Abstract
The tissues of the body are routinely subjected to various forms of mechanical vibration, the frequency, amplitude, and duration of which can contribute both positively and negatively to human health. The vocal cords, which are in close proximity to the thyroid, may also supply the thyroid with important mechanical signals that modulate hormone production via mechanical vibrations from phonation. In order to explore the possibility that vibrational stimulation from vocalization can enhance thyroid epithelial cell function, FRTL-5 rat thyroid cells were subjected to either chemical stimulation with thyroid stimulating hormone (TSH), mechanical stimulation with physiological vibrations, or a combination of the two, all in a well-characterized, torsional rheometer-bioreactor. The FRTL-5 cells responded to mechanical stimulation with significantly (p<0.05) increased metabolic activity, significantly (p<0.05) increased ROS production, and increased gene expression of thyroglobulin and sodium-iodide symporter compared to un-stimulated controls, and showed an equivalent or greater response than TSH only stimulated cells. Furthermore, the combination of TSH and oscillatory motion produced a greater response than mechanical or chemical stimulation alone. Taken together, these results suggest that mechanical vibrations could provide stimulatory cues that help maintain thyroid function.
Keywords: FRTL-5, Mechanobiology, Mechanotransduction, Low amplitude mechanical signals, Endocrine system
Highlights
-
•
Thyroid epithelial cells responded to mechanical vibrations similar to those from vocalization.
-
•
This response was equivalent or greater compared to chemical stimulation.
-
•
The combination of mechanical and chemical stimulation was synergistic.
-
•
It may be possible to influence thyroid function with mechanical vibrations.
1. Introduction
The tissues of the body are routinely subjected to various forms of mechanical vibration, the frequency, amplitude, and duration of which can contribute both positively and negatively to human health [1]. Small doses of vibration may promote tissue growth [2], while large doses can result in tissue damage [3]. An example of the later is hand-arm vibration syndrome (HAVS), where the operation of vibrating power tools can produce chronic and progressive dysfunction to the vascular, muscular, and neurological systems [4], [5]. Several studies on HAVS have demonstrated a connection between mechanical vibrations and an adverse cellular response, such as vascular complications in response to increased reactive oxygen species (ROS) production [6], [7], [8]. In contrast, whole body vibrations have been positively associated with substantial increases in hormone production [9], [10], [11], possibly through direct mechanical stimulation or enhanced biomolecular transport.
These studies suggest that mechanical vibrations could play an important role in regulating hormone production and ROS in the endocrine system. The most common endocrine disease, hypothyroidism, affects approximately 5% of the population [12]. It is characterized by a decrease in thyroid hormone production that stems in part from a reduction in thyroid epithelial cell sensitivity to thyroid stimulating hormone (TSH). In order for these hormones to be produced, iodine must be transported into the thyroid epithelial cells through the sodium iodine symporter (NIS). Iodine must then be oxidized by thyroid peroxidase (TPO) and the ROS hydrogen peroxide (H2O2) and added to the tyrosine residues of thyroglobulin (TG). The iodized tyrosine residues are then cleaved to form the thyroid hormones triiodothyronine (T3) and thyroxine (T4), which are critical for regulating cell metabolism, growth, and development [13].
There is some evidence that mechanical stimulation can positively influence TSH sensitivity. Several in vitro studies have demonstrated that thyroid epithelial cells are responsive to their mechanical environment, particularly in the context of altered gravity and space exploration [14], [15]. For example, Meli et al. found that Fischer rat thyroid line-5 (FRTL-5) cells had decreased sensitivity to thyroid stimulating hormone (TSH) after exposure to a hypogravity situation [15]. In contrast, FRTL-5 exposure to a 7g centrifugal force increased TSH receptor (TSHR) number and responsiveness to TSH [14]. The difference in outcomes could be in part due to an increase in reactive oxygen species (ROS) production in response to an altered mechanical environment, particularly as ROS are vital to thyroid hormone production. Too much ROS, however, can be detrimental to thyroid health [16], [17], with excess ROS implicated as the basis for the high rate of cancer found in the thyroid [18].
The vocal cords, which are in close proximity to the thyroid, may also supply the thyroid with important mechanical signals that modulate hormone and ROS production via mechanical vibrations from phonation. In order to explore the possibility that vibrational stimulation from vocalization can enhance thyroid epithelial cell function, we subjected FRTL-5 cells to physiological vibrations in a well-characterized, torsional rheometer-bioreactor [19], [20], [21] and compared their response to TSH stimulated cells.
2. Methods
2.1. Cell culture
Fischer rat thyroid line cells (FRTL-5, American Type Culture Collection, Rockville, MD, CRL 8305) derived from the normal thyroid gland of Fischer rats were maintained in a 37 °C humidified incubator supplied with 95%/5% air/CO2. FRTL-5 cells were cultured according to the supplier's recommendations in Ham’s F12K medium with 2 mM l-glutamine and adjusted to contain 1.5 g/L sodium bicarbonate. 0.5% bovine calf serum and six additional hormones required for FRTL-5 cells to proliferate and maintain thyroid function were added to the medium, as specified by the originators of this cell line [22], [23]. These six hormones included 1 mU/mL thyroid stimulating hormone (TSH), 0.01 mg/mL insulin, 10 nM hydrocortisone, 0.005 mg/mL transferrin, 10 ng/mL somatostatin, and 10 ng/mL glycyl-l-histidyl-l-lysine acetate (All reagents from Sigma, St Louis, MO) [24]. Growth medium was used for cell expansion and to acclimate the cells prior to initiating the experiments. For the experiments, growth medium was modified so that it either contained 10 mU/mL TSH or no TSH as indicated below. Cells were passaged at 70% confluency and harvested at passages four through eight for the experiments.
