Abstract
Inefficient delivery of drugs is a main cause of chemotherapy failure in hypo-perfused tumors. To enhance perfusion and drug delivery in these tumors, two strategies have been developed: vascular normalization, aiming at normalizing tumor vasculature and blood vessel leakiness; and stress alleviation, aiming at decompressing tumor vessels. Vascular normalization is based on anti-angiogenic drugs, whereas stress alleviation on stroma-depleting agents. Here, an alternatively approach to normalize vessels is presented, considering that malignant tumors tend to develop at chronic inflammation sites. Similarly to tumor vessel leakiness, inflammation is also characterized by vascular hyper-permeability. Therefore, testing the ability of anti-inflammatory agents, such as non-steroidal anti-inflammatory drugs or inflammation resolution mediators, as an alternative way to increase tumor drug delivery, might prove promising.
Keywords: stress alleviation, vascular normalization, tumor perfusion, anti-inflammatory agents, inflammation resolution
The Tumor microenvironment and drug delivery
Tumors are complex tissues consisting of cancer cells and their microenvironment [1, 2], which includes structural and cellular components. Structural components of the tumor microenvironment comprise tumor blood and lymphatic vessels, and the extracellular matrix (ECM) (see Glossary); while the stromal cell constituents [3] include angiogenic vascular cells (endothelial cells and pericytes), infiltrating immune cells, and cancer-associated fibroblasts (CAFs). Naturally, interactions between cancer cells and the various components of tumor microenvironment affect fundamental cancer cell properties such as proliferation, apoptosis, migration and invasion.
Glossary.
CAF: cancer-associated fibroblast, a highly enriched stromal cell population in the tumor microenvironment implicated in cancer cell invasion and fibrosis.
COX: cyclooxygenase, an enzyme responsible for the formation of prostanoids such as prostanglandin, inhibition of which can provide relief from pain and inflammation symptoms. There are two types of COX enzymes, COX-1 and COX-2, both of which produce prostaglandins. However, COX-1 enzymes produce baseline levels of prostaglandins that activate platelets and protect the lining of the gastrointestinal tract, while COX-2 enzymes produce prostaglandins in response to infection or injury.
Desmoplasia: also known as desmoplastic reaction, is the abnormal growth of fibrous tissue. It is often present around tumors and it is characterized by the accumulation of extracellular matrix.
ECM: extracellular matrix, is the non-cellular solid component that surrounds cells within tissues and organs providing support as well as basic biochemical and molecular signals needed for fundamental cellular processes such as development, differentiation, growth, homeostasis, and survival.
Edema: medical term for swelling caused by excess fluid within the tissues.
IFP: Interstitial Fluid Pressure, is the hydrostatic pressure of the fluid phase of a tissue.
Metronomic chemotherapy: More frequent and at lower doses administration of chemotherapy.
NSAIDs: Non-Steroidal Anti-Inflammatory Drugs, a class of drugs that includes drugs with analgesic and anti-pyretic effects. Although different NSAIDs have different structures, they all work by inhibiting COX enzymes.
PUFAs: polyunsaturated fatty acids, are fatty acids that contain more than one double bond in their backbone.
ROS: reactive oxygen species, are reactive molecules and free radicals derived from molecular oxygen, such as hydrogen peroxide, superoxide, hydroxyl ion and nitric oxide. They are mainly produced as byproducts during aerobic respiration and mitochondrial electron transport, in particular. They are implicating in cell signaling related to apoptosis and are associated with a number of deleterious events while elevated rates of ROS have been detected in almost all cancers.
TGF-β: transforming growth factor-β, a multifunctional secreted cytokine involved in processes such as cell proliferation, differentiation and apoptosis.
Vascular permeability: generally refers to the capacity of the blood vessel wall to allow the flow of molecules such as drugs or nutrients in and out of the vessel.
Vascular hyper-permeability: dramatic increase in vascular permeability observed in acute and chronic inflammation, wound healing and cancer.
VEGF: Vascular Endothelial Growth Factor, a growth factor known to promote angiogenesis.
It has been postulated that components and features of the tumor microenvironment may also set certain barriers with regard to the effective delivery of therapeutic agents, resulting in compromised therapeutic outcomes and decreased survival [4].
Chemotherapeutic drugs are, without a doubt, potent cytotoxic agents. However, they often fail to cure cancer due to the fact that they are unable to reach cancer cells within the tumor in sufficient amounts [5, 6]. Hence, the discovery of more efficient approaches to enhance tumor drug delivery is imperative. Here, the main obstacles in drug delivery to the tumor are presented along with the approaches currently used to overcome them. Furthermore, the use of anti-inflammatory agents is proposed as a promising alternative approach to normalize vessels and improve therapy.
