Abstract
Crk (C10 regulator of kinase) adaptor proteins are highly expressed in many types of human cancers and often contribute to aggressive cancer phenotypes. Crk II, a member of CRK family, has been reported to regulate cell migration and metastasis in breast cancer cells. However, its role in other cancer types has not been reported. In this study, we investigated the molecular function of Crk II in prostate cancer (PCa) cells (CWR-22rv1) in vitro and using a mouse tumor model. Results showed that Crk II knockdown by shRNA-mediated silencing (Crk II-shRNA) in the PCa cells significantly inhibited both cancer cell migration and invasion in cell culture study. Crk II-shRNA cancer cells also significantly decreased colony formation in vitro, but had no significant reduction of tumor volume after 4 weeks of cancer cell xenografting in vivo when compared to the scramble control. Interestingly, Crk II-shRNA cancer cells showed a greatly reduced level of insulin-like growth factor 1 receptor (IGF-1R) and decreased signaling of the IGF-1R/PI3K/Akt axis upon IGF-1 ligand stimulation. A close interaction between Crk II and IGF-1R was demonstrated upon co-immunoprecipitation of IGF-1R with Crk II protein. Further, treatment of cells with either proteosomal degradation or protein synthesis inhibitor showed higher proportion of ubiquitin-associated IGF-1R and faster degradation of IGF-1R in Crk II-shRNA cells in comparison with that in the control cancer cells. Taken together, these data suggest that Crk II plays an important role in the regulation of IGF-1R protein stability and affects downstream of IGF-1R signaling pathways. Therefore, targeting Crk-II can block IGF-1R growth signaling and suppress cancer cell invasion and progression.
Keywords: Crk II, Prostate cancer, IGF-1R
Highlights
-
•
Blocking Crk II inhibited cancer cell migration, invasion, and colony formation.
-
•
Knockdown Crk II decreased IGF-1R protein and its downstream signaling.
-
•
Crk II knockdown increased ubiquitination and degradation of IGF-1R.
1. Introduction
Prostate cancer (PCa) is the most common type of cancer in men and metastasis of treatment resistant cancer is a major cause of mortality [1], [2]. Thus, it is crucial to understand the molecular mechanisms involved in cancer cell motility and invasion that lead to PCa metastasis. It has been reported that the IGF-IR signaling plays a critical role in PCa progression and tumorigenesis [3], [4]. IGF-IR is a member of the type 1 family tyrosine kinase receptors and both IGF-1 and IGF-2 serve as IGF-1R ligands [3], [5]. Circulating IGF-1 levels showed positive correlation with disease progression of PCa, and high IGF-IR expression and activation were associated with both primary and metastatic PCa [4], [6]. Several IGF-IR targeting monoclonal antibodies are being evaluated in the clinic [7], [8], [9].
Crk (C10 regulator of kinase) belongs to the group of Src homology-2 (SH2) adaptor proteins and contains both SH2 and SH3 domains. Crk adaptor proteins bind to the phospho-tyrosine motifs of receptor tyrosine kinases (RTKs) and relay extracellular signals to intracellular pathways. Crk proteins have been well documented for overexpression in various cancers including PCa [10], [11], [12], [13], [14]. Crk II, a member of Crk adaptor proteins, has been reported to promote migration and invasion of breast cancer cells and induce anchorage-independent growth [15], [16], [17]. A study also showed that IGF-IR can interact with Crk II and induce downstream signaling through Crk II phosphorylation at tyrosine-221 in NIH-3T3 overexpressing IGF-IR cells [18]. However, little is known about the mechanisms of Crk II interaction with IGF-IR in cancer cells.
In this study, we investigated the effects of Crk II on cell migration, invasion and colony formation in vitro using a stable Crk knockdown (k/d) PCa cell line (CWR-22rv1) in comparison with cells transfected with scrambled control shRNA. The results demonstrated that Crk II k/d inhibited PCa cell motility and growth, and directly impacted IGF-IR protein stability and signaling by modulating ubiquitination and degradation pathway of IGF-IR.
2. Materials and methods
2.1. Cell Culture and antibodies used for detection
CWR-22Rv1 PCa cell line was obtained from ATCC (American Type Culture Collection) (Manassas, USA). Cells were cultured in a humidified CO2 incubator, 37 °C, in RPMI medium supplemented with 10% FBS (Hyclone) and 100 U/ml Penicillin/ Streptomycin from Sigma-Aldrich (St. Louis, MO, USA) as we reported before [19]. Antibodies for Western blotting of Crk II, IGFR-1β, phospho-Akt-473, Akt, phospho-Erk 44/42 and Erk 44/42 were purchased from Epitomics and other detection antibodies including phospho-IGFR-(1135/1136), p-4EBP1, p-mTOR, m-TOR, S6 kinase were from Cell signaling. Secondary detection antibodies were from Santa Cruz Biotechnology or other indicated sources.
