Abstract
Purpose
To investigate the pattern of referral of patients with superior mesenteric artery embolism (SMAE) and its effect on outcomes, and to evaluate the risk factors for bowel infarction.
Materials and Methods
This retrospective study included 66 consecutive patients diagnosed with acute SMAE between January 2001 and June 2016. Appropriate diagnosis by the referring physician was defined if the referral letter indicated that acute mesenteric ischemia was suspected or had been diagnosed at the referral center. Surgical delay was defined as the interval between symptom onset and surgery for definitive treatment.
Results
Among 54 patients transferred from other centers, 26 patients (48.1%) were diagnosed appropriately by the referring physician. The rate of appropriate diagnosis was differed significantly by the use of computed tomography (CT) scan at referral center (25/35 with CT and 1/19 without CT, P=0.00). The surgical delay was significantly longer in patients without appropriate diagnosis compared with the patients with appropriate diagnosis (53.5±52.3 hours vs. 28.8±23.6 hours, P=0.04). Initially, 56 patients received surgical treatment with 31 underwent bowel resection due to infarction, 6 received conservative treatment, and the remaining 4 patients refused any treatment. The surgical delay, abdominal distension, tenderness, rebound tenderness, and level of C-reactive protein were associated with bowel infarction at initial operation. Overall in-hospital mortality was 32%.
Conclusion
A high index of suspicion with appropriate diagnostic modality, such as CT scan is crucial in patients with SMAE for reducing surgical delay as a risk factor of bowel infarction.
Keywords: Mesenteric artery, superior, Embolism, Atrial fibrillation, Intestines, Infarction
INTRODUCTION
Acute superior mesenteric artery (SMA) embolism (SMAE) is implicated in 40% to 50% of cases of acute mesenteric ischemia and is considered to be a critical condition because of its high morbidity and mortality after treatment [1,2]. The reported mortality after surgical treatment has been reported as 20% to 80% without marked improvement over time [1–3].
One of major obstacles related to unfavorable outcomes in SMAE are ascribed to the delayed diagnosis and treatment. The diagnosis of SMAE is difficult to establish and often delayed because it is a relatively rare cause of abdominal pain and the clinical presentations are nonspecific. Therefore, clinical suspicion and proper diagnostic modality, such as computed tomography (CT) have been known to be highly important in these patients.
Traditional treatment of acute SMAE includes administration of heparin and surgical exploration. The surgery consists of SMA embolectomy, resection of the gangrenous bowel, and a second-look operation if necessary [2]. Recently, selected endovascular approaches, such as aspiration thrombectomy combined with thrombolysis or stent placement have been reported to have acceptable results with respect to morbidity and mortality [4–7]. However, the remaining concerns about an endovascular-first approach are the difficulty of identifying bowel infarction by clinical and radiologic evaluation, and reperfusion injury in cases of bowel infarction after endovascular revascularization of the SMA. Therefore, the identification of bowel infarction prior to an endovascular approach is important in making decisions about the treatment modality. Although the risk factors for survival after SMAE have been reported [8,9], the risk factors for bowel infarction have not been fully investigated.
The purpose of this study was to investigate the pattern of referral of patients with SMAE to a tertiary referral center and its effect on their outcomes, and to evaluate the early outcomes including risk factors for bowel infarction.
MATERIALS AND METHODS
Between January 2001 and June 2016, 66 consecutive patients diagnosed with acute SMAE at a single tertiary referral center were eligible for this study and retrospectively reviewed. Patients suffering chronic mesenteric ischemia, acute SMA thrombosis, SMA occlusion related to aortic or isolated SMA dissection, and trauma-associated SMA occlusion were excluded.
Medical records were examined for the referring physician’s suspected or definite diagnosis of acute mesenteric ischemia based on the referral letter to Kyungpook National University Hospital (KNUH). In addition, the patient’s clinical symptoms and comorbidities, the treatment approach, operative findings including bowel resection (BR), and in-hospital mortality were examined. The duration of symptoms and laboratory test values were recorded at the time of initial presentation to KNUH. The duration from symptom onset to surgery was also recorded and the time interval between admission to KNUH and surgery was calculated from the electronic medical records.