2.2. Application of physiological vibrations with a torsional rheometer-bioreactor
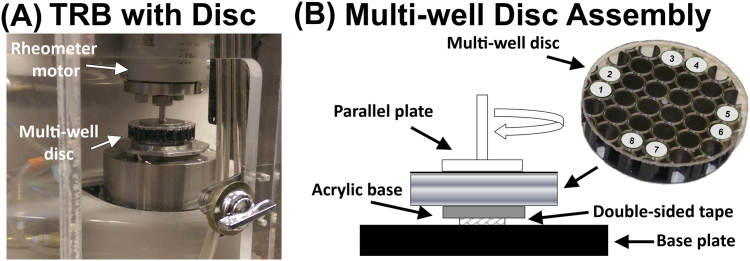
With the Torsional Rheometer-Bioreactor (TRB) previously developed in our lab [19], physiological forces can be applied to adherent cells in a multi-well disc by specifying the frequency, amplitude, and duration of vibration (Fig. 1). Briefly, 96 well tissue-culture plates were cut into 57 mm diameter, multi-well discs so that the plates could be positioned in the TRB. The plates, which were previously sterilized by the manufacturer, were covered with a sterile adhesive to prevent debris from entering the wells during cutting with a jigsaw and to preserve sterility. Next, trypsin/EDTA (Life Technologies, Grand Island, NY) was used to release FRTL-5 cells from the tissue culture flasks. The cells were centrifuged at 1500 rpm for 5 min, re-suspended in complete media, and then plated at a density of 10,000 cells/well to each of the eight outer wells of the disc, each of equal radial distance from the disc center. FRTL-5 cells were also re-plated at the same density in wells of a 96-well plate to serve as controls (n=8). Cellular attachment was allowed to occur in all wells for 48 h in growth medium (i.e., with 1 mU/mL TSH) before TSH was completely removed from the growth medium for an additional 48 h of culture. TSH deprivation between 1 and 10 days is frequently employed (e.g., [25], [26]) prior to beginning an experiment because it greatly increases FRTL-5 sensitivity to the reintroduction of TSH [22], and because deprivation is thought to control cell synchronization [27]. We chose a deprivation period of 48 h followed by re-exposure to 10 mU/mL TSH based on other studies that used similar parameters [28], [29]. In particular, we used the study by Bjorkman et al. [28] as a guide, where they showed a significant increase in hydrogen peroxide production with two days of TSH deprivation followed by 10 mU/mL of TSH. Immediately following this 48 h TSH-free culture period, 10 mU/mL of TSH was added back to growth medium supplied to half of the wells in the multi-well disc (n=4) and to half of the wells in the control plate (n=4). The other half of the wells received growth medium without any TSH. The multi-well discs were then mounted onto the TRB and subjected to four conditions: (1) no oscillation and no TSH; (2) no oscillation and TSH; (3) oscillation and no TSH, (4) oscillation and TSH. The oscillatory conditions for this study were selected based on vibrational parameters related to vocalization [30]. The TRB was set up to apply inertial forces to the adherent cells in the form of oscillatory accelerations of 2 m/s2, based on accelerations measured on the skin in front of the thyroid [31]. In addition, the experiments were conducted at an torsional frequency of 126 Hz, a frequency within the range of a typical adult male voice and corresponding to the characteristics of American speech and the daily voice usage of public school teachers [30]. Finally, the stimulation was applied constantly (i.e., a duty ratio of 1) for the full duration of the experiment (i.e., four hours) in order to produce for this pilot study what we hypothesized would be a maximum effect from mechanical stimulation. Four hours was selected in order to be consistent with prior experiments conducted with vocal fold fibroblasts [19] and because t-tests from a preliminary study indicated significant differences in metabolic activity (p=0.0154) between TSH deprived and TSH stimulated groups after only four hours.
Fig. 1.
Torsional Rheometer Bioreactor (A) Image of the TRB enclosed in an environmentally controlled chamber. (B) Schematic of the components of the multi-disc assembly, including the locations of the eight wells used in each study. Four of the wells were chemically stimulated with TSH in addition to receiving oscillatory mechanical signals. The other four wells received no TSH.
2.3. Quantification of metabolic activity
Alamar Blue (alamarBlue®, Life Technologies, Grand Island, NY) was used to quantify differences in metabolic activity in response to exposure to TSH and oscillatory accelerations [32]. A 10x stock solution of Alamar Blue was diluted in calcium and magnesium free PBS to achieve a 1x final concentration. At the conclusion of each experiment, medium was removed, the wells were washed gently with PBS, and 100 µL of Alamar Blue solution was added to each well. Both the multi-well discs and controls were returned to the incubator for 1 h. The Alamar Blue solution was then transferred to a black 96-well plate and the fluorescence was measured (Ex/Em=560/590 nm) with a fluorometer (FLOUstar, BMG LABTECH Ltd., Ortenberg, Germany). Results are reported in arbitrary fluorescence units (AFU) with the blank value subtracted.
2.4. Quantification of cell number
Hoechst 33342 (H1399, Life Technologies, Grand Island, NY) was used to quantify cell number. After removal of Alamar Blue, the wells were washed with PBS and 100 µL of 5 μg/mL Hoechst in PBS was added to each well, followed by a 30 min incubation at 37 °C. Fluorescence was measured with a flourometer (Ex/Em =380/460 ηm) and reported as AFU.
2.5. Quantification of reactive oxygen species (ROS) levels
A dichlorofluorescein (DCF) assay (D399, ThermoFisher) was conducted on a separate set of experiments that were subjected to identical experimental conditions as those for the previous assay in order to measure differences in ROS production [33]. After stimulation, media was removed from the wells and replaced with 50 µL of 10 μM DCF in PBS solution and incubated at 37 °C for 30 min. ROS production was measured with a fluorometer (Ex/Em=495/520 nm) and reported as AFU.
