Abstract
Background
Inadequate representation of the human tissue environment during a preclinical screen can result in inaccurate predictions of compound effects. Consequently, pharmaceutical investigators are searching for preclinical models that closely resemble original tissue for predicting clinical outcomes.
Methods
The current research aims to compare the impact of using serum-free medium instead of complete culture medium during the last step of psoriatic skin substitute reconstruction. Skin substitutes were produced according to the self-assembly approach.
Results
Serum-free conditions have no negative impact on the reconstruction of healthy or psoriatic skin substitutes presented in this study regarding their macroscopic or histological appearances. ATR-FTIR results showed no significant differences in the CH2 bands between psoriatic substitutes cultured with or without serum, thus suggesting that serum deprivation did not have a negative impact on the lipid organization of their stratum corneum. Serum deprivation could even lead to a better organization of healthy skin substitute lipids. Percutaneous analyses demonstrated that psoriatic substitutes cultured in serum-free conditions showed a higher permeability to hydrocortisone compared to controls, while no significant differences in benzoic acid and caffeine penetration profiles were observed.
Conclusions
Results obtained with this 3D-psoriatic skin substitute demonstrate the potential and versatility of the model. It could offer good prediction of drug related toxicities at preclinical stages performed in order to avoid unexpected and costly findings in the clinic.
General significance
Together, these findings offer a new approach for one of the most important challenges of the 21st century, namely, prediction of drug toxicity.
Keywords: Pathogenesis, 3D-psoriatic skin model, Culture conditions, Physical characterization of the stratum corneum, FTIR experiments, Percutaneous absorption
Highlights
-
•
Impact of serum-free conditions during psoriatic skin substitutes reconstruction.
-
•
Lipids disorganization of healthy and psoriatic skin substitutes.
-
•
Permeation profiles of healthy skin substitutes.
-
•
Permeation profiles of psoriatic skin substitutes.
-
•
Potential and veratility of a 3d-reconstructed model to perform dermatological testing.
1. Introduction
Skin, the largest organ of the body, ensures several complex functions such as thermoregulation, metabolism and perception [1], [2], [3]. However, the main function of the skin is to act as a barrier against the external environment and to protect the body from chemical, physical and biological aggressions [4], [5]. The epidermis, particularly the stratum corneum, provides this barrier function due to its compact structure [6]. In fact, this layer is composed of corneocytes (dead and keratinized keratinocytes), surrounded by a lipid bilayer [7], [8]. Corneocytes ensure protection against mechanical injuries, while the lipid matrix prevents the penetration of chemical compounds and microorganisms [9], [10]. Nevertheless, this structure may be disorganized in certain skin diseases such as psoriasis, thus causing a decrease in skin barrier function [3], [11].
Psoriasis is a chronic inflammatory skin disease characterized by red plaques on the skin [12]. These plaques are the result of keratinocyte hyperproliferation, immune cell infiltration, and an increase in angiogenesis [13], [14]. The hyperproliferation of cells leads to a poor differentiation of the epidermis, which causes a dysfunctional barrier function and an increased permeability of the psoriatic skin [11], [15]. Currently, there is no curative treatment available, and the development of more specific treatments with fewer side effects is a major concern in major's pharmaceutical industry [16]. However, the lack of highly predictive and representative psoriatic skin models has slowed the discovery of new antipsoriatic drugs [17]. Thus, the development of new psoriatic skin models represents a key component in the fight against psoriasis [17].
Over the past years, our team has been developing a three-dimensional psoriatic skin model reconstructed according to a self-assembly approach. These self-assembled skin substitutes, which are free of exogenous material, retain several psoriasis-like features, such as disorganization of the stratum corneum lipids [18], hyperproliferation and abnormal differentiation of the keratinocytes [19], as well as increased angiogenesis [20]. Moreover, it has been demonstrated that a retinoic acid treatment performed on this in vitro skin model exhibited similar results to that of psoriatic skin in vivo [21].
Serum is recognized as an undefined mixture which contains many growth factors and inflammation mediators that can introduce unpredictable reactions and interfere with many tests while using it during the culture process [22]. It can contain various quantities of haemoglobin, pathological agents and/or high levels of protein, which can affect analyses (such as protein test purification), or favour the growth of undesired cells [23], [24], [25]. However, this mixture is essential for cell culture in order to: 1) stimulate cell proliferation and differentiation; 2) regulate cell membrane permeability; 3) provide attachment and spreading factors; 4) maintain physical properties of the culture system and 5) inhibit proteases through stabilizing and detoxifying factors [24], [26], [27].
In 2005, Black et al. demonstrated that deprivation of serum at an advanced state of culture did not result in any negative effects on their collagen gel model [28]. In 2011, our group reported that a self-assembled human skin model, composed of healthy fibroblasts and keratinocytes, could produce a well-differentiated epidermis independently of the presence or absence of serum during the culture process [29]. These results suggest that using calf serum at an early state during the production of the substitutes is suitable to allow cell growth while using serum-deprived medium at an advanced state is acceptable to reduce unforeseen interactions and to avoid individual differences between serum lots [30].
On the other hand, it is important to note that inadequate representation of the human tissue environment during a preclinical screen can result in inaccurate predictions of compound effects. Moreover, because modeling pathological processes through skin tissue engineering could face different challenges than healthy substitutes reconstruction, the impact of using a serum-free medium instead of a complete culture medium at the air-liquid interface step has been evaluated during the reconstruction process of psoriatic skin substitutes and compared with that of healthy skin substitutes. Since pharmaceutical investigators are searching for preclinical models that closely resemble original tissue for predicting clinical outcomes, a specific control on culture conditions has been considered in the reconstruction of psoriatic skin substitutes. This model will allow researchers to perform dermopharmaceutical testing and pathology studies in a human context to gain further insight into physiological processes.
2. Materials and methods
2.1. Patients
Four patients with plaque psoriasis were recruited from the dermatology department of the Hôpital de l’Enfant-Jésus, Québec, QC, Canada. The subjects were Caucasian females aged between 46 and 65 years (cell populations: ♀46, ♀47, ♀51 and ♀65). Six-millimeter punch biopsies were taken under local anesthesia from lesional skin. Skin samples used to generate healthy primary human cell banks were derived from breast reduction surgeries from a non-psoriatic patient (cell populations: ♀18, ♀38 and ♀42). Normal human skin was obtained from breast reduction surgeries (Dermatology department of CHU de Québec, Québec, QC) and psoriatic skin was from NDRI (National Disease Research Interchg, Philadelphia, PA). This study was conducted in agreement with the Helsinki declaration and performed under the guidelines of the research ethics committee of the “CHU de Québec”. All patients were given adequate information to provide written consent.
2.2. Cell culture media
Fibroblasts were cultured in the Dulbecco-Vogt modification of Eagle's medium (DMEM) supplemented with 10% fetal calf serum (Invitrogen, Burlington, ON, Canada), 100 UI/ml penicillin G (Sigma, Oakville, ON, Canada) and 25 μg/ml gentamicin (Schering, Pointe-Claire, QC, Canada). Keratinocytes were cultured in a combination of DMEM with Ham's F12 (3:1) supplemented with 5% Fetal Clone II serum (Hyclone, Scarborough, ON, Canada), 5 μg/ml insulin (Sigma), 0.4 μg/ml hydrocortisone (Calbiochem, EMD Biosciences, Gibbstown, NJ, USA), 10-10 M cholera toxin (MP Biomedicals, Montréal, QC, Canada), 10 ng/ml human epidermal growth factor (EGF) (Austral Biological, San Ramon, CA, USA), 100 UI/ml penicillin G (Sigma) and 25 μg/ml gentamicin (Schering).