Causes of insufficient drug delivery to tumors
Inefficient drug delivery may arise due to barriers posed by abnormal structure and function of tumor stroma, known as desmoplasia. Desmoplastic tumors are characterized by a dense ECM, containing increased levels of total fibrillar collagen, hyaluronan, fibronectin, proteoglycans and tenascin C [4], which also provides the tumor with a reservoir of growth factors eventually promoting its growth. The dense fiber composition of these tumors along with the abundance of stromal cells generate forces that compress intratumoral blood vessels, reducing tumor blood vessel functionality and thus, the systemic administration of the drug to the tumor. Additionally, desmoplasia impedes the homogeneous penetration of therapeutic agents into the tumor due to the excessive accumulation of ECM fibers [6]. High collagen and cellular densities reduce the size of the pores of the tumor interstitial space that are available for drug penetration and result in increased resistance to interstitial fluid flow. This, in turn, further enhances the uniform elevation of the interstitial fluid pressure (IFP) and renders diffusion the dominant transport mechanism in the tumor interior. Therefore, when therapeutics extravasate from the hyper-permeable tumor vessels, they are not able to effectively penetrate deep into the tumor [5].
A second cause of inefficient drug delivery is the presence of hyper-permeable (leaky) blood vessels occurring due to upregulation of pro-angiogenic factors that subsequently leads to formation of immature vessels with structural abnormalities such as wide junctions between the endothelial cells, and large numbers of fenestrae and intercellular openings, resulting in the formation of tumor vessels whose pores can be up to two orders of magnitude larger than normal [7, 8]. Consequently, the blood supply reaching the tumor is decreased due to excessive fluid loss from the vascular to the extravascular space of the tumor, as well as due to the state of dysfunction in which tumor blood vessels are in, being characterized by lack of hierarchy, formation of vascular shunts and partially collapsed lumen [9, 10].
This in turn leads to hypoxia and lowering of the microenvironment pH, which further promotes tumor progression. At the same time hypo-perfusion and hypoxia compromise normal immune response, thus rendering the tumor impervious to the immune system [11, 12] with an invasive and metastatic phenotype [13]. As expected, reduced blood supply also interferes with effective drug delivery to the tumor [9].
Additionally, tumor vessel hyper-permeability drives IFP elevation to values comparable to microvascular pressure, thus, diminishing pressure gradients across the tumor vessel wall and hindering convective transport of drugs [4–6].
Strategies to improve drug delivery
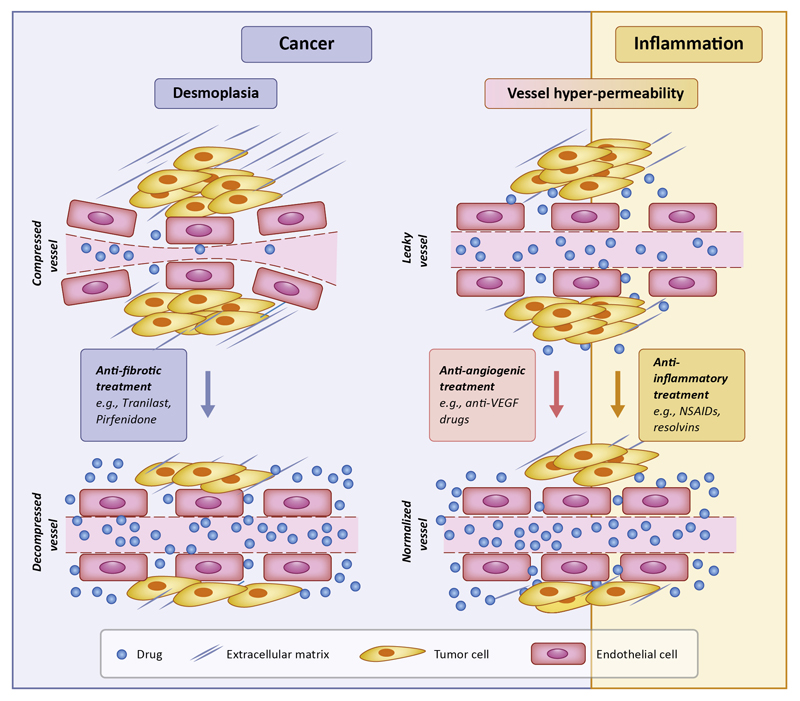
Two approaches that have been proposed to date to bypass the causes of inefficient drug delivery are: i) Stress-alleviation strategies targeting the ECM or CAFs, and ii) Vascular normalization strategies targeting the tumor vasculature (Figure 1, Key Figure). Stress alleviation strategies are based on the concept that desmoplasia hinders proper drug delivery by compressing intratumoral blood vessels, and thus agents that relief the stress accumulated by ECM components will facilitate vessel decompression, improve perfusion, and enhance delivery of chemotherapy [2, 14, 15]. In fact, repurposing of common anti-fibrotic drugs to reduce collagen and/or hyaluronan levels when combined with cytotoxic drugs has shown to cause stress alleviation and improve overall survival of mice-bearing tumors [16–18]. Similarly, pharmacologic depletion of CAFs has shown to reduce intratumoral stresses, improving perfusion, drug delivery and overall survival in pancreatic and breast tumor models [19, 20]. However, genetic deletion of CAFs might enhance tumor progression [21, 22].