2.2. Construction of shRNA knockdown cells
Lentiviral pLKO.1 shRNA from Open Biosystems was used for construction of a stable shRNA-Crk II knockdown cancer cell lines. Three shRNA sequences were used to establish the Crk II knockdown cell lines: shRNA 1 (TRCN0000021847): TTGTCCCGGATTCTCAAGATG; shRNA 2 (TRCN0000021844) TTAAAGTCAAAGAGGGCTCGC; and shRNA 3 (TRCN0000021848): TTCCCATTAAAGTCAAAGAGG. Scrambled shRNA in pLKO.1 vector was used as a control. Viral packaging and cancer cell transfection were performed according to the manufacturer's instructions where the stable Crk II knockdown or control cells were selected and maintained in RPMI medium containing 1 µg/ml puromycin as previously reported [20].
2.3. Quantitative polymerase chain reaction (qPCR)
RNA was extracted using Trizol (Thermo Fischer Scientific) and total RNA was used for cDNA synthesis using SuperScript™ III First-Strand Synthesis kit (Invitrogen) as described before [19]. Using SYBR green dye, qPCR was conducted using CFX96 Touch™ real-time PCR detection system (Bio-Rad Laboratories). Crk II mRNA expression was quantified with GAPDH as a control using 2-ΔΔCt method [20].
2.4. Migration and invasion assays
Migration assays were conducted as described previously [21]. Briefly, cells were seeded in serum-free culture medium in a transwell plate (6 wells, 8 µm pore) (Corning, NY) and the lower chamber containing complete medium. Cell migration was determined after 48 h of culturing the cells at 37 °C. Similarly, for the invasion experiments, cells were plated in the transwells with precoated matrigel (BD Biosciences) in 0.01 M Tris (pH 8.0) and 0.7% NaCl solution. After 48 h in culture, cells on the upper side of the membrane were removed with the cotton swab and the cells on the lower surface were stained using 0.5% crystal violet. Images were captured using a phase-contrast microscope and five images were taken per well and replicate wells (n=3) were included in each experiment. The assays were repeated 2 times. Quantification of the number of migratory cells per image was performed using image J software.
2.5. Colony formation assays
Cancer cells (1000 or 100 per well) were seeded in 6 well plates, cultured for two weeks in a humidified incubator at 37 °C and 5% CO2, and culture medium was refreshed every 3–4 days during the experiment. Cells were fixed with 4% paraformaldehyde, stained using 0.01% crystal violet solution overnight and washed with PBS as reported before [20]. Colonies were imaged and counted with triplicates. Growth inhibition was computed as a percentage of the scrambled control.
2.6. In vivo xenograft studies
The animal study protocol was approved by the Animal Welfare Committee at The University of Texas Medical School at Houston and the studies were conducted in accordance with the guidelines of animal care as described before [22]. Athymic nu/ nu male mice (Charles River Laboratories) were used for in vivo tumor growth study with Crk II-shRNA PCa cells in comparison with the scrambled control cells. Procedures for tumor implantation and monitoring of tumor growth were as reported previously [22]. Briefly, cancer cells (5×106/ mouse) were implanted subcutaneously into mice at 7 weeks of age and tumor size was monitored weekly using an electronic caliper. The tumor volume was calculated using a formula: tumor volume (mm3)=(width)2xlength/2. Tumor tissues were harvested at the end of study by snap freezing in liquid nitrogen for ex vivo analysis of CRK-II and its related signaling molecules.
2.7. Western blotting (WB)
Cancer cells were cultured to 70–80% confluency in normal culture conditions before the analysis. For IGF stimulation study, cells were cultured in a low serum (1% FBS) overnight, then treated with IGF-1 (10 ng/ml) for different time periods. Cell lysates were prepared by adding lysing the cells in RIPA buffer (Calbiochem) containing protease and phosphatase inhibitor cocktail. The protein concentration was determined using Bio-Rad protein assay reagents based on the manufacturer's instruction and 30–50 µg of protein lysates was used for SDS-PAGE gel separation and WB was conducted as described previously [20]. WB detection was performed with an enhanced chemiluminescence kit (Denville Scientific), imaged using a FluorChem M imager and quantified based on the staining density by Image J software.