2.3. Cell culture
As previously described [19], keratinocytes were extracted using the isolation method with thermolysin and trypsin [31]. According to this method, keratinocytes (passage 1) were seeded at 8×103 cells/cm2 on a feeder layer of irradiated 3T3 mouse fibroblasts. Fibroblasts were extracted using the isolation method with thermolysin and collagenase [32], [33]. They were seeded at 4×103 cells/cm2 and used at passage 6 for skin substitute's production. All cultures were incubated at 37 °C in an 8% CO2 air atmosphere and changed three times a week with the media previously described in the cell culture media section. Cells were frozen at −150 °C until needed.
2.4. Production of tissue-engineered skin substitutes
Skin substitutes were produced according to the self-assembly method, as previously described [19]. Briefly, the fibroblasts were cultured in the presence of ascorbic acid at a concentration of 50 µg/ml (Sigma, Mississauga, ON, Canada) to form manipulatable sheets. After 28 days, dermal sheets were carefully detached from flasks with curved forceps. Then, dermal sheets were superimposed and a Merocel® sponge was placed on the top of the substitutes to facilitate the natural fusion of dermal sheets leading to the formation of a new dermal layer [34]. After seven days, the keratinocytes (passage 2) were seeded on the dermal equivalent to form a new epidermal layer. After seven days of growth, the substitutes were raised to the air-liquid interface. Deprivation of serum was initiated at day 0 of the air-liquid interface culture before the beginning of the differentiation process [35]. Substitutes were cultured in serum-free medium (DMEM with Ham's F12+ additives) over a period of 21 days and compared to the controls, which were cultured in the presence of serum over the complete experiment. After 21 days of culture at the air-liquid interface, biopsies of the skin substitutes were analyzed.
2.5. Histological analysis
Two biopsies of each combination were fixed in HistoChoice® solution and embedded in paraffin wax. Five-micrometer thick sections were cut and stained with Masson's Trichrome.
2.6. Immunolabelling analyses
After 21 days of culture at the air–liquid interface, biopsies were taken and the samples were embedded in Tissue-Tek OCT compound (Somagen Diagnostics, Edmonton, Alberta, Canada) to be stored at –80 °C until needed. Indirect immunofluorescence analyses were performed on acetone-fixed cryosections (5 µm thick). The antibodies used were: rabbit polyclonal anti-ki67 (dilution 1:400; Abcam, Cambridge, United Kingdom); mouse monoclonal (IgG1) anti-involucrin (dilution 1:800; Sigma-Aldrich, Saint-Louis, Missouri, United States); mouse monoclonal (IgG1) anti-human filaggrin (dilution 1:800; Abcam, Cambridge, United Kingdom). The second antibody was goat anti-rabbit IgG (H+L) Alexa 594 (dilution 1:800; Life Technologies, Carlsbad, California, United States); or rabbit anti-mouse IgG (H+L) Alexa 488 (dilution 1:2000, Life Technologies, Carlsbad, California, United States). The nuclei were labelled with Hoechst reagent 33258 (dilution 1:100; Sigma) and added to the secondary antibody solution. For cell proliferation, ki67-positive stained cells were counted for each combination. Twenty fields of each combination were counted.
2.7. Isolation of the stratum corneum sample
For skin substitutes, stratum corneum was mechanically separated from the epidermis after 21 days of culture at the air–liquid interface. For substitutes, epidermis was easily removable by a mechanical technique. The isolated stratum corneum was kept at 4 °C until analyses.
2.8. FTIR experiments
Infrared spectra of the stratum corneum of the skin substitutes were obtained using a Golden Gate single reflection attenuated total reflection system (Specac, Pleasantville, NY, USA) fitted with a diamond crystal. The infrared spectra were recorded with a Nicolet Magna 860 Fourier transform spectrometer (Thermo-Nicolet, Madison, WI, USA) equipped with a narrow band mercury–cadmium–telluride (MCT) detector and a germanium-coated KBr beam splitter. At each temperature, a total of 128 interferograms were acquired, co-added and Fourier transformed, using a Happ–Genzel apodization function to give a spectral resolution of 4 cm−1 in the spectral range of 4000–750 cm−1. All data were processed with the Grams 386 software (Galactic Industries Corporation, Salem, MA, USA). The spectral region corresponding to the CH2 stretching band vibrations was baseline-corrected using a cubic function and the frequency peak position was determined using the centre of gravity at the top 10% of the bands.
2.9. Percutaneous absorption
Percutaneous absorption testing was performed using standard Franz diffusion cell technique as described by Franz [36], [37], [38]. Briefly, samples were clamped tightly between the two glass chambers of the diffusion cell (FDC-100 Standard, 0.63 cm2 surface area O-ring (Crown Glass, Somerville, NJ, USA). Receiver compartment was filled with media (DMEM supplemented with penicillin G (Sigma), gentamicin (Schering) and 0.5 µg/ml of fungizone (Bristol-Myers Squibb Canada, Montréal, QC, Canada)) and maintained at 37 °C with a water jacket. 100 µl of 14Cbenzoic acid (0,005 mCi/100 µl/chamber) (MP Biomedicals), or 14Ccaffeine (0.0005 mCi/100 µl/chamber) (Perkin Elmer, Whaltham, MA, USA) or 3H-hydrocortisone (0.0025 mCi/100 µl/chamber) (Perkin Elmer) were deposited on the substitute. Samples were taken at selected intervals (1, 2, 4, 6, 8 and 24 h) with a 5 ml syringe prolonged by a catheter (3½ FR Tom Cat Length 4½) and were conserved at 4 °C until needed. Radioactivity was determined with a scintillation counter Beckman LS 6000 SC (Beckman Instruments Inc., Fullerton, CA, USA) by adding 0.5 ml of sample to 4.5 ml of scintillation fluid Scintisafe™ 30% (Fischer Scientific ltd., Québec, QC, Canada).
2.10. Statistical analysis
Data were analyzed using the t-test: paired two samples for means, 0.05 significance level, using data analysis Excel package and expressed as means±standard deviation.
3. Results
3.1. Macroscopic aspect of skin substitutes
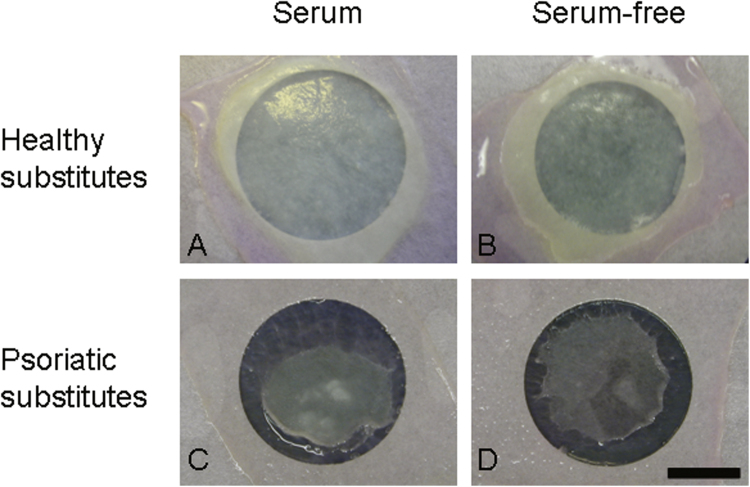
Healthy substitutes cultured with or without serum showed a uniform, smooth and white surface (Fig. 1A and B). Psoriatic substitutes cultured with serum were less uniform showing a smaller spread out of cells compared with healthy substitutes (Fig. 1C vs 1A). Serum-free culture conditions did not seem to have a strong impact on the macroscopic aspect of both kinds of substitutes reconstructed compared with their respective counterparts (Fig. 1B vs 1A and 1 D vs 1C).
