Abstract
Intrauterine growth restriction (IUGR) induced by placental restriction (PR) in the sheep negatively impacts lung and pulmonary surfactant development during fetal life. Using a sheep model of low birth weight (LBW), we found that there was an increase in mRNA expression of surfactant protein (SP)-A, -B and -C in the lung of LBW lambs but no difference in the protein expression of SP-A or -B. LBW also resulted in increased lysosome-associated membrane glycoprotein (LAMP)-3 mRNA expression, which may indicate an increase in either the density of type II Alveolar epithelial cells (AEC) or maturity of type II AECs. Although there was an increase in glucocorticoid receptor (GR) and 11β-hydroxysteroid dehydrogenase (11βHSD)-1 mRNA expression in the lung of LBW lambs, we found no change in the protein expression of these factors, suggesting that the increase in SP mRNA expression is not mediated by increased GC signalling in the lung. The increase in SP mRNA expression may, in part, be mediated by persistent alterations in hypoxia signalling as there was an increase in lung HIF-2α mRNA expression in the LBW lamb. The changes in the hypoxia signalling pathway that persist within the lung after birth may be involved in maintaining SP production in the LBW lamb.
Introduction
Intrauterine lung development and maturation are critically important for postnatal survival of the neonate. An important step in fetal lung development in late gestation is the maturation of the pulmonary surfactant system, which consists of a complex mixture of ∼90% lipids and 8–10% surfactant proteins (SP) [1]. Pulmonary surfactant is synthesised in type II alveolar epithelial cells (AEC), stored in lamellar bodies and under appropriate conditions is secreted via exocytosis into the alveolar space [2]. SP-B and -C, which are hydrophobic surfactant proteins, interact with the lipid component to facilitate adsorption of the surfactant film to the air-liquid interface of the alveoli. This film reduces the work of breathing by maintaining stability between alveolar units and preventing adherence of the alveolar walls during expiration [3]. SP-A and -D play important roles as effectors of the pulmonary innate immune system [4,5].
SP expression by type II AECs is under the control of a range of factors, including glucocorticoids (GC) [6,7], hypoxia [8] and peroxisome proliferator-activated receptor γ (PPARγ) [9]. GC increase the mRNA expression of SP-A, -B and -C indirectly through transcription factors such as thyroid transcription factor-1 (TTF-1) and cofactors such as GATA binding protein 6 (GATA-6) [10,11]. Hypoxia plays an important role because SP expression in the lung is reduced in hypoxia inducible factor-2α (HIF-2α) knockout mice, which results in respiratory distress syndrome [12,13]. Other transcription factors also play a role because administration of rosiglitazone in rodents, a PPARγ agonist, increases lung maturation by increasing SP expression [9].
Intrauterine growth restriction (IUGR), defined as a birth weight less than the 10th centile for gestational age, affects more than 20 million newborns globally, and is particularly prevalent in developing countries [14,15]. Placental insufficiency is the most common cause of IUGR, which reduces both oxygen and nutrient transfer to the fetus [16,17]. There is a growing body of evidence that IUGR negatively impacts the growth and maturation of the fetal lung, with a decreased functional capacity persisting into later life [18,19]. Respiratory function is compromised in IUGR lambs as evidenced by lower functional residual capacity and total lung capacity compared with Control lambs at 8 weeks after birth [20]. IUGR alters lung structure with increased collagen deposition in rats [21] and reduced gas exchange surface in the late gestation fetal sheep [22]. An accumulation of glycogen in type II AECs in a maternal undernutrition model resulting in IUGR in rats suggests a delay in type II AEC differentiation [23]. Different sheep models of late gestation onset IUGR resulted in either increase SP-B mRNA expression, that is [24] or is not [13,25] associated with increased plasma cortisol concentration, or no change in SP mRNA expression [26] in late gestation. Late gestation onset IUGR also resulted in SP-B mRNA expression increase in 8 week old lambs [20]. In contrast, early gestation onset IUGR induced by PR is associated with delayed pulmonary surfactant maturation during late gestation, with a reduction in both surfactant protein mRNA and protein expression at 130 and 140 days (d) of gestation [27,28]. These changes are also accompanied by an increase in plasma cortisol concentrations [28] and there is evidence for a role of both hypoxia and glucocorticoid signalling [28]. In the present study, we aimed to determine whether early gestation onset IUGR would continue to supress SP expression in 21 day old lambs born with a low birth weight (LBW). Furthermore, we investigated potential molecular mechanisms, including GC and hypoxia signalling, cellular proliferation and PPARγ pathways, all of which regulate surfactant maturation in postnatal life.
Materials and methods
All procedures were approved by the University of Adelaide Animal Ethics Committee and conformed to the NHMRC Australian code of practice for the care and use of animals for scientific purposes.
Animals and surgery
Sixteen Merino ewes and their lambs were used in this study. Prior to mating, ewes were randomly divided into two treatment groups (Control and placental restriction (PR)). PR, leading to fetal growth restriction was induced in 6 ewes using carunclectomy [29–31]. General anaesthesia was induced in ewes with an intravenous injection of sodium thiopentone (1.25 g i.v., Pentothal, Rhone Merieux, Pinkenba, Queensland, Australia) and maintained with 2.5–4% halothane inhalation anaesthetic (Fluothane, ICI, Melbourne, Victoria, Australia) in oxygen. The uterus was incised and the majority of the caruncles, the sites of placentation, were removed from the uterus [27,29,32,33]. Antibiotics were administered intramuscularly to the ewe during surgery (153.5 mg procaine penicillin, 393 mg benzathine penicillin, 500 mg dihydrostreptomycin, Lyppards, South Australia, Australia). These ewes were allowed to recover for a period of at least 10–12 weeks before entering a mating program along with Control ewes [30].
Lamb birth and postnatal monitoring
Ewes gave birth spontaneously at term in individual pens after acclimatisation of at least two weeks [30]. The definition of average birth weight (ABW) and low birth weight (LBW) lambs was determined using a frequency distribution curve of birth weights of Control Merino singleton lambs from a separate cohort of 45 animals born from 1995–2004 under investigation by our laboratory. Birth weight of ABW lambs was 5.91±0.17 kg (n = 9, male = 6, female = 3) and that of LBW lambs was 3.78±0.17 kg (n = 7, male = 4, female = 3), which is 2 standard deviations below the average cohort mean. Information on the phenotype of the lambs and studies of cardiac growth and insulin signalling in skeletal muscles for this cohort have been published previously [30,34–38].
Post mortem and tissue collection
Twenty-one days after birth, the lambs (ABW, n = 9; LBW, n = 7) were humanely killed with an overdose of sodium pentobarbitone injected into the jugular vein (Virbac Pty Ltd, Peakhurst, New South Wales, Australia). Lungs were dissected, weighed and samples were snap frozen in liquid nitrogen within 15 min and stored at -80°C for molecular analysis.
Quantification of gene expression
Total RNA extraction
All essential information regarding our procedure is included as per the MIQE guidelines (7). Total RNA was extracted from ~50 mg lung samples (ABW, n = 9; LBW, n = 6) using Invitrogen Trizol Reagent Solution (Invitrogen Australia Pty. Ltd., Victoria, Australia) and RNeasy Mini Kit (QIAGEN, Victoria, Australia) according to the manufacturer’s instructions as previously described [39,40]. Total RNA was quantified by spectrophotometric measurements at 260 and 280nm in an Eppendorf BioPhotometer (Crown Scientific, NSW, Australia) and the 260/280nm ratio results were checked for protein and DNA contamination in each sample. Integrity of purified RNA was verified by assessment of the RNA bands run on a 1% agarose gel. cDNA was synthesised as previously described [39]. Controls containing either no Superscript III (No Amplification Control (NAC)) or no RNA transcript (No Template Control (NTC)) were used to test for genomic DNA and reagent contamination, respectively.