Figure 1. Key Figure: Main strategies to improve drug delivery to tumors.
Stress alleviation decompresses tumor blood vessels by targeting tumor ECM (desmoplasia); whereas vascular normalization reduces tumor vessel wall permeability. Both strategies can improve perfusion and drug delivery. Anti-inflammatory treatment is proposed as an alternative to anti-angiogenic treatment to induce vascular normalization.
The vascular normalization strategy on the other hand, which has been used in the clinic during the last 10 years [9, 23], is based on the notion that the tumor vasculature needs to be brought closer to a more “normal” state to be functional. Vascular normalization is achieved with judicious doses of anti-angiogenic drugs, targeting mainly Vascular Endothelial Growth Factor (VEGF) or its receptors. Thus, vascular normalization restores tumor perfusion by fortifying the vessel wall and the vascular network structure, which also decreases interstitial fluid pressure due to the reduced amount of fluid leaking from the vessels. Anti-angiogenic treatments worked well in preclinical models but failed in clinical trials [24], and combinatorial approaches have been employed extensively both in preclinical and clinical studies with varying degrees of success [9, 25] whereby the anti-angiogenic drug aims to normalize and not destroy the vessels and it is combined with chemotherapy to eradicate the tumor.
Apart from anti-angiogenic treatment, tumor perfusion and tumor vessel function can be significantly improved using metronomic chemotherapy [26], in which lower doses of the chemotherapeutic agent are administered more frequently. In fact, metronomic administration is currently being tested in clinical trials, as it was shown to be more efficacious than maximum tolerated dose treatment in preclinical studies [27].
Strikingly, evidence from mathematical modeling shows that the combined use of vascular normalization and stress-alleviation strategies may be more beneficial for certain tumor types [28]. For instance, while vascular normalization is considered to be more effective in tumors with highly permeable and uncompressed vessels, such as a subset of glioblastomas; stress-alleviation should be more effective in tumors with less permeable and highly compressed vessels such as pancreatic cancers. Thus, it is only plausible to suggest that a combined strategy will be ideal for tumors with highly permeable but compressed vessels, such as certain types of breast cancer.
Cancer development at inflammation sites
It has been noted that malignant tumors often develop at sites of chronic injury, infection, or inflammation [29]. In fact, although the first connection between inflammation and cancer was made as early as 1983, the concept is still (but progressively) gaining ground [30]. It has become evident that many malignancies originate in areas of infection and inflammation, as a result of normal host response [31], which induces chronic inflammation. Immune cells generate reactive oxygen species (ROS) and nitrogen species to fight infection leading to DNA damage in proliferating cells, which, when occurring repeatedly, induces permanent genomic alterations that promote cancer. Indeed, mutations in the tumor suppressor p53 gene are equally frequent in tumors and in chronic inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease [29]. Similarly, patients with inflammatory bowel disease or chronic ulcerative colitis are prone to developing colon cancer [32–34]; hepatitis C patients are predisposed to developing liver carcinoma [35]; chronic Helicobacter pylori infection is the world’s leading cause of stomach cancer [36]; and pancreatitis is associated with development of pancreatic cancer [32]. Lastly, obesity, which is considered as a condition of low level chronic inflammation, has been associated with accelerated tumor progression, decreased response to treatment, and poor prognosis. This observation demonstrates that dysfunctional angiogenesis and inflammation in the adipose tissue of obese patients promotes tumor progression and chemotherapy resistance [37].
Can targeting inflammation improve tumor drug delivery?
It has been long known that a hallmark of inflammation is increased vascular permeability, which leads not only to extravasation of inflammatory and immune cells by a yet unknown mechanism [38], but also to the escape of a protein-rich fluid into the extravascular tissue. This, in turn, results in increased pressure of the interstitial fluid which ultimately causes fluid accumulation in the tumor interstitial space. This increased extravascular fluid is known as edema and is one of the main characteristics of inflammation [39]. Thus, the leaky nature of blood vessels in inflammation bears similarities to the leaky vessels seen in tumors, providing another link between inflammation and cancer.
The key differential concept between leaky vessels in inflammation and cancer is that vessel leakiness in inflammation is self-limiting being resolved by itself via the action of certain inflammation resolution mediators, whereas cancer is associated with further deregulation that is not easily resolved. Hence, targeting inflammation may be an alternative approach to increase tumor drug delivery (Figure 1, Key Figure) either by targeting the molecular mediators of inflammation resolution, or via the use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs).