Fig. 1.
Macroscopic aspects of healthy and psoriatic substitutes cultured with or without serum (scale bar=2 cm). The results were confirmed with cells from 3 (healthy substitutes) or 4 (psoriatic substitutes) patients (n=10 substitutes for each condition).
3.2. Histological aspect
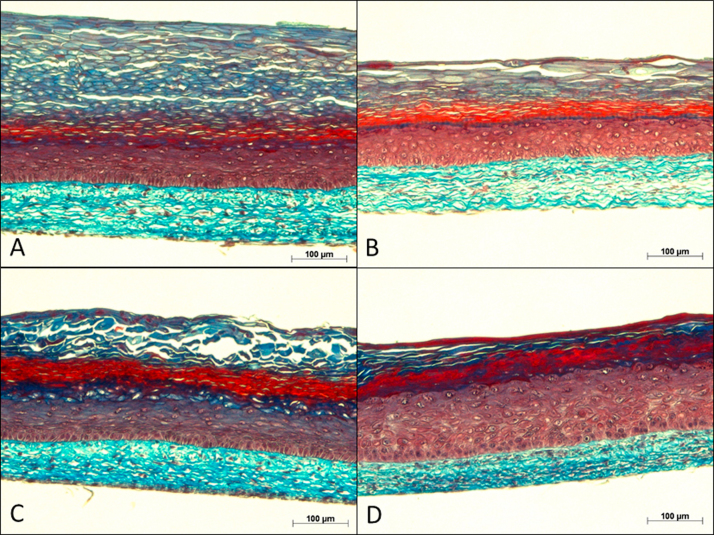
Masson's trichrome staining images of 5-μm-thick biopsies show the structure of the skin substitutes. These histological analyses confirm that the self-assembly method allows one to produce substitutes with a structure resembling that of human skin. In fact, the characteristic skin layers are easily distinguishable, with the dermis being identified by the presence of collagen fibers, appearing in light blue, and by the nuclei of the fibroblasts that are stained in purple. In addition, the cytoplasm of cells in the living epidermis displays a red color. Basophilic substances found in the stratum corneum appear in dark blue. A well-differentiated epidermis was observed in healthy substitutes cultured with serum (Fig. 2A). As previously observed by our group, psoriatic substitutes cultured in complete medium depicted a more disorganized epidermis compared with healthy substitutes (Fig. 2C vs 2A). The serum-free condition did not seem to have a strong impact on the histologic features of the skin substitutes compared with their respective counterparts. However, the stratum corneum of healthy substitutes appeared thinner with deprivation of serum, nevertheless, the living epidermis was similar (Fig. 2B vs 2A).
Fig. 2.
Masson's trichrome staining obtained from healthy and psoriatic substitutes cultured with or without serum. The results were confirmed with cells from 3 (healthy substitutes) or 4 (psoriatic substitutes) patients (n=3 substitutes for each condition) (scale bar=100 µm).
3.3. Immunolabelling analyses
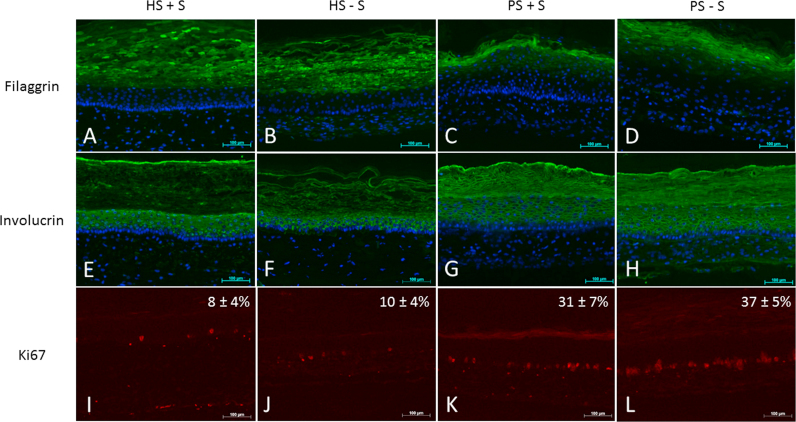
In psoriatic skin in vivo, the expression of filaggrin is diminished or totally absent compared with normal human skin. As previously demonstrated, these features were also maintained in our psoriatic skin model compared with healthy skin substitutes [19]. In the present study, this pattern was still observed for the conditions with (Fig. 3C vs 3A) or without serum (Fig. 3D vs 3B). In normal human skin, the expression of involucrin appears in the spinous layer of the epidermis, whereas in psoriatic skin, these markers are overexpressed or appeared earlier in terms of localization. This pattern was confirmed in the psoriatic substitutes (Fig. 3G vs 3 E) and even for those cultured in a serum-free medium (Fig. 3H vs 3F). These results are in accordance with previous results obtained from our group [19] and confirmed that the psoriatic phenotype was not altered following deprivation of serum. The absence of serum at the end of the reconstruction process, which is the air-liquid phase, did not seem to impact the epidermal differentiation of the substitutes (Fig. 3: HS+S vs HS−S and PS+S vs PS−S).
Fig. 3.
Proliferation and Differentiation markers. Ki-67, filaggrin and involucrin stainings of healthy substitutes cultured with (HS+S) or without (HS−S) serum, psoriatic substitutes cultured with (HS+S) or without (HS−S) serum; scale bar=100 µm. The results were confirmed with 3 experiments (total of six samples for each condition). Nuclei were stained with Hoechst. (Magnification×10). Percentage of positive Ki-67 cells are presented as mean±SD.
3.4. Cell proliferation
Immunolabelling of basal layer Ki67 positive cells was performed and cells were counted to quantify cell proliferation in the skin substitutes (Figure 3I–3L). Psoriatic skin substitutes, both, with or without serum (Figs. 3K and 3L), showed higher cell proliferation, more than 3 times, compared with healthy skin substitutes (Figs. 3I and 3J). These results are in accordance with the hyperproliferation observed in psoriatic skin. Hyperproliferating keratinocytes is a category of anomalies that contributes to the symptoms of psoriasis [39].
3.5. ATR-FTIR spectroscopy
ATR-FTIR is a well-established method to evaluate the order of hydrocarbon chains in terms of the population of trans and gauche conformers, the packing behaviour, and phase transitions. Of particular interest for studying stratum corneum lipids are the bands between 2800 and 3000 cm−1 which are due to C–H stretching vibrations primarily associated with the lipid alkyl chains. These absorbances occur near 2920 and 2850 cm−1 for the asymmetric νas(CH2) and symmetric νs(CH2) vibrations, respectively. CH2 bands at low frequencies are generally characteristic of a high content of trans conformers while bands at higher frequencies are associated with the presence of gauche conformers [40], [41]. By studying the frequency variation as a function of temperature, we observe a phase transition in which the system goes from an ordered to a disordered conformation.
