Abstract
Comprehensively investigate the association of CT morphology and clinical findings of adenocarcinoma with EGFR mutation status. Retrospectively included 282 patients who was pathologically proved as lung adenocarcinoma with known EGFR mutation status (mutations: 138 patients, female: 86, median age: 66 years; wildtype: 144 patients, female: 67, median age: 62 years) and their pre-treatment CT scans were analyzed. CT findings and clinical information were collected. Univariate and multivariable logistic regression analysis were performed. Adjusted for age, gender and smoking history of two groups, significantly more patients with pleural tags, pleural and liver metastases were found in the EGFR mutated group (P = 0.007, 0.004, and 0.043, respectively). Multivariable logistic regression analysis found that the model included age, gender, smoking history, air bronchogram, pleural tags, pleural and liver metastasis had a moderate predictive value for EGFR mutation status (AUC = 0.741, P < .0001). Exon-19 deletion was associated with air bronchogram which adjusted for age, gender and smoking history (P = 0.007, OR: 2.91, 95%CI: 1.25–7.79). The evidence of pleural tags, pleural and liver metastases go along with a higher probability of EGFR mutation in adenocarcinoma patients and air bronchogram is positively associated with Exon-19 deletion mutation.
Introduction
Lung cancer remains the leading cause of cancer deaths for both men and women in the worldwide [1]. Many advances have been made in the understanding of the pathogenesis and management of lung cancer, particularly of adenocarcinoma (ADC). Specifically, the discovery of epidermal growth factor receptor (EGFR) mutations has changed lung cancer treatment. EGFR mutation are associated with a dramatic clinical response to the EGFR tyrosine kinase inhibitors (EGFR TKIs) gefitinib and erlotinib [2–4].
EGFR mutation testing is usually based on formaline fixed and paraffin embedded tumor specimens [5]. Approximately two thirds of non-small cell lung cancer (NSCLC) patients are diagnosed at an advanced stage of the disease [6] where only limited tumor specimens (biopsies, cytology) can be obtained in contrast to complete tumor resection. These limited tissue/cytologic samples are not always available or evaluable for diagnosis and mutation testing, leaving some patients unable to have the EGFR mutation status of their tumors determined [7]. Tumor heterogeneity [8] and the presence of lesions that are inaccessible to needle biopsy challenge the tumor biopsies as well. These challenges are accentuated in a later line setting because re-biopsy may not be feasible and tumor heterogeneity may be greater.
Therefore, a less invasive procedure to increase the pre-test probability for EGFR mutation analysis would be helpful. It has already been shown that non-smoking status, female and East Asian ethnicity are correlated with EGFR mutation, but they are not sufficient to select or exclude patients for EGFR mutation testing [9–10]. Several studies have demonstrated that circulating free tumor-derived DNA (ctDNA), which can be isolated from the plasma or serum of patients with NSCLC, is feasible to assess EGFR mutation status [11–12]. However, ctDNA analysis is technically challenging, the suitability and performance of ctDNA testing varies significantly between different geographic regions and different laboratories [13–14].
Computed tomography (CT) is wildly used in clinic to evaluate lung cancer patients and few studies have been carried out to investigate the imaging features of ADC with EGFR mutations. The results are controversial and the correlation of imaging features with EGFR mutation is unclear [15–18]. We hypothesize that there were some CT characteristics might correlate with EGFR mutation status and those CT characteristics might serve as a complementary way to suggest the EGFR mutation status.
In order to identify imaging characteristics of EGFR mutated ADC, we retrospectively analyzed computed tomography (CT) images of a cohort suffering from ADC with known EGFR mutation status.
Materials and methods
This study was in compliance with the Health Insurance Portability and Accountability Act (HIPAA) regulations. Informed written consent for examinations (including CT, PET-CT and pathology examinations) were obtained from all patients. Clinical records of included patients suffering from ADC admitted to our 3rd level throacic hospital between February 2006 and October 2013 were reviewed retrospectively. The retrospective analysis was approved by the ethics committee of the medical school of the University of Heidelberg (IRB approval number S-048/2012). All patient records were anonymized and de-identified prior to analysis.
Patients and clinical assessment
1575 consecutive ADC patients have been analyzed for EGFR mutation status (Exon 18–21), demographics and tumor histopathology. 271 patients (17%) showed EGFR mutations and a similar number of patients (n = 280) with EGFR wildtype from the same database was selected randomly for comparison. According to the inclusion criterion (available CT images before surgery, chemo- and radiotherapy in the Picture Archiving and Communication System, PACS [Synapse, Fuji Medical System]), 282 patients (male: female: 129: 153, mean age: 64 years) were included into the analysis. In a few cases (n = 6), the only pre-treatment imaging available was a positron emission tomogram with a non-enhanced CT (PET-CT) which was deemed adequate for lesion interpretation and characterization.
Gender, age, smoking status (non-smokers were defined as having smoked <100 cigarettes/life, former and active smokers were designated as smokers), malignant tumor history were retrieved from clinical documents (Table 1).