Inflammation resolution mediators
Inflammation resolution mechanisms are in place to ensure subsiding of inflammation-related symptoms, including reversal of vessel leakiness. In fact, for successful resolution of inflammation, normal vascular permeability has to be restored first [40]. However, although the mediators and signaling mechanisms that lead to inflammation are well-characterized, little is known about the factors that mediate resolution of inflammation.
During inflammation, a number of pro-inflammatory lipid mediators, primarily derived from arachidonic acid (AA), are produced. AA is converted to various potent lipid mediators by cyclooxygenases (COX) and lipoxygenases to produce prostaglandins and leukotrienes. Indeed, COX enzymes convert AA into 10 subclasses of prostaglandins that are important in inflammation, while excessive production of prostaglandins and leukotrienes is correlated with progression from acute to chronic inflammation [41].
Several studies indicated that inflammation resolution depends greatly on the expression of specific cytokines [42–44] such as interleukin (IL)-10 and transforming growth factor-β (TGF-β), and lipid mediators such as lipoxins [43], protectins, and resolvins [45–47]. Moreover, self-limited inflammation resolution following acute inflammation is a co-ordinated event initiated by an active “class switch” from prostaglandins and leukotrienes inflammatory mediators to resolvins, lipoxins, protectins, and maresins through complex processes involving multiple enzymes and cell types [48]. Lipoxin A4 (LXA4) is formed from AA, D resolvins, maresins and protectins are formed from Docosahexaenoic acid (DHA), and E resolvins from eicosapentaenoic acid. Both, DHA and eicosapentanoic acid are Ω-3 polyunsaturated fatty acids (PUFAs). Interestingly, despite the fact that inflammation resolution (and hence the production of inflammation resolution mediators) is a physiological process, aspirin-triggered inflammation resolution mediators have been reported (LXA4) [41]. Therefore, resolution mediators are not only produced during the physiological independent co-ordinated inflammation resolution process, but can also be triggered by NSAIDs such as aspirin.
NSAIDs may be beneficial not only by inhibiting COX, but also by triggering inflammation resolution mediators. However, evidence on the use of resolution mediators in cancer animal models is sparse, and its use in cancer patients has not been investigated yet [47]. A summary of the main characteristics of the most important inflammation resolution mediators is shown in Table 1.
Table 1. Characteristics of inflammation resolution mediators.
| Name of inflammation resolution mediator [41,77] | Other pertinent information |
|---|---|
| Lipoxin LXA4 | ↓Angiogenesis and cell proliferation, ROS, adhesion, and pro-inflammatory cytokines |
| Resolvin RvD1 | ↓ adhesion receptors, neovascularization, ROS and pro-inflammatory cytokines |
| DHA | Precursor of D resolvins with antixodant, anti-proliferative, and anti-metastatic effects |
| Resolvin RvE1 | ↓ DC IL-12 production and migration, inflammatory actions of COX-2, ↑ LXA4 production and polymorphonuclear detachment, blocks polymorphonuclear chemotaxis |
| Resolvin RvD2 | ↓ polymorphonuclear adhesion to endothelial cells |
| Maresin-1 | ↓ Neutrophil number in exudates, transendothelial polymorphonuclear cell migration |
NSAIDs
Several studies showed that long-term users of aspirin and NSAIDs have lower risk of developing cancers of the colon, lung, oesophagus, and stomach [49, 50]. Thus, it is not surprising that NSAIDs, and especially COX-2 inhibitors [51], have been proposed as potential anti-cancer agents [49, 51, 52]. However, the exact mechanism of NSAIDs’ action on tumors is not known [53].
In a rat glioma model, NSAID's were found to diminish tumor-induced protein extravasation caused by intracranial gliomas [54], suggesting that NSAIDs inhibit tumor-related vascular hyper-permeability. In another study, vascular permeability assays and mouse corneal models of angiogenesis were used to examine the efficacy of systemic treatment with different NSAIDs on vascular hyper-permeability [55]. This study showed that systemic application of most NSAIDs, except for acetaminophen (a non NSAID with antipyretic, analgesic but no anti-inflammatory properties), blocked VEGF-induced permeability in mice, suggesting, although not proving, that NSAIDs are effective at suppressing vascular leakiness [55]. A summary of the main characteristics of the most widely-used NSAIDs is shown in Table 2. In brief, NSAIDs are divided into selective and non-selective COX inhibitors. Selective are those targeting mostly COX-2 that is inducible and activated in inflammatory settings, and non-selective are those targeting both COX-1 (constitutively expressed/housekeeping) and COX-2. All NSAIDs (Table 2), have analgesic and anti-pyretic as well as anti-inflammatory properties), and constitute different chemical classes namely, acetic acid derivatives, propionic acid derivatives, para-aminophenol derivatives, oxicams, fenamates, and salicylates, which comprise the non-selective NSAIDs. Selectivity of these NSAIDS to COX-1/COX-2 is variable, and COX-1 inhibition is considered a “side effect” bringing gastrointestinal mucosa irritation and anti-platelet activity. Some of them inhibit COX-1 and COX-2 with comparable potency (e.g. ibuprofen and ketoprofen-propionic acid derivatives), some have intermediate to higher potency for COX-2 selectivity than COX-1 (meloxicam/oxicam, diclofenac/acetic acid), and others are highly selective for COX-2 (rofecoxib). Finally, acetyl-salicylic acid is more selective for COX-1 than COX-2 [56].
