] [PubMed] [Google Scholar]
- Legradi A, Varszegi S, Szigeti C, Gulya K. Adult rat hippocampal slices as in vitro models for neurodegeneration Studies on cell viability and apoptotic processes. Brain Res Bull. 2011;84(1):39–44. doi: 10.1016/j.brainresbull.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH. Organotypic Monolayer Cultures of Nervous Tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH. Organotypic cultures of neural tissue. Trends Neurosci. 1988;11(11):484–489. doi: 10.1016/0166-2236(88)90007-0. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Frey JU, Behnisch T. LTP in cultured hippocampal-entorhinal cortex slices from young adult (P25-30) rats. J Neurosci Methods. 2003;130(1):19–32. doi: 10.1016/s0165-0270(03)00228-0. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim E, Park M, Lee E, Namkoong K. Organotypic hippocamal slice culture from the adult mouse brain: A versatile tool for translational neuropsychopharmacology. Prog Neuropsychopharmacol Biol Psychiatry. 2013;41:36–43. doi: 10.1016/j.pnpbp.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Hrabetova S, et al. Long-term maintenance of mature hippocampal slices in vitro. J Neurosci Methods. 2000;98:145–154. doi: 10.1016/s0165-0270(00)00197-7. [DOI] [PubMed] [Google Scholar]
- Adamchik Y, Frantseva MV, Weisspapir M, Carlen PL, Perez Velazquez JL. Methods to induce primary and secondary traumatic damage in organotypic hippocampal slice cultures. Brain rRes Brain Res Protoc. 2000;5:153–158. doi: 10.1016/s1385-299x(00)00007-6. [DOI] [PubMed] [Google Scholar]
- Cho S, Wood A, Bowlby MR. Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Curr Neuropharmacol. 2007;5(1):19–33. doi: 10.2174/157015907780077105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noraberg J, Poulsen FR, et al. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4(4):435–452. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- De Simoni A, Griesinger CB, Edwards F. a Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol. 2003;550(Pt 1):135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindrom P, Piguet P, Förster E. New Methods for Culturing Cells from Nervous Tissues. Basel, Switzerland: BioValley Karger; 2005. [Google Scholar]
- Berger T, Frotscher M. Distribution and morphological characteristics of oligodendrocytes in the rat hippocampus in situ and in vitro: An immunocytochemical study with the monoclonal Rip antibody. J Neurocytol. 1994;23(1):61–74. doi: 10.1007/BF01189817. [DOI] [PubMed] [Google Scholar]
- Haber M, Vautrin S, Fry EJ, Murai KK. Subtype-specific oligodendrocyte dynamics in organotypic culture. Glia. 2009;57(9):1000–1013. doi: 10.1002/glia.20824. [DOI] [PubMed] [Google Scholar]
- Bonde C, Sarup A, Schousboe A, Gegelashvili G, Zimmer J, Noraberg J. Neurotoxic and neuroprotective effects of the glutamate transporter inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) during physiological and ischemia-like conditions. Neurochem Int. 2003;43(4-5):371–380. doi: 10.1016/s0197-0186(03)00024-x. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Heimrich B, Frotscher M. Loss of layer-specific astrocytic glutamine synthetase immunoreactivity in slice cultures of hippocampus. Eur J Neurosci. 1993;5(2):122–127. doi: 10.1111/j.1460-9568.1993.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Hailer NP, Jarhult JD, Nitsch R. Resting microglial cells in vitro: analysis of morphology and adhesion molecule expression in organotypic hippocampal slice cultures. Glia. 1996;18(4):319–331. doi: 10.1002/(sici)1098-1136(199612)18:4<319::aid-glia6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hailer NP, Wirjatijasa F, Roser N, Hischebeth GTR, Korf HW, Dehghani F. Astrocytic factors protect neuronal integrity and reduce microglial activation in an in vitro model of N-methyl-D-aspartate-induced excitotoxic injury in organotypic hippocampal slice cultures. Eur J Neurosci. 2001;14(2):315–326. doi: 10.1046/j.0953-816x.2001.01649.x. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Kessler M, et al. Stable maintenance of glutamate receptors and other synaptic components in long-term hippocampal slices. Hippocampus. 1995;5(5):425–439. doi: 10.1002/hipo.450050505. [DOI] [PubMed] [Google Scholar]
- Holopainen IE. Organotypic hippocampal slice cultures: A model system to study basic cellular and molecular mechanisms of neuronal cell death, neuroprotection, and synaptic plasticity. Neurochem Res. 2005;30(12):1521–1528. doi: 10.1007/s11064-005-8829-5. [DOI] [PubMed] [Google Scholar]