2.8. Co-immunoprecipitation (co-IP)
Cell lysates were incubated overnight with 1.5 µg of Crk II or 2.5 µg of IGF-IRβ antibody (Epitomics) and Dynabeads® protein G (Novex Biotechnologies) were subsequently added for 4 h at 4 οC with slow mixing. The supernatant was removed using a magnetic separator and the magnetic beads were washed with the sample buffer. 2X SDS Laemmli sample buffer (Biorad) with β-mercaptoethanol was added to the beads, heated at 80 οC for 8 min and then subjected to WB for detection of IGF-IR and Crk II [20].
2.9. Proteosomal inhibitor treatment and ubiquitination assays
Cells were treated with 10 µm of MG-132 (EMD Millipore, MA, USA) for 4 h in culture before lysate preparation. For detection of ubiquitination of IGF-IR, pull-down using IGF-IR antibody and WB using anti-ubiquitin PD-4 antibody (Cell Signaling Technology) was performed to detect ubiquitination as described previously [20].
2.10. Cycloheximide treatment
Cells were seeded in a 6-well culture plate a day before and cycloheximide (EMD Millipore, MA, USA) at 100 µg/ml was added into the culture for various time periods. Cell lysates were collected at 0, 8, 12 and 24 h after the cycloheximide treatment and evaluated for IGF-IR by WB [20].
3. Results
3.1. Crk II knockdown inhibited cancer CWR-22rv1 cell migration and invasion
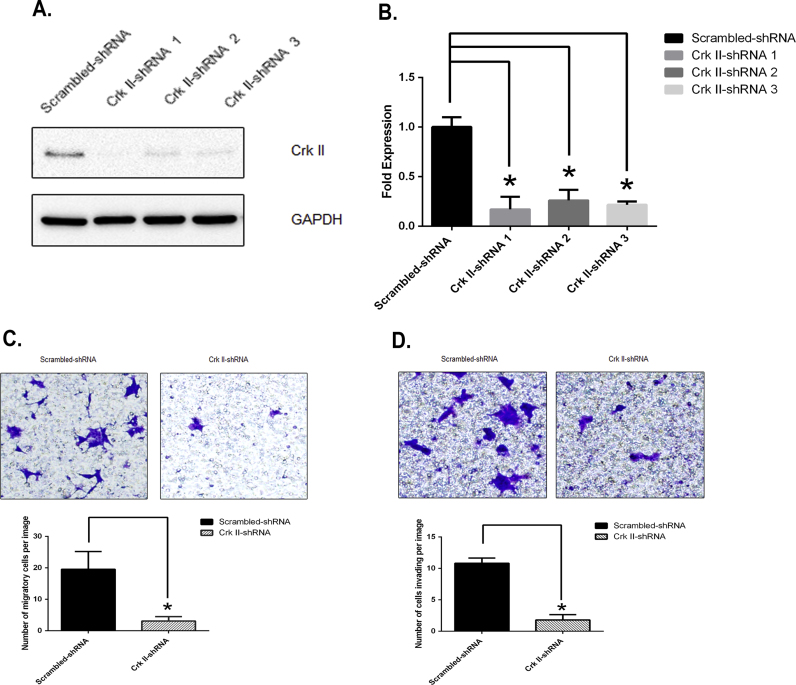
In order to examine the functional role of Crk II in PCa, we abrogated Crk II expression in CWR-22rv1 (an androgen-independent PCa cell line), and established stable Crk II knockdown cells (Crk II-shRNA) using a lentiviral vector system. In comparison with the vehicle control cells (Scramble-shRNA), the Crk II-shRNA cells had low to no detectable levels of CRK II by western blotting (Fig. 1 A). Quantitative analysis using q-PCR showed a significant decrease of Crk II RNA transcripts in all three Crk-II shRNA knockdown cell lines in comparison with that of the scramble control cells (Fig. 1 B). In order to compare the effects of Crk II knockdown on cancer cell migration and invasion, we determined both cell migration and invasion of Crk II-shRNA cells in comparison with the scramble control cells using transwell assays. Crk II-shRNA cells exhibited significantly lower motility than that in the scrambled control cells (p<0.05) (Fig. 1 C). Crk II-shRNA cancer cells also showed a significant decrease of invasiveness in comparison with the Scrambled- control (p<0.05); (Fig. 1 D). As all three shRNA cell lines showed similar results, the data from the shRNA 2 knockdown cell line is shown in Fig. 1 C and D. Therefore, following studies were carried out using the shRNA 2 knockdown cancer cells.
Fig. 1.