Table 2. Characteristics of representative NSAIDs drugs.
| Name of NSAID [78] | Target COX | Chemical Class |
|---|---|---|
| Indomethacin | COX1 & COX2 | Acidic acid derivative |
| Naproxen | COX1 & COX2 | Propionic acid derivative |
| Celecoxib | COX2 | Diaryl heterocyclic compound |
| Ibuprofen | COX1 & COX2 | Propionic acid derivative |
| Aspirin | COX1 & COX2 | Salicylates |
| Ketoprofen | COX1 & COX2 | Propionic acid derivative |
| Pyrox | COX1 & COX2 | Enolic acids (Oxicams) |
| Mephenamic acid | COX1 & COX2 | Fenamates |
It should also be noted that macrophages constitute a major component of tumor stroma and, in fact, tumor-associated macrophages are known to show anti- (M1) or pro- (M2) tumor functions depending on the cytokine milieu of the tumor microenvironment. Indeed, this macrophage shift from pro-tumor M2 phenotype to anti-tumor M1 phenotype has been proposed as a beneficial anti-cancer therapeutic target [57, 58]. Importantly, there is evidence indicating that COX-2 is an important participant in macrophage polarization [59]. In that regard, inhibition of COX-2 activity inhibits hypoxic cancer cell-induced M2-polarization of macrophages [59], and use of the selective COX-2 inhibitor celecoxib (Table 2) changes macrophage phenotype from M2 to M1 in a mouse model of colon cancer [60]. Moreover, similar effect was recently seen using the lipid mediator lipoxin LXA4, (Table 1) [61]. Thus, all of the above suggest that COX-2 inhibition or inflammation resolution mediators have a great potential as possible anti-cancer treatment modality.
In conclusion, NSAIDs may have the potential to inhibit vascular hyper-permeability by inhibiting COX enzymes thus reducing prostaglandin synthesis, while inflammation resolution mediators may also play a role in restoring vascular permeability by their actions.
Additionally, NSAIDs have also been shown to be potential chemopreventive agents. However, it has not been shown whether co-administration of NSAIDs with chemotherapy improves drug delivery, survival or reduces metastasis.
We reviewed two highly desmoplastic cancer types, breast and pancreatic cancer, to demonstrate what is already known with regard to NSAID use in these cancers.
NSAIDs in breast and pancreatic cancer
The idea of using NSAIDs in breast cancer is not exactly new, as many studies use aspirin and other NSAIDs or even precursors of resolution inflammation mediators, such as PUFA including DHA as chemopreventive agents against breast cancer [62, 63]. In fact, regular use of aspirin and possibly other NSAIDs seem to decrease breast cancer risk [64], while ibuprofen exerts a protective but marginal effect regarding recurrence [65]. The fact that increased expression of COX-2 has been found in breast cancer [66] also indicates that NSAIDs could be beneficial for breast cancer treatment. However, evidence of the clinical benefits of NSAIDs use in breast cancer are currently too limited [66–68], while epidemiological studies on use of NSAIDs in breast cancer are conflicting, rendering further experimental investigation imperative [65].
Regarding pancreatic cancer, little is known about the effect of NSAIDs on the progression of the disease. A recent study in pancreatic cancer patients indicated that high-dose aspirin, rather than low-dose aspirin, might be associated with decreased risk for pancreatic cancer [69] while another meta-analysis study did not show any association between NSAIDs and pancreatic cancer [70]. Other studies suggest though that NSAIDs in pancreatic cancer need further investigation as the COX-2 gene is expressed in 67-90% of pancreatic cancer tumors [71, 72]. Furthermore, COX-2 inhibitors have been shown to inhibit the growth and metastasis of established tumors [73], while the mechanisms involved have not been fully elucidated.
As mentioned above, NSAIDs are known to inhibit angiogenesis [74] and vascular permeability, but little is known regarding their effect on ECM. A study showed that NSAIDs also inhibit production of fibrillar collagen as well as cell migration [75] but evidence is limited. Accordingly, use of NSAIDs and possibly other anti-inflammatory agents could target both the vascular and the desmoplastic component of tumors.
