Abstract
The ER forms a contiguous structure of interconnected sheets and tubules that spreads from the nuclear envelope to the cell cortex. Through its attachment to the cytoskeleton, the ER undergoes dynamic rearrangements, such as tubule extension and movement. ER shaping proteins (reticulons and DP1/Yop1p) play key roles in generating and maintaining the unique reticular morphology of the ER. Atlastin and its yeast homologue, Sey1p, mediate homotypic ER membrane fusion, which leads to the formation of new three-way junctions within the polygonal network. At these junctions, the Lunapark protein, Lnp1p, works in conjunction with the reticulons, DP1/Yop1p, and in antagonism to atlastin/Sey1p to maintain the network in a dynamic equilibrium. Defects in ER morphology have been linked to certain neurological disorders.
Introduction
The endoplasmic reticulum (ER), typically the largest membrane-bound organelle in a eukaryotic cell, plays a critical role in many cellular processes, including protein synthesis, protein modification, lipid synthesis and the regulation of Ca2+ homeostasis and secretion [1]. At an ultrastructural level it can be classified into two types, smooth ER (SER) and rough ER (RER). The RER has a sheet-like morphology and is characterized by the presence of ribosomes associated with the biosynthesis of secretory and membrane proteins. Conversely, the SER is devoid of ribosomes and tends to be more tubular in structure. Specialized regions of the ER, termed ER exit site (ERES), have been defined by their unique role in the assembly of the COPII vesicles that mediate ER-to-Golgi traffic [2]. Little, however, is known about the biogenesis and organization of these sites. The ER closely interacts with many organelles including mitochondria, Golgi, endosomes, lysosomes, peroxisomes and the plasma membrane to allow the transfer of lipids and intracellular signals [3], and interactions with the cytoskeleton play key roles in its dynamics and distribution.
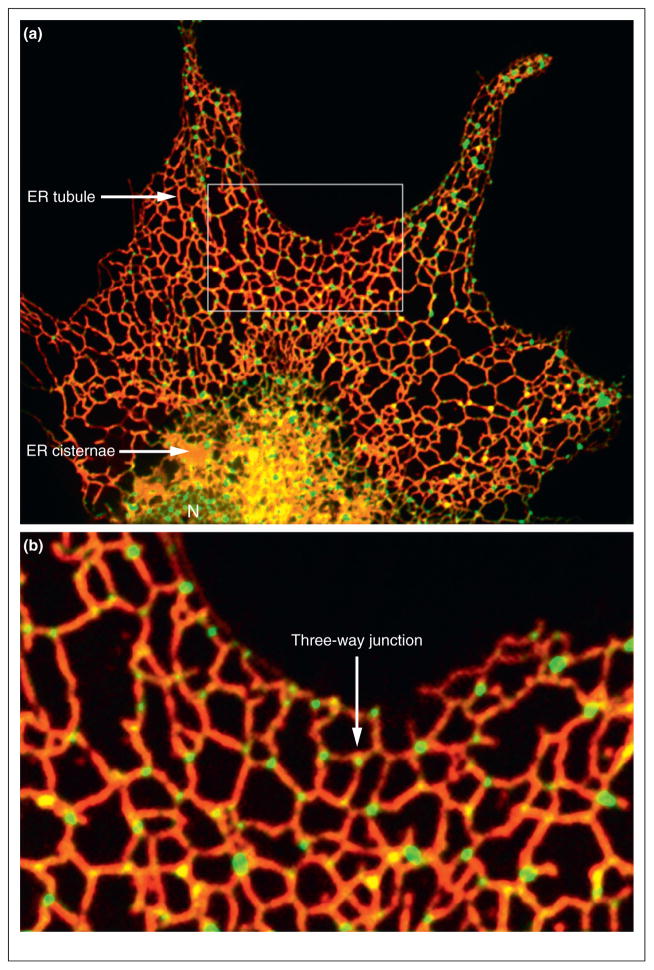
At the cellular level the ER consists of an extended polygonal network of tubules connecting to sheet-like cisternae and the nuclear envelope (NE) to form one contiguous membrane network with a common luminal space [1,4] (Figure 1a). In budding yeast the network of interconnected ER tubules lies just beneath the plasma membrane (cortical ER) with several tubules traversing the cytoplasm to connect the cortical ER to the NE [5]. The ER is a highly dynamic organelle, continuously undergoing rearrangements that include tubule branching, branchpoint sliding, tubule retraction, tubule–tubule membrane fusion, ring closure and ER partitioning during cell division [6–9]. Through these movements and shape changes, an irregular polygonal ER network is maintained that extends throughout the entire cell volume (Figure 1b). The analysis of mutants defective in ER morphogenesis convincingly demonstrate that ER morphology is essential for many intracellular events [10,11••,12,13,14•,15••,16••,17••]. These studies provide new and important insights into how ER morphology and dynamics serve its multifunctional roles.