Table 1. Clinical characteristics of EGFR mutation (M) and wildtype (wt) cohorts.
| Characteristics |
EGFR mutation (n = 138) |
EGFR wildtype (n = 144) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age (years) | |||||
| Median | 66 | 62 | 0.056 | ||
| Range | 33–87 | 40–84 | |||
| Sex | |||||
| Female | 86 | 62% | 67 | 47% | 0.008* |
| Smoking status | |||||
| Non-Smokers | 106 | 84% | 94 | 67% | 0.002* |
| N/A | 11 | 8% | 4 | 3% | |
| Malignant tumor history | 19 | 15% | 24 | 17% | 0.628 |
| N/A | 11 | 8% | 4 | 3% | |
| UICC stage# | 0.066 | ||||
| I | 4 | 3% | 1 | 1% | |
| II | 2 | 1% | 5 | 4% | |
| III | 24 | 18% | 40 | 28% | |
| IV | 104 | 78% | 96 | 68% | |
| N/A | 4 | 3% | 2 | 1% | |
| N stage | 0.983 | ||||
| N0 | 41 | 29% | 41 | 30% | |
| N1 | 17 | 12% | 17 | 12% | |
| N2 | 47 | 33% | 42 | 30% | |
| N3 | 39 | 27% | 38 | 28% | |
| Distant metastases | 0.063 | ||||
| M0 | 30 | 22% | 46 | 32% | |
| M1 | 104 | 78% | 96 | 68% | |
| N/A | 4 | 3% | 2 | 1% | |
Abbreviations: UICC: International Union Against Cancer; N/A: not applicable, LDH: lactat dehydrogenase
#: 69 patients were staged by pathology and the rest of patients (n = 207) by clinical criteria.
*: P<0.05 was considered as statistically significant.
Histopathologic and EGFR analysis
Pathological diagnosis of the surgical specimens (n = 40), biopsies (n = 235) and cytological specimens (n = 7) were performed by board-certified pathologists according to the criteria of the 2004 WHO and 2011 IASLC/ATS/ERS classification [19]. EGFR mutations in exons 18–21 were determined by direct DNA sequencing as described previously in detail [20].
CT evaluation
All included patients underwent chest CT or PET-CT, which had been conducted within one-month prior treatment and were interpreted on a PACS reading workstation retrospectively by two experienced chest radiologists (Z.J and D.J) in consensus blinded to the EGFR analysis results. CT examinations were performed at multiple institutions with a variety of helical scanners. The median section thickness used was 3mm (range from 0.5mm to 7mm).
Detailed imaging characteristics of the primary lesions, corresponding lymph nodes and distant metastases were recorded (Fig 1), including [21–26]:
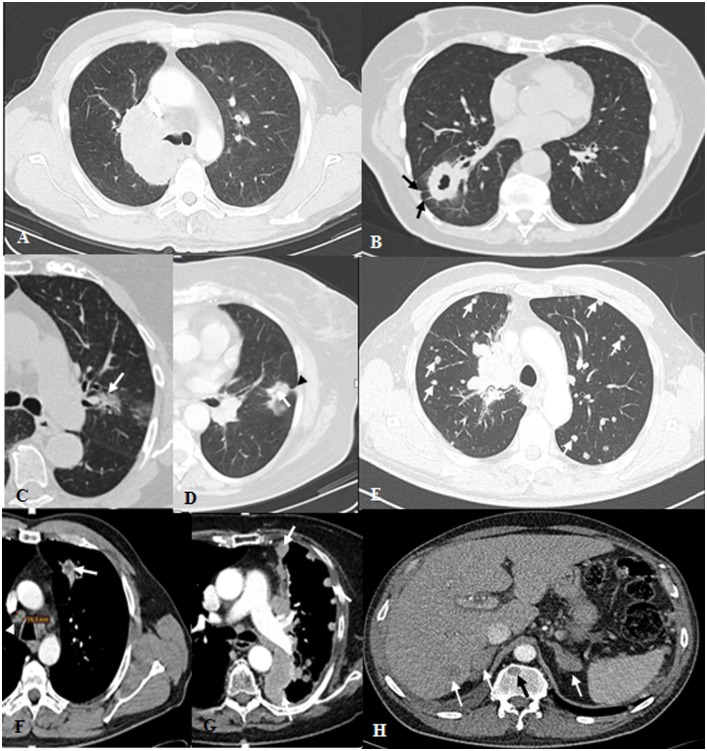
Fig 1. Transversal CT images of pulmonary adenocarcinoma with examples of the morphological features under investigation.