Concluding remarks
In this article, we discuss a different approach to improve drug delivery to solid tumors, taking into account the fact that malignant tumors tend to develop at sites of chronic injury, infection or inflammation [29, 32]. In fact, similarly to vessel leakiness observed in tumors, inflammation is also characterized by vascular hyper-permeability which leads to edema. However, the key difference between the two conditions is that acute inflammation (and hence vessel leakiness) is self-limiting, being easily resolved through mechanisms involving lipoxins, protectins and resolvins [48], which is not the case for cancer. Therefore, as both cancer and inflammation lead to leaky blood vessels, which compromises delivery of chemotherapy in cancer, an alternative approach proposed in this opinion article would be to test the ability of anti-inflammatory agents as an alternative way to increase drug delivery to the tumor. Of course, several questions remain to be addressed (see “Outstanding Questions”), opening the way to repurposing NSAIDs and inflammation resolution mediators for optimizing drug delivery to the tumor. First, it would be important to investigate whether administration of known NSAIDs or inflammation resolution mediators along with chemotherapy improves vessel leakiness and subsequently, chemotherapy delivery. Such an effect would mean that we can achieve more effective treatment with lower doses of the cytotoxic drug. Moreover, it should be further investigated whether anti-inflammatory drugs can enhance the effectiveness of anti-angiogenic agents to normalize the tumor vasculature or whether they act by a different mechanism. Furthermore, the exact molecular mechanism of NSAID action needs to be elucidated to determine whether COX inhibition is fundamental or other mechanisms are involved. Revelation of this implicated mechanism will shed more light into relevant research and also provide the basis for other therapeutic agents that need to be investigated in the same context. For instance, it would be quite significant to test if PUFAs could also act as chemopreventive agents by producing inflammation resolution mediators and normalizing tumor vasculature, or if other anti-inflammatory agents, including anti-diabetic drugs (e.g., metformin and rosiglitazone) can be used alone or in combination with common vascular normalization agents to improve drug delivery. Along the same line, the study of the role of corticosteroids needs not to be neglected, as they comprise an important group of potent anti-inflammatory drugs. In addition, corticosteroids have immunosuppressive and other properties of palliative nature, which are useful in cancer. All these properties, though, are mediated via the nuclear glucocorticoid receptors, making the study of these drugs more difficult and complex than other anti-inflammatory agents. However, they may be promising for facilitating cancer treatment as they inhibit tumor growth via downregulation of tumor associated inflammation [76].
Outstanding Questions.
Can inflammation resolution mediators and NSAIDs reverse vascular leakiness and improve tumor drug delivery?
Can anti-inflammatory drugs enhance the effectiveness of anti-angiogenic agents to normalize the tumor vasculature?
Is the effect of selected NSAIDs due to COX inhibition or other novel mechanisms?
Can PUFA act as chemopreventive agents by resolving inflammation and normalizing tumor vasculature?
How aspirin is different from other NSAIDs in cancer?
Can other anti-inflammatory agents, such as corticosteroids, or anti-diabetic drugs (e.g., metformin and rosiglitazone) be used alone or in combination with already used agents for vascular normalization?
Thus, the avenues that are being opened are immense with many exciting questions waiting to be addressed potentially leading to improved intratumoral drug delivery and subsequently, a better therapeutic outcome.
Trends.
Drug delivery to the tumor is often compromised due to vascular hyper-permeability (leakiness) of tumor blood vessels or vessel compression, both of which can lead to inefficient delivery of the cytotoxic drug and therapeutic failure.
Methods of vascular normalization aim to fortify the tumor vessel wall reducing vessel leakiness and thus, they may improve drug delivery to the tumor enhancing the efficacy of therapeutic agents.
Vascular normalization is usually achieved with the use of anti-angiogenic agents.
Vessel leakiness can be potentially reversed by NSAIDs and/or inflammation resolution mediators, inducing vessel normalization and improving tumor drug delivery.