Sixty-three patients were admitted through the emergency department via referral from other local medical centers or direct attendance at KNUH with acute-onset abdominal pain within the previous 2 weeks, while the remaining 3 patients were transferred from other departments because of abdominal pain during an admission for another disease. The main diagnostic method in the majority of patients was intravenous contrast-enhanced CT without oral contrast at referral center or KNUH. Two patients underwent a non-enhanced CT scan, and 3 patients did not receive a CT scan and the diagnosis was made during abdominal exploration. The diagnosis of SMAE was retrospectively verified based on all available information: clinical data, CT findings, and surgical records. All CT findings were re-evaluated by an experienced vascular surgeon (H.K.) and radiologist (J.L.). Embolism was indicated by a filling defect of contrast material in a segment of the SMA distal to SMA orifice. A very acute onset of symptoms, atrial fibrillation, CT or echocardiographic findings of cardiac thrombi, and the presence of other synchronous embolic events (e.g., solid abdominal organ infarction, stroke, or limb embolism) were also indicative of embolic etiology.
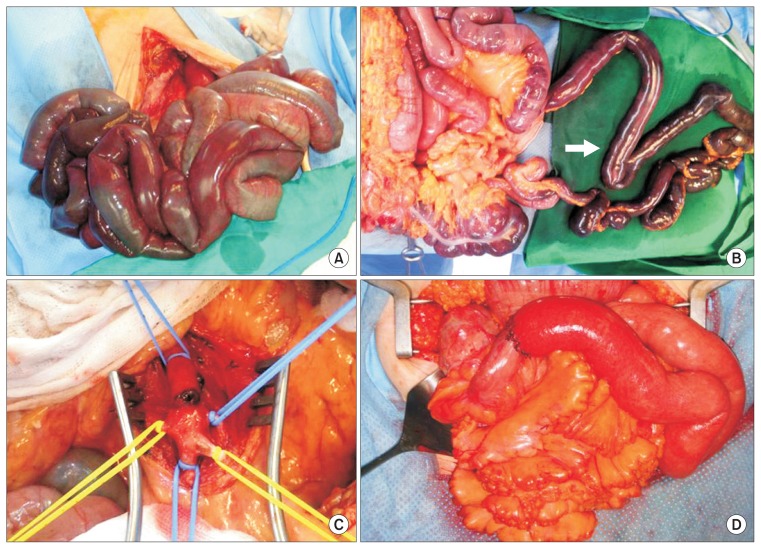
The general protocol for management of patients with severe abdominal pain or suspected bowel infarction after clinical and radiologic evaluation called for intravenous heparin (3,000 to 5,000 IU as a bolus injection) at the time of diagnosis. In patients with mild symptoms and no definite signs of bowel infarction, such as nonenhancement of bowel wall on CT scan, conservative management including bowel rest and anticoagulation therapy was initiated with close monitoring for clinical deterioration. Surgical exploration was first made by midline abdominal incision. After entrance to the peritoneal cavity, the viability of the bowel was explored and clinically evaluated (Fig. 1A). In cases of definite bowel infarction, the mesentery of the gangrenous bowel segment was divided prior to revascularization to prevent the potential reperfusion injury caused by perfusion of gangrenous bowel (Fig. 1B). In patients without a definite bowel infarction, mesenteric revascularization with SMA embolectomy was performed after assessment of the bowel status. A Harmonic scalpel (Ethicon Endo-Surgery, Cincinnati, OH, USA) or LigaSure (Medtronic, Boulder, CO, USA) was used for rapid division of the bowel mesentery. After division of the mesentery, SMA embolectomy using a Fogarty catheter was performed via transverse arteriotomy on the main SMA trunk (Fig. 1C), and then irrigation with warm saline was performed, and the viability of the bowel was reassessed via bowel color, motility and Doppler examination. After final assessment, BR was performed with end-to-end anastomosis of viable bowel using the method of hand-sewn Gambee anastomosis (Fig. 1D). For patients who were selected to undergo surgical exploration, low-molecular-weight or unfractionated heparin was injected after surgery if there were no signs of active bleeding. Unless contraindicated, all patients subsequently received heparin for anticoagulation, which prior to discharge was replaced by warfarin for indefinite use as tolerated.
Fig. 1.