2.6. Quantification of cyclic adenosine monophosphate (cAMP) Levels
A direct cAMP assay kit (Enzo Life Sciences, Farmingdale, NY) was used to measure intracellular cAMP concentration (also on a separate set of experiments under identical experimental conditions) because TSH stimulates thyroid cells primarily through the cAMP signaling cascade [34]. Three independent experiments were conducted for each condition. However, due to the low cell densities in this experiment, all four wells for a given experiment and condition were pooled together for one measurement, thus giving a sample size of n=3 for each condition. At the conclusion of each experiment, the FRTL-5 cells were lysed with 100 µL 0.1 M HCl for 10 min, centrifuged at 5000 rpm for 5 min, and then frozen so that all experiments could be analyzed simultaneously. Following the manufacturer's protocol, an acetylation step was used to increase sensitivity. A 1:2 mix of acetic anhydride: trimethylamine was added at a 5% concentration to the samples and the standards, which ranged from 0.078 pmol/mL to 20 pmol/mL. Neutralizing solution (50 µL) was added to each well of the 96-well cAMP ELISA plate. An additional 100 µL of sample or standard was then added to each well, followed by 50 µL of blue conjugate and 50 µL of yellow antibody. The wells were mixed at 280 rpm for 2 h. The solution was then removed and the wells were washed 3 times. 200 µL of substrate solution was added to each well and incubated at room temperature for 1 h. 50 µL of stop solution was then added and the absorbance was measured at 405 ηm with a Spectramax i3x (Molecular Devices, Sunnyvale, CA).
2.7. Thyroglobulin (TG) and sodium/iodine symporter (NIS) gene expression assessed with qPCR
TG and NIS gene expression was quantified using qPCR. TG is a precursor protein for thyroid hormone production, and NIS is important for trafficking of iodine into thyroid epithelial cells. Immediately following the stimulation period, FRTL-5 cells were removed from the multi-disc and control plates with trypsin/EDTA. Cells were then centrifuged and wash with PBS before being stored at −20 °C for later analysis. Upon thawing, total RNA was isolated using an RNeasy Mini Kit (Qiagen, Austin, TX, USA) according to the manufacturer's directions. A total of 100 ηg of RNA was converted to cDNA via RT-PCR by using a high capacity cDNA reverse transcription kit (Applied Biosystems, MA, USA). The RT-PCR protocol was 10 min at 25 °C followed by 37 °C for 2 h. The resultant cDNA was diluted 1:5 in RNAase free water, then 4.5 µL of the diluted cDNA was mixed with 5.5 µL of TG (Rn00578496_m1) or NIS (Rn00583900_m1) TaqMan miRNA primer and probe mix (1:10 of NIS, TG primer: TaqMan probe solution) (Life Technologies, MA, USA). These reactions were carried out in triplicate. Gene expression levels were measured with an Applied Biosystems 7300 Real Time PCR System (Applied Biosystems, MA, USA) in a 96 well plate with thermal cycling parameters set at 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. Steady-state mRNA levels were normalized to 18 s rRNA and calculated relative to untreated controls by the relative quantitation using comparative CT. Because RNA yields were low, all four wells for a given condition were pooled together for one measurement run in triplicate. Three independent experiments were conducted for each condition (i.e., n=3).
2.8. Statistical analysis
All data are presented as mean values±standard deviation. Except for the gene expression data and the cAMP ELISA (sample sizes detailed above), these values represent 4 independent experiments, each containing four samples per group (n=16). The oscillation and TSH condition for the alamar blue and Hoechst assays represents n=15 samples because one sample was discarded due to a sample processing error. Anderson-Darling tests were conducted first to confirm that the data were not from non-normal distributions. One-way analysis of variance (ANOVA) with post hoc Tukey tests (Prism 7, GraphPad) were then used to determine statistical significance.
3. Results
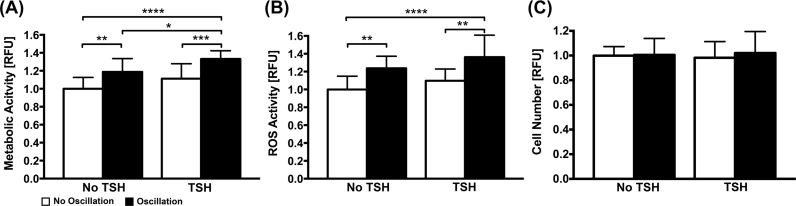
Changes in FRTL-5 cell metabolic activity were affected in a stimulation dependent manner (Fig. 2A). The addition of TSH increased metabolic activity 11% (though not significantly, p=0.107) compared to the unstimulated control (i.e., no TSH and no oscillation). Oscillation alone also had a significant effect on metabolism (p=0.0014), increasing it by 20% compared to control. The combination of oscillation and TSH significantly increased metabolic activity further (33%) compared to control (p<0.001). Relative levels of ROS produced in response to TSH and oscillatory stimulation mirrored the trends observed for metabolic activity (Fig. 2B). TSH alone increased ROS production 10% compared to controls. Oscillation alone and oscillation with TSH increased significantly ROS production 24% (p=0.0077) and 36% (p<0.0001) compared to controls, respectively. For all testing conditions, the FRTL-5 cells grew into clustered multicellular aggregates over the course of the experiment. No significant differences in cell number were observed amongst the conditions tested (Fig. 2C), indicating that mechanical and chemical treatments did not affect cell attachment to the substrate.
Fig. 2.
FRTL-5 Metabolic Activity, ROS production, and Cell Number in Response to Treatment. (A). Oscillation significantly increased FRTL-5 cell metabolic activity compared to no oscillation both with and without TSH (* p=0.0244, ** p=0.0014, *** p=0.0002, **** p<0.0001). (B) Oscillation significantly increased ROS compared to no oscillation both with (** p=0.0026) and without (** p=0.0077) TSH (p****< 0.0001). (C) Cell number was not significantly affected by TSH or oscillation.