Quantitative real-time PCR
Based on the geNorm component of the qBase 2.0 relative quantification model (Biogazelle, Belgium), ribosomal protein P0 (RpP0, [30]), β actin (ACTB, [39]) and cyclophilin (CYCLO, [27]) were selected from a panel of eight candidate reference genes [41]. These reference genes were used as they were stably expressed across both groups (ABW vs. LBW) [42,43]. Primer pairs for plasminogen activator inhibitor (PAI)-1, DNA methyltransferase (DNMT) -1, -3a and -3b, fatty acid synthase (FAS) and sphingosine kinase (SPHK)-1 were designed (Table 1). Primers were validated to generate a single transcript as confirmed by melt-curve/dissociation curve and sequenced by the Australian Genome Research Facility Ltd. The mRNA expression of surfactant proteins (SP-A, -B, -C and -D [27,39]), GC signalling genes (glucocorticoid receptor (GR), mineralocorticoid receptor (MR) [39], 11β-hydroxysteroid dehydrogenase (11βHSD) -1 and -2 [39], GATA-binding protein (GATA) -6 [39], thyroid transcription factor (TTF) -1 [39]), hypoxia signalling and feedback (hypoxia inducible factor (HIF) -1α and -2α, prolyl hydroxylase domain (PHD) -1, -2 and -3) [44], hypoxia responsive genes (Vascular endothelial growth factor (VEGF) [44], jumanji demethylases1A (JMJD1A) [44], insulin-like growth factor (IGF) -2 [45], fms-related tyrosine kinase (FLT) -1 [44] and angiotensin converting enzyme (ACE) -1 [45]), peroxisome proliferator-activated receptors gamma (PPARγ) [46], PPARγ responsive genes (SPHK-1 and PAI-1), genes involved in proliferation (proliferating cell nuclear antigen (PCNA, KI-67 [28]), genes regulating the cell cycle (p27 and cyclin D1 (CCND-1) [47]), marker of lamellar bodies in type II AECs (lysosomal associated membrane protein (LAMP-3) [28]), surfactant lipid transporter (ATP-binding cassette, sub-family A, member 3 (ABCA3) [48]), markers of pulmonary surfactant lipid synthesis (phosphate cytidylyl transferase 1, choline, alpha (PCYT1A) [48] and FAS) and reference genes in lung samples were measured using Fast SYBR® Green Master Mix (Applied Biosystems, California, USA) on a ViiA7 Fast Real-time PCR system (Applied Biosystems, California, USA) as previously described [40]. The abundance of each transcript relative to the abundance of stable reference genes was calculated using DataAssist 3.0 analysis software (Applied Biosystems, California, USA) [43] and expressed as mRNA mean normalised expression (MNE) ± SEM [39,40].
Table 1. qRT-PCR primer sequences, concentrations and accession numbers for target genes that were designed and validated for this study.
| Primer Name | Sequence 5’ → 3’ | Primer Conc. (μM) |
Melting point (°C) | Size (bp) | Accession No. |
Reference |
|---|---|---|---|---|---|---|
| SP-A | 80 | 90 | AF211856 | [27] | ||
| Forward | AGCTCCAGGGCACACTCCATG | 0.3 | ||||
| Reverse | CTCCCACTTCCAGCATGGAC | 0.3 | ||||
| SP-B | 84 | 153 | AF107544 | [27] | ||
| Forward | GGGCCCCACATTCTGGTGC | 0.3 | ||||
| Reverse | TCCTTGGCCATCTTGGTGAGG | 0.3 | ||||
| SP-C | 84 | 149 | AF076634 | [27] | ||
| Forward | GCAAAGAGGTCTTGATGGAG | 0.3 | ||||
| Reverse | CAGGGCTCCTACGATCACC | 0.3 | ||||
| SP-D | 76 | 62 | AJ133002.1 | [39] | ||
| Forward | GGCCACAGCCCAGAACAA | 0.3 | ||||
| Reverse | AAGTACCCTCCTTCCTGGTATCG | 0.3 | ||||
| SPHK-1 | 82 | 70 | XM_002696204.2 | |||
| Forward | CCACTGCCCCCACCTGGTCTATGTG | 0.45 | ||||
| Reverse | CACACCCTTCCCATCCTTGG | 0.45 | ||||
| PAI-1 | 84 | 182 | NM_001174114 | |||
| Forward | TCGACAGCAGATCCAAGAGGCAAT | 0.45 | ||||
| Reverse | TGAAGAAGTTGGGCATGAAACCGC | 0.45 | ||||
| FAS | 81 | 95 | AF479289.1 | |||
| Forward | CCCAGCTCAACGAAACCA | 0.45 | ||||
| Reverse | GACGAGGTCAACACCCTTCC | 0.45 | ||||
| DNMT-1 | 79 | 164 | NM_001009473.1 | |||
| Forward | AGCAAGCGGAGACCAGAAGAGAAA | 0.45 | ||||
| Reverse | TCGGTCTTGGACACCACTGCTATT | 0.45 | ||||
| DNMT-3a | 81 | 80 | HQ202740.1 | |||
| Forward | TTTCCAATGTGCCATGACAGCGAC | 0.45 | ||||
| Reverse | GGCCCACTCGATCATCTGTTTGTT | 0.45 | ||||
| DNMT-3b | 81 | 146 | HQ202741.1 | |||
| Forward | CCAGTGGTTTGGTGATGGCAAGTT | 0.45 | ||||
| Reverse | TGGCTTTCTCCAGAGCATGGTACA | 0.45 |
Accession numbers refer to the published cDNA sequences from which the primer sequences were designed.
Quantification of protein extraction
Protein extraction
∼50–100 mg of a subset of lung samples (ABW = 7, LBW = 6) were cut and sonicated (Kinematica PT-MR-3100, Lucerne, Switzerland) in 500 μL of homogenising buffer. Homogenates were centrifuged, the supernatant was recovered and the pellets were discarded.
Total protein quantification
The amount of protein in each extraction was determined using a micro Bicinchoninic acid (BCA) protein assay kit (PIERCE, Thermo Fisher Scientific Inc, Rockford, USA) with bovine serum albumin to generate a standard curve. The extracted protein was diluted with protein sample buffer in aliquots to a concentration of 5 mg/mL. Prior to Western blot analysis, samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and gels stained with Coomassie blue reagent (Thermo Fisher Scientific Inc., USA). This is a quality control step performed before Western Blotting to confirm equal loading of the proteins as performed previously [36,40,49–51].
Western blotting
Protein from each sample was subjected to SDS-PAGE as described above and transferred onto a nitrocellulose membrane using a wet transfer system [28,44]. The membranes were stained with 0.1% Ponceau S in 7% acetic acid to confirm proper transfer and washed with 7% acetic acid to remove the stain. The membranes were blocked in either 5% BSA in Tris-buffered saline with 1% Tween-20 (TBS-T; 10 mM Tris, 0.9% NaCl, pH 7.4, with 0.1% Tween 20) or 5% skim milk in TBS-T and then incubated with the respective primary antibody; goat anti-human SP-A antibody (Chemicon International, Billerica, MA) [28], mouse anti-human monoclonal antibody to SP-B (produced by Dr Y. Suzuki, Kyoto University, Japan and kindly donated by Prof Fred Possmayer, University of Western Ontario, Canada) [27], rabbit anti-human polyclonal antibody to 11βHSD-1 (Cayman Chemicals, MI, USA) [28], rabbit anti-human polyclonal antibody to 11βHSD-2 (Cayman Chemicals, MI, USA) [28], rabbit anti-mouse polyclonal antibody to GR (generously donated by Prof Vicki Clifton, Mater Medical Research Institute Limited, Australia) [28], goat anti-human polyclonal antibody GATA-6 (Santa Cruz Biotechnology, Inc, Texas, USA) [28], rabbit anti-human polyclonal antibody to TTF-1 (Santa Cruz Biotechnology, Inc, Texas, USA) [28], rabbit anti-human polyclonal antibody to PHD-1 (Novus Biologicals, CO, USA) [28,44] and rabbit anti-human polyclonal antibody to PHD-2 (Novus Biologicals, CO, USA) [28,44]. Membranes were then incubated with their respective horseradish peroxidase (HRP)-conjugated secondary IgG antibody (Cell Signalling Technology, Inc., Massachusetts, USA) and antigen-antibody complexes were detected by enhanced chemiluminescence using SuperSignal® West Pico chemiluminescent substrate (Thermo Fisher Scientific Inc., USA) using an ImageQuantTM LAS 4000 imager (GE Healthcare, Australia). Specific bands were quantified by densitometry using ImageQuant TL software (GE Healthcare, Australia) and data is expressed as arbitrary units (AU).
Plasma cortisol radioimmunoassay
Total plasma cortisol concentration was measured using an 125I radioimmunoassay kit (GE Healthcare, Sydney, Australia) as previously described [27,52] in a blood sample collected from the lamb on the morning of the 20th day after birth. The average efficiency of recovery of 125I cortisol using dichloromethane extraction was 90%. The sensitivity of the assay was 0.39 nmol/L. The rabbit anti-cortisol antibody cross-reacted 1% with cortisone and 17-hydroxyprogesterone and 0.01% with aldosterone, pregnenolone, estradiol, and progesterone. The inter- and intra-assay coefficients of variation (CV) were less than 10%.