Example of operative procedures in patients with superior mesenteric artery embolism and bowel infarction. (A) Initial operative photography showing profound bowel infarction at initial abdominal exploration. (B) The mesentery of the gangrenous bowel segment was divided with a Harmonic scalpel prior to revascularization (arrow). (C) Superior mesenteric artery embolectomy was performed via transverse arteriotomy on the main trunk. (D) Final photograph after end-to-end anastomosis of viable bowel using the method of Gambee anastomosis.
There were 2 main outcomes of interest in our study. Firstly, the referral patterns from a local medical center to KNUH and whether or not the diagnosis of SMAE was made at the time of the initial visit. We also analyzed the effect of appropriate diagnosis of SMAE by the referring physician on the early outcomes, including bowel infarction. Secondly, we analyzed the risk factors for bowel infarction at initial presentation to guide the determination of the possible treatment method; for example, an endovascular approach.
To assess the referral patterns and their effects, we analyzed whether the referring clinicians’ referral letter indicated a suspicion of mesenteric ischemia. If the referral letter indicated that acute mesenteric ischemia was suspected or had been diagnosed after various evaluations including a CT scan, we defined this as an appropriate diagnosis by the referring physician.
To guide the determination of the treatment method, the patients were divided retrospectively into 2 groups. The BR group included all those patients who underwent BR due to advanced bowel infarction during abdominal exploration at the time of initial presentation, and the non-BR group included those patients who did not undergo BR at initial presentation, including patients who were conservatively managed and those underwent only SMA embolectomy during abdominal exploration because there was no definite bowel infarction. Presentation delay was defined as the interval between symptom onset and presentation to KNUH. Surgical delay was defined as the interval between symptom onset and surgery for definitive treatment.
Student’s independent t-test was applied for between-group comparisons of age, presentation delay, and white blood cell count. Given the potential for a skewed distribution of values, group comparison of surgical delay and the level of C-reactive protein relied on the nonparametric Mann–Whitney U-test (also known as the Wilcoxon test for independent measures). The 18 categorical variables tested were subjected to chi-square analysis (if sample size was adequate) or Fisher’s exact test (for smaller samples). Multivariate analysis could not be performed because of the overall small sample size. All calculations relied on standard IBM SPSS Statistics ver. 20.0 software (IBM Co., Armonk, NY, USA), with statistical significance set at P<0.05.
RESULTS
1) Patient characteristics
The average patient age was 71.9 years (range, 39–90 years), and 28 patients (42.4%) were male. All patients suffered from abdominal pain, and the mean duration of presentation delay was 15.3 hours (range, 1–192 hours). Concomitant symptoms included vomiting in 37 patients, diarrhea in 22, and hematochezia in 16. Abdominal distension was observed in 13 patients. Abdominal tenderness was present in 55 patients and rebound tenderness in 14. Combined comorbidities included hypertension in 34 patients, hyperlipidemia in 23, diabetes in 18, and current smoking in 18. At the time of admission, atrial fibrillation (AF) was noted in 46 patients (69.7%), and congestive heart failure was present in 21 patients. A history of suspected embolism including cerebral infarction was present in 17 patients (25.8%), and combined embolism was noted in 16 patients (24.2%) at the time of first admission. Details of patient characteristics and embolic events are summarized in Table 1.
Table 1.
Characteristics of patients with acute SMAE (n=66)
| Characteristic | Value |
|---|---|
| Age (y) | 71.9 (39–90) |
| Male | 28 (42.4) |
| Duration (h) | |
| Presentation delay | 15.3 (1–192) |
| Surgical delaya | 24.5 (6.5–240) |
| Initial presentation to operationa | 5.0 (1–212) |
| Coexisting medical condition | |
| Atrial fibrillation | 46 (69.7) |
| Hypertension | 34 (51.5) |
| Diabetes mellitus | 18 (27.3) |
| Smoking (current smoker) | 18 (27.3) |
| Hyperlipidemia | 23 (34.8) |
| Ischemic heart disease | 8 (12.1) |
| Congestive heart failure | 21 (31.8) |
| Cerebral infarction | 11 (16.7) |
| Renal insufficiency (s-Cr>1.5 mg/dL) | 14 (21.2) |
| History of embolismb | 17 (25.8) |
| Combined embolismc | 16 (24.2) |
| Clinical presentations | |
| Vomiting | 37 (56.1) |
| Diarrhea | 22 (33.3) |
| Hematochezia | 16 (24.2) |
| Abdominal distension | 13 (19.7) |
| Abdominal tenderness | 55 (83.3) |
| Rebound tenderness | 14 (21.2) |
| Laboratory findings | |
| White blood cell count (×103/μL) | 16.83 (1.47–54.51) |
| C-reactive protein (mg/dL) | 7.32 (0.08–35.39) |
Values are presented as mean (range) or number (%).