(A) Central lesion with tumor-bearing lobe atelectasis; (B) peripheral cavitary solid mass with ovoid shape, predominant spiculated margins and associated pleural tags (arrow); (C) central irregular semi-solid lesion in smooth margin with air bronchogram (arrow), GGO account for 25%-50%; (D) peripheral lesion with irregular shape, air bronchogram (arrow) and pleural tag (arrowhead); (E) central solid lesion with irregular shape and predominant lobulated margins with bilateral pulmonary metastases (arrows); (F) Irregular shape tumor after contrast enhancement showed central necrosis (arrow) and contralateral mediastinal lymph node metastasis (arrowhead); (G) tumor-side pleural metastases (arrows); (H) liver, bone, and bilateral adrenal gland metastases (arrows).
Primary tumor: location (peripheral [outer perimeter within 1 cm of the pleura], middle and central); maximal axial size; shape (with or without atelectasis [obstructive, compressive and combining]; if without, the shape was classified as round, ovoid, lobulated or irregular); margin (smooth, spiculated, lobulated, concave and the predominant one); attenuation (solid, ground glass opacity [GGO], or semisolid with recording the percentage of GGO); presence of cavitation, air bronchogram (AB), calcification within the tumor; number of pleural tags; number of satellite nodules, including the maximum size of the biggest one; number of nodules in different ipsilateral lobes; pleural contacts (slight pleural contact, visceral pleural invasion, parietal pleural invasion); tumor enhancement (homogeneous, heterogeneous, large necrosis [more than 50% of the tumor area]).
Lymph node (LN): According to the 7th Edition TNM classification of lung cancer, LN were divided into three levels: N1 –N3). Short axial diameter of the biggest LN in each level was recorded if more than 5 mm as well as the attenuation of the corresponding LN after contrast enhancement (fatty, isodense, hyperdense, necrosis, mixed). A LN with a short axis diameter of more than 10mm was rated as metastasis [27–28].
Distant metastases: a) Presence of nodule(s) in the contralateral lung (recording the maximal axial diameter of the biggest nodule and the distribution of pulmonary metastases [regional or random and diffuse]); presence of lymphatic carcinomatosis or pleural carcinosis (all the pleural metastases were either proven by histology or clinical criteria (pleura with obvious irregular thickening or nodules of the pleura which became more irregular and thicker during the follow up); b) Nearly all included patients (n = 267/282) underwent additional imaging studies (abdominal CT or ultrasound, brain CT or magnetic resonance (MR) imaging and whole-body bone scanning with technetium 99m medronate) for tumor staging. All available images and reports were reviewed. The specific distant metastatic organ (brain, liver, adrenal gland etc), the number of corresponding metastases and the total number of distant metastases which were divided into two groups (less or more than five) were recorded.
Statistical analysis
Initially, differences between categorical clinical and CT features were compared by χ2 test, Fisher’s exact test and Kruskal-Wallis test. Quantitative continuous variables in clinical and CT data were compared by Mann-Whitney-U test. Cases like “the size of satellite nodule and minor size of N1LN” with single missing data points too much were not included into the logistic regression analysis. Univariate logistic and multivariable logistic regression analysis which adjusted for age, gender and smoking history of two groups were employed to evaluate the relationship between clinical and CT features with EGFR mutation status. The optimized combination of different CT and clinical features to predicting the EGFR mutation status was performed by logistic regression analysis. P < 0.05 was considered to be statistically significant. Bonferroni adjustment method will be performed in multiple comparisons if necessary. The statistical software (SPSS 16.0; SPSS, Chicago, Ill) was used to perform the analysis and create graphs.
Results
EGFR mutation status and clinical characteristics
Our cohort included 282 patients. 138 patients (female: male: 86: 52, median age 66y) had EGFR mutations (M) while 144 patients (female: male: 67: 77, median age 62y) were EGFR wildtype (wt). The EGFR mutation group was constituted by 62 patients (45%) who had exon 19 deletions, 39 patients (28%) who harbored p.L 858R mutations and 27% of patients with other types of mutations (Fig 2).
Fig 2. The percentage composition of the detected EGFR mutations.
- Note:- Combined EGFR mutation stands for mutation at least was found in two exons (18–21), for example point mutation in 18 and 20 exons.
- - Seldom mutation means at least two combination of the way of EGFR mutation, such as deletion combined insertion in exon 19.
- - There was one patients had both deletion in exon 19 and p.L858R.
All the clinical characteristics with EGFR mutation status were recorded in Table 1.
EGFR mutation status and CT features
Primary tumor
No statistically significant differences between EGFR mutation status (mutation: M and wildtype: WT) in tumor location (peripheral, middle and central), size, shape, margin, attenuation, pleural contact and enhancement. However, AB was found in 44% (nM = 55) of the EGFR mutated patients and 31% (nWT = 39) of the patients with wt (P = 0.033). The percentage of EGFR mutated patients (nM = 42 (33%) vs. nWT = 26 (20%), P = 0.013) with more than one pleural tag was significantly higher. EGFR mutated patients showed a significantly higher incidence of satellite nodule (nM = 71 [56%] vs. nWT = 50 [38%], P = 0.004) while the median size of the biggest one was smaller (7mm vs. 9mm, P = 0.034), (Table 2) (S1 Table).