Acknowledgements
We thank Prof. Rakesh K. Jain and Dr. John D. Martin for insightful comments on the manuscript. This work has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007–2013)/ERC Grant Agreement No. 336839-ReEngineeringCancer.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Martin JD, et al. Reengineering the Tumor Microenvironment to Alleviate Hypoxia and Overcome Cancer Heterogeneity. Cold Spring Harb Perspect Med. 2016;6(12) doi: 10.1101/cshperspect.a027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Gkretsi V, et al. Remodeling Components of the Tumor Microenvironment to Enhance Cancer Therapy. Front Oncol. 2015;5:214. doi: 10.3389/fonc.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan VP, et al. Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Annu Rev Chem Biomol Eng. 2011;2:281–98. doi: 10.1146/annurev-chembioeng-061010-114300. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–64. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashizume H, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156(4):1363–80. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobbs SK, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95(8):4607–12. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–22. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pries AR, et al. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer. 2010;10(8):587–93. doi: 10.1038/nrc2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casazza A, et al. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2014;33(14):1743–54. doi: 10.1038/onc.2013.121. [DOI] [PubMed] [Google Scholar]
- 12.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39(1):61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nature reviews Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 14.Stylianopoulos T. The Solid Mechanics of Cancer and Strategies for Improved Therapy. J Biomech Eng. 2017;139(2) doi: 10.1115/1.4034991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stylianopoulos T, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci U S A. 2012;109(38):15101–8. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chauhan VP, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papageorgis P, et al. Tranilast-induced stress alleviation in solid tumors improves the efficacy of chemo- and nanotherapeutics in a size-independent manner. Sci Rep. 2017;7:46140. doi: 10.1038/srep46140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polydorou C, Mpekris F, Papageorgis P, Voutouri V, Stylianopoulos T. Pirfenidone normalizes the tumor microenvironment to improve chemotherapy. Oncotarget. 2017;8(15):24506–24517. doi: 10.18632/oncotarget.15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mpekris F, et al. Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy. J Control Release. 2017;261:105–112. doi: 10.1016/j.jconrel.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozdemir BC, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–34. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhim AD, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25(6):735–47. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–18. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 25.Goel S, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mpekris F, et al. Role of vascular normalization in benefit from metronomic chemotherapy. Proc Natl Acad Sci U S A. 2017;114(8):1994–1999. doi: 10.1073/pnas.1700340114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andre N, et al. Metronomics: towards personalized chemotherapy? Nat Rev Clin Oncol. 2014;11(7):413–31. doi: 10.1038/nrclinonc.2014.89. [DOI] [PubMed] [Google Scholar]
- 28.Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc Natl Acad Sci U S A. 2013;110(46):18632–7. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 31.Kuper H, et al. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248(3):171–83. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 32.Cannon J. Colorectal Neoplasia and Inflammatory Bowel Disease. Surg Clin North Am. 2015;95(6):1261–9, vii. doi: 10.1016/j.suc.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Jawad N, et al. Inflammatory bowel disease and colon cancer. Recent Results Cancer Res. 2011;185:99–115. doi: 10.1007/978-3-642-03503-6_6. [DOI] [PubMed] [Google Scholar]
- 34.Kristo I, et al. Incidental adenocarcinoma in patients undergoing surgery for stricturing Crohn's disease. World J Gastroenterol. 2017;23(3):472–477. doi: 10.3748/wjg.v23.i3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeda H, et al. Genetic basis of hepatitis virus-associated hepatocellular carcinoma: linkage between infection, inflammation, and tumorigenesis. J Gastroenterol. 2017;52(1):26–38. doi: 10.1007/s00535-016-1273-2. [DOI] [PubMed] [Google Scholar]
- 36.Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615–40. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 37.Fukumura D, et al. Obesity and Cancer: An Angiogenic and Inflammatory Link. Microcirculation. 2016;23(3):191–206. doi: 10.1111/micc.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuppers V, et al. Locking endothelial junctions blocks leukocyte extravasation, but not in all tissues. Tissue Barriers. 2013;1(1):e23805. doi: 10.4161/tisb.23805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claesson-Welsh L. Vascular permeability--the essentials Ups. J Med Sci. 2015;120(3):135–43. doi: 10.3109/03009734.2015.1064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadl A, Leitinger N. The role of endothelial cells in the resolution of acute inflammation. Antioxid Redox Signal. 2005;7(11–12):1744–54. doi: 10.1089/ars.2005.7.1744. [DOI] [PubMed] [Google Scholar]
- 41.Freire MO, Van Dyke TE. Natural resolution of inflammation. Periodontol 2000. 2013;63(1):149–64. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilroy DW, et al. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3(5):401–16. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 43.Lawrence T. Inflammation and cancer: a failure of resolution? Trends Pharmacol Sci. 2007;28(4):162–5. doi: 10.1016/j.tips.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Lawrence T, et al. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2(10):787–95. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 45.Janakiram NB, et al. Role of lipoxins, resolvins, and other bioactive lipids in colon and pancreatic cancer. Cancer Metastasis Rev. 2011;30(3–4):507–23. doi: 10.1007/s10555-011-9311-2. [DOI] [PubMed] [Google Scholar]