Lung tissue cortisol enzyme-linked immunosorbent assay (ELISA)
A subset of lung samples (~50–60 mg; ABW = 5, LBW = 6) were cut and sonicated (Kinematica PT-MR-3100, Lucerne, Switzerland) as previously described [53]. The samples were centrifuged at 1,500rcf (Beckman J6 Centrifuge), the supernatant removed from each sample and the pellets were discarded. Cortisol was extracted from the supernatant by combining supernatant with extraction buffer (Oxford Biomedical Research Inc., Michigan, USA) and ethyl ether (VWR, Qld, Australia) and vortexed for 1 min followed by 5 min phase separation. The organic phase was transferred into a glass tube and dried at 37°C with air for 30 min and re-suspended in extraction buffer.
Test samples and the cortisol standards were assayed in duplicate on a Cortisol Enzyme Immunoassay according to the manufacturer’s guidelines (EA65, Oxford Biomedical Research Inc., Michigan, USA) [54]. The plate was read on a standard 96-well colorimetric plate reader (450 nm; Victor3 Multilabel Plate Reader 1420, Perkin Elmer, Massachusetts, USA) and total cortisol concentration was determined from the standard curve. Results are expressed as total cortisol in lung tissue (pg/mg).
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Two-way ANOVA was performed to determine the effect of treatment (ABW vs LBW), the effect of sex (M vs F) and the interaction between the effects of treatment and sex on all parameters (Statistical Package for Social Sciences (SPSS) v17.0, Chicago, USA). In this cohort we saw no effect of sex and no interaction between the effects of treatment and sex for any parameter. Therefore, data for male and female animals were pooled and analysed using a Student’s unpaired t-test (ABW vs LBW). A probability level of 5% (P<0.05) was considered statistically significant.
Results
Effect of LBW on body and lung weight, plasma cortisol concentration and lung tissue cortisol in lambs at 21d after birth
LBW lambs had significantly decreased body weight both at birth and 21d after birth as previously published [30]. LBW lambs had a decrease in absolute lung weight (ABW, 206.86±10.47 g; LBW, 163.84±9.09 g; P<0.05). However, there was no change in relative lung weight (ABW, 15.68±0.64g/kg; LBW, 15.56±1.68 g/kg; P>0.05). There was also no significant difference in plasma cortisol concentration (ABW, 16,200 ± 4,900 pmol/L; LBW, 22,700 ± 5,600 pmol/L; P>0.05) or lung tissue cortisol concentration (ABW, 2.99 ± 0.60 pg/mg; LBW, 4.56 ± 1.0 pg/mg; P>0.05) on the day of post mortem between ABW and LBW lambs.
Effect of LBW on SP mRNA and SP-A and -B protein expression in the lung of LBW lambs 21d after birth
There was higher SP-A, -B and -C mRNA expression in the lungs of LBW compared with ABW lambs, however, there was no difference in SP-D mRNA expression (Fig 1A, 1B, 1C and 1D). There was no difference in SP-A and -B protein expression (Fig 1E and 1F) in the lung between the ABW and LBW lambs. There was a significant negative correlation between birth weight and SP-A mRNA expression, but there was no significant relationship between birth weight and SP-B, -C or -D mRNA expression (Table 2).
Fig 1.
There was higher mRNA expression of SP-A (A), -B (B) and -C (C), but not SP-D (D), in the lungs of the LBW lambs (n = 6; Closed bars) compared with ABW lambs (n = 9; Open bars). There was no difference in the protein expression of SP-A (E), -B (F) and SP-D (G) in the lungs of the LBW lambs (n = 6; Closed bars) compared with ABW lambs (n = 7; Open bars). Representative Western blot images from animals in each treatment group for SP-A (G),–B (H) and–D (J) are presented. MNE, mean normalised expression; ABW, Average birth weight; LBW, Low birth weight; *P<0.05 from ABW.
Table 2. Regression analyses of SP, GR, 11βHSD mRNA expression against birth weight and weight at 21 days and SP mRNA expression against plasma cortisol concentrations on postnatal day 20.
| Variables | Equation | N | Adjusted r2 | P |
|---|---|---|---|---|
| SP mRNA expression (MNE) vs. Birth weight (kg) | ||||
| SP-A | y = 1.84x – 0.23 | 14 | 0.565 | 0.001 |
| SP-B | 14 | 0.205 | 0.059 | |
| SP-C | 14 | 0.108 | 0.135 | |
| SP-D | 14 | 0.099 | 0.145 | |
| GR, 11βHSD mRNA expression (MNE) and plasma cortisol vs. Birth weight (kg) | ||||
| GR | y = 0.19x – 0.015 | 14 | 0.489 | 0.003 |
| 11βHSD-1 | 13 | 0.191 | 0.076 | |
| 11βHSD-2 | 13 | 0.016 | 0.292 | |
| Plasma cortisol concentration | 9 | 0.413 | 0.062 | |
| Lung tissue cortisol concentration | 11 | -0.0120 | 0.372 | |
| SP mRNA expression (MNE) vs. Weight at 21 days (kg) | ||||
| SP-A | y = 2.09x – 0.012 | 15 | 0.356 | 0.011 |
| SP-B | 15 | 0.169 | 0.071 | |
| SP-C | 15 | 0.049 | 0.212 | |
| SP-D | 15 | -0.026 | 0.436 | |
| GR, 11βHSD mRNA expression (MNE) and plasma cortisol vs. Weight at 21 days (kg) | ||||
| GR | y = 0.228x – 0.001 | 14 | 0.582 | 0.001 |
| 11βHSD-1 | 13 | 0.092 | 0.292 | |
| 11βHSD-2 | 14 | 0.051 | 0.217 | |
| Plasma cortisol concentration | 10 | 0.079 | 0.219 | |
| Lung tissue cortisol concentration | 11 | -0.0976 | 0.747 | |
| SP mRNA expression (MNE) vs plasma cortisol concentration | ||||
| SP-A | 10 | 0.051 | 0.259 | |
| SP-B | 10 | 0.006 | 0.830 | |
| SP-C | 10 | 0.156 | 0.259 | |
| SP-D | 10 | 1.34E-005 | 0.992 | |
| SP mRNA expression (MNE) vs lung tissue cortisol concentration | ||||
| SP-A | 10 | 0.146 | 0.150 | |
| SP-B | 10 | 0.038 | 0.278 | |
| SP-C | y = 0.34x + 2.27 | 10 | 0.446 | 0.021 |
| SP-D | y = 0.0092x + 0.026 | 10 | 0.4522 | 0.020 |
Regression equations are only provided in case of significant relationships. MNE, mean normalised expression.
Effect of LBW on GC signalling and cofactors involved in GC signalling in the lung of lambs 21d after birth
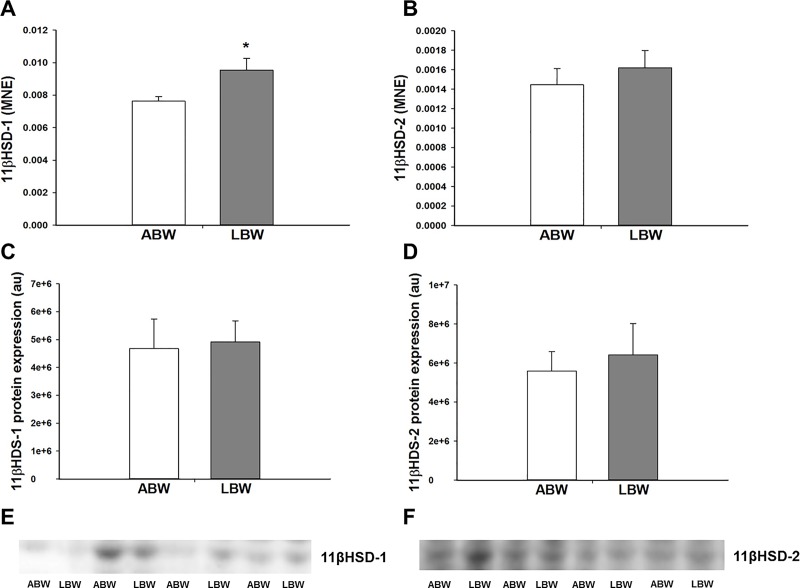
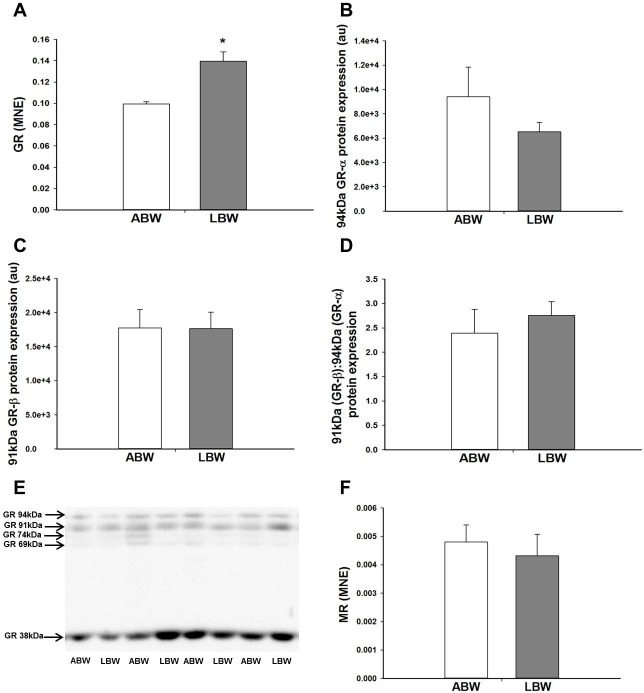
There was an increase in the mRNA expression of the GC activating isoform 11βHSD-1, but not the GC deactivating isoform 11βHSD-2 in the lung of the LBW compared with the ABW lambs (Fig 2A and 2B). However, there was no difference in the protein expression of 11βHSD-1 and 11βHSD-2 between the lungs of ABW and LBW lambs (Fig 2C and 2D). LBW was associated with a significant increase in lung GR mRNA expression (Fig 3A) that resulted in a significant negative correlation between birth weight and GR mRNA expression (Table 2), but not in the lung protein abundance of GR. Furthermore, there was no difference in the protein expression of the 94kDa GR-α, 91kDa GR-β, 74kDa GR-P, 69kDA GR, 39kDA GR or ratio of GR-β to GR-α isoforms between LBW and ABW lambs (Fig 3B, 3C and 3D). There was also no difference in the mRNA expression of the MR (Fig 3F). LBW was also associated with a decrease in GC signalling cofactor GATA-6 mRNA expression in the lungs (Fig 4A). However, there was no difference in the protein expression of GATA-6 in the lungs of the LBW and the ABW lambs (Fig 4C). There was no difference in either mRNA or protein expression of TTF-1 (Fig 4B and 4D).