SMAE, superior mesenteric artery embolism; s-Cr, serum creatinine.
Data analyzed for 56 patients received surgical exploration at initial presentation,
History of embolism include cerebral infarction in 8 patients, embolism of extremity in 5 patients, and embolism of visceral artery in 4 patients,
Combined embolism include embolism of visceral artery in 8 patients, embolism of extremity in 7 patients, and 1 cerebral infarction.
2) Referral patterns and their effect
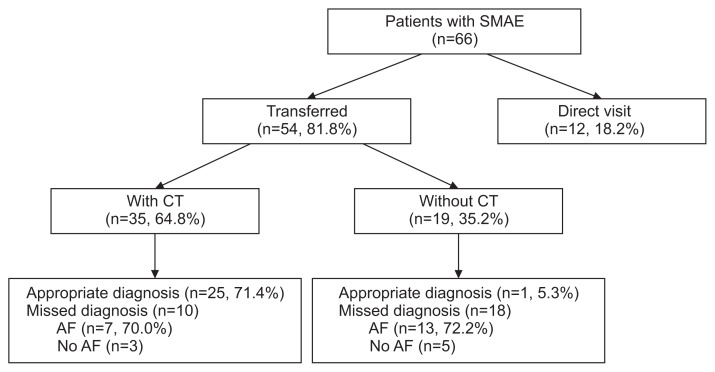
Fifty-four patients were transferred from other hospitals by referring physicians, and the remaining 12 patients, including 3 patients transferred from other departments, directly attended KNUH. Among the 54 transferred patients, CT was performed at the referring center for 35 patients, while 19 patients were transferred without CT. In 35 patients for whom a CT scan was performed at the referring center, 25 were considered to be appropriately diagnosed with acute mesenteric ischemia; however, only 1 was considered to have been appropriately diagnosed with acute mesenteric ischemia in the 19 patients for whom a CT scan was not performed at the referring center (P=0.000). Therefore, 26 (48.1%) of 54 patients referred from other hospitals were considered to have received an appropriate diagnosis (Fig. 2). Among 28 patients with inappropriate diagnosis at referral center, AF was present in 20 patients (7 in patients with CT and 13 in patients without CT at referring center).
Fig. 2.
Algorithm of transfer pattern in patients with SMAE. SMAE, superior mesenteric artery embolism; CT, computed tomography; AF, atrial fibrillation.
The mean presentation delay was similar for both with and without appropriate diagnosis by the referring physician (25.2±23.5 hours vs. 35.5±42.8 hours, P=0.285). However, the surgical delay was significantly longer in patients without an appropriate diagnosis compared with the patients with an appropriate diagnosis (53.5±52.3 hours vs. 28.8±23.6 hours, P=0.040).
3) Early outcomes and risk factors for bowel resection
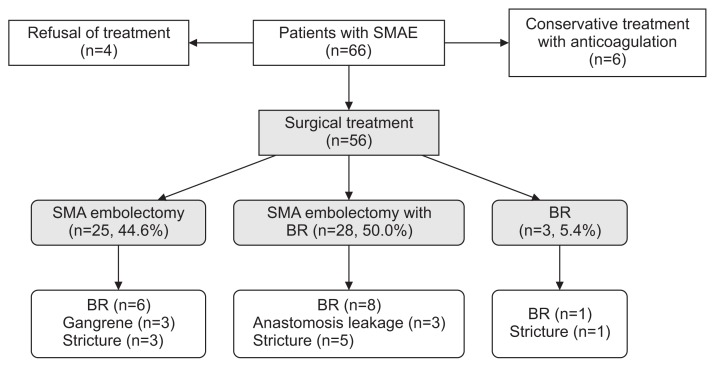
Four patients with suspected bowel infarction of the 66 patients refused any treatment, and all died within 1-month after diagnosis. Six patients with mild clinical symptoms and no definite sign of bowel infarction on CT scan received anticoagulation therapy without any revascularization procedures, and the remaining 56 patients underwent surgical exploration. Of these 56 patients, 25 underwent SMA embolectomy without BR at initial exploration, 28 underwent BR combined with SMA embolectomy because of advanced bowel infarction, and 3 underwent BR for infarcted bowel only. Therefore, 35 (53.0%) of 66 patients, including 4 with suspected bowel infarction who refused treatment, were considered to have bowel infarction at the time of their initial presentation. After exclusion of the 4 patients who received no treatment, half of the 62 remaining patients received BR as initial treatment and were categorized as the BR group, while the remaining 31 patients were classified as the non-BR group (Fig. 3).