Table 2. Statistically significant imaging characteristics comparison between EGFR mutation statuses.
| TNM | CT Features | EGFR wildtype (wt) | EGFR mutation (M) | P* | P# (OR, 95%CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | No. | % | Total | No. | % | |||||
| Primary tumor | Air bronchogram1 | 126 | 39 | 31% | 125 | 55 | 44% | 0.033 | 0.059 | |
| Pleural tags (>1)2 | 133 | 26 | 20% | 127 | 42 | 33% | 0.013 | 0.007(2.27, 1.25–4.14) | ||
| SN3 | Number (≥1) | 131 | 50 | 38% | 126 | 71 | 56% | 0.004 | - | |
| Size(mm) | 50 | - | - | 71 | - | - | 0.034 | - | ||
| Median | 9 | - | - | 7 | - | - | - | - | ||
| Range | 3–29 | - | - | 3–34 | - | - | - | - | ||
| LN | Biggest N14 LN size | 90 | - | - | 88 | - | - | 0.023 | - | |
| Median (mm) | 14 | - | - | 12 | - | - | - | - | ||
| Range | 7–33 | - | - | 6–22 | - | - | - | - | ||
| Metastases | M1a | Pleural | 144 | 30 | 21% | 138 | 50 | 36% | 0.004 | 0.004(2.30, 1.30–4.06) |
| M1b | Liver5 | 140 | 8 | 6% | 132 | 20 | 15% | 0.010 | 0.043(2.54, 1.03–6.28) | |
| Bilateral adrenal5 | 140 | 9 | 6% | 130 | 0 | 0% | 0.002 | NA | ||
LN: lymph node, EGFR: epidermal growth factor receptor, SN: satellite nodules
OR: odd ratio, CI: confidence interval.
1: 31 patients (M: wt = 13: 18) were excluded from this specific analysis, because the primary tumor of 10 patients could not be identified and the tumor-bearing lobe of the rest of patients were atelectasis, thus, the contour of the primary tumor was barely clearly recognized.
2: 22 patients (M: wt = 11:11) were excluded from this specific analysis, because the primary tumor of 10 patients could not be identified and the tumor associated atelectasis hides the pleural relation of the tumor in these patients.
3: Satellite nodules in 25 patients (M:wt = 12:13) could not be counted due to atelectasis and the not-identified primary tumors.
4: N1: ipsilateral peribronchial and/or ipsilateral hilar LN and intrapulmonary nodes, only included the short size of LN more than 5mm.
5: Information concerning liver and adrenal gland metastases was missing for 10 and 12 patients, respectively.
#: The P value was calculated by multivariable logistic regression analysis which adjusted for age, gender and smoking history.
*: P < 0.05 was considered as statistically significant.
Lymph nodes
The median short axis diameter of the biggest N1 LN in EGFR mutated patients was smaller (12 mm vs. 14 mm; P = 0.023) (Table 2). Other comparisons such as the short axis of the biggest LN in the other two levels and LN staging status were not statistically different.
Metastases
While random and diffuse, pulmonary metastases were more frequent in the EGFR mutation group (nM: 11 [8%] vs. nWT: 4 [3%], P = 0.052), this observation did not reach significance. The frequency of pleural metastases, of which 63 patients were confirmed by pathology and 17 patients were diagnosed according to clinical criteria, was higher in the EGFR mutation group (nM = 50 [36%] vs. nWT = 30 [21%], P = 0.004). With distant metastases, the overall incidence and the patients with more than five metastases were similar between the two groups. However, liver metastases were found significantly more often in EGFR mutated patients (P = 0.010) while bilateral adrenal gland metastases were found only in EGFR wt patients (P = 0.002). Interestingly, half of the adrenal metastases patients in EGFR wt group were manifested as bilateral metastases (Table 2) (S1 Table).
Univariate and multivariable logistic regression analysis
Univariate logistic analysis showed that gender, smoking history, AB, pleural tags, pleural metastasis and liver metastasis were significantly different in two groups and the P values were 0.008, 0.002, 0.033, 0.014, 0.004 and 0.013, respectively. (Table 2).
Adjusted for age, gender and smoking history of two groups respectively, we found that the incidence of pleural tags (P = 0.007), pleural (P = 0.004) and liver metastases (P = 0.043) are significantly higher in EGFR mutated patients. (Table 2) However, the incidence of AB was nearly to show the significant difference in two groups (P = 0.059). Further, multivariable regression analysis found that combined age, gender, smoking history, AB, pleural tags, pleural and liver metastasis together which showed the highest predictive value (AUC = 0.741) (Fig 3).
Fig 3. Receiver operator characteristic curve for the model which composed by age, gender, smoking history, air bronchogram, pleural tags, pleural and liver metastasis to predict EGFR mutation status.
The area under the curve is 0.741.