Fig 2.
There was higher mRNA expression of 11βHSD-1 (A) in the lungs of the LBW lambs (n = 6; Closed bars) compared with the ABW lambs (n = 8; Open bars). There was no difference in the mRNA expression of 11βHSD-2 (B) in the lungs of the LBW lambs (n = 6; Closed bars) compared with the ABW lambs (n = 8; Open bars). There was no difference in the protein expression of 11βHSD-1 (C) and 11βHSD-2 (D) between the lungs of the LBW lambs (n = 6; Closed bars) and ABW lambs (n = 7; Open bars). Representative Western blot images from animals in each treatment group for 11βHSD-1 (E) and 11βHSD-2 (F) are presented. MNE, mean normalised expression; ABW, Average birth weight; LBW, Low birth weight; *P<0.05 from ABW.
Fig 3.
There was higher mRNA expression of the GR (A) in the lungs of the LBW lambs (n = 7; Closed bars) compared with the ABW lambs (n = 6; Open bars). However, there was no difference in the protein expression of 94kDa GR-α (B), 91kDa GR-β (C) between the lungs of the LBW lambs (n = 6; Closed bars) and ABW lambs (n = 7; Open bars). There was no difference in the ratio of 91kDa GR-β to 94kDA GR-α protein expression (D) between the lungs of the LBW lambs (Closed bars) and ABW lambs (Open bars). A representative Western blot image from animals in each treatment group for GR (E) is presented. There was no difference in the mRNA expression of MR between the lungs of the LBW lambs (n = 6; Closed bars) and ABW lambs (n = 9; Open bars) (F). MNE, mean normalised expression; ABW, Average birth weight; LBW, Low birth weight; *P<0.05 from ABW.
Fig 4.
There was lower mRNA expression of GATA-6 (A) in the lungs of the LBW lambs (n = 6; Closed bars) compared with the ABW lambs (n = 9; Open bars). There was no difference in the mRNA expression of TTF-1 (B) in the lungs of the LBW lambs (n = 5; Closed bars) compared with the ABW lambs (n = 8; Open bars). There was no difference in the protein expression of GATA-6 (C) and TTF-1(D) between the lungs of the LBW lambs (n = 6; Closed bars) and ABW lambs (n = 7; Open bars). Representative Western blot images from animals in each treatment group for GATA-6 (E) and TTF-1 (F) are presented. MNE, mean normalised expression; ABW, Average birth weight; LBW, Low birth weight; *P<0.05 from ABW.
Effect of LBW on hypoxia signalling and feedback in the lungs of lambs 21d after birth
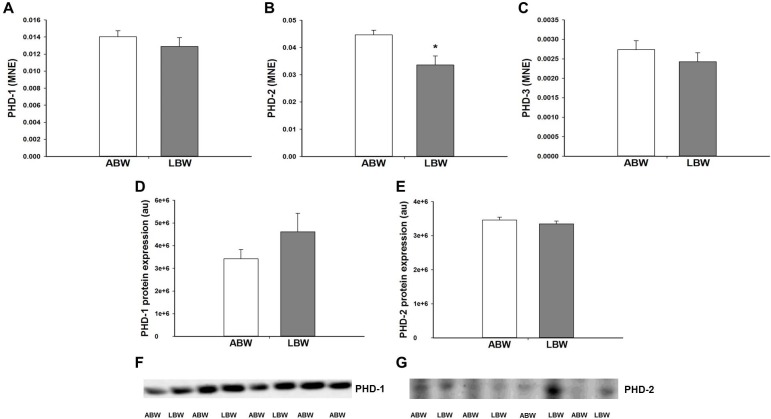
There was a decrease in PHD-2 mRNA expression in the lung of LBW lambs (Fig 5B). However, there was no difference in PHD-1 or PHD-3 mRNA expression (Fig 5A and 5C) between the lungs of LBW and ABW lambs. There was also no difference in the protein expression of PHD-1 and PHD-2 in the lungs of LBW and ABW lambs (Fig 5D and 5E). There was an increase in HIF-2α, but not HIF-1α mRNA expression in the lungs of LBW lambs (Fig 6A and 6B). There was no effect of LBW on the mRNA expression of genes with hypoxia response elements (VEGF, JMJD1A, IGF-2, FLT-1 and ACE-1; Table 3).
Fig 5.
There was no difference in the mRNA expression of PHD-1 (A) and PHD-3 (C) in the lungs of the LBW lambs (n = 6; Closed bars) compared with the ABW lambs (n = 9; Open bars). There was lower mRNA expression of PHD-2 (B) in the lungs of the LBW lambs (n = 6; Closed bars) compared with the ABW lambs (n = 8; Open bars). There was no difference in the protein expression of PHD-1 (D) and PHD-2 (E) in the lungs of the LBW lambs (n = 6; Closed bars) compared with the ABW lambs (n = 7; Open bars). Representative Western blot images from animals in each treatment group for PHD-1 (F) and PHD-2 (G) are presented. MNE, mean normalised expression; ABW, Average birth weight; LBW, Low birth weight; *P<0.05 from ABW.
Fig 6.
There was no difference in the mRNA expression of HIF-1α (A) in the lungs of the LBW lambs (n = 6; Closed bars) compared with the ABW lambs (n = 9; Open bars). There was higher mRNA expression of HIF-2α (B) in the lungs of the LBW lambs (n = 6; Closed bars) compared with the ABW lambs (n = 8; Open bars). MNE, mean normalised expression; ABW, Average birth weight; LBW, Low birth weight; *P<0.05 from ABW.
Table 3. mRNA expression of genes with hypoxia response elements in the lung of ABW and LBW lambs.
| Gene Name (MNE) | ABW (n = 9) | LBW (n = 7) |
|---|---|---|
| VEGF | 0.070 ± 0.004 | 0.084 ± 0.017 |
| JMJD1A | 0.026 ± 0.002 | 0.024 ± 0.003 |
| IGF-2 | 1.147 ± 0.086 | 1.011 ± 0.145 |
| FLT-1 | 0.081 ± 0.004 | 0.093 ± 0.015 |
| ACE-1 | 0.044 ± 0.003 | 0.053 ± 0.008 |
Data (mean ± SEM) were analysed using Student’s unpaired t-test. MNE, mean normalised expression; ABW, average birth weight; LBW, low birth weight.
Effect of LBW on mRNA expression of PPARγ and genes with PPARγ response elements in the lungs 21d after birth
There was no change in lung mRNA expression of PPARγ or PAI-1, a gene with a PPARγ response element, between ABW and LBW lambs (Table 4). However, there was higher SPHK-1 mRNA expression, another gene that has a PPARγ response element, in the lungs of LBW compared with ABW lambs.