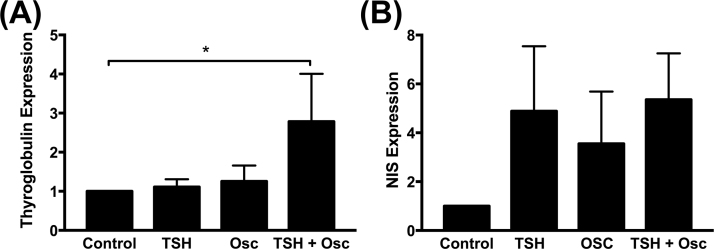
Gene expression was also dependent on the stimulation conditions. Relative TG gene expression remained unchanged in response to TSH alone (Fig. 3A). TG expression increased 25% with oscillation alone and nearly tripled (p=0.0406) with the combination of oscillation and TSH compared to control. NIS expression (Fig. 3B) was highly variable and increased approximately three to five-fold in response to all forms of treatment compared to control.
Fig. 3.
Gene expression normalized to 18 s and to control (i.e., no oscillation, no TSH). (A) The combination of TSH and oscillation (TSH+OSC) resulted in a 100% increase in thyroglobulin expression (p=0.0406). (B) Sodium Iodide Symporter (NIS) expression increased approximately 3 to 5-fold (though not significantly) for all three cases of stimulation compared to controls.
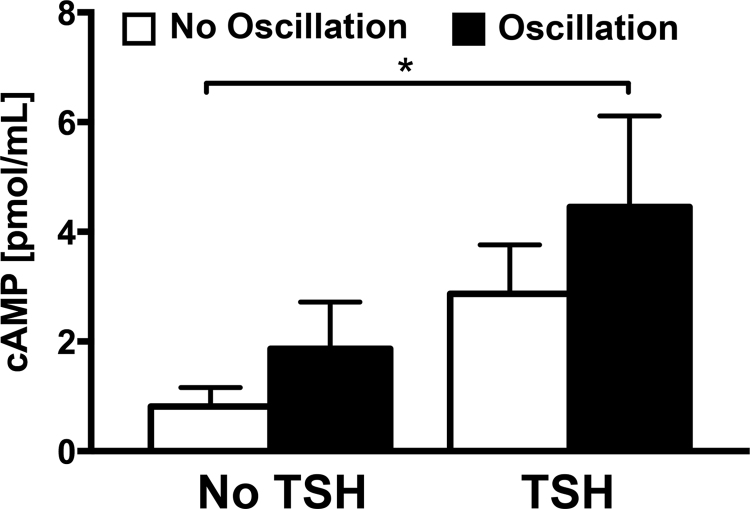
Because TSH acts primarily through the cAMP signaling cascade [34], cAMP levels for each condition were also measured in order to determine if oscillation also triggers an increase in cAMP (Fig. 4). The baseline cAMP concentration measured in the unstimulated control group was 0.816±0.344 pmol/mL. The concentration increased 2.3 fold in response to oscillation alone (1.866±0.853 pmol/mL) and 3.5 fold in response to TSH alone (2.870±0.894 pmol/mL) compared to controls. Together, the combination of TSH and oscillation significantly (p=0.0121) increased cAMP levels 5.5 fold at 4.452±1.663 pmol/mL compared to controls.
Fig. 4.
cAMP Levels in Response to Treatment. cAMP levels increased in response to treatments with either TSH or oscillation. TSH and oscillation produced the highest levels of cAMP, which were significantly greater than control (* p=0.0121, n =3).
4. Discussion
Given that mechanical vibrations have been associated with a positive increase in hormone production [9], [10], [11], and that the thyroid is in close proximity to the vocal cords, we hypothesized that thyroid hormone production could also be positively stimulated by the vibrational forces of phonation. In order to begin to test this hypothesis, we subjected adherent FRTL-5 cells to oscillatory inertial forces from rotational oscillatory motion that approximates the accelerations thyroid cells might experience during typical phonation.
We then measured changes in metabolism, ROS production, and TG and NIS gene production as indicators of enhanced thyroid function because each of these components is vital to thyroid hormone production, and because each has been shown to increase in response to hormonal stimulation with TSH [28], [35], [36]. We reasoned that if inertial forces from oscillation are capable of providing the same (or similar) stimulatory effects as TSH, one should expect to see comparable increases to those from TSH stimulation alone. The FRTL-5 cells responded to mechanical stimulation with increased metabolic activity, increased ROS production, and increased gene expression of NIS and TG compared to un-stimulated controls, and showed an equivalent or greater response than TSH only stimulated cells. Taken together, these results suggest that mechanical vibrations could provide stimulatory cues that help maintain thyroid function.
Which signaling pathways are activated by these mechanical cues is unclear. TSH stimulates rat and human thyroid cells primarily via an increase in intracellular cAMP, which in turn activates several additional signaling pathways [34]. In order to determine if mechanical stimulation also operates through the same cAMP signaling cascade as TSH does, we measured intracellular cAMP levels and found that they too increased with oscillation. The cAMP trends did, however, differ some from the trends for metabolic activity and ROS. Specifically, cAMP was lower when only oscillations were applied than when only TSH was administered. Although not significantly different from each other, this trend was flipped with respect to the trends found for metabolic activity and ROS.
It is possible that a difference in cAMP levels between the oscillation and TSH groups exists, and that this difference would become significant with a larger samples size. If this is the case, this difference might indicate that other signaling mechanisms are also operating that could be differentially regulated depending on whether the stimulus is mechanical, hormonal, or a combination of the two. Such mechanisms could operate parallel to the cAMP cascade, such as the phosphoinositide phospholipase C (PLC) pathway [34], [37], or they could interact further downstream of cAMP, such as MAPK [34], [38]. For example, modulation of the MAPK/ERK pathway through mechanical stimulation has been demonstrated in other cell types [39], and could play some role here. Further investigation will be required to determine precisely which pathways are activated by vibrational stimulation.