Fig. 3.
Treatment results of SMAE after surgical treatment. SMAE, superior mesenteric artery embolism; BR, bowel resection.
Among the clinical factors seen at the time of initial presentation, abdominal distension, tenderness, and rebound tenderness were more frequently found in the BR group compared with the non-BR group (35.5% vs. 3.2% for abdominal distension, P=0.001; 93.5% vs. 74.2% for abdominal tenderness, P=0.038; 32.3% vs. 9.7% for rebound tenderness, P=0.029). The surgical delay was significantly longer in the BR group compared with the non-BR group (57.7±59.3 hours vs. 27.1±20.0 hours, P=0.044). C-reactive protein was significantly higher in the BR group compared with the non-BR group (11.6±11.1 mg/dL vs. 4.3±6.3 mg/dL, P=0.010) (Table 2).
Table 2.
Characteristics of patients and risk factors for BR at initial presentation in acute SMAE (n=62)
| Characteristic | Non-BR group (n=31) | BR group (n=31) | P-value |
|---|---|---|---|
| Age (y) | 70.7 (39–87) | 72.4 (43–89) | 0.564 |
| Male | 12 (38.7) | 14 (45.2) | 0.607 |
| Duration (h) | |||
| Presentation delay | 21.7 (1–120) | 36.6 (2–192) | 0.099 |
| Overall surgical delay | 27.1 (7–85)a | 57.7 (7–240) | 0.044 |
| In-hospital surgical delay | 6.2 (2–18)a | 21.1 (1–212) | 0.685 |
| Coexisting medical condition | |||
| Atrial fibrillation | 22 (71.0) | 21 (67.7) | 0.783 |
| Hypertension | 12 (38.7) | 19 (61.3) | 0.075 |
| Diabetes mellitus | 9 (29.0) | 8 (25.8) | 0.776 |
| Smoking (current smoker) | 9 (29.0) | 9 (29.0) | 1.000 |
| Hyperlipidemia | 12 (38.7) | 9 (29.0) | 0.421 |
| Ischemic heart disease | 5 (16.1) | 3 (9.7) | 0.707 |
| Congestive heart failure | 11 (35.5) | 8 (25.8) | 0.409 |
| Cerebral infarction | 6 (19.4) | 4 (12.9) | 0.490 |
| Renal insufficiency (s-Cr>1.5 mg/dL) | 4 (12.9) | 10 (32.3) | 0.068 |
| History of embolism | 10 (32.3) | 6 (19.4) | 0.246 |
| Combined embolism | 10 (32.3) | 6 (19.4) | 0.246 |
| Clinical presentations | |||
| Vomiting | 18 (58.1) | 17 (54.8) | 0.798 |
| Diarrhea | 12 (38.7) | 9 (29.0) | 0.421 |
| Hematochezia | 9 (29.0) | 6 (19.4) | 0.374 |
| Abdominal distension | 1 (3.2) | 11 (35.5) | 0.001 |
| Abdominal tenderness | 23 (74.2) | 29 (93.5) | 0.038 |
| Rebound tenderness | 3 (9.7) | 10 (32.3) | 0.029 |
| Laboratory findings | |||
| White blood cell count (×103/μL) | 17.91 (5.45–54.51) | 16.09 (1.47–42.39) | 0.450 |
| C-reactive protein (mg/dL) | 4.3 (0.2–22.9) | 11.6 (0.1–35.4) | 0.010 |
Values are presented as mean (range) or number (%).