Table 4. mRNA expression of PPARγ and genes with PPARγ response elements in the lung of ABW and LBW lambs.
| Gene name (MNE) | ABW (n = 9) | LBW (n = 7) |
|---|---|---|
| PPARγ | 0.005 ± 0.0004 | 0.006 ± 0.0005 |
| SPHK-1 | 0.00038 ± 0.00003 | 0.00049 ± 0.00004* |
| PAI-1 | 0.137 ± 0.02 | 0.142 ± 0.01 |
Data (mean ± SEM) were analysed using Student’s unpaired t-test. MNE, mean normalised expression; ABW, average birth weight; LBW, low birth weight
*P < 0.05.
Effect of LBW on markers of proliferation, cell cycle regulators, markers of type II AECs and markers of pulmonary surfactant lipid synthesis in the lungs of lambs 21d after birth
There was no difference in the mRNA expression of markers of proliferation (PCNA and KI-67) or regulators of cell cycle progression (p27 and CCND-1) in the lungs of LBW and ABW lambs (Table 5). However, LBW was associated with an increase in FAS mRNA expression, an enzyme involved in surfactant lipid synthesis, but there was no difference in the mRNA expression of PCYT1A, a rate limiting enzyme involved in surfactant phosphatidylcholine synthesis in the lung. LBW was associated with higher mRNA expression of LAMP-3, a marker of lamellar bodies present in type II AECs but there was no effect on surfactant lipid transporter ABCA3 mRNA expression (Table 5).
Table 5. mRNA expression of markers of cellular proliferation and regulators of cell cycle progression, marker of lamellar bodies in type II AECs, pulmonary surfactant lipid synthesis and transport in the lung of ABW and LBW lambs.
| Gene name (MNE) | ABW (n = 9) | LBW (n = 7) |
|---|---|---|
| Proliferation and cell cycle progression | ||
| PCNA | 0.015 ± 0.002 | 0.020 ± 0.006 |
| KI-67 | 0.016 ± 0.001 | 0.024 ± 0.005 |
| p27 | 0.027 ± 0.002 | 0.025 ± 0.002 |
| CCND-1 | 0.017 ± 0.004 | 0.016 ± 0.002 |
| Marker of lamellar bodies in type II AECs | ||
| LAMP-3 | 0.088 ± 0.015 | 0.129 ± 0.024* |
| Pulmonary surfactant lipid synthesis and transport | ||
| PCYT1A | 0.041 ± 0.001 | 0.040 ±0.004 |
| FAS | 0.035 ± 0.001 | 0.045 ±0.002* |
| ABCA3 | 0.033 ± 0.002 | 0.037 ± 0.002 |
Data (mean ± SEM) were analysed using Student’s unpaired t-test. MNE, mean normalised expression; ABW, average birth weight; LBW, low birth weight
*P < 0.05.
Discussion
In this study, we have shown that there is an increase in lung mRNA expression of SP-A, -B and -C in LBW compared with ABW lambs at 21 days after birth which is in contrast to the decrease in SP-A, SP-B and SP-C mRNA expression and protein abundance in the lung of the PR fetus in late gestation [27,28]. Despite the an absolute fold change of ~2.0-fold increase in SP-Aand absolute fold change of ~1.7-fold increase in -B mRNA expression in the lung of LBW lambs in this study, there was no difference in the protein expression of either SP-A or -B. Hence, the deficit in lung tissue SP in the PR fetus observed in late gestation was normalised by 21d after birth in the LBW lamb, possibly as a result of the persistent elevation in SP mRNA expression. In this study, we have further examined the role of a range of intrapulmonary signalling pathways, including regulation by GCs, hypoxia, PPARγ, cellular proliferation and global methylation, which may be responsible for the altered regulation of surfactant maturation in the LBW lamb.
The dramatic increase in fetal plasma cortisol during late gestation in the normally grown fetus [55,56]coincides with important steps in lung structural and pulmonary surfactant maturation in preparation for postnatal life [57,58]. GCs increase the mRNA expression of SP-A, -B and -C [39,59,60]. In sheep, the PR fetus has higher plasma cortisol concentrations across late gestation compared with Control fetuses [27,61]. However, despite increased plasma cortisol concentrations in the PR fetus, there is reduced surfactant mRNA and protein expression in late gestation [27], which is associated with reduced GC signalling and altered hypoxia signalling [28]. As adult men born with LBW have higher plasma cortisol concentrations than men born ABW, we measured cortisol concentration and factors regulating glucocorticoid availability in the LBW lambs [62,63]. However, in the present study, we found that the higher plasma cortisol concentrations observed in the hypoxic PR fetus in late gestation were not sustained in the 21d old LBW lambs. In this study, we have measured the expression of regulators of cortisol availability; 11βHSD-1, which converts inactive cortisone to active cortisol, and 11βHSD-2, which converts active cortisol into inactive cortisone [64]. Despite an increase in the mRNA expression of 11βHSD-1, we found no change in the 11βHSD-1 protein abundance in the lungs of the LBW lamb and this may be due to a small absolute fold change in mRNA expression of 11βHSD-1 of only ~1.18-fold Although there was an absolute fold change of ~1.3-fold increase in mRNA abundance of the GR, the intracellular mediator of GC action, this was not associated with an increase in the protein abundance of GR. Therefore, GC is unlikely to be responsible for the increased surfactant protein mRNA expression in the lung.
We found no change in the expression of key markers of cellular proliferation including PCNA and KI-67 as well as genes involved in the regulation of cell cycle progression, p27 and CCND-1, suggesting that there was no change in cell proliferation in lung tissue. In the present study we were unable to determine the numerical density of SP producing cells in the alveolar epithelium of lung tissue due to lack of fixed tissue availability and we therefore used LAMP-3, which is expressed in lamellar bodies, as a marker of type II AEC maturation. In normal developing sheep lungs, an increase in LAMP-3 correlates with a decrease in glycogen storage, a substrate for surfactant lipid synthesis, in type II AEC cells, which indicates cellular maturation [65]. In the present study, there was an increase in mRNA expression of LAMP-3 (absolute fold change of ~1.73-fold) in the lungs of the LBW lambs, which may indicate either an increase in the number of type II AEC, or an increase in the number of lamellar bodies in the existing type II AEC, suggesting an increase in the maturity and synthetic capacity for surfactant production. Overall, the reasons for the lack of increase in the SP protein expression, despite an increase in SP mRNA expression, is unknown but may be due to either epigenetic changes in the lung or an increase in secretion of SP into the alveolar space. Moreover, to date neither we, nor to our knowledge, others have examined potential changes in the lipid component of surfactant in this lamb model of LBW following PR. A limitation of the current study is that we were unable to perform lung functional studies in these lambs or analyse surfactant composition in lung lavage. In 140d gestation fetuses, PR decreased total phosphatidylcholine content in lung lavage [41] while in a rat undernutrition model of IUGR, there was a decrease in lung tissue saturated phosphatidylcholine on postnatal day 1, which was normalised by 1 week of age [66]. Here we examined the mRNA expression of two key enzymes involved in the synthesis of pulmonary surfactant lipids, PCYT1A and FAS [67]. Interestingly, we found an increase in the mRNA expression of FAS, which may indicate that there is an increase in surfactant lipid synthesis in the lungs of the LBW lambs.
A further key regulator of surfactant development is the intracellular hypoxia signalling pathway, which is essential for normal lung development [68]. Although the fetus is hypoxemic during normal development relative to the newborn or adult [69], PR fetuses experience chronic hypoxemia compared with the normally grown fetus [16,70]. The hypoxia inducible factor (HIF)-α subunits mediate transcription of a range of genes involved in metabolism, angiogenesis, erythropoiesis, mitogenesis and apoptosis during periods of hypoxia [71]. Regulation of hypoxia signalling by the HIF-α subunit is negatively regulated by the prolyl hydroxylase domain (PHD) enzyme family during periods of normoxemia [71,72]. However, there is a change in this feedback mechanism during chronic hypoxemia resulting in increased PHD activity [73]. We have recently reported that PR increases both PHD-2 and PHD-3 in the lung of fetuses at 130 and 140d gestation [28] and increases in PHD-2 in the heart of 140d gestation fetuses [44]. These findings suggest that the exposure of the PR fetus to chronic hypoxemia in utero dynamically regulates hypoxia signalling in the lung in late gestation. In the present study, although there was no difference in the mRNA expression of HIF-1α, there an increase in absolute fold change of ~2-fold in HIF2-α.There was a small decreased in absolute fold change of ~0.75-fold in PHD-2 mRNA expression in the lungs of LBW lambs and this may be the reason why the decrease is not reflected in the protein expression of PHD-2. Loss of HIF-2α in mice leads to decreased SP-A, -B and -D mRNA expression at birth, indicating the importance of HIF-2α in lung development and the production of SP [12]. Interestingly, the increase in HIF-2α but not HIF-1α mRNA expression was observed in LBW lambs and is in direct contrast to the observation in lungs of the late gestation PR fetus [28], but may explain the increase in SP mRNA expression in the lungs of LBW lambs. Interestingly, the changes observed in this study provide evidence for reduced feedback by PHDs to downregulate HIF-α expression in the postnatal normoxemic environment. This suggests that there may be a persistence of the upregulation of the hypoxia signalling pathway in the lung of the LBW lamb.