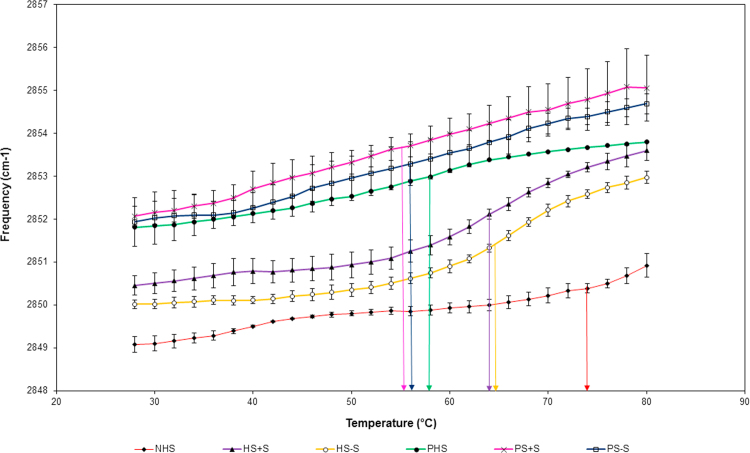
The lipid organization of the stratum corneum was analyzed by ATR-FTIR. The temperature-dependent frequencies of the symmetric CH2 mode of vibration associated to stratum corneum lipids were measured between the temperature range of 28–80 °C. Results showed that higher frequencies are obtained for psoriatic stratum corneum of the two conditions tested (with or without serum) compared with their respective healthy controls (Fig. 4: pink line compared with purple line) and (Fig. 4: blue line compared with yellow line). These results are in accordance with those obtained for both kinds of skin in vivo (Fig. 4: green line compared with red line). At lower temperatures, frequencies around 2850 cm−1 are observed for healthy stratum corneum compared with 2852 cm−1 for psoriatic stratum corneum. Moreover, the lipid phase transition occurred between 50 and 60 °C for psoriatic skin and around 65–75 °C for healthy skin (Fig. 4). These results suggest a more disorganized lipidic system of psoriatic stratum corneum, which correlates with a more permeable skin of these patients such as reported in the literature [42]. It is interesting to note that there is a slight increase in the order of the lipid chains for the healthy substitutes cultured without serum (Fig. 4, yellow line) compared with their controls (healthy substitutes cultured in the complete medium; Fig. 4, purple line) suggesting that deprivation of serum could ever have a positive impact on the organization of the stratum corneum lipids of substitutes. These results are in accordance with a previous study of our group [29]. Moreover, psoriatic skin substitutes did not appear to be affected by a serum-free culture in regards to their lipid chain order (Fig. 4: pink line compared with blue line).
Fig. 4.
Stratum corneum lipid organization results obtained following ATR-FTIR spectroscopy analyses. Healthy substitutes with serum (HS+S), psoriatic substitutes with serum (PS+S), healthy substitutes cultured in serum-free condition (HS−S), psoriatic substitutes cultured in serum-free condition (PS−S), normal human skin (NHS) and psoriatic human skin (PHS). The results were confirmed with cells from 3 (healthy substitutes) or 4 (psoriatic substitutes) patients (n=3 substitutes for each condition).
3.6. Percutaneous absorption
3.6.1. Kinetics of percutaneous absorption
3.6.1.1. Hydrocortisone
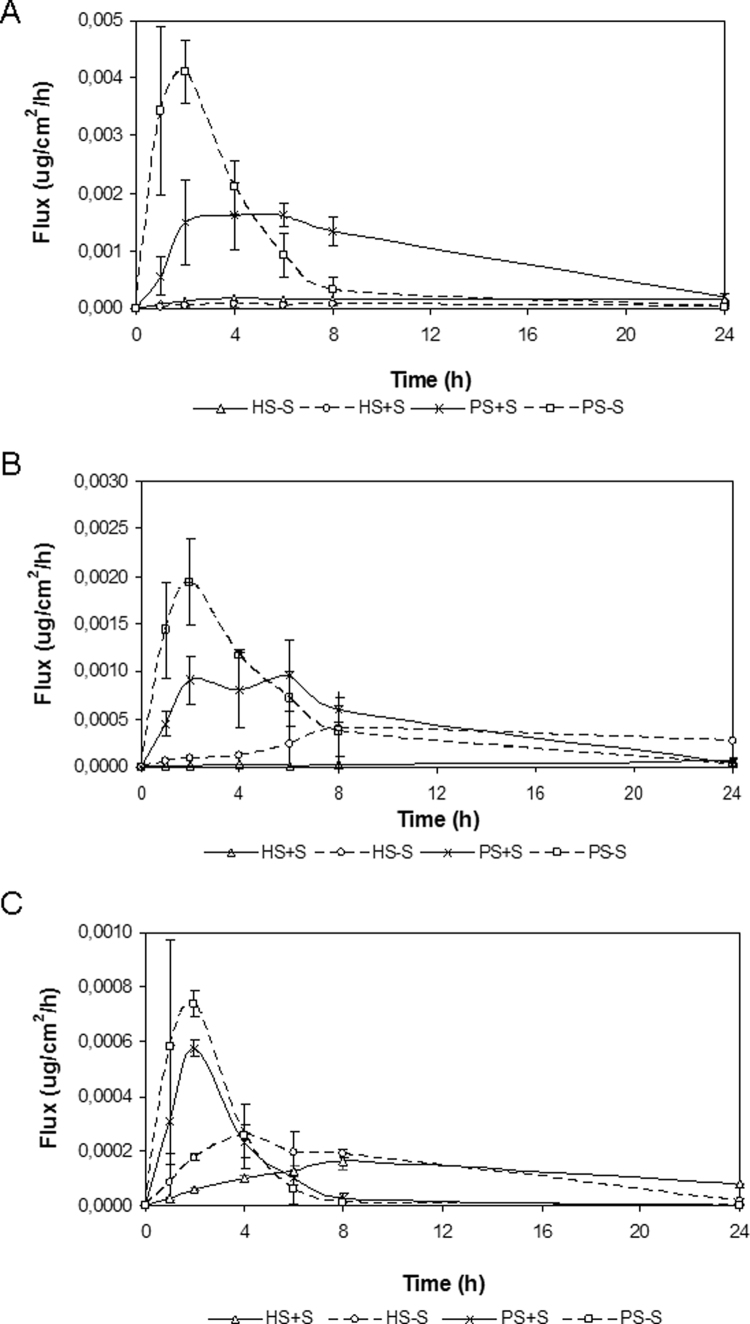
In healthy skin substitutes cultured with serum (HS+S), hydrocortisone mean fluxes increased for 6 h (6.11×10−5 ±2.20×10−5 µg/cm2/h) and then reached a steady state until 24 h. Healthy substitutes cultured without serum (HS-S) showed an increase of hydrocortisone mean fluxes for 4 h (1.64×10−4 ±9.50×10−5 µg/cm 2/h) before reaching a steady state until 24 h. A significant difference was observed between HS+S and HS-S at the 24 h time point only. In psoriatic skin substitutes cultured with serum (PS+S) hydrocortisone mean fluxes rapidly increased at 1 h, reached a steady state between 2 h and 6 h (1.49×10-3 ±7.35×10-4 µg/cm2/h; 1.61×10−3 ±1.98×10−4 µg/cm2/h) and then decreased until 24 h. From 2 h, significant differences were observed between PS+S and HS+S. Mean fluxes of psoriatic skin substitutes cultured in serum-free condition (PS-S) rapidly increased at 1 h, reached a peak at 2 h (4.10×10−3 ±5.55×10−4 µg/cm2/h), where a significant difference appeared from the three other conditions (PS+S, HS-S, HS+S), and then, a decrease took place until 24 h (2.15×10−5 ±1.86×10−5 µg/cm2/h). At 24 h, hydrocortisone mean fluxes were still significantly different between the PS−S and PS+S conditions (Fig. 5).