- 46.Lee HJ, et al. Resolvin D1 inhibits TGF-beta1-induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32. Int J Biochem Cell Biol. 2013;45(12):2801–7. doi: 10.1016/j.biocel.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Q, et al. Resolution of Cancer-Promoting Inflammation: A New Approach for Anticancer Therapy. Front Immunol. 2017;8:71. doi: 10.3389/fimmu.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serhan CN, et al. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu Rev Med. 2000;51:511–23. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12(1):88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Williams CS, et al. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18(55):7908–16. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 52.Allaj V, et al. Non-steroid anti-inflammatory drugs, prostaglandins, and cancer. Cell Biosci. 2013;3(1):8. doi: 10.1186/2045-3701-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sahin IH, et al. Impact of non-steroidal anti-inflammatory drugs on gastrointestinal cancers: current state-of-the science. Cancer Lett. 2014;345(2):249–57. doi: 10.1016/j.canlet.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Reichman HR, et al. Effects of steroids and nonsteroid anti-inflammatory agents on vascular permeability in a rat glioma model. J Neurosurg. 1986;65(2):233–7. doi: 10.3171/jns.1986.65.2.0233. [DOI] [PubMed] [Google Scholar]
- 55.Pakneshan P, et al. Differential suppression of vascular permeability and corneal angiogenesis by nonsteroidal anti-inflammatory drugs. Invest Ophthalmol Vis Sci. 2008;49(9):3909–13. doi: 10.1167/iovs.07-1527. [DOI] [PubMed] [Google Scholar]
- 56.Brune K, Patrignani P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J Pain Res. 2015;8:105–18. doi: 10.2147/JPR.S75160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allavena P, et al. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 58.Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32(6):463–88. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 59.Dubey P, et al. Cyclooxygenase-2 inhibition attenuates hypoxic cancer cells induced m2-polarization of macrophages. Cell Mol Biol (Noisy-le-grand) 2014;60(3):10–5. [PubMed] [Google Scholar]
- 60.Nakanishi Y, et al. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis. 2011;32(9):1333–9. doi: 10.1093/carcin/bgr128. [DOI] [PubMed] [Google Scholar]
- 61.Simoes RL, et al. Lipoxin A4 selectively programs the profile of M2 tumor-associated macrophages which favour control of tumor progression. Int J Cancer. 2017;140(2):346–357. doi: 10.1002/ijc.30424. [DOI] [PubMed] [Google Scholar]
- 62.Bougnoux P, et al. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br J Cancer. 2009;101(12):1978–85. doi: 10.1038/sj.bjc.6605441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merendino N, et al. Dietary omega -3 polyunsaturated fatty acid DHA: a potential adjuvant in the treatment of cancer. Biomed Res Int. 2013;2013:310186. doi: 10.1155/2013/310186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takkouche B, et al. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst. 2008;100(20):1439–47. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 65.Moris D, et al. The Role of NSAIDs in Breast Cancer Prevention and Relapse: Current Evidence and Future Perspectives. Breast Care (Basel) 2016;11(5):339–344. doi: 10.1159/000452315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Regulski M, et al. COX-2 inhibitors: a novel strategy in the management of breast cancer. Drug Discov Today. 2016;21(4):598–615. doi: 10.1016/j.drudis.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Ferlay J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 68.Liu W, et al. Combination of radiation and celebrex (celecoxib) reduce mammary and lung tumor growth. Am J Clin Oncol. 2003;26(4):S103–9. doi: 10.1097/01.COC.0000074147.22064.67. [DOI] [PubMed] [Google Scholar]
- 69.Cui XJ, et al. High-dose aspirin consumption contributes to decreased risk for pancreatic cancer in a systematic review and meta-analysis. Pancreas. 2014;43(1):135–40. doi: 10.1097/MPA.0b013e3182a8d41f. [DOI] [PubMed] [Google Scholar]
- 70.Capurso G, et al. Meta-analysis: the use of non-steroidal anti-inflammatory drugs and pancreatic cancer risk for different exposure categories. Aliment Pharmacol Ther. 2007;26(8):1089–99. doi: 10.1111/j.1365-2036.2007.03495.x. [DOI] [PubMed] [Google Scholar]
- 71.Molina MA, et al. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999;59(17):4356–62. [PubMed] [Google Scholar]
- 72.Okami J, et al. Overexpression of cyclooxygenase-2 in carcinoma of the pancreas. Clin Cancer Res. 1999;5(8):2018–24. [PubMed] [Google Scholar]
- 73.Zalatnai A. Novel therapeutic approaches in the treatment of advanced pancreatic carcinoma Cancer. Treat Rev. 2007;33(3):289–98. doi: 10.1016/j.ctrv.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 74.Dermond O, Ruegg C. Inhibition of tumor angiogenesis by non-steroidal anti-inflammatory drugs: emerging mechanisms and therapeutic perspectives. Drug Resist Updat. 2001;4(5):314–21. doi: 10.1054/drup.2001.0219. [DOI] [PubMed] [Google Scholar]
- 75.Lyons TR, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17(9):1109–15. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Banciu M, et al. Utility of targeted glucocorticoids in cancer therapy. J Liposome Res. 2008;18(1):47–57. doi: 10.1080/08982100801893978. [DOI] [PubMed] [Google Scholar]
- 77.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177(4):1576–91. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burke A, Smyth E, FitzGerald GA. Analgesic-Antipyretic and Anti-inflammatory Agents; Pharmacotherapy of Gout. In: Brunton LL, Parker KL, editors. Goodman and Gilman's Manual of Pharmacology and Therapeutics. McGraw-Hill; 2008. pp. 430–460. [Google Scholar]