Association of the CT features with EGFR exon-19 deletion or p.L858R
After adjusting for age, gender and smoking history, multivariable logistic regression analysis showed that AB strongly associated with exon-19 deletion mutation (OR, 2.91; 95%CI: 1.25–7.79; P = 0.011), as compared to the rest of EGFR mutations. Whereas, compared with the rest of EGFR mutated patients, patients with p.L858R mutation had significant less AB (OR, 3.12; 95%CI: 1.25–7.79, P = 0.015) (Table 3). With other imaging features, there were no significant differences were found when associated them with EGFR exon-19 deletion or p.L858R.
Table 3. Within EGFR mutation group, significant correlations of deletion in exon 19 or p.L 858R with clinical and CT features.
| Characteristics | Deletion in exon 191 | P* | P #(OR, 95%CI) | p.L858R1 | P* | P #(OR, 95%CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | % | No | % | Yes | % | No | % | |||||
| Female (n = 138) |
45/62 | 73% | 41/76 | 54% | 0.025 | >0.05 | 23/39 | 59% | 63/99 | 64% | 0.611 | - |
| Air bronchogram (n = 125) |
33/59 | 56% | 22/66 | 33% | 0.011 | 0.007(2.91, 1.25–7.79) | 9/34 | 27% | 46/91 | 51% | 0.016 | 0.015(3.12, 1.25–7.79) |
OR: odd ratio, CI: confidence interval.
#: The P value was calculated by multivariable logistic regression analysis which adjusted for age, gender and smoking history.
*: P < 0.05 was considered as statistically significant.
1: 13 patients were excluded out in this specific analysis, because the primary tumor of 5 patients could not be identified and the tumor-bearing lobe in 8 patients were atelectasis and the contour of the primary tumor was barely recognized.
Discussion
This study has identified certain clinical and imaging characteristics which were correlated with EGFR mutations. According to previous reports [9, 10], we found that women and non-smokers tended to have EGFR mutation more often. To our knowledge, no study has previously been done with the objective of a comprehensive comparison of CT features of ADC patients with different EGFR mutation status, hence only single pattern such as GGO [16, 17, 29–32] have been evaluated so far. In addition, there were two studies have showed that ADC patients with malignant pleural effusion (MPE) had a higher incidence of EGFR mutation [33, 34].
Our study has showed that, several radiological features associated with EGFR mutation in ADC: the number of patients with AB, pleural tags, pleural and liver metastases was significantly higher if EGFR is mutated. Logistic regression analysis showed that the model composed by age, gender, smoking history, AB, pleural tag (n≥1), pleural and liver metastasis have a moderate predictive value for EGFR mutation. These could enable radiologists to better understand the imaging features which correlated with EGFR mutation and to applying this understanding into clinical practice by allowing radiologists to raise clinical suspicion for EGFR mutation.
Lepidic predominant ADC were already described to show AB frequently [35], while a correlation of EGFR mutations with the lepidic pattern has also been demonstrated [20]. This might explain why EGFR mutated patients in our cohort showed more AB. Koenigkam Santos M et al. [36] found that ADC was more commonly associated with pleural tags, compared to squamous cell carcinomas. In our study, mutated ADC had more pleural tags than wild type ADC.
Several reports [33, 37] found that overall survival (OS) could be prolonged in EGFR positive ADC patients with MPE undergoing EGFR-TKI therapy. Meanwhile, several retrospective studies [33, 34] discovered that patients with ADC and MPE had a higher rate of EGFR mutation. A functional variant of the EGFR promoter, 216G/T (rs712829), was associated with pleural spread of ADC which is usually accompanied with MPE [38]. This might explain the significantly higher number of pleural metastases in EGFR mutated patients. The accompanied high incidence of pleural effusion might cause more compressive atelectasis (nM = 12 vs. nWT = 6) in ADC with EGFR mutation.
In contrast to the previous findings [18, 39–41], neither significant differences in LN staging, pulmonary, nor brain metastases were noticed, while satellite metastases were significantly more frequent and of smaller size in EGFR mutated tumors. The latter findings were probably associated with the abundant angiogenesis of EGFR pathways [42] since this important mediator supports local spread resulting in multiple metastases in the same lobe or the whole lung. This mechanism might also explain the few necrosis in EGFR mutated tumors (nM = 1; nWT = 7, S1 Table). In addition, the short axis of N1 LN in EGFR mutated tumors was smaller. A possible explanation for this maybe EGFR was expressed in almost all cells with the only exception of the mature lymphohematopoietic cells [42].
The majority of detected EGFR mutations were either p.L858R or exon-19 deletions (39 + 62 = 101, 73%, Table 3). An association of CT features and OS with p.L858R or exon-19 deletions might be different. It has been demonstrated that p.L858R were correlated with the CT feature ‘invasive solid pattern’ [29], had not prolonged OS after EGFR-TKI therapy [43]. While exon-19 deletion, in contrast, had a longer progression-free survival (PFS) [44] and OS [43,45] after TKI treatment. Therefore, it is useful to know the correlation of significant clinical features and CT pattern with EGFR p.L858R or exon-19 deletions. As we found exon-19 deletion mutations were significantly more frequent in women and this was consistent with the finding that EGFR mutation favoring female patients. Even excitingly, we found exon-19 deletion is correlated with a significantly greater number of AB, whereas tumors with p.L858R mutation had significantly less frequent AB (Table 3).