Fig. 5.
Fluxes of hydrocortisone (A), benzoic acid (B) and caffeine (C) through healthy and psoriatic skin substitutes. The samples were taken after 0, 1, 2, 4, 6, 8 and 24 h. Healthy substitutes with serum (HS+S), healthy substitutes cultured in serum-free condition (HS−S), psoriatic substitutes with serum (PS+S), and psoriatic substitutes cultured in serum-free condition (PS−S). The results were confirmed with cells from 3 (healthy substitutes) or 4 (psoriatic substitutes) patients (n=3 substitutes for each condition).
3.6.1.2. Benzoic acid
In healthy skin substitutes cultured with serum (HS+S) benzoic acid mean fluxes were of linear aspect showing no peak. The maximum value was obtained at 24 h (5.65×10−5±4.15×10−6 µg/cm2/h). Benzoic acid mean fluxes of healthy skin substitutes cultured without serum (HS-S) increased for 8 h, then reached a steady state until 24 h (2.70×10−4 ±1.10×10−4 µg/cm2/h). At this point, a significant difference was observed between HS-S and HS+S. In psoriatic skin substitutes cultured with serum (PS+S) benzoic acid mean fluxes increased while it stagnated at 2 h (9.11×10−4 ±2.47×10−4 µg/cm2/h) to decrease until 24 h (4.73×10−5 ±4.01×10−5 µg/cm2/h). Significant differences were observed at 1, 2, 4, 6 and 8 h compared with HS+S. Psoriatic substitutes cultured without serum (PS-S) showed a very rapid increase of the mean fluxes, a peak at 2 h (1.94×10−3 ±4.64×10−4 µg/cm2/h) and a decrease until 24 h (2.42×10−5 ±2.03×10−5 µg/cm2/h). No significant difference was observed between PS−S and PS+S whereas significant differences were observed between PS−S and HS−S at 1, 2, 4 and 24 h (Fig. 5).
3.6.1.3. Caffeine
In healthy skin substitutes cultured with serum (HS+S) caffeine mean fluxes were of linear and continuous aspect. The maximum value was obtained at 8 h (1.64×10−4 ±3.23×10−5 µg/cm2/h) and then decreased until 24 h (7.66×10−5 ±2.43×10−6 µg/cm2/h). Healthy substitutes cultured without serum (HS-S) showed an increase of caffeine mean fluxes and a peak at 4 h (2.56×10−4 ±1.33×10−5 µg/cm2/h). Significant differences were observed at 2, 4, 6 and 24 h. In psoriatic skin substitutes cultured with serum (PS+S) caffeine mean fluxes rapidly increased with a peak observed at 2 h (5.77×10−4 ±3.28×10−5 µg/cm2/h) and then decreased until 24 h (1.15×10−6 ±7.50×10−7 µg/cm2/h). Significant differences were observed between PS+S and HS+S at 2, 4, 8 and 24 h. Psoriatic substitutes cultured without serum (PS-S) showed a very rapid increase of mean fluxes, a peak at 2 h (7.38×10−4 ±4.95×10−5 µg/cm2/h) and a decrease until 24 h (2.93×10−7 ±1.62×10−7 µg/cm2/h). Significant differences were observed at 2 h between: PS−S and PS+S; PS−S and HS−S at 2, 6 and 8 h (Fig. 5).
3.6.2. Permeability of healthy and psoriatic skin substitutes
As shown in Table 1, healthy skin substitutes cultured without serum (HS-S) showed higher permeability to benzoic acid and caffeine (5–3 folds higher, respectively) compared with healthy skin substitutes cultured with serum (HS+S) after 2 h, while no significant difference was noted for hydrocortisone. Similar results were observed at 4 h (*significant). Our results showed that psoriatic skin substitutes are more permeable to the three molecules tested than healthy skin substitutes. More precisely, after 2 h, it appeared that the permeability of psoriatic skin substitutes cultured with serum (PS+S) was more important than what was observed for healthy skin substitutes (HS+S) regarding hydrocortisone, benzoic acid and caffeine (30, 46 and 10 folds higher, respectively). Similar results were observed at 4 h (27, 40 and 4 folds, respectively) and 24 h (16 and 6 folds, respectively except for caffeine where no significant differences were noted at this time point) (**significant). Finally, no significant differences were observed between psoriatic skin substitutes cultured with (PS+S) or without (PS−S) serum for benzoic acid and caffeine at any time points. However, a more important permeability to hydrocortisone was observed at 2 and 4 h (4 and 2 folds higher, respectively) when psoriatic skin substitutes were cultured in serum-free conditions (***significant). Although there was clear evidence that serum-free conditions increased the permeation profiles of all three molecules tested, the order of magnitude of their respective cumulative amounts correlated for each kind of substitutes and their controls.
Table 1.
Cumulative amount of hydrocortisone, benzoic acid and caffeine through the healthy and psoriatic skin substitutes cultured with or without serum. All data presented are the mean±standard deviation. The results were confirmed with cells from 3 (healthy substitutes) or 4 (psoriatic substitutes) patients (n=3 substitutes for each condition).
| Condition |
Cumulative amount (×10-5µg) |
||
|---|---|---|---|
| 2 h | 4 h | 24 h | |
| Hydrocortisone | |||
| HS+S | 4.24±2.27 | 12.20±5.92 | 57.50±10.80 |
| HS-S | 12.90±6.10 | 33.60±18.10 | 227.00±97.80 |
| PS+S | 129.00±67.30** | 332.00±140.00** | 896.00±99.40** |
| PS-S | 476.00±118.00*** | 741.00±73.90*** | 920.00±29.50 |
| Benzoic acid | |||
| HS+S | 1.87±0.84 | 4.71±1.16 | 68.30±3.59 |
| HS-S | 9.61±4.59* | 24.70±11.60* | 377.00±172.00 |
| PS+S | 86.00±23.50** | 189.00±75.40** | 433.00±141.00** |
| PS-S | 213.00±60.50 | 360.00±58.60 | 521.00±31.60 |
| Caffeine | |||
| HS+S | 5.38±0.75 | 17.90±1.95 | 132.00±7.40 |
| HS-S | 16.20±5.22* | 48.50±6.86* | 115.00±28.80 |
| PS+S | 55.90±16.00** | 85.20±22.10** | 102.00±24.90 |
| PS-S | 83.20±25.60 | 115.00±11.20 | 124.00±2.99 |
significant between HS+S and HS-S),
significant between PS+S and HS+S), and
significant between PS+S and PS-S).
4. Discussion
Fetal calf serum is an ill-defined mixture constituted of various nutrients, growth factors and hormones, which are crucial for growth and cell differentiation [43]. However, previous research had demonstrated the possibility to culture healthy substitutes in serum-free conditions [28], [29], [44]. This alternative is suitable to reduce production costs and to increase reproducibility as well as unforeseen interactions following the use of undefined serum mixtures [28], [29]. The present study was designed to compare the effects encountered on psoriatic skin substitutes while using serum-free instead of complete culture medium at the air-liquid interface.