Figure 1.
The ER network. (a) The ER network of COS-7 cells is labeled with mCherry-KDEL (red) and the junctions are labeled with Lnp1-GFP (green). Also marked: nucleus (N), ER cisternae and ER tubules. (b) The box area marked in the top panel is magnified below. Interconnected ER tubules form a network with three-way junctions marked by Lnp1-GFP.
The medical importance of ER morphogenesis is underscored by the identification of a class of neurologic disorders known as hereditary spastic paraplegias (HSPs). Approximately, 60% of afflicted HSP individuals suffer from autosomal dominant mutations in one of three proteins: spastin (SPG4), atlastin (SPG3A), and REEP1 (SPG31) [18,19], which mediate ER morphology and localize to the ER. With several families of ER shaping proteins identified [11••,13,14•,15••,16••,17••], a more complete picture of ER morphogenesis is rapidly evolving. The present review will discuss some of the recent discoveries regarding the formation and maintenance of the ER network.
ER dynamics and mobility
Live cell imaging studies have revealed that ER tubule extension is driven by the sliding of ER tubules along the microtubule (MT) cytoskeleton. This is achieved in two mechanistically distinct ways: tip attachment complexes (TAC) and sliding dynamics [9,20,21]. ER sliding driven by MT motors is more predominant and faster than TAC dynamics [20,21]. TACs are formed by the physical interaction between the ER-resident protein STIM1 and the MT plus end-binding protein EB1 [22]. In this way, the ER attaches to the tips of MTs through TACs and ER tubules grow or retract in concert with MT movement. As STIM1 has a MT-binding domain and is concentrated at ER tubule tips, depletion of STIM1 reduces ER tubule movement along MTs. Depletion of EB1 leads to a similar reduction of ER tubule movement [22]. Recently, Rab10 has been identified as an ER-specific Rab GTPase that accumulates at the leading edge of dynamic ER tubules and regulates ER tubule extension along MTs [23].
Although MTs are involved in ER tubule extension and movement, they are probably not essential for ER tubule and network formation, as these structures can be generated in vitro without the cytoskeleton [24]. Nonetheless, the ER-MT connection plays an important role in ER remodeling and ER distribution throughout the cytoplasmic space. Accordingly, depolymerization of the MT with nocodazole leads to the collapse of the ER network towards the cell center and the formation of sheet-like structures [8,25]. In vivo, the ER network is dependent on stable interactions between the ER and MTs mediated by ER-associated proteins, such as REEP1, p180, p22, and CLIMP63 [26–29]. These proteins may help to align nascent ER tubules with MTs after they have been pulled from a pre-existing ER structure.
The movement of the nucleus and associated nuclear ER during the cell cycle in yeast is dependent on MTs, as it is in mammalian cells; however, cortical ER morphology and mobility in yeast require the actin cytoskeleton rather than MTs [5]. Perturbation of the actin cytoskeleton using actin-disrupting drugs results in alterations in cortical ER morphology and dynamics [30]. More importantly, Estrada et al. [6] showed that cortical ER inheritance relies on actin, a myosin V motor Myo4p and an adaptor protein She3p. ER tubules become anchored at the bud tip [31] in a process that requires the function of Sec3p, a component of exocyst tethering complex [32]. In growing buds, the extension of ER tubules from the bud tip to the cell periphery is regulated by the cell wall integrity (CWI) MAP kinase pathway. Inactivation of the MAP kinase Slt2p by the phosphatase Ptc1p is essential for the successful delivery of ER tubules to the periphery of daughter cells [33,34]. These findings revealed that ER inheritance is a multistage process, which is tightly regulated to ensure the accurate delivery of ER into daughter cells during cell division.