SMAE, superior mesenteric artery embolism; BR, bowel resection; s-Cr, serum creatinine.
The duration was analyzed for 26 patients after exclusion of 5 patients with conservative treatment.
After the initial treatment, subsequent BR was performed in 15 (24.2%) of 62 patients for various reasons. Of 6 patients treated conservatively with anticoagulation, none underwent subsequent BR. In 25 patients who underwent only SMA embolectomy at the initial exploration, 6 (24.0%) underwent subsequent BR, including 3 patients with a delayed bowel stricture and 3 patients with progressive bowel gangrene. In 28 patients who underwent SMA embolectomy and BR at initial exploration, 8 (28.6%) underwent subsequent BR because of anastomotic leakage in 3 patients and delayed bowel stricture in 5. Of 3 patients who underwent BR alone at the initial exploration, 1 received additional BR because of a delayed stricture. Therefore, a total of 37 (59.7%) of 62 patients treated for SMAE underwent BR, and 67% of 56 patients who underwent surgical exploration ultimately underwent BR because of advanced bowel infarction or the sequelae of bowel ischemia caused by SMAE (Fig. 3).
During the index admission, 21 patients died and overall in-hospital mortality was 32%, including the 4 patients who refused treatment. The most common cause of death was sepsis with multiorgan failure (14 patients) followed by pneumonia (4 patients), and 1 patient each for acute myocardial infarction, acute renal failure, and gastrointestinal bleeding. For the 62 treated patients, BR was not associated with in-hospital mortality (32.3% in the non-BR group vs. 22.6% in the BR group; P=0.393).
DISCUSSION
The purpose of our study was to investigate the pattern of referral of patients with SMAE to a tertiary hospital and its effect on outcomes, and to evaluate the risk factors for bowel infarction. In our study, the surgical delay was a risk factor of BR and significantly longer compared with those in the non-BR group. Therefore, we can suspect that early revascularization in patients with SMAE can reduce the rate of BR caused by progressive bowel infarction. To achieve early revascularization, clinical suspicion and appropriate diagnosis by the primary referring physician were critical. In our series, the rate of appropriate diagnosis by the referring physician was 48%. The presentation delay did not differ significantly for patients with and without an appropriate diagnosis by the referring physician. However, the surgical delay was significantly longer in patients without an appropriate diagnosis by the referring physician compared with patients with an appropriate diagnosis. This difference could reflect the fact that patients without clinical suspicion or without appropriate diagnosis of SMAE by the referring physician were eventually recognized at our department for definitive treatment after additional time for diagnosis.
Notably, of 35 patients for whom a CT scan was performed at the referring center, 25 were considered to be appropriately diagnosed; however, only 1 was considered to have been appropriately diagnosed in the 19 patients for whom a CT scan was not performed at the referring center. Therefore, above findings re-emphasize that a high index of suspicion with respect to sudden-onset abdominal pain in patients with AF and early use of appropriate diagnostic modality, such as CT scan are crucial in the management of patients with acute SMAE.
There are also previous reports focusing on the importance of clinical suspicion by the referring physician [6,10]. Recently, Lehtimäki et al. [10] reported the importance of clinical suspicion in patients with acute mesenteric ischemia. In that study, the rate of suspected acute mesenteric ischemia prior to imaging was 31%, and the crucial findings of acute mesenteric ischemia could have been detected in 97% of the radiology reports if the clinician had mentioned suspected acute mesenteric ischemia in the referral; when it was not mentioned, the rate was 81% (P=0.04). Also, patients in their series in whom acute mesenteric ischemia was not suspected prior to CT were more likely to require BR.
Recently, with the advancement of endovascular techniques and devices, transcatheter embolus aspiration with adjunctive thrombolysis and stenting has been used with acceptable results in patients with SMAE, with a reported mortality in these series of 10% to 40% [5,7,11–13]. SMAE usually occurs in older patients who have a high incidence of combined medical comorbidities [14,15]. Therefore, in patients without definite bowel infarction at the time of presentation, an endovascular approach can be a better-tolerated option because surgical trauma and general anesthesia can be minimized. However, in patients with definite bowel infarction at the time of presentation, an endovascular-first approach with subsequent on-demand laparotomy after close observation can theoretically result in clinical deterioration because of reperfusion injury caused by revascularization of necrotic bowel and untreated residual bowel infarction.