In this current study, we demonstrate that it is possible to produce 3D-psoriatic skin substitutes using a serum-deprived medium, which show similar psoriasis-like features such as their counterparts cultured in a complete medium. Hyperproliferation and abnormal differentiation of the epidermal cells as well as lipid disorganization and higher permeability of the stratum corneum compared with healthy substitutes were observed. The possibility of culturing 3D-psoriatic skin substitutes in serum-free medium can be explained in part by the fact that starvation of serum takes place at the air-liquid interface, which is an advanced state of culture, meaning that keratinocytes had finished their proliferation and have begun their differentiation [35].
The functionality of the skin barrier is assured by its outermost layer, the stratum corneum, which is a complex mosaic, composed of corneocytes embedded in a specialized lipid matrix [7], [45], [46], [47]. The permeation of a molecule through skin can be measured by a percutaneous absorption technique and its pathway (intercellular and/or transcellular routes) can be determined regarding its physicochemical characteristics [45]. In this study, we followed the penetration profiles of three molecules: hydrocortisone, benzoic acid and caffeine. The three penetrants were chosen because they covered a wide polarity range. Hydrocortisone, although usually referred to as a polar steroid, is a relatively non-polar material and is likely, therefore, to penetrate the skin mainly by a lipid route with a small fraction partitioning into the polar pathway. Benzoic acid, a highly polar molecule, should permeate preferentially through the polar pathways in the stratum corneum. And caffeine, as an amphiphilic molecule, should permeate via both routes [48], [49], [50], [51].
Percutaneous absorption analyses showed that healthy skin substitutes cultured in serum-free conditions are significantly more permeable to benzoic acid and caffeine (5–3 folds higher, respectively) than their respective controls after 2 and 4 h (Table 1*). Hydrocortisone diffusion results were not significantly different at these same time points suggesting that a slight increase in the lipid chains order of the healthy skin substitutes cultured without serum (ATR-FTIR results, Fig. 4, yellow line) has an impact mainly on molecules that permeate via the lipid route and less on molecules that could follow the polar route, even as a partial combination of permeation pathways, such as for caffeine. A possible explanation for this is probably related to corneocyte lipid envelopes. In some regions of the stratum corneum, intercellular lamellae are absent. This most often occurs at the periphery of the corneocytes, in regions where cell-to-cell cohesion is maintained by desmosomes. However, where the paired bilayers are absent, the lipid envelopes of the apposing corneocytes are not in direct contact but are separated from each other by a lipid monolayer. In fact, sphingosine chains are everted to form an intervening mutual monolayer. We believe that there is a lack of important categories of lipids in our substitutes such as reported by other groups in the field of tissue engineering [52], which belong to the lipids involved in the cohesion of the corneocyte envelope. Even if ATF-FTIR spectroscopy reports an increased order of lipid chains, it is probably related to the intercellular lamellae of the lipid route instead of the lipids of the corneocyte envelopes, which are more likely to be involved in the permeation pathways of benzoic acid and caffeine than hydrocortisone. The significant increases in the cumulative amounts of benzoic acid and caffeine suggest that the pathways they both follow are more altered than the one followed by hydrocortisone for which no significant differences were reported (Table 1).
The percutaneous absorption profiles of psoriatic skin substitutes were very rapid, reaching a peak at 2 h for all molecules tested compared with healthy skin substitutes (Fig. 5). When psoriatic substitutes were cultured in a complete medium, the diffusion results after 2 and 4 h of hydrocortisone, benzoic acid and caffeine were 30–27, 45–40, 10–4 folds higher respectively than those observed for healthy skin substitutes, demonstrating that the cutaneous barrier of psoriatic skin substitutes is less functional than the healthy skin substitutes such as skin in vivo (Table 1**). These results are in agreement with the ATR-FTIR results, which showed an important intercellular lipid disorganization of the stratum corneum of the psoriatic skin substitutes cultured with serum compared with healthy substitutes cultured in the same condition (Fig. 4). For the psoriatic cells, a serum-deprived medium was comparable to a complete medium since both culture conditions showed psoriasis-like lipid disorganization of the stratum corneum (Fig. 4). Moreover, psoriatic skin substitutes cultured without serum showed no significant difference for benzoic acid and caffeine permeation profiles whereas significant differences were noted for hydrocortisone after 2 and 4 h (Table 1***), suggesting a better lipid organization of the non-polar region but a slight disorganization of the polar region of the stratum corneum lipids. The high skin barrier permeability of the psoriatic skin substitute can be explained by an abnormal differentiation process of its epidermis, thus causing a poor cohesion with a lipid disorganization of its stratum corneum (polar and non-polar regions)[53], [54]. These results confirm that substitutes reconstructed according to the self-assembly method presented in this study have the possibility to selectively make a difference in their barrier function towards the different physicochemical properties of compounds tested, which appears to be similar to their respective counterparts. Nevertheless, healthy and psoriatic substitutes showed differences in that matter: lipophilic molecules profiles appeared to be more affected than hydrophilic and amphiphilic molecules profiles by deprivation of serum for psoriatic skin substitutes compared with healthy skin substitutes. In fact, the opposite was observed for the latter: benzoic acid and caffeine were significantly more permeable than hydrocortisone while using serum-free medium.
In conclusion, we have clearly shown that serum-free conditions have no negative impacts on the reconstruction of healthy or psoriatic skin substitutes presented in this study regarding their macroscopic or histological appearances. ATR-FTIR spectroscopy results even showed that this condition could lead to a better organization of healthy skin substitute lipids. However, since the permeability barrier not only depends on organization of lipids in the stratum corneum, but also on their composition, our next goal will be to improve the stratum corneum lipid properties by increasing the resemblance of their lipid content and composition to that of native human stratum corneum. In that matter, psoriatic and healthy substitutes will probably need different modulations of their respective culture conditions since different penetration profiles were observed depending on the physicochemical properties of the molecules tested. Indeed, optimization of culture conditions is still a focus of future research in the field of tissue engineering since it has been reported that under the used conditions of our actual protocols, native human skin will not be able to form a stratum corneum with lipid properties similar to that observed in vivo [55]. Modulation of culture conditions, such as the use of defined media and/or the addition of specific media supplements, will offer the opportunity to further improve the culture medium and these advancements are necessary to develop healthy or pathological substitutes, which can mimic closely their in vivo counterparts. Finally, results obtained with this 3D-psoriatic skin substitute demonstrate the potential and versatility of the model. It could offer good prediction of drug related toxicities at preclinical stages performed in order to avoid unexpected and costly findings in the clinic.
Author disclosure statement
No competing financial interests exist.
Acknowledgements
This study was supported by the Canadian Institutes of Health Research (CIHR) (MOP-86498; MOP-90283). A. D-F. and I. G. received studentships from the Fonds d’Enseignement et de Recherche (FER) of the Faculté de Pharmacie, Université Laval, Québec, QC, Canada. R. P. is a career award scholar from the Fonds de Recherche du Québec-Santé (FRQS) (30848).
Footnotes
Transparency data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2016.09.012.
Contributor Information
Alexandra Duque-Fernandez, Email: alexandra_df@yahoo.com.
Lydia Gauthier, Email: lydiagauthier@gmail.com.
Mélissa Simard, Email: melissa.simard.6@ulaval.ca.
Jessica Jean, Email: jessica.jean@pha.ulaval.ca.