Shaping ER tubules and membrane curvature
Ground breaking work by Voeltz et al. [17••] identified the reticulon (RTN) and DP1/Yop1p family members as necessary and sufficient for shaping ER tubules. RTN and DP1/Yop1p proteins are ubiquitously expressed in eukaryotes, and are enriched in tubular subcompartments of the ER as well as the sharply curved edges of ER cisternal sheets [17••,35]. Although RTN and DP1/Yop1p are not related by sequence, they each contain two closely spaced transmembrane domains (TMDs). The TMDs have been proposed to form a wedge-like structure within the outer leaflet of the lipid bilayer and thereby generate positive curvature in the ER membrane. The overexpression of RTN or DP1 in COS-7 cells leads to pronounced tubule formation [17••,36], while immunodepletion of Rtn4a blocks tubule formation [17••]. Disruption of RTN1 in budding yeast abolishes the cortical ER network and results in a proliferation of cisternal ER [11••,13]. Moreover, purified yeast Rtn1p or Yop1p, when reconstituted into phospholipid vesicles, leads to the formation of tubular structures in vitro. RTN and DP1/Yop1p have been shown to form immobile arc-shaped oligomers that promote membrane tubulation [36]. Together, these studies show that RTN and DP1/Yop1p are responsible for tubule formation both in vitro and in vivo.
In addition to forming ER tubules, RTN and DP1/Yop1p are thought to be involved in other membrane shaping events, such as generating the sharply curved edges of ER sheets and nuclear pores. By lining the edges of ER sheets with open arcs that possess a small radius of curvature, RTN and DP1/Yop1p are able to bring the two apposing faces of the sheets into close proximity [35]. Given that RTN and DP1/Yop1p are essentially excluded from the flat faces of ER sheets and that the levels of these proteins can shift the balance between the tubular ER network and membrane sheets [17••], additional mechanisms may exist to regulate the local abundance of RTN and DP1/Yop1p.
The induction of membrane curvature by membrane-associated proteins is a common mechanistic theme for cellular events that involve membrane deformation. One such event is COPII vesicle formation and budding at ERES. Although COPII vesicle assembly has been well studied [37], the higher order structure of ERES remains elusive. A recent study by Okamoto et al. [38] demonstrated that ERES localization in budding yeast is restricted to highly curved domains of the ER, such as ER tubules and the edges of ER sheets, where Rtn1p is concentrated. Combined deletions of RTN1, RTN2 and YOP1 were also shown to alter ERES clusters, which still localize to the edges of the aberrantly expanded ER sheets [38]. Apparently, highly curved regions of the ER are preferred sites for ERES formation. With the ability to generate high membrane curvature, RTN and DP1/Yop1p may provide the underlying architecture necessary to promote the local ERES organization and integrity that facilitates efficient COPII vesicle assembly.
Nuclear pores (where the inner and outer nuclear membranes are fused) represent domains of high curvature. Some studies have suggested that RTN and DP1/Yop1p are involved in NE assembly [10,39]. Dawson et al. [12] showed that, in yeast, the rtn1Δyop1Δ mutant exhibits severe defects in the structure and distribution of nuclear pore complexes, which in turn perturbs nuclear import capacity. The rtn1Δyop1Δ mutant is also synthetically lethal or sick when combined with mutants that harbor mutations in Nup (nucleoporin) subcomplexes, and antibody against Rtn4a specifically inhibits nuclear pore formation in vitro [12]. These findings implicate RTN and DP1/Yop1p in NPC assembly and suggest that the membrane-shaping properties of these proteins play a role in nuclear pore formation and stability.
Three-way junctions
Three-way junctions are key elements of the ER network. They are formed when the tip of an ER tubule fuses to the side of another tubule, forming a new polygon within the network (Figure 1). Two parallel studies revealed that atlastin, a dynamin-like GTPase, is a critical mediator of homotypic ER fusion [15••,16••]. Consistent with its role in ER tubule fusion, atlastin displays a punctate distribution along ER tubules and is enriched at three-way junctions [23,29]. Anti-atlastin antibodies were shown to inhibit ER network formation in vitro, suggesting a critical role for this protein in shaping the ER [15••]. Orso and colleagues [16••] provided the first direct evidence that Drosophila atlastin is sufficient to catalyze membrane fusion in vitro. In vivo depletion studies in Drosophila neurons revealed that the loss of atlastin causes discontinuity of the ER lumen and a fragmented ER network [16••], whereas overexpression of a GTPase deficient form of atlastin leads to long unbranched ER tubules in mammalian cells [15••].