Besides, our study has its own limitations. This was a retrospective study which would induce patient selection bias and the case included in the study were not enough, even we have already included as many cases as we could. However, these results might serve as a basement for our further international cooperative study and all those results still need be further validated by our prospective study.
Conclusion
Clinical and CT-derived imaging characteristics are associated with activating EGFR mutations. Especially, the presence of AB, pleural tag, pleural and liver metastases may help to increase pretest probability for EGFR mutation. In addition, the presence of AB positively associated with EGFR exon-19 mutation.
Supporting information
(DOC)
(DOCX)
Acknowledgments
We would like to extend our thanks to Dr. Elizabeth Chang Xu for the English editing of the manuscript. Jing Zhao, Julien Dinkel, Arne Warth, Niels Reinmuth, Philipp Schnabel, Thomas Muley, Michael Meister, Martin Steins, Heinz-Peter Schlemmer, Felix JF Herth, Hans-Ulrich Kauczor and Claus Peter Heussel are a member of German Center for Lung Research (DZL).
Data Availability
All relevant data are available from the Figshare Repository, URL: https://figshare.com/s/fb1fcb01a8b869863eef, DOI: 10.6084/m9.figshare.5297065.
Funding Statement
1. The funder (LungenClinic Grosshansdorf GmbH) provided support in the form of salaries for authors [N.R.], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. 2. The funders we mentioned in conflict of interests have provided support in the form of salaries for authors, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59:225–249. doi: 10.3322/caac.20006 [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–1500. doi: 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139. doi: 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- 4.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004; 101:13306–13311 doi: 10.1073/pnas.0405220101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. : Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors:guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013; 8(7):823–59. doi: 10.1097/JTO.0b013e318290868f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics.CA Cancer J Clin 2011; 61:69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 7.Fenizia F, De Luca A, Pasquale R, Sacco A, Forgione L, Lambiase M, et al. EGFR mutations in lung cancer: from tissue testing to liquid biopsy. Future Oncol. 2015;11: 1611–23. doi: 10.2217/fon.15.23 [DOI] [PubMed] [Google Scholar]
- 8.Vignot S, Frampton GM, Soria JC, Yelensky R, Commo F, Brambilla C, et al. : Next-generation sequencing reveals high concordance of recurrent somatic alterations between primary tumor and metastases from patients with non-small-cell lung cancer. J Clin Oncol 2013; 31: 2167–2172. doi: 10.1200/JCO.2012.47.7737 [DOI] [PubMed] [Google Scholar]
- 9.Girard N, Sima CS, Jackman DM, Sequist LV, Chen H, Yang JC, et al. : Nomogram to predict the presence of EGFR activating mutation in lung adenocarcinoma. Eur Respir J 2012; 39(2):366–72. doi: 10.1183/09031936.00010111 [DOI] [PubMed] [Google Scholar]
- 10.Dogan S, Shen R, Ang DC, Johnson ML, D'Angelo SP, Paik PK, et al. : Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res 2012; 18(22):6169–77. doi: 10.1158/1078-0432.CCR-11-3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douillard JY, Ostoros G, Cobo M, Ciuleanu T, Cole R, McWalter G, et al. Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. J Thorac Oncol. 2014;9:1345–53. doi: 10.1097/JTO.0000000000000263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009; 361:958–67. doi: 10.1056/NEJMoa0904554 [DOI] [PubMed] [Google Scholar]
- 13.Han B, Tjulandin S, Hagiwara K, Normanno N, Wulundari L, Konstantin Konstantinovich L, et al. Determining the prevalence of EGFR mutations in Asian and Russian patients (pts) with advanced non-small-cell lung cancer (aNSCLC) of adenocarcinoma (ADC) or non-ADC histology: IGNITE study. Ann Oncol. 2015; 26 (Suppl 1): i29–i30. [Google Scholar]
- 14.Reck M, Hagiwara K, Han B, Tjulandin S, Grohe C, Yokoi T, et al. Investigating the utility of circulating-free tumour-derived DNA (ctDNA) in plasma for the detection of epidermal growth factor receptor (EGFR) mutation status in European and Japanese patients (pts) with advanced non-small-cell lung cancer (aNSCLC). Ann Oncol. 2015; 26 (Suppl 1): i58–i59. [Google Scholar]