The positive effects of vibrational stimulation have been noted by others, particularly as a potential therapy for fostering an anabolic response in the musculoskeletal system [40], [41]. Of particular relevance to this study are the reports on high frequency, low-amplitude mechanical signals (LMS) and their role in regulating osteogenesis, musculogenesis, and other adaptive cellular responses [42], [43], [44], [45], [46], [47], [48], [49]. LMS is characterized by strains so low as to be considered negligible (i.e. peak strains are on the order of 1–2 microstrain) [42]. In our experiments, the FRTL-5 cells were attached to stiff tissue culture polystyrene (TCP), which we assumed also experienced negligible deformations during oscillation. Without a deformable substrate, gene transcription cannot ostensibly be triggered by the well-characterized outside-in signaling pathway in which mechanical cues are transmitted from the ECM to the cytoskeleton via transmembrane focal adhesions and on to the nucleus [42]. The underlying mechanisms responsible for an adaptive cellular response to LMS remain unclear. Recently, Uzer et al. found that the LINC (linker of nucleoskeleton and cytoskeleton) complex, a collection of proteins that mechanically couples the cytoskeleton to the nucleus, appears to play an important role in the cellular response to high frequency, LMS [46]. They observed that LMS applied to mesenchymal stem cells facilitated focal adhesion kinase (FAK) and Akt phosphorylation, actin cytoskeletal reorganization, and force transmission to the nucleus via the LINC complex [46]. When the LINC complex was disabled these adaptive responses to LMS disappeared. Others have also provided evidence that LINC helps maintain a balance of forces between the nucleus and the actin cytoskeleton, and that dynamic changes in the homeostatic state of stress in the cytoskeleton can transfer localized deformations/forces to the nucleus, which in turn mechanoregulates gene transcription and other cellular activities [50], [51], [52], [53]. In the context of LMS, it appears feasible that force generation can originate internally from the mechanical deformations created by the passive response of the denser nucleus [46], [54] due to acceleration from the oscillatory rotational motion imposed on the multi-well disc by the TRB.
Another possible source of mechanical stimulation is from fluid shear stress. In our system, each well was filled completely with culture medium in order to reduce shear stresses caused by sloshing of the culture medium. Smooth particle hydrodynamic computational simulations predicted that shear stresses decrease with increasing fluid height in the well, and that for similar TRB operating conditions the peak shear stresses should be less than 0.01 Pa [19]. Although these shear stresses are quite small in the TRB, it is still possible that they also are a source of mechanical stimulation in vivo when movement is less confined. For example, similar to our study, computational simulations on the effect of LMS on osteocytes in vitro conclude that membrane deformations from fluid shear stresses are too low to be stimulatory [55]. However, simulations of LMS on trabecular bone in vivo estimate median shear stresses between 0.3 and 1.1 Pa that are considered high enough to produce an anabolic effect [56]. Clearly more investigation is required to determine which mechanical mechanisms might be responsible for LMS's effect on cell behavior.
In this initial study, we chose to apply a vibration frequency, acceleration, and duration that was comparable to our previous work with vocal fold fibroblasts for an adult male voice with a teacher-student contact time of 4–6 h [19]. However, the parameter space mimicking human phonation is quite large. As such, it should be noted that the current study does not indicate if higher or lower frequencies, accelerations, duty cycles, or durations, as may occur in other settings (e.g., musicians and high vocal professionals), causes more or less metabolic activity, ROS production, and TG/NIS gene expression, or whether such activities are beneficial or harmful to thyroid and overall health.
The culture condition parameter space for FRTL-5 cells is also quite large. Although FRTL-5 cells are a well-characterized cell line for studying thyroid function, culture conditions, including the length of TSH deprivation, the concentration of TSH during re-exposure, and the duration of re-exposure for the experiment, vary widely [34], [57]. For this pilot study, our culture conditions were within the ranges reported in the literature (e.g., [28]). However, the four hour TSH re-exposure time we chose was shorter than the 24–48 h more commonly employed (though not outside the range of reported values). We picked four hours in order to be consistent with prior experiments conducted in our lab with vocal fold fibroblasts [19]. Future studies will also explore longer re-exposure times, which could increase significantly FRTL-5 metabolism, iodide uptake, and gene expression in response to TSH, as others have reported (e.g., [22], [58], [59]), compared to the four hours used here.
In addition to exploring the vibrational and culture condition parameter space further, future investigations on the effects of vibratory stimulation of FRTL-5 cells will also require the development of a more physiological culture system that better reflects the in vivo structure and physiology of the follicle [60]. Although FRTL-5 cells are well characterized, and they replicate many of the functions of the thyroid, including TSH sensitivity and thyroglobulin production/secretion [15], the cells used in this study were maintained in monolayer on hard TCP, the stiffness of which greatly exceeds that of the ECM of soft tissues. Several recent studies have demonstrated that many cell activities, including differentiation, can be regulated by mechanical cues such as substrate stiffness [61], [62], [63] and dimensionality (2D vs. 3D) [64]. In fact, thyroid epithelial cells cultured in static 3D collagen gels do form follicles with structural polarity [60], [65], [66], presumably because of favorable mechanical and compositional cues. Our next steps will be to translate our findings here to a similar 3D collagen gel system [67].
In conclusion, these pilot data are supportive of the possibility of using vibrations from phonation to stimulate thyroid hormone production and for augmenting existing treatments for some thyroid-related diseases. A phonation-based treatment could also be beneficial for offsetting the negative effects of microgravity on the thyroid, a situation of particular importance to those subjected to extended space travel.
Acknowledgements
Support was provided by the National Science Foundation (CAREER 1452728). The authors also would like to acknowledge James Ankrum for assistance and fruitful discussions.
Footnotes
Transparency document associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2016.10.008.
Appendix A. Transparency document
Supplementary material
.