Isabelle Gendreau, Email: isabelle.gendreau.1@ulaval.ca.
Alexandre Morin, Email: alexandre.morin.12@ulaval.ca.
Jacques Soucy, Email: jfsoucy@videotron.ca.
Michèle Auger, Email: michele.auger@chm.ulaval.ca.
Roxane. Pouliot, Email: roxane.pouliot@pha.ulaval.ca.
Appendix A. Transparency document
Supplementary material
.
References
- 1.A. Mélissopoulos, C. Levacher, La peau: Structure et physiologie., Lavoisier, 2012.
- 2.E. Arens, H. Zhang, The skin’s role in human thermoregulation and comfort, in: Thermal and Moisture Transport in Fibrous Materials, Woodhead Publishing Limited, 2006.
- 3.Bouwstra J.A., Ponec M. The skin barrier in healthy and diseased state. Biochim. Et. Biophys. Acta (BBA)-Biomembr. 2006;1758:2080–2095. doi: 10.1016/j.bbamem.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Nemes Z., Steinert P.M. Bricks and mortar of the epidermal barrier. Exp. Mol. Med. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- 5.Groen D., Berthaud F., Bouwstra J.A., Chapuis C., Gooris G.S., Boncheva M. In vitro model systems for studying the impact of organic chemicals on the skin barrier lipids. Biochim. Et. Biophys. Acta (BBA) - Biomembr. 2014;1838:310–318. doi: 10.1016/j.bbamem.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Feingold K.R., Elias P.M. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim. Et. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2014;1841:280–294. doi: 10.1016/j.bbalip.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 7.van Smeden J., Janssens M., Gooris G.S., Bouwstra J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Et. Biophys. Acta. 2014;1841:295–313. doi: 10.1016/j.bbalip.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Oguri M., Gooris G.S., Bito K., Bouwstra J.A. The effect of the chain length distribution of free fatty acids on the mixing properties of stratum corneum model membranes. Biochim. Et. Biophys. Acta (BBA)-Biomembr. 2014;1838:1851–1861. doi: 10.1016/j.bbamem.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Elias P.M. Skin Barrier Function. Curr. Allergy Asthma Rep. 2008;8:299–305. doi: 10.1007/s11882-008-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feingold K., Elias P. The important role of lipids in the epidermis and their role in the formation and maintenance of the cutaneous barrier. BBA-Mol. Cell Biol. Lipids. 2014;(3):279. doi: 10.1016/j.bbalip.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H., Tsuji H., Minami-Hori M., Miyauchi Y., Iizuka H. Defective barrier function accompanied by structural changes of psoriatic stratum corneum. J. Dermatol. 2014;41:144–148. doi: 10.1111/1346-8138.12393. [DOI] [PubMed] [Google Scholar]
- 12.Boehncke W.H., Boehncke S., Schon M.P. Managing comorbid disease in patients with psoriasis. BMJ. 2010;340:b5666. doi: 10.1136/bmj.b5666. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths C.E., Barker J.N. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 14.Nestle F.O., Kaplan D.H., Barker J. Psoriasis, The new England. J. Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 15.Motta S., Monti M., Sesana S., Mellesi L., Ghidoni R., Caputo R. Abnormality of Water Barrier Function in Psoriasis: role of Ceramide Fractions. Arch. Dermatol. 1994;130:452–456. [PubMed] [Google Scholar]
- 16.Fadzil M.H., Ihtatho D., Affandi A.M., Hussein S.H. Area assessment of psoriasis lesions for PASI scoring. J. Med Eng. Technol. 2009;33:426–436. doi: 10.1080/07434610902744066. [DOI] [PubMed] [Google Scholar]
- 17.Jean J., Pouliot R. In vivo and in vitro models of psoriasis. In: Eberli D., editor. Tissue engineering. 2010. pp. 359–382. [Google Scholar]
- 18.Bernard G., Auger M., Soucy J., Pouliot R. Physical characterization of the stratum corneum of an in vitro psoriatic skin model by ATR-FTIR and Raman spectroscopies. Biochim. Et. Biophys. Acta. 2007;1770:1317–1323. doi: 10.1016/j.bbagen.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Jean J., Lapointe M., Soucy J., Pouliot R. Development of an in vitro psoriatic skin model by tissue engineering. J. Dermatol. Sci. 2009;53:19–25. doi: 10.1016/j.jdermsci.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Ayata R.E., Bouhout S., Auger M., Pouliot R. Study of in vitro capillary-like structures in psoriatic skin substitutes. BioRes. Open Access. 2014;3:197–205. doi: 10.1089/biores.2014.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jean J., Soucy J., Pouliot R. Effects of retinoic acid on keratinocyte proliferation and differentiation in a psoriatic skin model. Tissue Eng. Part A. 2011;17:1859–1868. doi: 10.1089/ten.TEA.2010.0463. [DOI] [PubMed] [Google Scholar]
- 22.K.J. Lambert, J.R. Birch, Cell growth media, in: G.J. Spier RE (Ed.) Animal Cell Biotechnology, vol. 1, Academic Press, 1985, pp. 85–112.
- 23.Rossi C.R., Kiesel G.K. Factors affecting the production of bovine type I interferon on bovine embryonic lung cells by polyriboinosinic-polyribocytidylic acid. Am. J. Vet. Res. 1980;41:557–560. [PubMed] [Google Scholar]
- 24.van der Valk J., Mellor D., Brands R., Fischer R., Gruber F., Gstraunthaler G., Hellebrekers L., Hyllner J., Jonker F.H., Prieto P., Thalen M., Baumans V. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol. Vitr. 2004;18:1–12. doi: 10.1016/j.tiv.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Docherty R.J., Hamilton A.O., Pattison N., Mounsey G., Foster R., Hodgson P., Benny G. Low IgG FBS: applications to monoclonal antibody and vaccine production. Focus. 1994;16:14–17. [Google Scholar]
- 26.Froud S.J. The development, benefits and disadvantages of serum-free media. Dev. Biol. Stand. 1999;99:157–166. [PubMed] [Google Scholar]
- 27.Mazlyzam A.L., Aminuddin B.S., Saim L., Ruszymah B.H. Human serum is an advantageous supplement for human dermal fibroblast expansion: clinical implications for tissue engineering of skin. Arch. Med Res. 2008;39:743–752. doi: 10.1016/j.arcmed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Black A.F., Bouez C., Perrier E., Schlotmann K., Chapuis F., Damour O. Optimization and characterization of an engineered human skin equivalent. Tissue Eng. 2005;11:723–733. doi: 10.1089/ten.2005.11.723. [DOI] [PubMed] [Google Scholar]
- 29.Jean J., Bernard G., Duque-Fernandez A., Auger F.A., Pouliot R. Effects of serum-free culture at the air-liquid interface in a human tissue-engineered skin substitute. Tissue Eng. Part A. 2011;17:877–888. doi: 10.1089/ten.TEA.2010.0256. [DOI] [PubMed] [Google Scholar]
- 30.Chabaud S., Simard M., Gendreau I., Pouliot R., Bolduc S. Origin of Serum Affects Quality of Engineered Tissues Produced by the Self-Assembly Approach. Scientifica. 2016;2016 doi: 10.1155/2016/3825645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Germain L., Rouabhia M., Guignard R., Carrier L., Bouvard V., Auger F.A. Improvement of human keratinocyte isolation and culture using thermolysin. Burn.: J. Int. Soc. Burn Inj. 1993;19:99–104. doi: 10.1016/0305-4179(93)90028-7. [DOI] [PubMed] [Google Scholar]
- 32.L. Germain, F.A. Auger, Tissue engineered biomaterials: biological and mechanical characteristics In: T.D. Wise DL, D.A. Altobelli, M.J. Yaszemski, J.D. Gresser, E.R. Schwartz,(Eds.), Encyclopedic Handbook of Biomaterials and Bioengineering 1995 Marcel Dekker New York pp. 699–734.