Crk II knockdown inhibited CWR22rv1 cancer cell migration and invasion. (A) Western blot detection of Crk II protein levels in Crk-II-shRNA cells; (B) qRT-PCR detection of Crk II mRNA in Scrambled and Crk II-shRNA cells; (C) Representative images and quantification of average numbers of cells detected in migration using a transwell assay, n=3; (D) Representative images and quantification of cells invading through a matrigel-coated membrane insert after 48 h cell culture. Experiments were repeated three times (n=3), and * indicates p value <0.05. The data in C&D show the results from the shRNA 2 stable cancer cell line. The original images were 1360 pixels*1024 pixels at 72 dpi, (length of 18’’ *14’’) and a portion of the image (4’’ *4’’) from the original 72 dpi image (288 pixels *288 pixels) is shown. The resolution of the images was adjusted from 72 to 300 dpi using Adobe photoshop program. For the bar graph, 10 separate images were taken from 3 experimental replications and the error bars represent the standard deviation (sd) calculated from the representative images.
3.2. Crk II knockdown suppressed colony formation in vitro but did not significantly inhibit tumor volume in vivo
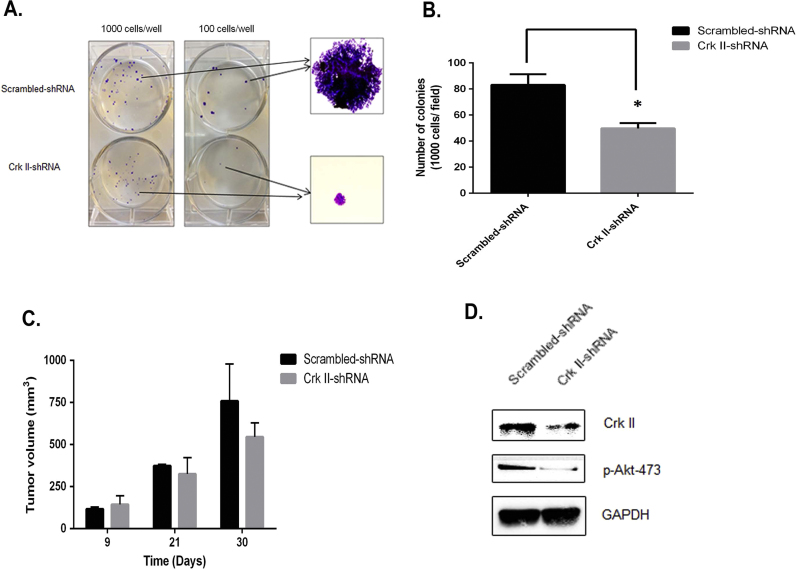
To investigate the role of Crk II in CWR22rv1 cell proliferation, we performed colony formation assays using cell culture study. Crk II-shRNA cells showed both reduced numbers and the size of colonies in comparison with that in the scramble control cells (Fig. 2 A). Number of colonies formed from a fixed number of seeding cells (both 100 and 1000 cells/ well) decreased by 40% in Crk II knockdown cells as compared to that in control cells (Fig. 2 B). Crk II-shRNA cells also had slightly smaller tumors as compared to the scrambled-shRNA control after 4 weeks post implantation of cancer cells in vivo, even though the differences in the tumor volume were not statistically significant (Fig. 2 C). The smaller impact on tumor growth in vivo than that in the cell culture study may be caused by the differences of assay sensitivity and physiological conditions in in vivo and in vitro studies. Ex vivo assay of tumor lysates showed that Crk II and p-Akt levels were significantly decreased and IGF-IR was reduced in Crk II shRNA tumors in comparison with that in the scrambled control tumors (Fig. 2 D).
Fig. 2.
Crk II knockdown inhibited colony formation in vitro but had less significant effects on tumor growth in vivo. (A) Representative images of colony formation by Crk II knockdown cells in comparison with the scramble control cancer cells; (B) Reduced numbers of colonies formed by Crk II-shRNA cancer cells in comparison with the that by the scrambled control cells. The cancer cells (seeding density at 1000 cells/well) were cultured for 10 days at 37 °C cell culture incubator and medium were refreshed every 3 days; (C) Tumor volumes of Crk II knockdown cells (Crk II-shRNA) were not significantly affected in comparison with that in the Scrambled-shRNA control cells n=3. (D) WB detection of Crk II in ex vivo tumor lysates.