References
- 1.Beck B.R. Vibration Therapy to prevent bone loss and falls: mechanisms and efficacy. Curr. Osteoporos. Rep. 2015;13(6):381–389. doi: 10.1007/s11914-015-0294-8. [DOI] [PubMed] [Google Scholar]
- 2.Ozcivici E., Luu Y.K., Rubin C.T., Judex S. Low-level vibrations retain bone marrow’s osteogenic potential and augment recovery of trabecular bone during reambulation. PLoS One. 2010;5(6):e11178. doi: 10.1371/journal.pone.0011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCann M.R., Patel P., Pest M.A., Ratneswaran A., Lalli G., Beaucage K.L. Repeated exposure to high-frequency low-amplitude vibration induces degeneration of murine intervertebral discs and knee joints. Arthritis Rheumatol. 2015;67(8):2164–2175. doi: 10.1002/art.39154. [DOI] [PubMed] [Google Scholar]
- 4.Rolke R., Rolke S., Vogt T., Birklein F., Geber C., Treede R.D. Hand-arm vibration syndrome: clinical characteristics, conventional electrophysiology and quantitative sensory testing. Clin. Neurophysiol.: Off. J. Int. Feder. Clin. Neurophysiol. 2013;124(8):1680–1688. doi: 10.1016/j.clinph.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Heaver B., Hutton S.B. Keeping an eye on the truth? Pupil size changes associated with recognition memory. Memory. 2011;19(4):398–405. doi: 10.1080/09658211.2011.575788. [DOI] [PubMed] [Google Scholar]
- 6.Hughes J.M., Wirth O., Krajnak K., Miller R., Flavahan S., Berkowitz D.E. Increased oxidant activity mediates vascular dysfunction in vibration injury. J. Pharmacol. Exp. Ther. 2009;328(1):223–230. doi: 10.1124/jpet.108.144618. [DOI] [PubMed] [Google Scholar]
- 7.White C.R., Haidekker M.A., Stevens H.Y., Frangos J.A. Extracellular signal-regulated kinase activation and endothelin-1 production in human endothelial cells exposed to vibration. J. Physiol. 2004;555(Pt 2):565–572. doi: 10.1113/jphysiol.2003.059899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krajnak K., Dong R.G., Flavahan S., Welcome D., Flavahan N.A. Acute vibration increases alpha2C-adrenergic smooth muscle constriction and alters thermosensitivity of cutaneous arteries. J. Appl. Physiol. 1985;2006(4) doi: 10.1152/japplphysiol.00761.2005. (1230-7) [DOI] [PubMed] [Google Scholar]
- 9.Bosco C., Iacovelli M., Tsarpela O., Cardinale M., Bonifazi M., Tihanyi J. Hormonal responses to whole-body vibration in men. Eur. J. Appl. Physiol. 2000;81(6):449–454. doi: 10.1007/s004210050067. [DOI] [PubMed] [Google Scholar]
- 10.Couto B.P., Silva H.R., Filho A.G., da Silveira Neves S.R., Ramos M.G., Szmuchrowski L.A. Acute effects of resistance training with local vibration. Int. J. Sports Med. 2013;34(9):814–819. doi: 10.1055/s-0032-1331198. [DOI] [PubMed] [Google Scholar]
- 11.Di Loreto C., Ranchelli A., Lucidi P., Murdolo G., Parlanti N., De Cicco A. Effects of whole-body vibration exercise on the endocrine system of healthy men. J. Endocrinol. Investig. 2004;27(4):323–327. doi: 10.1007/BF03351056. [DOI] [PubMed] [Google Scholar]
- 12.Vanderpump M.P. The epidemiology of thyroid disease. Br. Med. Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 13.Barrett E.J. The thryoid gland. In: Boron W.F.B, Boulpaep E.L., editors. Medical Physiology: A Cellular And Molecular Approaoch. Elsevier; 2012. pp. 1044–1056. [Google Scholar]
- 14.Albi E., Curcio F., Lazzarini A., Floridi A., Cataldi S., Lazzarini R. A firmer understanding of the effect of hypergravity on thyroid tissue: cholesterol and thyrotropin receptor. PLoS One. 2014;9(5):e98250. doi: 10.1371/journal.pone.0098250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meli A., Perrella G., Curcio F., Ambesi-Impiombato F.S. Response to hypogravity of normal in vitro cultured follicular cells from thyroid. Acta Astronaut. 1998;42(1–8):465–472. doi: 10.1016/s0094-5765(98)00139-8. [DOI] [PubMed] [Google Scholar]
- 16.Krohn K., Maier J., Paschke R. Mechanisms of disease: hydrogen peroxide, DNA damage and mutagenesis in the development of thyroid tumors. Nat. Clin. Pract. Endocrinol. Metab. 2007;3(10):713–720. doi: 10.1038/ncpendmet0621. [DOI] [PubMed] [Google Scholar]
- 17.Ohye H., Sugawara M. Dual oxidase, hydrogen peroxide and thyroid diseases. Exp. Biol. Med. 2010;235(4):424–433. doi: 10.1258/ebm.2009.009241. [DOI] [PubMed] [Google Scholar]
- 18.Chen A.Y., Jemal A., Ward E.M. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115(16):3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 19.Klemuk S.A., Vigmostad S., Endapally K., Wagner A.P., Titze I.R. A multiwell disc appliance used to deliver quantifiable accelerations and shear stresses at sonic frequencies. Processes. 2014;2(1):71–88. [Google Scholar]
- 20.Klemuk S.A., Jaiswal S., Titze I.R. Cell viability viscoelastic measurement in a rheometer used to stress and engineer tissues at low sonic frequenciesa. J. Acoust. Soc. Am. 2008;124(4):2330–2339. doi: 10.1121/1.2973183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titze I.R., Klemuk S.A., Gray S. Methodology for rheological testing of engineered biomaterials at low audio frequencies. J. Acoust. Soc. Am. 2004;115(1):392–401. doi: 10.1121/1.1631941. [DOI] [PubMed] [Google Scholar]
- 22.F.S. Ambesi-Impiombato, Living, fast-growing thyroid cell strain, FRTL-5. Google Patents, 1986
- 23.L. Kohn, W. Valente, E. Grollman-Wolff, S. Aloj, P. Vitti, Clinical determination and/or quantification of thyrotropin and a variety of thyroid stimulatory or inhibitory factors performed in vitro with an improved thyroid cell line, FRTL-5, Google Patents, 1986.