Recent structural studies suggest that two atlastin molecules, residing on opposing membranes, cross-link those membranes by dimerizing with each other in a GTP-dependent manner [40]. Consistent with this model, X-ray crystallography data revealed two independent dimeric forms of atlastin that correspond to either the prefusion or post-fusion state [41••,42••]. Fusion requires the hydrolysis of GTP and the interaction of atlastin with the lipid bilayer [43,44].
The functional orthologue of atlastin in the yeast Saccharomyces cerevisiae, Sey1p, like atlastin, localizes to the three-way junctions of the ER network [11••,15••]. The sey1 mutant displays expanded ER sheets at the expense of branched ER tubules [11••] and a significant delay in ER membrane fusion [11••,45]. Additionally in yeast, the t-SNARE Ufe1p provides an alternate fusion pathway. Consistent with this proposal, the sey1Δufe1-1 double mutant exhibits an exaggerated delay in ER membrane fusion [45].
A screen of the yeast deletion library for defects in ER morphology uncovered a role for Lnp1p, a member of the Lunapark family, in morphogenesis of the ER network. Lunapark is evolutionarily conserved in eukaryotes and localizes to the three-way junctions of the peripheral ER network in yeast and mammalian cells [11••]. In its absence, the cortical ER network becomes more densely reticulated [11••]. The deletion of LNP1 in rtnΔyop1Δ mutants also results in a severe growth defect and an enhanced ER morphology defect. Interestingly, these defects are rescued by the loss of SEY1. Thus, Lnp1p acts in synergy with the reticulons and Yop1p, but in antagonism to Sey1p. We speculate that Lnp1p may promote polygon loss through ring closure, or act as an inhibitor of Sey1p function [11••].
ER morphology-related neurological diseases
Defects in ER morphology are believed to be the major pathogenic cause of hereditary spastic paraplegias (HSP), a class of human neurological disorders subject to length-dependent axonophathy of corticospinal motor neurons. Dominant mutations in spastin, atlastin or REEP1, and their family members, account for most cases of HSP [18,19,46]. Lunapark was first identified and linked to neuronal function in a gene-mapping study by Spitz and colleagues [47], who observed that the gene encoding this protein is transcribed in various domains of the central nervous system during embryo development in mice and chickens. In Caenorhabditis elegans, Lunapark is expressed in neuronal structures and is involved in synaptic vesicle transport [48]. Residing at the junctions of the ER network, Lnp1p works in conjunction with HSP-related family members (Rtn1p, Yop1p and Sey1p) to maintain ER morphology [11••] (Figure 2a). These findings imply that Lunapark may play an essential role in the development and function of neurons which, being highly elongated, are more sensitive to defects in ER morphology.
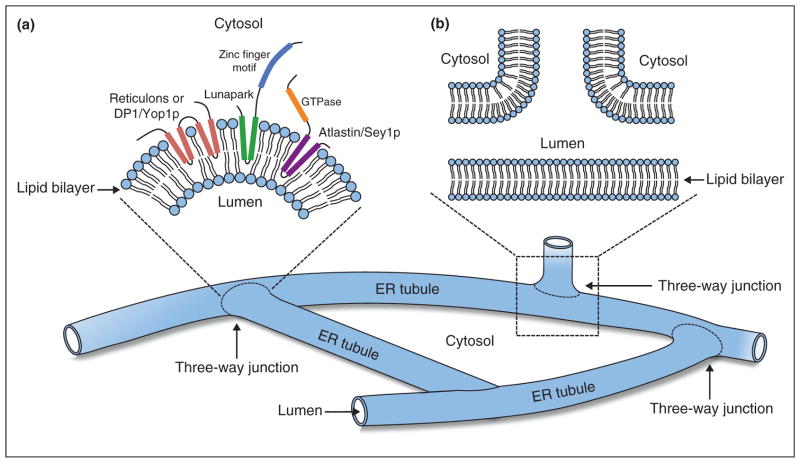
Figure 2.
Schematic diagram of the ER shaping proteins at three-way junctions. (a) Membrane topology around a three-way junction where reticulons (coral), DP1/Yop1p (coral), Lunapark (green and blue) and atlastin (purple and orange) insert into the outer leaflet of the phospholipid bilayer from the cytosolic side of the membrane. (b) A cross-section view of a three-way junction.