As described in our series, BR because of definite bowel infarction was necessary in the half of patients at initial presentation after exclusion of treatment refusal. In addition, 67% of patients who underwent operation ultimately required BR because of definite bowel infarction or the sequelae of bowel ischemia. These results are similar to the results recentaly reported by Raupach et al. [5] for an endovascular first approach, who reported their 12-year experience with primary endovascular therapy with subsequent on-demand laparotomy. According to their report, total in-hospital mortality was 27% and subsequent explorative laparotomy was performed in 73% of patients with necrotic BR in 41%. Therefore, preoperative identification of definite bowel infarction is an important issue for the management of SMAE.
Currently, the mainstay of diagnostic modalities in SMAE is the biphasic CT scan for vessel evaluation in the arterial phase and intestinal evaluation in the delayed phase [16]. In patients with specific intestinal findings, such as intestinal pneumatosis, portomesenteric venous gas, and nonenhancement of the bowel wall in CT scan, advanced bowel infarction can be strongly suspected [10,17]. However, as described by some investigators, CT protocol was optimal for acute mesenteric ischemia (with contrast enhancement in arterial and venous phases) in only 35% of cases [10], and intestinal features were more difficult to detect than vascular features [10,16]. Additionally, as in our series, many patients are transferred with a CT scan that was checked at the referring center, therefore, the precise evaluation of current status of bowel ischemia from a previous CT scan without additional contrast enhanced CT scan is difficult matter. In our series, the overall surgical delay, abdominal distension, abdominal tenderness, rebound tenderness, and a high level of CRP were associated clinical factors with BR. Therefore, in these cases, immediate surgical exploration after an endovascular approach or a direct surgical approach with BR and SMA embolectomy should be considered to prevent delay in the treatment of bowel infarction.
In addition, we experienced 15 (22%) additional BRs in 62 patients after initial treatment because of stricture, anastomotic leakage, and progressive bowel infarction with gangrene. Therefore, awareness of the possible development of intestinal complications and close monitoring after treatment is mandatory in the treatment of SMAE.
The major limitation of this study is its retrospective nature and the small patient sample that was acquired over a long period because SMAE is an uncommon disease. The majority of our patients were treated with a surgical approach, therefore, direct comparison of the rate of BR and in-hospital mortality after surgical and endovascular approaches was impossible. Furthermore, although we included all patients with SMAE registered in our department, some patients who present at the emergency department in poor general condition and with high comorbidity can die without treatment because the family refuses treatment. This particular limitation prevented us from assessing the exact rate of BR and in-hospital mortality, meaning that selection bias may be present. Finally, we did not assess the long-term outcomes according to BR, such as short bowel syndrome, quality of life, and long-term mortality. In our series, bowel infarction related to SMAE was not associated with in-hospital mortality. It is somewhat unexpected and may lessen the importance of bowel necrosis or surgical delay. Therefore, more long-term outcomes regarding the effect of BR are necessary.
In conclusion, a high index of suspicion with early abdominal CT scanning is crucial for an early diagnosis of SMAE to reduce surgical delay as a risk factor of bowel infarction. Overall surgical delay, abdominal distension, abdominal tenderness, rebound tenderness, and a high level of CRP were associated clinical factors with BR.
Footnotes
Conflict of interest: None.
This article was presented as an oral presentation in 64th Congress of Korean Society of Vascular Surgery.