3.3. Crk II knockdown downregulated IGF-IR levels and suppressed PI3K/Akt signaling pathway in CWR-22rv1 cells
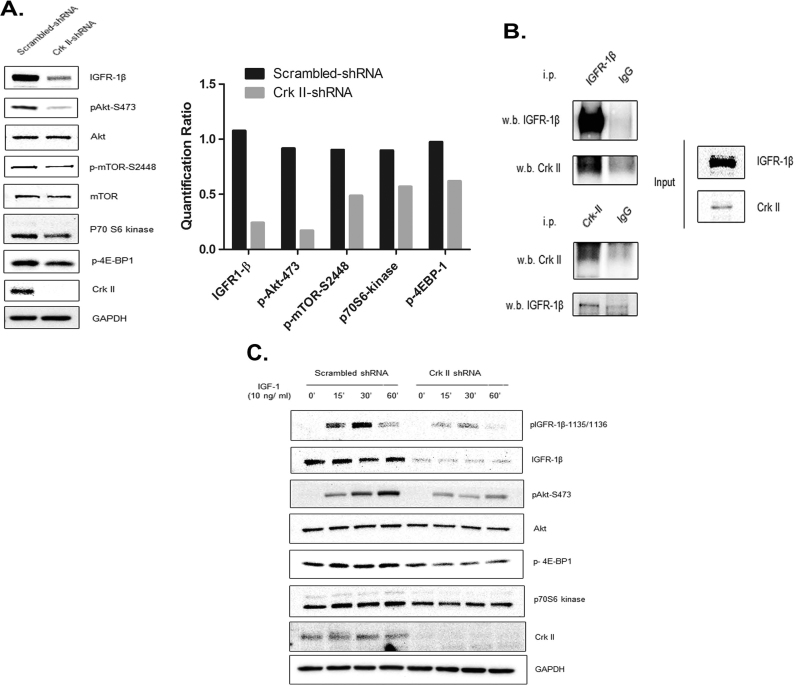
To understand the Crk II-mediated signaling pathways that are crucial in the cell migration and invasion, we compared expression levels of a panel of RTKs including IGF-1R and epidermal growth factor receptor (EGFR) family receptors in the Crk II-shRNA and scramble-shRNA cells. Results showed that IGF-IR expression was significantly reduced and its downstream key signaling molecules such as p-Akt, p-mTOR, p70S6K and p4EBP1 were also impacted as determined by WB (Fig. 3 A). The right bar graph in Fig. 3. A shows quantification of the WB staining intensity. But the levels of EGFR family members showed no changes (Suppl. Fig. S1). To determine if Crk II interacts directly with IGF-IR as an adaptor protein, we conducted co-ip using IGF-IR and Crk II antibodies and WB detection. The results revealed that IGF-IR was directly associated with Crk II (Fig. 3 B). Further, we determined IGF-IR levels and its downstream signaling proteins in Crk II-shRNA cells in comparison with that in the scramble control upon IGF-1 stimulation. As expected, p-IGF-IR-1135/1136, p-Akt-473, p-mTOR, p70S6K and p4EBP1 levels decreased in Crk II-shRNA cells when compared with Scrambled-control cells in the presence of IGF-1 for 15 30 and 60 min (Fig. 3 C). At 30 min, IGF-1-induction of p-IGF-IR-1135/1136 is maximum, after which it reduces at 60 minThese results indicate that Crk II can modulate the levels of IGF-IR and impact IGF-IR downstream signaling through PI3K/ Akt in PCa cells.
Fig. 3.
Crk II knockdown decreased levels of IGF-1R and its downstream signaling in CWR22rv1 cancer cells. (A) WB detection (30 μg protein loaded in each well) of IGF-1R (antibody against IGF-1R β subunit) and the downstream signaling molecules of PI3K-Akt pathway. Bar graph shows the quantification of the staining intensities of each protein using the software of the FluorChem M imager (ProteinSimple); (B) Interaction of Crk II and IGF-1R by co-immunoprecipitation (i.p.). The CWR22rv1 cancer cell lysates were used to conduct pull down with either anti-Crk II antibody or anti-IGF-1Rβ antibody and isotype IgG (IgG) was used as a control; (C) Effects of IGF 1 stimulation on IGF-1R signaling in Crk II-shRNA knockdown cancer cells in comparison with the scramble control. WB detection was conducted similarly as in (A). Cancer cells were cultured in a low serum medium (1% FBS) over night and stimulated with IGF (10 ng/ml) for different times (15 and 30 min).