- Guy Y, Rupert AE, Sandberg M, Weber SG. A simple method for measuring organotypic tissue slice culture thickness. J Neurosci Methods. 2011;199(1):78–81. doi: 10.1016/j.jneumeth.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabiec U, Koch M, et al. The endocannabinoid N-arachidonoyldopamine (NADA) exerts neuroprotective effects after excitotoxic neuronal damage via cannabinoid receptor 1 (CB 1) Neuropharmacology. 2012;64(4):1797–1807. doi: 10.1016/j.neuropharm.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Daviaud N, Garbayo E, Schiller PC, Perez-Pinzon M, Montero-Menei CN. Organotypic cultures as tools for optimizing central nervous system cell therapies. Exp Neurol. 2013;248:429–440. doi: 10.1016/j.expneurol.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Ebrahimi F, Hezel M, Koch M, Ghadban C, Korf HW, Dehghani F. Analyses of neuronal damage in excitotoxically lesioned organotypic hippocampal slice cultures. Ann Anat. 2010;192(4):199–204. doi: 10.1016/j.aanat.2010.06.002. [DOI] [PubMed] [Google Scholar]
- He S, Shao LR, Wang Y, Bausch SB. Synaptic and extrasynaptic plasticity in glutamatergic circuits involving dentate granule cells following chronic N-methyl-D-aspartate receptor inhibition. J Neurophysiol. 2013;109(6):1535–1547. doi: 10.1152/jn.00667.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O, Taubenberger N, et al. Muscarinic receptor activation determines the effects of store-operated Ca2+-entry on excitability and energy metabolism in pyramidal neurons. Cell Calcium. 2012;51(1):40–50. doi: 10.1016/j.ceca.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Pérez-Gómez A, Tasker RA. Enhanced neurogenesis in organotypic cultures of rat hippocampus after transient subfield-selective excitotoxic insult induced by domoic acid. Neuroscience. 2012;208:97–108. doi: 10.1016/j.neuroscience.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Rietschin L, Gradwohl G, Guillemot F, Gähwiler BH. Neurogenesis in hippocampal slice cultures. Mol Cell Neurosc. 2004;26(2):241–250. doi: 10.1016/j.mcn.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Pozzo Miller LD, Mahanty NK, Connor JA, Landist DMD. Pyramidal Cell Death in Slice Cultures From Rat Hippocampus Is Prevented By Glutamate Receptor Antagonists. Neuroscience. 1994;63(2) doi: 10.1016/0306-4522(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Koch M, Kreutz S, et al. The cannabinoid WIN 55,212-2-mediated protection of dentate gyrus granule cells is driven by CB 1 receptors and modulated by TRPA1 and Ca v2.2 channels. Hippocampus. 2011;21(5):554–564. doi: 10.1002/hipo.20772. [DOI] [PubMed] [Google Scholar]
- Pukrop T, Dehghani F, et al. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia. 2010;58(12):1477–1489. doi: 10.1002/glia.21022. [DOI] [PubMed] [Google Scholar]
- Kohl A, Dehghani F, Korf HW, Hailer NP. The bisphosphonate clodronate depletes microglial cells in excitotoxically injured organotypic hippocampal slice cultures. Exp Neurol. 2003;181(1):1–11. doi: 10.1016/s0014-4886(02)00049-3. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Ohnishi T, Kanemura Y, Maruno M, Yoshimine T. Quantitative analysis of glioma cell invasion by confocal laser scanning microscopy in a novel brain slice model. Biochem Biophys Res Commun. 2000;269(2):513–520. doi: 10.1006/bbrc.2000.2332. [DOI] [PubMed] [Google Scholar]
- Aaberg-Jessen C, Nørregaard A, Christensen K, Pedersen CB, Andersen C, Kristensen BW. Invasion of primary glioma- and cell line-derived spheroids implanted into corticostriatal slice cultures. Int J Clin Exp Pathol. 2013;6(4):546–560. [PMC free article] [PubMed] [Google Scholar]
- Vinci M, Box C, Eccles SA. Three-dimensional (3D) tumor spheroid invasion assay. J Vis Exp. 2015. p. e52686. [DOI] [PMC free article] [PubMed]
- Hagemann C, Fuchs S, et al. Impact of MACC1 on human malignant glioma progression and patients ' unfavorable prognosis. Neuro Oncol. 2013;15(12):1696–1709. doi: 10.1093/neuonc/not136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann T, Grabiec U, Ghadban C, Feese K, Dehghani F. The Influence of Biomechanical Properties and Cannabinoids on Tumor Invasion. Cell Adh Migr. 2016;11(1):54–67. doi: 10.1080/19336918.2016.1183867. [DOI] [PMC free article] [PubMed] [Google Scholar]