- 33.Auger F.A., Lopez Valle C.A., Guignard R., Tremblay N., Noel B., Goulet F., Germain L. Skin equivalent produced with human collagen, In vitro cellular & developmental biology. Animal. 1995;31:432–439. doi: 10.1007/BF02634255. [DOI] [PubMed] [Google Scholar]
- 34.D. Larouche, J. Jean, F. Berthod, L. Germain, R. Pouliot, Markers for an in vitro skin substitute, in: T.M.a.E. Novik (Ed.) Alternative technologies to animal testing, Boston, 2010, pp. 283.
- 35.Robitaille H., Simard-Bisson C., Larouche D., Tanguay R.M., Blouin R., Germain L. The small heat-shock protein Hsp27 undergoes ERK-dependent phosphorylation and redistribution to the cytoskeleton in response to dual leucine zipper-bearing kinase expression. J. Invest Dermatol. 2010;130:74–85. doi: 10.1038/jid.2009.185. [DOI] [PubMed] [Google Scholar]
- 36.Franz T.J. Percutaneous absorption on the relevance of in vitro data. J. Invest. Dermatol. 1975;64:190–195. doi: 10.1111/1523-1747.ep12533356. [DOI] [PubMed] [Google Scholar]
- 37.Michel M., L’Heureux N., Auger F.A., Germain L. From newborn to adult: phenotypic and functional properties of skin equivalent and human skin as a function of donor age. J. Cell Physiol. 1997;171:179–189. doi: 10.1002/(SICI)1097-4652(199705)171:2<179::AID-JCP8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 38.Michel M., L’Heureux N., Pouliot R., Xu W., Auger F.A., Germain L. Characterization of a new tissue-engineered human skin equivalent with hair, In vitro cellular & developmental biology. Animal. 1999;35:318–326. doi: 10.1007/s11626-999-0081-x. [DOI] [PubMed] [Google Scholar]
- 39.M.H. Tan, M. Lebwohl, Psoriasis, Drugs of today (Barcelona, Spain: 1998), 3, 4, 1998, pp. 641–647. [DOI] [PubMed]
- 40.Mendelsohn R., Flach C.R., Moore D.J. Determination of molecular conformation and permeation in skin via IR spectroscopy. Microsc. Imaging, Biochim. Et. Biophys. Acta (BBA)-Biomembr. 2006;1758:923–933. doi: 10.1016/j.bbamem.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Ongpipattanakul B., Francoeur M.L., Potts R.O. Polymorphism in stratum corneum lipids. Biochim. Et. Biophys. Acta (BBA)-Biomembr. 1994;1190:115–122. doi: 10.1016/0005-2736(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 42.Chiang A., Tudela E., Maibach H.I. Percutaneous absorption in diseased skin: an overview. J. Appl. Toxicol. 2012;32:537–563. doi: 10.1002/jat.1773. [DOI] [PubMed] [Google Scholar]
- 43.Enomoto M., Mori T., Park M. Influence of serum supplements in culture medium on gonadotropin-releasing hormone effects on colony formation. Life Sci. 2002;71:2153–2160. doi: 10.1016/s0024-3205(02)02015-5. [DOI] [PubMed] [Google Scholar]
- 44.Stark H.-J., Baur M., Breitkreutz D., Mirancea N., Fusenig N.E. Organotypic Keratinocyte Cocultures in Defined Medium with Regular Epidermal Morphogenesis and Differentiation. J. Invest. Dermatol. 1999;112:681–691. doi: 10.1046/j.1523-1747.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- 45.Nino M., Calabro G., Santoianni P. Topical delivery of active principles: the field of dermatological research. Dermatol. Online J. 2010;16:4. [PubMed] [Google Scholar]
- 46.van Smeden J., Boiten W.A., Hankemeier T., Rissmann R., Bouwstra J.A., Vreeken R.J. Combined LC/MS-platform for analysis of all major stratum corneum lipids, and the profiling of skin substitutes. Biochim. Et. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 1841;014:70–79. doi: 10.1016/j.bbalip.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Mojumdar E., Kariman Z., van Kerckhove L., Gooris G., Bouwstra J. The role of ceramide chain length distribution on the barrier properties of the skin lipid membranes. Biochim. Et. Biophys. Acta (BBA)-Biomembr. 2014;1838:2473–2483. doi: 10.1016/j.bbamem.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 48.Rougier A., Rallis M., Krien P., Lotte C. In vivo percutaneous absorption: a key role for stratum corneum/vehicle partitioning. Arch. Dermatol. Res. 1990;282:498–505. doi: 10.1007/BF00371943. [DOI] [PubMed] [Google Scholar]
- 49.Barry B., Bennett S. Effect of penetration enhancers on the permeation of mannitol, hydrocortisone and progesterone through human skin. J. Pharm. Pharmacol. 1987;39:535–546. doi: 10.1111/j.2042-7158.1987.tb03173.x. [DOI] [PubMed] [Google Scholar]
- 50.Reifenrath W.G., Hawkins G.S., Kurtz M.S. Percutaneous penetration and skin retention of topically applied compounds: an in vitro-in vivo study. J. Pharm. Sci. 1991;80:526–532. doi: 10.1002/jps.2600800605. [DOI] [PubMed] [Google Scholar]
- 51.Hawkins G.S., Reifenrath W.G. Influence of skin source, penetration cell fluid, and, partition coefficient on in vitro skin penetration. J. Pharm. Sci. 1986;75:378–381. doi: 10.1002/jps.2600750411. [DOI] [PubMed] [Google Scholar]
- 52.Thakoersing V.S., Smeden J., Boiten W.A., Gooris G.S., Mulder A.A., Vreeken R.J., El Ghalbzouri A., Bouwstra J.A. Modulation of stratum corneum lipid composition and organization of human skin equivalents by specific medium supplements. Exp. Dermatol. 2015;24:669–674. doi: 10.1111/exd.12740. [DOI] [PubMed] [Google Scholar]
- 53.Školová B., Janůšová B., Vávrová K. Ceramides with a pentadecasphingosine chain and short acyls have strong permeabilization effects on skin and model lipid membranes. Biochim. Et. Biophys. Acta (BBA)-Biomembr. 2016;185(8):220–232. doi: 10.1016/j.bbamem.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Pullmannová P., Staňková K., Pospíšilová M., Školová B., Zbytovská J., Vávrová K. Effects of sphingomyelin/ceramide ratio on the permeability and microstructure of model stratum corneum lipid membranes. Biochim. Et. Biophys. Acta (BBA)-Biomembr. 2014;1838:2115–2126. doi: 10.1016/j.bbamem.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Thakoersing V.S., Danso M.O., Mulder A., Gooris G., El Ghalbzouri A., Bouwstra J.A. Nature versus nurture: does human skin maintain its stratum corneum lipid properties in vitro? Exp. Dermatol. 2012;21:865–870. doi: 10.1111/exd.12031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material