3.4. Crk II knockdown increased ubiquitination and degradation of IGF-IR
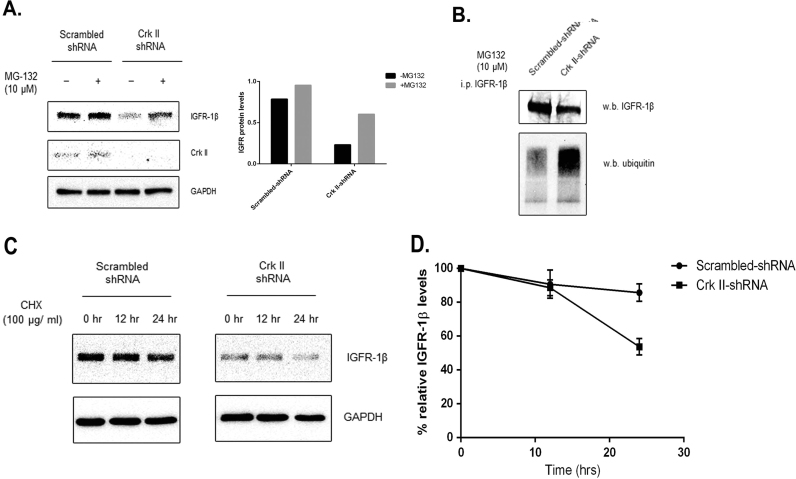
To investigate the mechanism of IGF-IR downregulation by Crk II, we determined the effects of Crk II on IGF-IR degradation pathway. Results showed that IGF-IR levels were increased in Crk II-shRNA cells after treatment with a proteosomal inhibitor (MG132, at 10 µm) (Fig. 4 A), suggesting that the effect of Crk II on IGF-IR levels involves post-translational regulation through proteosomal degradation pathway. As ubiquitination of IGF-1R is important for protein proteosomal degradation, we further investigated IGF-IR ubiquitination in Crk II knockdown cells. Our results demonstrated that ubiquitinated IGF-IR increased in Crk II knockdown cells in comparison with that in scrambled controls when the comparable levels of IGF-IR were used for the detection (Fig. 4 B). We also determined the effect of Crk II knockdown on IGF-IR by treatment with cycloheximide, an inhibitor of protein synthesis. Results showed that IGF-IR levels were declined faster in the Crk II knockdown cancer cells (close to 50% of IGFR-IR remaining) in comparison with that in the control cells (80% IGFR-IR remaining) after 24 h in the presence of cycloheximide (Fig. 4 C and D). These data indicate that Crk II regulates IGF-IR at the post-translational level by stabilizing IGF-IR and preventing it from ubiquitination and degradation.
Fig. 4.
Crk II knockdown increased ubiquitination and decreased stability of IGF-1R. (A) WB detection of IGF-1R in Crk II knockdown (Crk II-shRNA) cells in comparison with the scramble control after treatment with the proteosomal inhibitor MG132 (10 µm) for 4 h in culture medium; (B) WB detection of IGF-1R ubiquitination using an antibody against ubiquitination. IGF-1R protein was pulled down by immunoprecipitation using anti-IGF-1Rβ antibody before loading on gel for WB; (C) WB detection shows the reduced stability of IGF-1R in Crk II knockdown cancer cells when compared to the scramble control cells. The cells were treated with a protein synthesis inhibitor cycloheximide (100 μg/ml) for 0, 12 and 24 h. The experiments were repeated twice, n=2; (D) Quantitation of the staining intensity of IGF-1R shown in the WB. The staining intensity at 0 h is used as reference control (100%) in the calculation and error bars in the bar graph indicate the standard deviations calculated from the two independent experimental repeats.
4. Discussion
In this study, we revealed an important role of Crk II as an adaptor protein of IGF-IR in invasion and migration of androgen-independent PCa cells. Crk family proteins are one of the crucial regulators in the signaling of many RTKs [10], [15], [17], [23]. Crk II overexpression in breast and lung cancer patients is shown to be correlated with the adverse tumor grade [12], [13]. In this study, we demonstrated for the first time that Crk II plays an important role in migration and invasion of CWR22Rv1 prostate cancer cells. The results are consistent with the reported roles of Crk II in breast and lung cancer cells [14].
It has been reported that IGF-IR signaling is important for PCa progression and development [3]. Up-regulated IGFR mRNA and protein levels have been noted in both primary PCa and patients with bone metastases [6]. Increased IGF-IR expression and signaling were reported in vitro and in vivo xenografts in androgen-independent LNCaP/ C4-2 cells [24], [25]. Previous studies have shown that IGF-IR signaling was affected by Crk II association with IRS-4, a downstream IGF-IR signaling molecule [18], [26]. In this study, we demonstrated that Crk II modulated IGF-IR levels by direct association of the two proteins. We also revealed that IGF-IR was modulated by Crk II through ubiquitination and proteosome-mediated IGF-IR degradation in PCa cells.
Crk II has a role in PI3-K activation and signaling through AKT/ mTOR pathway [27], [28], [29]. A previous study has shown that IGF-1 promoted migration of androgen-independent PCa cells through PI-3K-Akt signaling [30]. An mTOR inhibitor study revealed that mTOR regulated invasion gene signatures in PCa cells through 4EBP1 pathway [31]. This study using CWR22rv1 cells demonstrated an important role of Crk II in migration and invasion and a close link between Crk II and IGF-IR/ pAkt-mTOR signaling.