- 24.Bidey S., Chiovato L., Day A., Turmaine M., Gould R., Ekins R. Evaluation of the rat thyroid cell strain FRTL-5 as an in-vitro bioassay system for thyrotrophin. J. Endocrinol. 1984;101(3):269. doi: 10.1677/joe.0.1010269. (-NP) [DOI] [PubMed] [Google Scholar]
- 25.Lorenz S., Eszlinger M., Paschke R., Aust G., Weick M., Führer D. Calcium signaling of thyrocytes is modulated by TSH through calcium binding protein expression. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2010;1803(3) doi: 10.1016/j.bbamcr.2010.01.007. (352-60) [DOI] [PubMed] [Google Scholar]
- 26.Vainio M., Fredholm B.B., Törnquist K. Thyrotropin regulates adenosine A1 receptor expression in rat thyroid FRTL‐5 cells. Br. J. Pharmacol. 2000;130(2):471–477. doi: 10.1038/sj.bjp.0703325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambesi-Impiombato F.S., Villone G. The FRTL-5 thyroid cell strain as a model for studies on thyroid cell growth. Acta Endocrinol. 1987;116(Suppl. 1):S242–S245. doi: 10.1530/acta.0.114s242. [DOI] [PubMed] [Google Scholar]
- 28.Björkman U., Ekholm R. Hydrogen peroxide generation and its regulation in FRTL-5 and porcine thyroid cells. Endocrinology. 1992;130(1):393–399. doi: 10.1210/endo.130.1.1309340. [DOI] [PubMed] [Google Scholar]
- 29.Li X., Lu S., Miyagi E., Katoh R., Kawaoi A. Thyrotropin prevents apoptosis by promoting cell adhesion and cell cycle progression in FRTL-5 cells. Endocrinology. 1999;140(12):5962–5970. doi: 10.1210/endo.140.12.7183. [DOI] [PubMed] [Google Scholar]
- 30.Titze I.R., Hunter E.J., Švec J.G. Voicing and silence periods in daily and weekly vocalizations of teachers. J. Acoust. Soc. Am. 2007;121(1):469–478. doi: 10.1121/1.2390676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popolo P.S. Univeristy of Iowa; 2007. Relating Vocal Fold Vibration Amplitude to Skin Acceleration Level on the Anterior Neck. [Google Scholar]
- 32.Gloeckner H., Jonuleit T., Lemke H.D. Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar Blue. J. Immunol. Methods. 2001;252(1–2):131–138. doi: 10.1016/s0022-1759(01)00347-7. [DOI] [PubMed] [Google Scholar]
- 33.Wang H., Joseph J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999;27(5):612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 34.Rivas M., Santisteban P. TSH-activated signaling pathways in thyroid tumorigenesis. Mol. Cell. Endocrinol. 2003;213(1):31–45. doi: 10.1016/j.mce.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 35.Santisteban P., Kohn L., Di Lauro R. Thyroglobulin gene expression is regulated by insulin and insulin-like growth factor I, as well as thyrotropin, in FRTL-5 thyroid cells. J. Biol. Chem. 1987;262(9):4048–4052. [PubMed] [Google Scholar]
- 36.Spitzweg C., Joba W., Morris J., Heufelder A. Regulation of sodium iodide symporter gene expression in FRTL-5 rat thyroid cells. Thyroid. 1999;9(8):821–830. doi: 10.1089/thy.1999.9.821. [DOI] [PubMed] [Google Scholar]
- 37.Kimura T., Okajima F., Sho K., Kobayashi I., Kondo Y. Thyrotropin-induced hydrogen peroxide production in FRTL-5 thyroid cells is mediated not by adenosine 3′, 5′-monophosphate, but by Ca2+ signaling followed by phospholipase-A2 activation and potentiated by an adenosine derivative. Endocrinology. 1995;136(1):116–123. doi: 10.1210/endo.136.1.7828520. [DOI] [PubMed] [Google Scholar]
- 38.Pomerance M., Abdullah H.B., Kamerji S., Correze C., Blondeau J.P. Thyroid-stimulating hormone and cyclic AMP activate p38 mitogen-activated protein kinase cascade. Involvement of protein kinase A, rac1, and reactive oxygen species. J. Biol. Chem. 2000;275(51):40539–40546. doi: 10.1074/jbc.M002097200. [DOI] [PubMed] [Google Scholar]
- 39.Syedain Z.H., Weinberg J.S., Tranquillo R.T. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc. Natl. Acad. Sci. USA. 2008;105(18):6537–6542. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson W.R., Yen S.S., Rubin J. Vibration therapy: clinical applications in bone. Current opinion in endocrinology. Diabetes Obes. 2014;21(6):447. doi: 10.1097/MED.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan M.E., Uzer G., Rubin C.T. The potential benefits and inherent risks of vibration as a non-drug therapy for the prevention and treatment of osteoporosis. Curr. Osteoporos. Rep. 2013;11(1):36–44. doi: 10.1007/s11914-012-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garman R., Gaudette G., Donahue L.R., Rubin C., Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J. Orthop. Res. 2007;25(6):732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- 43.Castillo A.B., Alam I., Tanaka S.M., Levenda J., Li J., Warden S.J. Low-amplitude, broad-frequency vibration effects on cortical bone formation in mice. Bone. 2006;39(5):1087–1096. doi: 10.1016/j.bone.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 44.Rubin C., Capilla E., Luu Y., Busa B., Crawford H., Nolan D. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc. Natl. Acad. Sci. 2007;104(45):17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson W.R., Keller B.V., Davis M.L., Dahners L.E., Weinhold P.S. Low-magnitude, high-frequency vibration fails to accelerate ligament healing but stimulates collagen synthesis in the achilles tendon. Orthop. J. Sports Med. 2015;3(5) doi: 10.1177/2325967115585783. (2325967115585783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uzer G., Thompson W.R., Sen B., Xie Z., Yen S.S., Miller S. Cell mechanosensitivity to extremely low-magnitude signals is enabled by a LINCed nucleus. Stem Cells. 