- 15.Glynn C, Zakowski MF, Ginsberg MS. Are there imaging characteristics associated with epidermal growth factor receptor and KRAS mutations in patients with adenocarcinoma of the lung with bronchioloalveolar features? J Thorac Oncol 2010; 5(3):344–8. doi: 10.1097/JTO.0b013e3181ce9a7a [DOI] [PubMed] [Google Scholar]
- 16.Yano M, Sasaki H, Kobayashi Y, Yukiue H, Haneda H, Suzuki E, et al. : Epidermal growth factor receptor gene mutation and computed tomographic findings in peripheral pulmonary adenocarcinoma. J Thorac Oncol 2006; 1(5):413–6. [PubMed] [Google Scholar]
- 17.Aoki T, Hanamiya M, Uramoto H, Hisaoka M, Yamashita Y, Korogi Y. Adenocarcinomas with predominant ground-glass opacity: correlation of morphology and molecular biomarkers. Radiology 2012; 264(2):590–6. doi: 10.1148/radiol.12111337 [DOI] [PubMed] [Google Scholar]
- 18.Togashi Y, Masago K, Kubo T, Sakamori Y, Kim YH, Hatachi Y, et al. : Association of diffuse, random pulmonary metastases, including miliary metastases, with epidermal growth factor receptor mutations in lung adenocarcinoma. Cancer 2011; 117(4):819–25. doi: 10.1002/cncr.25618 [DOI] [PubMed] [Google Scholar]
- 19.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, et al. : International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society:international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc. 2011; 8(5):381–5. doi: 10.1513/pats.201107-042ST [DOI] [PubMed] [Google Scholar]
- 20.Warth A, Penzel R, Lindenmaier H, Brandt R, Stenzinger A, Herpel E, et al. : EGFR, KRAS, BRAF and ALK gene alterations in lung adenocarcinomas: patient outcome, interplay with morphology and immunophenotype. Eur Respir J 2014; 43(3):872–83. doi: 10.1183/09031936.00018013 [DOI] [PubMed] [Google Scholar]
- 21.Aoki T, Tomoda Y, Watanabe H, Nakata H, Kasai T, Hashimoto H, et al. : Peripheral lung adenocarcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology 2001; 220: 803–809. doi: 10.1148/radiol.2203001701 [DOI] [PubMed] [Google Scholar]
- 22.Gandara DR, Aberle D, Lau D, Jett J, Akhurst T, Heelan R, et al. : Radiographic imaging of bronchioloalveolar carcinoma: screening, patterns of presentation and response assessment. J Thorac Oncol 2006; 1: Suppl. 9, S20–6. [PubMed] [Google Scholar]
- 23.Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology 2009; 253: 606–622. doi: 10.1148/radiol.2533090179 [DOI] [PubMed] [Google Scholar]
- 24.Li F, Sone S, Abe H, Macmahon H, Doi K. Malignant versus benign nodules at CT screening for lung cancer: comparison of thin-section CT findings. Radiology 2004; 233: 793–798. doi: 10.1148/radiol.2333031018 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki K, Kusumoto M, Watanabe S, Tsuchiya R, Asamura H. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg 2006; 81: 413–419. doi: 10.1016/j.athoracsur.2005.07.058 [DOI] [PubMed] [Google Scholar]
- 26.Lee HJ, Goo JM, Lee CH, Park CM, Kim KG, Park EA, et al. : Predictive CT findings of malignancy in ground-glass nodules on thin-section chest CT: the effects on radiologist performance. Eur Radiol 2009; 19: 552–560. doi: 10.1007/s00330-008-1188-2 [DOI] [PubMed] [Google Scholar]
- 27.Mori K, Yokoi K, Saito Y, Tominaga K, Miyazawa N. Diagnosis of mediastinal lymph node metastases in lung cancer. Jpn J Clin Oncol 1992; 22(1):35–40. [PubMed] [Google Scholar]
- 28.Seely JM, Mayo JR, Miller RR, Müller NL. T1 lung cancer: prevalence of mediastinal nodal metastases and diagnostic accuracy of CT. Radiology 1993; 186(1):129–32. doi: 10.1148/radiology.186.1.8416552 [DOI] [PubMed] [Google Scholar]
- 29.Yoshida Y, Kokubu A, Suzuki K, Kuribayashi H, Tsuta K, Matsuno Y, et al. : Molecular markers and changes of computed tomography appearance in lung adenocarcinoma with ground-glass opacity. Jpn J Clin Oncol 2007; 37:907–912. doi: 10.1093/jjco/hym139 [DOI] [PubMed] [Google Scholar]
- 30.Chung JH, Choe G, Jheon S, Sung SW, Kim TJ, Lee KW, et al. : Epidermal growth factor receptor mutation and pathologic-radiologic correlation between multiple lung nodules with ground-glass opacity differentiates multicentric origin from intrapulmonary spread. J Thorac Oncol 2009; 4:1490–1495. doi: 10.1097/JTO.0b013e3181bc9731 [DOI] [PubMed] [Google Scholar]