Earlier studies have shown an interaction of Crk II with IGF-IR phosphotyrosine residues in IGF-IR-overexpressing NIH-3T3 cells [18], [26]. The co-immunoprecipitation data confirmed the interaction of Crk II and IGF-IR in PCa cells. Both Grb10 and β-Arrestin were reported as adaptor proteins for IGFR ubiquitination [32]. This study indicated that CRK II also was involved in modulation of IGF-IR levels and stability at post-translational level.
In summary, this study demonstrated an important role of Crk II in IGF-IR regulation in androgen-independent PCa cells. The data suggest that Crk II/ IGF-IR signaling axis can promote colony formation, migration and invasion in PCa cells. Thus, targeting Crk II/ IGF-IR axis may offer a valuable therapeutic strategy for treatment of androgen-independent metastatic prostate cancer.
Acknowledgements
We would like to thank Dr. XueJun Fan for helping with the animal study, Seema Mukherjee and Dr. Vidya Gopalkrishnan for their technical assistance in the study. This work was partially funded by Janssen R&D, LLC, the Texas Emerging Technology Fund to Dr. Z. An and Co-Investigator Dr. N. Zhang, and the Welch Foundation grant no. AU0042 to Dr. Z. An.
Footnotes
Transparency data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.10.009.
Appendix A. Transparency document
Supplementary material
.
Supplementary material
.
References
- 1.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics. CA Cancer J. Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Attar R.M., Takimoto C.H., Gottardis M.M. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin. Cancer Res. 2009;15:3251–3255. doi: 10.1158/1078-0432.CCR-08-1171. [DOI] [PubMed] [Google Scholar]
- 3.Monti S., Proietti-Pannunzi L., Sciarra A., Lolli F., Falasca P., Poggi M., Celi F.S., Toscano V. The IGF axis in prostate cancer. Curr. Pharm. Des. 2007;13:719–727. doi: 10.2174/138161207780249128. [DOI] [PubMed] [Google Scholar]
- 4.Stattin P., Bylund A., Rinaldi S., Biessy C., Dechaud H., Stenman U.H., Egevad L., Riboli E., Hallmans G., Kaaks R. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J. Natl. Cancer Inst. 2000;92:1910–1917. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- 5.Samani A.A., Yakar S., LeRoith D., Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr. Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 6.Hellawell G.O., Turner G.D., Davies D.R., Poulsom R., Brewster S.F., Macaulay V.M. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62:2942–2950. [PubMed] [Google Scholar]
- 7.Wu J.D., Odman A., Higgins L.M., Haugk K., Vessella R., Ludwig D.L., Plymate S.R. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin. Cancer Res. 2005;11:3065–3074. doi: 10.1158/1078-0432.CCR-04-1586. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann F., Garcia-Echeverria C. Blocking the insulin-like growth factor-I receptor as a strategy for targeting cancer. Drug Disco. Today. 2005;10:1041–1047. doi: 10.1016/S1359-6446(05)03512-9. [DOI] [PubMed] [Google Scholar]
- 9.Dean J.P., Sprenger C.C., Wan J., Haugk K., Ellis W.J., Lin D.W., Corman J.M., Dalkin B.L., Mostaghel E., Nelson P.S., Cohen P., Montgomery B., Plymate S.R. Response of the insulin-like growth factor (IGF) system to IGF-IR inhibition and androgen deprivation in a neoadjuvant prostate cancer trial: effects of obesity and androgen deprivation. J. Clin. Endocrinol. Metab. 2013;98:E820–E828. doi: 10.1210/jc.2012-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birge R.B., Kalodimos C., Inagaki F., Tanaka S. Crk and CrkL adaptor proteins: networks for physiological and pathological signaling. Cell Commun. Signal. 2009;7:13. doi: 10.1186/1478-811X-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sriram G., Birge R.B. Emerging roles for crk in human cancer. Genes Cancer. 2010;1:1132–1139. doi: 10.1177/1947601910397188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fathers K.E., Bell E.S., Rajadurai C.V., Cory S., Zhao H., Mourskaia A., Zuo D., Madore J., Monast A., Mes-Masson A.M., Grosset A.A., Gaboury L., Hallet M., Siegel P., Park M. Crk adaptor proteins act as key signaling integrators for breast tumorigenesis. Breast Cancer Res. 2012;14:R74. doi: 10.1186/bcr3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller C.T., Chen G., Gharib T.G., Wang H., Thomas D.G., Misek D.E., Giordano T.J., Yee J., Orringer M.B., Hanash S.M., Beer D.G. Increased C-CRK proto-oncogene expression is associated with an aggressive phenotype in lung adenocarcinomas. Oncogene. 2003;22:7950–7957. doi: 10.1038/sj.onc.1206529. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues S.P., Fathers K.E., Chan G., Zuo D., Halwani F., Meterissian S., Park M. CrkI and CrkII function as key signaling integrators for migration and invasion of cancer cells. Mol. Cancer Res. 2005;3:183–194. doi: 10.1158/1541-7786.MCR-04-0211. [DOI] [PubMed] [Google Scholar]