2015;33(6):2063–2076. doi: 10.1002/stem.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg N., Levy M., Francis M. Experimental model for stimulation of cultured human osteoblast-like cells by high frequency vibration. Cytotechnology. 2002;39(3):125–130. doi: 10.1023/A:1023925230651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jing D., Tong S., Zhai M., Li X., Cai J., Wu Y. Effect of low-level mechanical vibration on osteogenesis and osseointegration of porous titanium implants in the repair of long bone defects. Sci. Rep. 2015;5:17134. doi: 10.1038/srep17134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pongkitwitoon S., Weinheimer-Haus E.M., Koh T.J., Judex S. Low-intensity vibrations accelerate proliferation and alter macrophage phenotype in vitro. J. Biomech. 2016 doi: 10.1016/j.jbiomech.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 50.Versaevel M., Braquenier J.-B., Riaz M., Grevesse T., Lantoine J., Gabriele S. Super-resolution microscopy reveals LINC complex recruitment at nuclear indentation sites. Sci. Rep. 2014:4. doi: 10.1038/srep07362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iyer K.V., Pulford S., Mogilner A., Shivashankar G. Mechanical activation of cells induces chromatin remodeling preceding MKL nuclear transport. Biophys. J. 2012;103(7):1416–1428. doi: 10.1016/j.bpj.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang N., Tytell J.D., Ingber D.E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 53.Guilluy C., Osborne L.D., Van Landeghem L., Sharek L., Superfine R., Garcia-Mata R. Isolated nuclei adapt to force and reveal a mechanotransduction pathway within the nucleus. Nat. Cell Biol. 2014;16(4):376. doi: 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uzer G., Pongkitwitoon S., Ian C., Thompson W.R., Rubin J., Chan M.E. Gap junctional communication in osteocytes is amplified by low intensity vibrations in vitro. PLoS One. 2014;9(3):e90840. doi: 10.1371/journal.pone.0090840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X.T., Sun L.W., Qi H.Y., Shi H., Fan Y.B. The bio-response of osteocytes and its regulation on osteoblasts under vibration. Cell Biol. Int. 2016 doi: 10.1002/cbin.10575. [DOI] [PubMed] [Google Scholar]
- 56.Coughlin T.R., Niebur G.L. Fluid shear stress in trabecular bone marrow due to low-magnitude high-frequency vibration. J. Biomech. 2012;45(13):2222–2229. doi: 10.1016/j.jbiomech.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 57.Kimura T., Van Keymeulen A., Golstein J., Fusco A., Dumont J.E., Roger P.P. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr. Rev. 2001;22(5):631–656. doi: 10.1210/edrv.22.5.0444. [DOI] [PubMed] [Google Scholar]
- 58.Ajjan R., Watson P., Findlay C., Metcalfe R., Crisp M., Ludgate M. The sodium iodide symporter gene and its regulation by cytokines found in autoimmunity. J. Endocrinol. 1998;158(3):351–358. doi: 10.1677/joe.0.1580351. [DOI] [PubMed] [Google Scholar]
- 59.Noguchi Y., Harii N., Giuliani C., Tatsuno I., Suzuki K., Kohn L.D. Thyroglobulin (Tg) induces thyroid cell growth in a concentration-specific manner by a mechanism other than thyrotropin/cAMP stimulation. Biochem. Biophys. Res. Commun. 2010;391(1):890–894. doi: 10.1016/j.bbrc.2009.11.158. [DOI] [PubMed] [Google Scholar]
- 60.Toda S., Aoki S., Uchihashi K., Matsunobu A., Yamamoto M., Ootani A. Culture models for studying thyroid biology and disorders. ISRN Endocrinol. 2011;2011 doi: 10.5402/2011/275782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 62.Zarkoob H., Bodduluri S., Ponnaluri S.V., Selby J.C., Sander E.A. Substrate stiffness affects human keratinocyte colony formation. Cell. Mol. Bioeng. 2015;8(1):32–50. doi: 10.1007/s12195-015-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans N.D., Minelli C., Gentleman E., LaPointe V., Patankar S.N., Kallivretaki M. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur. Cells Mater. 2009;18:1–13. doi: 10.22203/ecm.v018a01. (discussion -4) [DOI] [PubMed] [Google Scholar]
- 64.Doyle A.D., Wang F.W., Matsumoto K., Yamada K.M. One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 2009;184(4):481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamashita K., Fujita H., Kitajima K., Nishii Y. Inter- and Intracellular luminal formation in porcine thyroid tissues cultured in a collagen substrate. Arch. Histol. Cytol. 1989;52(2):109–114. doi: 10.1679/aohc.52.109. [DOI] [PubMed] [Google Scholar]
- 66.Espanet H., Alquier C., Mauchamp J. Polarity reversal of inside-out thyroid follicles cultured on the surface of a reconstituted basement membrane matrix. Exp. Cell Res. 1992;200(2):473–480. doi: 10.1016/0014-4827(92)90198-h. [DOI] [PubMed] [Google Scholar]
- 67.De Jesus A.M., Aghvami M., Sander E.A. A combined in vitro imaging and multi-scale modeling system for studying the role of cell matrix interactions in cutaneous wound healing. PLoS One. 2016 doi: 10.1371/journal.pone.0148254. (10.1371/journal.pone.0148254) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material