Activation of PPARγ signalling increases SP-B and -C abundance in the lung [9]. In addition, PPARγ deficient mice have changes in lung structure, including enlarged air spaces, increased lung volume and decreased tissue resistance [74]. In this study, we evaluated the mRNA expression of PPARγ and two genes regulated by PPARγ, namely SPHK-1 [75] and PAI-1 [76]. Despite no change in PPARγ mRNA expression or its target PAI-1, we found an increase in SPHK-1 mRNA expression in LBW lambs, which is unlikely to be PPARγ-mediated. SPHK-1 expression can also be increased by HIF-2α [77], for which the mRNA was increased in the lungs of LBW lambs, providing further evidence for increased HIF-2α signalling.
In summary, although there is reduced surfactant maturation associated with altered regulation of factors important for lung maturation in the PR fetus in late gestation [27], surfactant maturation is normalised in the LBW lung by 21d after birth. It is unlikely that GC signalling is involved in regulating this postnatal surfactant maturation, as GC availability was not altered in the LBW lung. However, altered hypoxia signalling may be involved in the regulation of SP expression as we observed an increase in HIF-2α mRNA expression and decreased PHD-2 mRNA expression in addition to an increase in SPHK-1 mRNA expression, which may be driving this catch up in surfactant maturation observed in LBW lambs. SPHK-1 mRNA expression can be regulated by both PPARγ and HIF-2α, and in this study, we have found no changes in the mRNA expression of PPARγ and PAI-1, suggesting that the increase in SPHK-1 may be mediated by HIF-2α. Hence, our findings provide further evidence of altered molecular regulation, particularly associated with the hypoxia signalling pathway in postnatal life as a result of the compromised fetal development associated with IUGR.
Acknowledgments
We acknowledge the contribution of Jaime Duffield, Anne Jurisevic and Laura O’Carroll in carrying out the uterine carunclectomy surgery and in the characterisation of the LBW lambs used in this study. We thank Stacey Holman, Kimberley Wang, Darren Tosh and Robb Muirhead for assistance with Western blotting and qRT-PCR. We also acknowledge the assistance of Pamela Sim for performing the cortisol radioimmunoassay.
Abbreviations
- 11βHSD
11β hydroxysteroid dehydrogenase
- AEC
Alveolar epithelial cell
- ACE
Angiotensin converting enzyme
- ABCA3
ATP-binding cassette, sub-family A, member 3
- ABW
Average birth weight
- cDNA
Complementary deoxyribonucleic acid
- PCYT1A
phosphate cytidylyl transferase 1, choline, alpha
- CYCLO
Cyclophilin
- FAS
Fatty acid synthase
- FLT
Fms-related tyrosine kinase
- GATA
GATA binding protein
- GC
Glucocorticoid
- GR
Glucocorticoid receptor
- JMJD1A
Histone demethylase
- HIF
Hypoxia inducible factor
- IGF
Insulin-like growth factor
- IUGR
Intrauterine growth restriction
- LBW
Low birth weight
- LAMP
Lysosome-associated membrane glycoprotein
- MR
Mineralocorticoid receptor
- PAI
Plasminogen activator inhibitor
- PPARγA
Peroxisome proliferator-activated receptor gamma
- PR
Placental restriction
- PCNA
Proliferating cell nuclear antigen
- PHD
Prolyl hydroxylase domain
- RNA
Ribonucleic acid
- RpP0
Ribosomal protein P0
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEM
Standard error of the mean
- SPHK
sphingosine kinase
- SP
Surfactant protein
- TTF
Thyroid transcription factor
- UPE
umbilicoplacental embolisation
- VEGF
Vascular endothelial growth factor
- ACTB
β actin
Data Availability
All relevant data are within the paper.
Funding Statement
The animal component of the work was funded by a National Health and Medical Research Council Program Grant (https://www.nhmrc.gov.au/grants-funding/apply-funding/program-grants) (ICM). The molecular component of the work was funded by a South Australian Cardiovascular Research Network Fellowship (https://www.heartfoundation.org.au/research/research-networks/south-australian-cardiovascular-research-network) (CR10A4988; JLM) and a National Health and Medical Research Council Project Grant (https://www.nhmrc.gov.au/grants-funding/apply-funding/program-grants) (APP1030853; JLM and SO). JLM was funded by a South Australian Cardiovascular Research Network Fellowship (https://www.heartfoundation.org.au/research/research-networks/south-australian-cardiovascular-research-network) (CR10A4988) and a National Health and Medical Research Council Career Development Fellowship (https://www.nhmrc.gov.au/grants-funding/apply-funding/career-development-fellowships) (APP1066916). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
References
- 1.Orgeig S, Morrison JL, Sullivan LC, Daniels CB (2014) Chapter 9—The Development of the Pulmonary Surfactant System In: Pinkerton RHE, editor. The Lung (Second Edition). Boston: Academic Press; pp. 183–209. [Google Scholar]
- 2.Veldhuizen R, Nag K, Orgeig S, Possmayer F (1998) The role of lipids in pulmonary surfactant. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1408: 90–108. [DOI] [PubMed] [Google Scholar]
- 3.Possmayer F, Nag K, Rodriguez K, Qanbar R, Schurch S (2001) Surface activity in vitro: role of surfactant proteins. Comparative Biochemistry and Physiology-Part A: Molecular & Integrative Physiology 129: 209–220. [DOI] [PubMed] [Google Scholar]
- 4.Haagsman HP, Hogenkamp A, van Eijk M, Veldhuizen EJ (2008) Surfactant collectins and innate immunity. Neonatology 93: 288–294. doi: 10.1159/000121454 [DOI] [PubMed] [Google Scholar]
- 5.Kuroki Y, Takahashi M, Nishitani C (2007) Pulmonary collectins in innate immunity of the lung. Cellular microbiology 9: 1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x [DOI] [PubMed] [Google Scholar]
- 6.Cole TJ, Solomon NM, Van Driel R, Monk JA, Bird D, et al. (2004) Altered epithelial cell proportions in the fetal lung of glucocorticoid receptor null mice. American journal of respiratory cell and molecular biology 30: 613–619. doi: 10.1165/rcmb.2003-0236OC [DOI] [PubMed] [Google Scholar]
- 7.Garbrecht MR, Klein JM, Schmidt TJ, Snyder JM (2006) Glucocorticoid metabolism in the human fetal lung: implications for lung development and the pulmonary surfactant system. Neonatology 89: 109–119. [DOI] [PubMed] [Google Scholar]
- 8.Saini Y, Harkema JR, LaPres JJ (2008) HIF1α is essential for normal intrauterine differentiation of alveolar epithelium and surfactant production in the newborn lung of mice. Journal of Biological Chemistry 283: 33650–33657. doi: 10.1074/jbc.M805927200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Santos J, Sakurai R, Shin E, Cerny L, et al. (2009) Peroxisome Proliferator-Activated Receptor Gamma Agonists Enhance Lung Maturation in a Neonatal Rat Model. Pediatric research 65: 150–155. doi: 10.1203/PDR.0b013e3181938c40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison JL, Botting KJ, Soo PS, McGillick EV, Hiscock J, et al. (2012) Antenatal steroids and the IUGR fetus: are exposure and physiological effects on the lung and cardiovascular system the same as in normally grown fetuses? Journal of pregnancy 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendelson CR (2000) Role of transcription factors in fetal lung development and surfactant protein gene expression. Annual review of physiology 62: 875–915. doi: 10.1146/annurev.physiol.62.1.875 [DOI] [PubMed] [Google Scholar]
- 12.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, et al. (2002) Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nature medicine 8: 702–710. doi: 10.1038/nm721 [DOI] [PubMed] [Google Scholar]
- 13.McGillick EV, Orgeig S, Giussani DA, Morrison JL (2016) Chronic hypoxaemia as a molecular regulator of fetal lung development: implications for risk of respiratory complications at birth. Paediatric Respiratory Reviews. [DOI] [PubMed] [Google Scholar]
- 14.United Nations Children’s Fund, World Health Organization (2004) Low Birth Weight: Country, Regional and Global Estimates. New York: UNICEF.