Conclusions
At steady state, ring-closure must counterbalance polygon formation [7], thus building and remodeling the ER network require the involvement of a number of processes and components. Three-way ER junctions can be formed by tubule branching and fusion [7]; however, other mechanisms may be involved as well [49]. Despite the identification of atlastin and Lunapark as three-way junction associated proteins, the organization of these junctions at a more detailed level is unclear. The topological structure of the junction raises the question of how the membrane achieves the required complex curvature. Junction-specific components must exist to generate and stabilize local membrane curvature at the junction. These components could be either integral membrane proteins generating negative curvature within the outer leaflet or positive curvature within the inner leaflet of the lipid bilayer, or scaffold proteins shaping the membrane from either the cytoplasmic or lumenal side of the ER membrane (Figure 2b). It is tempting to speculate that such proteins might determine the site of tubule branching and fusion. Identifying the machinery that regulates junction-related events will be important for future studies.
Acknowledgments
The research in our laboratories was supported by a grant from the National Institutes of Health (GM073892) and the Howard Hughes Medical Institute. Salary support for SC and SF-N was provided by the Howard Hughes Medical Institute.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Baumann O, Walz B. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol. 2001;205:149–214. doi: 10.1016/s0074-7696(01)05004-5. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe R, Riezman H. Differential ER exit in yeast and mammalian cells. Curr Opin Cell Biol. 2004;16:350–355. doi: 10.1016/j.ceb.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Friedman JR, Voeltz GK. The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol. 2011;21:709–717. doi: 10.1016/j.tcb.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voeltz GK, Rolls MM, Rapoport TA. Structural organization of the endoplasmic reticulum. EMBO Rep. 2002;3:944–950. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Y, Ferro-Novick S, Novick P. Dynamics and inheritance of the endoplasmic reticulum. J Cell Sci. 2004;117:2871–2878. doi: 10.1242/jcs.01286. [DOI] [PubMed] [Google Scholar]
- 6.Estrada P, Kim J, Coleman J, Walker L, Dunn B, Takizawa P, Novick P, Ferro-Novick S. Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J Cell Biol. 2003;163:1255–1266. doi: 10.1083/jcb.200304030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C, Chen LB. Dynamic behavior of endoplasmic reticulum in living cells. Cell. 1988;54:37–46. doi: 10.1016/0092-8674(88)90177-8. [DOI] [PubMed] [Google Scholar]
- 8.Lu L, Ladinsky MS, Kirchhausen T. Cisternal organization of the endoplasmic reticulum during mitosis. Mol Biol Cell. 2009;20:3471–3480. doi: 10.1091/mbc.E09-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waterman-Storer CM, Salmon ED. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr Biol. 1998;8:798–806. doi: 10.1016/s0960-9822(98)70321-5. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DJ, Hetzer MW. Reshaping of the endoplasmic reticulum limits the rate for nuclear envelope formation. J Cell Biol. 2008;182:911–924. doi: 10.1083/jcb.200805140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Chen S, Novick P, Ferro-Novick S. ER network formation requires a balance of the dynamin-like GTPase Sey1p and the Lunapark family member Lnp1p. Nat Cell Biol. 2012;14:707–716. doi: 10.1038/ncb2523. This study identified a conserved Lunapark family member, Lnp1p that localizes to the three-way junctions of the ER and is essential for ER morphology and maintenance. The disruption of LNP1Δ in the rtn1Δyop1Δ mutant leads to synthetic sickness. This growth defect is rescued by the disruption of SEY1, suggesting that Lnp1p acts in synergy with the reticulons and Yop1p, but in antagonism to Sey1p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson TR, Lazarus MD, Hetzer MW, Wente SR. ER membrane-bending proteins are necessary for de novo nuclear pore formation. J Cell Biol. 2009;184:659–675. doi: 10.1083/jcb.200806174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Craene JO, Coleman J, Estrada de Martin P, Pypaert M, Anderson S, Yates JR, 3rd, Ferro-Novick S, Novick P. Rtn1p is involved in structuring the cortical endoplasmic reticulum. Mol Biol Cell. 2006;17:3009–3020. doi: 10.1091/mbc.E06-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Hu J, Shibata Y, Voss C, Shemesh T, Li Z, Coughlin M, Kozlov MM, Rapoport TA, Prinz WA. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science. 2008;319:1247–1250. doi: 10.1126/science.1153634. The authors provided direct evidence that purified yeast Rtn1p or Yop1p, when reconstituted into phospholipid vesicles, is sufficient to generate tubular structures in vitro. [DOI] [PubMed] [Google Scholar]