REFERENCES
- 1.Acosta S, Björck M. Modern treatment of acute mesenteric ischaemia. Br J Surg. 2014;101:e100–e108. doi: 10.1002/bjs.9330. [DOI] [PubMed] [Google Scholar]
- 2.Sise MJ. Acute mesenteric ischemia. Surg Clin North Am. 2014;94:165–181. doi: 10.1016/j.suc.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Yun WS, Lee KK, Cho J, Kim HK, Huh S. Treatment outcome in patients with acute superior mesenteric artery embolism. Ann Vasc Surg. 2013;27:613–620. doi: 10.1016/j.avsg.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Heiss P, Loewenhardt B, Manke C, Hellinger A, Dietl KH, Schlitt HJ, et al. Primary percutaneous aspiration and thrombolysis for the treatment of acute embolic superior mesenteric artery occlusion. Eur Radiol. 2010;20:2948–2958. doi: 10.1007/s00330-010-1859-7. [DOI] [PubMed] [Google Scholar]
- 5.Raupach J, Lojik M, Chovanec V, Renc O, Strýček M, Dvořák P, et al. Endovascular management of acute embolic occlusion of the superior mesenteric artery: a 12-year single-centre experience. Cardiovasc Intervent Radiol. 2016;39:195–203. doi: 10.1007/s00270-015-1156-6. [DOI] [PubMed] [Google Scholar]
- 6.Block TA, Acosta S, Björck M. Endovascular and open surgery for acute occlusion of the superior mesenteric artery. J Vasc Surg. 2010;52:959–966. doi: 10.1016/j.jvs.2010.05.084. [DOI] [PubMed] [Google Scholar]
- 7.Puippe GD, Suesstrunk J, Nocito A, Pfiffner R, Glenck M, Pfammatter T. Outcome of endovascular revascularisation in patients with acute obstructive mesenteric ischaemia: a single-centre experience. Vasa. 2015;44:363–370. doi: 10.1024/0301-1526/a000455. [DOI] [PubMed] [Google Scholar]
- 8.Acosta-Merida MA, Marchena-Gomez J, Hemmersbach-Miller M, Roque-Castellano C, Hernandez-Romero JM. Identification of risk factors for perioperative mortality in acute mesenteric ischemia. World J Surg. 2006;30:1579–1585. doi: 10.1007/s00268-005-0560-5. [DOI] [PubMed] [Google Scholar]
- 9.Park WM, Gloviczki P, Cherry KJ, Jr, Hallett JW, Jr, Bower TC, Panneton JM, et al. Contemporary management of acute mesenteric ischemia: factors associated with survival. J Vasc Surg. 2002;35:445–452. doi: 10.1067/mva.2002.120373. [DOI] [PubMed] [Google Scholar]
- 10.Lehtimäki TT, Kärkkäinen JM, Saari P, Manninen H, Paajanen H, Vanninen R. Detecting acute mesenteric ischemia in CT of the acute abdomen is dependent on clinical suspicion: review of 95 consecutive patients. Eur J Radiol. 2015;84:2444–2453. doi: 10.1016/j.ejrad.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Acosta S, Sonesson B, Resch T. Endovascular therapeutic approaches for acute superior mesenteric artery occlusion. Cardiovasc Intervent Radiol. 2009;32:896–905. doi: 10.1007/s00270-009-9559-x. [DOI] [PubMed] [Google Scholar]
- 12.Kärkkäinen JM, Lehtimäki TT, Saari P, Hartikainen J, Rantanen T, Paajanen H, et al. Endovascular therapy as a primary revascularization modality in acute mesenteric ischemia. Cardiovasc Intervent Radiol. 2015;38:1119–1129. doi: 10.1007/s00270-015-1064-9. [DOI] [PubMed] [Google Scholar]
- 13.Arthurs ZM, Titus J, Bannazadeh M, Eagleton MJ, Srivastava S, Sarac TP, et al. A comparison of endovascular revascularization with traditional therapy for the treatment of acute mesenteric ischemia. J Vasc Surg. 2011;53:698–704. doi: 10.1016/j.jvs.2010.09.049. discussion 705. [DOI] [PubMed] [Google Scholar]
- 14.Acosta S, Ogren M, Sternby NH, Bergqvist D, Björck M. Incidence of acute thrombo-embolic occlusion of the superior mesenteric artery--a population-based study. Eur J Vasc Endovasc Surg. 2004;27:145–150. doi: 10.1016/j.ejvs.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Wyers MC. Acute mesenteric ischemia: diagnostic approach and surgical treatment. Semin Vasc Surg. 2010;23:9–20. doi: 10.1053/j.semvascsurg.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Kirkpatrick ID, Kroeker MA, Greenberg HM. Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experience. Radiology. 2003;229:91–98. doi: 10.1148/radiol.2291020991. [DOI] [PubMed] [Google Scholar]
- 17.Costa AF, Chidambaram V, Lee JJ, Asquith J, Skaff ER, Thipphavong S. Multidetector computed tomography of mesenteric ischaemia. Insights Imaging. 2014;5:657–666. doi: 10.1007/s13244-014-0361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]