- 15.Hilder L, Zhichao Z, Parker M, Jahan S, GM C (2014) Australia’s mothers and babies 2012. In: AIHW, editor. Perinatal statistics series no. 30. Cat. no. PER 69 ed. Canberra.
- 16.Morrison JL (2008) Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clinical and Experimental Pharmacology and Physiology 35: 730–743. doi: 10.1111/j.1440-1681.2008.04975.x [DOI] [PubMed] [Google Scholar]
- 17.Resnik R (2002) Intrauterine growth restriction. Obstetrics & Gynecology 99: 490–496. [DOI] [PubMed] [Google Scholar]
- 18.Harding R, Maritz G (2012) Maternal and fetal origins of lung disease in adulthood. Seminars in Fetal & Neonatal Medicine 17: 67–72. [DOI] [PubMed] [Google Scholar]
- 19.Kotecha SJ, Watkins WJ, Heron J, Henderson J, Dunstan FD, et al. (2010) Spirometric Lung Function in School-Age Children. American journal of respiratory and critical care medicine 181: 969–974. doi: 10.1164/rccm.200906-0897OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyce BJ, Louey S, Davey MG, Cock ML, Hooper SB, et al. (2001) Compromised respiratory function in postnatal lambs after placental insufficiency and intrauterine growth restriction. Pediatric research 50: 641–649. doi: 10.1203/00006450-200111000-00018 [DOI] [PubMed] [Google Scholar]
- 21.Alcazar MAA, Östreicher I, Appel S, Rother E, Vohlen C, et al. (2011) Developmental regulation of inflammatory cytokine-mediated Stat3 signaling: the missing link between intrauterine growth restriction and pulmonary dysfunction? Journal of Molecular Medicine: 1–13. [DOI] [PubMed] [Google Scholar]
- 22.Lipsett J, Tamblyn M, Madigan K, Roberts P, Cool JC, et al. (2006) Restricted fetal growth and lung development: a morphometric analysis of pulmonary structure. Pediatric pulmonology 41: 1138–1145. doi: 10.1002/ppul.20480 [DOI] [PubMed] [Google Scholar]
- 23.Curle D, Adamson I (1978) Retarded development of noenatal rat lung by maternal malnutrition. Journal of Histochemistry & Cytochemistry 26: 401. [DOI] [PubMed] [Google Scholar]
- 24.Gagnon R, Langridge J, Inchley K, Murotsuki J, Possmayer F (1999) Changes in surfactant-associated protein mRNA profile in growth-restricted fetal sheep. American Journal of Physiology-Lung Cellular and Molecular Physiology 276: L459–L465. [DOI] [PubMed] [Google Scholar]
- 25.Brain KL, Allison BJ, Niu Y, Cross CM, Itani N, et al. (2015) Induction of controlled hypoxic pregnancy in large mammalian species. Physiological reports 3: e12614 doi: 10.14814/phy2.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cock ML, Albuquerque CA, Joyce BJ, Hooper SB, Harding R (2001) Effects of intrauterine growth restriction on lung liquid dynamics and lung development in fetal sheep. American journal of obstetrics and gynecology 184: 209–216. doi: 10.1067/mob.2001.108858 [DOI] [PubMed] [Google Scholar]
- 27.Orgeig S, Crittenden TA, Marchant C, McMillen IC, Morrison JL (2010) Intrauterine growth restriction delays surfactant protein maturation in the sheep fetus. American Journal of Physiology-Lung Cellular and Molecular Physiology 298: L575–L583. doi: 10.1152/ajplung.00226.2009 [DOI] [PubMed] [Google Scholar]
- 28.Orgeig S, McGillick EV, Botting KJ, Zhang S, McMillen IC, et al. (2015) Increased lung prolyl hydroxylase and decreased glucocorticoid receptor are related to decreased surfactant protein in the growth restricted sheep fetus. American Journal of Physiology-Lung Cellular and Molecular Physiology: ajplung. 00275.02014. [DOI] [PubMed] [Google Scholar]
- 29.Alexander G (1964) Studies on the placenta of the sheep (Ovis aries L.). Journal of reproduction and fertility 7: 289–305. [DOI] [PubMed] [Google Scholar]
- 30.Duffield JA, Vuocolo T, Tellam R, McFarlane JR, Kauter KG, et al. (2009) Intrauterine Growth Restriction and the Sex Specific Programming of Leptin and Peroxisome Proliferator-Activated Receptor &ggr;(PPAR&ggr;) mRNA Expression in Visceral Fat in the Lamb. Pediatric research 66: 59–65. [DOI] [PubMed]
- 31.Danielson L, McMillen IC, Dyer JL, Morrison JL (2005) Restriction of placental growth results in greater hypotensive response to α‐adrenergic blockade in fetal sheep during late gestation. The Journal of physiology 563: 611–620. doi: 10.1113/jphysiol.2004.080523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, et al. (2007) Restriction of placental function alters heart development in the sheep fetus. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 293: R306–R313. doi: 10.1152/ajpregu.00798.2006 [DOI] [PubMed] [Google Scholar]
- 33.Edwards LJ, Simonetta G, Owens JA, Robinson JS, McMillen IC (1999) Restriction of placental and fetal growth in sheep alters fetal blood pressure responses to angiotensin II and captopril. The Journal of Physiology 515: 897–904. doi: 10.1111/j.1469-7793.1999.897ab.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang KC, Zhang L, McMillen IC, Botting KJ, Duffield JA, et al. (2011) Fetal growth restriction and the programming of heart growth and cardiac insulin‐like growth factor 2 expression in the lamb. The Journal of physiology 589: 4709–4722. doi: 10.1113/jphysiol.2011.211185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muhlhausler BS, Duffield JA, Ozanne SE, Pilgrim C, Turner N, et al. (2009) The transition from fetal growth restriction to accelerated postnatal growth: a potential role for insulin signalling in skeletal muscle. The Journal of physiology 587: 4199–4211. doi: 10.1113/jphysiol.2009.173161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang KC, Lim CH, McMillen IC, Duffield JA, Brooks DA, et al. (2013) Alteration of cardiac glucose metabolism in association to low birth weight: Experimental evidence in lambs with left ventricular hypertrophy. Metabolism 62: 1662–1672. doi: 10.1016/j.metabol.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 37.Wang KC, Brooks DA, Summers‐Pearce B, Bobrovskaya L, Tosh DN, et al. (2015) Low birth weight activates the renin–angiotensin system, but limits cardiac angiogenesis in early postnatal life. Physiological reports 3: e12270 doi: 10.14814/phy2.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang KC, Tosh DN, Zhang S, McMillen IC, Duffield JA, et al. (2015) IGF-2R-Gαq signaling and cardiac hypertrophy in the low-birth-weight lamb. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 308: R627–R635. doi: 10.1152/ajpregu.00346.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGillick EV, Orgeig S, McMillen IC, Morrison JL (2013) The fetal sheep lung does not respond to cortisol infusion during the late canalicular phase of development. Physiological reports 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soo PS, Hiscock J, Botting KJ, Roberts CT, Davey AK, et al. (2012) Maternal undernutrition reduces P-glycoprotein in guinea pig placenta and developing brain in late gestation. Reproductive Toxicology 33: 374–381. doi: 10.1016/j.reprotox.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 41.McGillick EV, Orgeig S, Morrison JL (2016) Regulation of lung maturation by prolyl hydroxylase domain inhibition in the lung of the normally grown and placentally restricted fetus in late gestation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 310: R1226–R1243. doi: 10.1152/ajpregu.00469.2015 [DOI] [PubMed] [Google Scholar]
- 42.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome biology 8: R19 doi: 10.1186/gb-2007-8-2-r19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology 3: research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Botting KJ, McMillen IC, Forbes H, Nyengaard JR, Morrison JL (2014) Chronic Hypoxemia in Late Gestation Decreases Cardiomyocyte Number but Does Not Change Expression of Hypoxia‐Responsive Genes. Journal of the American Heart Association 3: e000531 doi: 10.1161/JAHA.113.000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Rattanatray L, MacLaughlin SM, Cropley JE, Suter CM, et al. (2010) Periconceptional undernutrition in normal and overweight ewes leads to increased adrenal growth and epigenetic changes in adrenal IGF2/H19 gene in offspring. The FASEB Journal 24: 2772–2782. doi: 10.1096/fj.09-154294 [DOI] [PubMed] [Google Scholar]