- 15••.Hu J, Shibata Y, Zhu PP, Voss C, Rismanchi N, Prinz WA, Rapoport TA, Blackstone C. A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell. 2009;138:549–561. doi: 10.1016/j.cell.2009.05.025. This study demonstrated a critical role for mammalian atlastin and Sey1p, a yeast ortholog of atlastin, in the maintenance of peripheral ER morphology. Residing on the ER, atlastin/Sey1p was shown to interact with the reticulons and DP1/Yop1p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, Micaroni M, Egorova A, Martinuzzi A, McNew JA, et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature. 2009;460:978–983. doi: 10.1038/nature08280. This work provided the first direct evidence that Drosophila atlastin is sufficient to catalyze homotypic ER membrane fusion in vitro. In vivo studies revealed that atlastin is required to maintain the continuity of the ER network, as the depletion of atlastin causes discontinuity of the ER lumen and a fragmented network. [DOI] [PubMed] [Google Scholar]
- 17••.Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. This study provided convincing evidence that ER tubules are shaped by the reticulons and DP1/Yop1p. [DOI] [PubMed] [Google Scholar]
- 18.Blackstone C, O’Kane CJ, Reid E. Hereditary spastic paraplegias: membrane traffic and the motor pathway. Nat Rev Neurosci. 2011;12:31–42. doi: 10.1038/nrn2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renvoise B, Blackstone C. Emerging themes of ER organization in the development and maintenance of axons. Curr Opin Neurobiol. 2010;20:531–537. doi: 10.1016/j.conb.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J Cell Biol. 2010;190:363–375. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wozniak MJ, Bola B, Brownhill K, Yang YC, Levakova V, Allan VJ. Role of kinesin-1 and cytoplasmic dynein in endoplasmic reticulum movement in VERO cells. J Cell Sci. 2009;122:1979–1989. doi: 10.1242/jcs.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigoriev I, Gouveia SM, van der Vaart B, Demmers J, Smyth JT, Honnappa S, Splinter D, Steinmetz MO, Putney JW, Jr, Hoogenraad CC, et al. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr Biol. 2008;18:177–182. doi: 10.1016/j.cub.2007.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.English AR, Voeltz GK. Rab10 GTPase regulates ER dynamics and morphology. Nat Cell Biol. 2013;15:169–178. doi: 10.1038/ncb2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreier L, Rapoport TA. In vitro formation of the endoplasmic reticulum occurs independently of microtubules by a controlled fusion reaction. J Cell Biol. 2000;148:883–898. doi: 10.1083/jcb.148.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terasaki M, Chen LB, Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986;103:1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrade J, Zhao H, Titus B, Timm Pearce S, Barroso M. The EF-hand Ca2+-binding protein p22 plays a role in microtubule and endoplasmic reticulum organization and dynamics with distinct Ca2+-binding requirements. Mol Biol Cell. 2004;15:481–496. doi: 10.1091/mbc.E03-07-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klopfenstein DR, Kappeler F, Hauri HP. A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 1998;17:6168–6177. doi: 10.1093/emboj/17.21.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa-Goto K, Tanaka K, Ueno T, Tanaka K, Kurata T, Sata T, Irie S. p180 is involved in the interaction between the endoplasmic reticulum and microtubules through a novel microtubule-binding and bundling domain. Mol Biol Cell. 2007;18:3741–3751. doi: 10.1091/mbc.E06-12-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SH, Zhu PP, Parker RL, Blackstone C. Hereditary spastic paraplegia proteins REEP1, spastin, and atlastin-1 coordinate microtubule interactions with the tubular ER network. J Clin Invest. 2010;120:1097–1110. doi: 10.1172/JCI40979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prinz WA, Grzyb L, Veenhuis M, Kahana JA, Silver PA, Rapoport TA. Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Biol. 2000;150:461–474. doi: 10.1083/jcb.150.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fehrenbacher KL, Davis D, Wu M, Boldogh I, Pon LA. Endoplasmic reticulum dynamics, inheritance, and cytoskeletal interactions in budding yeast. Mol Biol Cell. 2002;13:854–865. doi: 10.1091/mbc.01-04-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiederkehr A, Du Y, Pypaert M, Ferro-Novick S, Novick P. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol Biol Cell. 2003;14:4770–4782. doi: 10.1091/mbc.E03-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du Y, Walker L, Novick P, Ferro-Novick S. Ptc1p regulates cortical ER inheritance via Slt2p. EMBO J. 2006;25:4413–4422. doi: 10.1038/sj.emboj.7601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Du Y, Siegel S, Ferro-Novick S, Novick P. Activation of the mitogen-activated protein kinase, Slt2p, at bud tips blocks a late stage of endoplasmic reticulum inheritance in Saccharomyces cerevisiae. Mol Biol Cell. 2010;21:1772–1782. doi: 10.1091/mbc.E09-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibata Y, Shemesh T, Prinz WA, Palazzo AF, Kozlov MM, Rapoport TA. Mechanisms determining the morphology of the peripheral ER. Cell. 2010;143:774–788. doi: 10.1016/j.cell.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata Y, Voss C, Rist JM, Hu J, Rapoport TA, Prinz WA, Voeltz GK. The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum. J Biol Chem. 2008;283:18892–18904. doi: 10.1074/jbc.M800986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2011;14:20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto M, Kurokawa K, Matsuura-Tokita K, Saito C, Hirata R, Nakano A. High-curvature domains of the ER are important for the organization of ER exit sites in Saccharomyces cerevisiae. J Cell Sci. 2012;125:3412–3420. doi: 10.1242/jcs.100065. [DOI] [PubMed] [Google Scholar]
- 39.Kiseleva E, Morozova KN, Voeltz GK, Allen TD, Goldberg MW. Reticulon 4a/NogoA locates to regions of high membrane curvature and may have a role in nuclear envelope growth. J Struct Biol. 2007;160:224–235. doi: 10.1016/j.jsb.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daumke O, Praefcke GJ. Structural insights into membrane fusion at the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2011;108:2175–2176. doi: 10.1073/pnas.1019194108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Bian X, Klemm RW, Liu TY, Zhang M, Sun S, Sui X, Liu X, Rapoport TA, Hu J. Structures of the atlastin GTPase provide insight into homotypic fusion of endoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 2011;108:3976–3981. doi: 10.1073/pnas.1101643108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Byrnes LJ, Sondermann H. Structural basis for the nucleotide-dependent dimerization of the large G protein atlastin-1/SPG3A. Proc Natl Acad Sci U S A. 2011;108:2216–2221. doi: 10.1073/pnas.1012792108. This study and Ref. [41••] solved two X-ray structures of human atlastin that correspond to the prefusion or post-fusion states. These studies suggested that atlastin molecules residing on opposing membranes cross-link membranes by dimerizing with each other in a GTP-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu TY, Bian X, Sun S, Hu X, Klemm RW, Prinz WA, Rapoport TA, Hu J. Lipid interaction of the C terminus and association of the transmembrane segments facilitate atlastin-mediated homotypic endoplasmic reticulum fusion. Proc Natl Acad Sci U S A. 2012;109:E2146–E2154. doi: 10.1073/pnas.1208385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pendin D, Tosetto J, Moss TJ, Andreazza C, Moro S, McNew JA, Daga A. GTP-dependent packing of a three-helix bundle is required for atlastin-mediated fusion. Proc Natl Acad Sci U S A. 2011;108:16283–16288. doi: 10.1073/pnas.1106421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anwar K, Klemm RW, Condon A, Severin KN, Zhang M, Ghirlando R, Hu J, Rapoport TA, Prinz WA. The dynamin-like GTPase Sey1p mediates homotypic ER fusion in S. cerevisiae. J Cell Biol. 2012;197:209–217. doi: 10.1083/jcb.201111115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blackstone C. Cellular pathways of hereditary spastic paraplegia. Annu Rev Neurosci. 2012;35:25–47. doi: 10.1146/annurev-neuro-062111-150400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spitz F, Gonzalez F, Duboule D. A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell. 2003;113:405–417. doi: 10.1016/s0092-8674(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 48.Ghila L, Gomez M. The evolutionarily conserved gene LNP-1 is required for synaptic vesicle trafficking and synaptic transmission. Eur J Neurosci. 2008;27:621–630. doi: 10.1111/j.1460-9568.2008.06049.x. [DOI] [PubMed] [Google Scholar]
- 49.Griffing LR. Networking in the endoplasmic reticulum. Biochem Soc Trans. 2010;38:747–753. doi: 10.1042/BST0380747. [DOI] [PubMed] [Google Scholar]