- 31.Hsu KH, Chen KC, Yang TY, Yeh YC, Chou TY, Chen HY, et al. : Epidermal growth factor receptor mutation status in stage I lung adenocarcinoma with different image patterns. J Thorac Oncol 2011; 6(6):1066–72. doi: 10.1097/JTO.0b013e31821667b0 [DOI] [PubMed] [Google Scholar]
- 32.Usuda K, Sagawa M, Motono N, Ueno M, Tanaka M, Machida Y, et al. : Relationships between EGFR mutation status of lung cancer and preoperative factors—are they predictive? Asian Pac J Cancer Prev 2014; 15(2):657–62. [DOI] [PubMed] [Google Scholar]
- 33.Wu SG, Yu CJ, Tsai MF, Liao WY, Yang CH, Jan IS, et al. : Survival of lung adenocarcinoma patients with malignant pleural effusion. Eur Respir J 2013; 41(6):1409–18. doi: 10.1183/09031936.00069812 [DOI] [PubMed] [Google Scholar]
- 34.Smits AJ, Kummer JA, Hinrichs JW, Herder GJ, Scheidel-Jacobse KC, Jiwa NM, et al. : EGFR and KRAS mutations in lung carcinomas in the Dutch population: increased EGFR mutation frequency in malignant pleural effusion of lung adenocarcinoma. Cell Oncol (Dordr) 2012; 35(3):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lederlin M, Puderbach M, Muley T, Schnabel PA, Stenzinger A, Kauczor HU, et al. : Correlation of radio- and histomorphological pattern of pulmonary adenocarcinoma. Eur Respir J 2013; 41(4):943–51. doi: 10.1183/09031936.00056612 [DOI] [PubMed] [Google Scholar]
- 36.Koenigkam Santos M, Muley T, Warth A, de Paula WD, Lederlin M, Schnabel PA, et al. : Morphological computed tomography features of surgically resectable pulmonary squamous cell carcinomas: Impact on prognosis and comparison with adenocarcinomas. Eur J Radiol 2014; 83(7):1275–81. doi: 10.1016/j.ejrad.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 37.Guo H, Wan Y, Tian G, Liu Q, Kang Y, Li Y, et al. : EGFR mutations predict a favorable outcome for malignant pleural effusion of lung adenocarcinoma with Tarceva therapy. Oncol Rep 2012; 27(3):880–90. doi: 10.3892/or.2011.1559 [DOI] [PubMed] [Google Scholar]
- 38.Guo H, Xing Y, Liu R, Chen S, Bian X, Wang F, et al. : -216G/T (rs712829), a functional variant of the EGFR promoter, is associated with the pleural metastasis of lung adenocarcinoma. Oncol Lett 2013; 6(3):693–698. doi: 10.3892/ol.2013.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enomoto Y, Takada K, Hagiwara E, Kojima E. Distinct features of distant metastasis and lymph node stage in lung adenocarcinoma patients with epidermal growth factor receptor gene mutations. Respir Investig 2013; 51(3):153–7. doi: 10.1016/j.resinv.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 40.Shin DY, Na II, Kim CH, Park S, Baek H, Yang SH. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014; 9(2):195–9. doi: 10.1097/JTO.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 41.Laack E, Simon R, Regier M, Andritzky B, Tennstedt P, Habermann C, et al. : Miliary never-smoking adenocarcinoma of the lung: strong association with epidermal growth factor receptor exon 19 deletion. J Thorac Oncol 2011; 6(1):199–202. doi: 10.1097/JTO.0b013e3181fb7cf1 [DOI] [PubMed] [Google Scholar]
- 42.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, et al. : The role of the EGFR signaling in tumor microenvironment. J Cell Physiol 2008; 214:559–67. doi: 10.1002/jcp.21260 [DOI] [PubMed] [Google Scholar]
- 43.J. C.-H. Yang, L. V. Sequist, M. Schuler, T. Mok, N. Yamamoto, K. O’Byrne, et al: Overall survival in patients with advanced NSCLC harboring common (Del19/L858R) EGFR mutations: analysis of two large, open-label phase III studies of afatinib vs chemotherapy, LUX-Lung 3 and LUX-Lung 6. Presented at the American society of clinical oncology meeting, Chicago, Illinois, May30- Jun 3, 2014.
- 44.Choi CM, Kim MY, Lee JC, Kim HJ. Advanced lung adenocarcinoma harboring a mutation of the epidermal growth factor receptor: CT findings aftertyrosine kinase inhibitor therapy. Radiology 2014; 270(2):574–82. doi: 10.1148/radiol.13121824 [DOI] [PubMed] [Google Scholar]
- 45.Jackman DM, Yeap BY, Sequist LV, Lindeman N, Holmes AJ, Joshi VA, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006; 12:3908–14. doi: 10.1158/1078-0432.CCR-06-0462 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Data Availability Statement
All relevant data are available from the Figshare Repository, URL: https://figshare.com/s/fb1fcb01a8b869863eef, DOI: 10.6084/m9.figshare.5297065.