- 46.Muhlhausler BS, Duffield JA, McMillen IC (2007) Increased maternal nutrition stimulates peroxisome proliferator activated receptor-γ, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology 148: 878–885. doi: 10.1210/en.2006-1115 [DOI] [PubMed] [Google Scholar]
- 47.Lie S, Sim S, McMillen I, Williams-Wyss O, MacLaughlin S, et al. (2013) Maternal undernutrition around the time of conception and embryo number each impact on the abundance of key regulators of cardiac growth and metabolism in the fetal sheep heart. Journal of developmental origins of health and disease 4: 377–390. doi: 10.1017/S2040174413000354 [DOI] [PubMed] [Google Scholar]
- 48.McGillick EV, Orgeig S, Morrison JL (2015) Structural and molecular regulation of lung maturation by intratracheal VEGF administration in the normally grown and placentally restricted fetus. The Journal of physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang KC, Brooks DA, Thornburg KL, Morrison JL (2012) Activation of IGF-2R stimulates cardiomyocyte hypertrophy in the late gestation sheep fetus. The Journal of physiology 590: 5425–5437. doi: 10.1113/jphysiol.2012.238410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lie S, Duffield J, McMillen I, Morrison J, Ozanne S, et al. (2013) The effect of placental restriction on insulin signaling and lipogenic pathways in omental adipose tissue in the postnatal lamb. Journal of Developmental Origins of Health and Disease 4: 421–429. doi: 10.1017/S2040174413000202 [DOI] [PubMed] [Google Scholar]
- 51.Nicholas LM, Rattanatray L, MacLaughlin SM, Ozanne SE, Kleemann DO, et al. (2013) Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. The FASEB Journal 27: 3786–3796. doi: 10.1096/fj.13-227918 [DOI] [PubMed] [Google Scholar]
- 52.Warnes K, Coulter CL, Robinson JS, McMillen IC (2003) The effect of intrafetal infusion of metyrapone on arterial blood pressure and on the arterial blood pressure response to angiotensin II in the sheep fetus during late gestation. The Journal of physiology 552: 621–633. doi: 10.1113/jphysiol.2003.049437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGillick EV, Orgeig S, Allison BJ, Brain KL, Niu Y, et al. (2017) Maternal chronic hypoxia increases expression of genes regulating lung liquid movement and surfactant maturation in male fetuses in late gestation. The Journal of Physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monau TR, Vargas VE, Zhang L, Myers DA, Ducsay CA (2010) Nitric oxide inhibits ACTH-induced cortisol production in near-term, long-term hypoxic ovine fetal adrenocortical cells. Reproductive Sciences 17: 955–962. doi: 10.1177/1933719110376092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz J, Rose JC (1998) Development of the pituitary adrenal axis in fetal sheep twins. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 274: R1–R8. [DOI] [PubMed] [Google Scholar]
- 56.Fowden A, Silver M (1988) Induction of labour in domestic animals: endocrine changes and neonatal viability In: Jensen A, Künzel W, editors. The endocrine control of the fetus physiologic and pathophysiologic aspects. Berlin, Heidelberg: Springer; pp. 401–411 [Google Scholar]
- 57.Kitterman J, Liggins G, Campos G, Clements J, Forster C, et al. (1981) Prepartum maturation of the lung in fetal sheep: relation to cortisol. Journal of Applied Physiology 51: 384–390. [DOI] [PubMed] [Google Scholar]
- 58.Bolt RJ, Van Weissenbruch M, Lafeber H, Delemarre‐van de Waal H (2001) Glucocorticoids and lung development in the fetus and preterm infant. Pediatric pulmonology 32: 76–91. [DOI] [PubMed] [Google Scholar]
- 59.Floros J, Gross I, Nichols KV, Veletza SV, Dynia D, et al. (1991) Hormonal effects on the surfactant protein B (SP-B) mRNA in cultured fetal rat lung. Am J Respir Cell Mol Biol 4: 449–454. doi: 10.1165/ajrcmb/4.5.449 [DOI] [PubMed] [Google Scholar]
- 60.Liley HG, White RT, Benson BJ, Ballard PL (1988) Glucocorticoids both stimulate and inhibit production of pulmonary surfactant protein A in fetal human lung. Proceedings of the National Academy of Sciences 85: 9096–9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phillips ID, Simonetta G, Owens JA, Robinson JS, Clarke IJ, et al. (1996) Placental restriction alters the functional development of the pituitary-adrenal axis in the sheep fetus during late gestation. Pediatric research 40: 861–866. doi: 10.1203/00006450-199612000-00014 [DOI] [PubMed] [Google Scholar]
- 62.Gerbal-Chaloin S, Daujat M, Pascussi J-M, Pichard-Garcia L, Vilarem M- J, et al. (2002) Transcriptional Regulation of CYP2C9 Gene Role of Glucocorticoid Receptor and Constitutive Androstane Receptor. Journal of Biological Chemistry 277: 209–217. doi: 10.1074/jbc.M107228200 [DOI] [PubMed] [Google Scholar]
- 63.Phillips DI, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, et al. (2000) Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension 35: 1301–1306. [DOI] [PubMed] [Google Scholar]
- 64.Tomlinson JW, Stewart PM (2001) Cortisol metabolism and the role of 11β-hydroxysteroid dehydrogenase. Best Practice & Research Clinical Endocrinology & Metabolism 15: 61–78. [DOI] [PubMed] [Google Scholar]
- 65.Meyerholz DK, DeGraaf JA, Gallup JM, Olivier AK, Ackermann MR (2006) Depletion of alveolar glycogen corresponds with immunohistochemical development of CD208 antigen expression in perinatal lamb lung. Journal of Histochemistry & Cytochemistry 54: 1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen CM, Wang LF, Su B (2004) Effects of maternal undernutrition during late gestation on the lung surfactant system and morphometry in rats. Pediatric research 56: 329–335. doi: 10.1203/01.PDR.0000134254.83113.8E [DOI] [PubMed] [Google Scholar]
- 67.Rooney SA, Young SL, Mendelson CR (1994) Molecular and cellular processing of lung surfactant. The FASEB Journal 8: 957–967. [DOI] [PubMed] [Google Scholar]
- 68.Shimoda LA, Semenza GL (2011) HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. American journal of respiratory and critical care medicine 183: 152–156. doi: 10.1164/rccm.201009-1393PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, et al. (2001) Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Developmental Dynamics 220: 175–186. doi: 10.1002/1097-0177(20010201)220:2<175::AID-DVDY1101>3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 70.McMillen IC, Adams MB, Ross JT, Coulter CL, Simonetta G, et al. (2001) Fetal growth restriction: adaptations and consequences. Reproduction 122: 195–204. [DOI] [PubMed] [Google Scholar]
- 71.Appelhoff RJ, Tian Y-M, Raval RR, Turley H, Harris AL, et al. (2004) Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. Journal of Biological Chemistry 279: 38458–38465. doi: 10.1074/jbc.M406026200 [DOI] [PubMed] [Google Scholar]
- 72.Huang LE, Arany Z, Livingston DM, Bunn HF (1996) Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its α subunit. Journal of Biological Chemistry 271: 32253–32259. [DOI] [PubMed] [Google Scholar]
- 73.Ginouves A, Ilc K, Macías N, Pouysségur J, Berra E (2008) PHDs overactivation during chronic hypoxia “desensitizes” HIFα and protects cells from necrosis. Proceedings of the National Academy of Sciences 105: 4745–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Tsai LW, et al. (2006) Epithelial cell PPARγ contributes to normal lung maturation. The FASEB Journal 20: 1507–1509. doi: 10.1096/fj.05-5410fje [DOI] [PubMed] [Google Scholar]
- 75.Koch A, Völzke A, Wünsche C, Meyer zu Heringdorf D, Huwiler A, et al. (2012) Thiazolidinedione‐dependent activation of sphingosine kinase 1 causes an anti‐fibrotic effect in renal mesangial cells. British journal of pharmacology 166: 1018–1032. doi: 10.1111/j.1476-5381.2012.01824.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marx N, Bourcier T, Sukhova GK, Libby P, Plutzky J (1999) PPARγ activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression PPARγ as a potential mediator in vascular disease. Arteriosclerosis, thrombosis, and vascular biology 19: 546–551. [DOI] [PubMed] [Google Scholar]
- 77.Anelli V, Gault CR, Cheng AB, Obeid LM (2008) Sphingosine Kinase 1 Is Up-regulated during Hypoxia in U87MG Glioma Cells ROLE OF HYPOXIA-INDUCIBLE FACTORS 1 AND 2. Journal of Biological Chemistry 283: 3365–3375. doi: 10.1074/jbc.M708241200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.