- 15.Spencer K.S., Graus-Porta D., Leng J., Hynes N.E., Klemke R.L. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J. Cell Biol. 2000;148:385–397. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamorte L., Kamikura D.M., Park M. A switch from p130Cas/Crk to Gab1/Crk signaling correlates with anchorage independent growth and JNK activation in cells transformed by the Met receptor oncoprotein. Oncogene. 2000;19:5973–5981. doi: 10.1038/sj.onc.1203977. [DOI] [PubMed] [Google Scholar]
- 17.Lamorte L., Royal I., Naujokas M., Park M. Crk adapter proteins promote an epithelial-mesenchymal-like transition and are required for HGF-mediated cell spreading and breakdown of epithelial adherens junctions. Mol. Biol. Cell. 2002;13:1449–1461. doi: 10.1091/mbc.01-10-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koval A.P., Blakesley V.A., Roberts C.T., Jr., Zick Y., Leroith D. Interaction in vitro of the product of the c-Crk-II proto-oncogene with the insulin-like growth factor I receptor. Biochem J. 1998;330(2):923–932. doi: 10.1042/bj3300923. (Pt) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y., Fan X., Meng W., Deng H., Zhang N., An Z. Engagement of immune effector cells by trastuzumab induces HER2/ERBB2 downregulation in cancer cells through STAT1 activation. Breast Cancer Res. 2014;16:R33. doi: 10.1186/bcr3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Z.Huang, B.K.Choi, K.Mujoo, X.Fan, M.Fa, S.Mukherjee, N.Owiti, N.Zhang, Z.An, The E3 ubiquitin ligase NEDD4 negatively regulates HER3/ErbB3 level and signaling, Oncogene, 2014. [DOI] [PubMed]
- 21.Choi B.K., Fan X., Deng H., Zhang N., An Z. ERBB3 (HER3) is a key sensor in the regulation of ERBB-mediated signaling in both low and high ERBB2 (HER2) expressing cancer cells. Cancer Med. 2012;(1):28–38. doi: 10.1002/cam4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan X., Brezski R.J., Deng H., Dhupkar P.M., Shi Y., Gonzalez A., Zhang S., Rycyzyn M., Strohl W.R., Jordan R.E., Zhang N., An Z. A novel therapeutic strategy to rescue the immune effector function of proteolytically inactivated cancer therapeutic antibodies. Mol. Cancer Ther. 2015;14:681–691. doi: 10.1158/1535-7163.MCT-14-0715. [DOI] [PubMed] [Google Scholar]
- 23.Hempstead B.L., Birge R.B., Fajardo J.E., Glassman R., Mahadeo D., Kraemer R., Hanafusa H. Expression of the v-crk oncogene product in PC12 cells results in rapid differentiation by both nerve growth factor- and epidermal growth factor-dependent pathways. Mol. Cell Biol. 1994;14:1964–1971. doi: 10.1128/mcb.14.3.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickerson T., Chang F., Lorimer D., Smeekens S.P., Sawyers C.L., Pollak M. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR) Cancer Res. 2001;61:6276–6280. [PubMed] [Google Scholar]
- 25.Krueckl S.L., Sikes R.A., Edlund N.M., Bell R.H., Hurtado-Coll A., Fazli L., Gleave M.E., Cox M.E. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64:8620–8629. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
- 26.Karas M., Koval A.P., Zick Y., LeRoith D. The insulin-like growth factor I receptor-induced interaction of insulin receptor substrate-4 and Crk-II. Endocrinology. 2001;142:1835–1840. doi: 10.1210/endo.142.5.8135. [DOI] [PubMed] [Google Scholar]
- 27.Brown E.J., Beal P.A., Keith C.T., Chen J., Shin T.B., Schreiber S.L. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377:441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 28.Gingras A.C., Kennedy S.G., O'Leary M.A., Sonenberg N., Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goh E.L., Zhu T., Yakar S., LeRoith D., Lobie P.E. CrkII participation in the cellular effects of growth hormone and insulin-like growth factor-1. Phosphatidylinositol-3 kinase dependent and independent effects. J. Biol. Chem. 2000;275:17683–17692. doi: 10.1074/jbc.M001972200. [DOI] [PubMed] [Google Scholar]
- 30.Montagnani Marelli M., Moretti R.M., Procacci P., Motta M., Limonta P. Insulin-like growth factor-I promotes migration in human androgen-independent prostate cancer cells via the alphavbeta3 integrin and PI3-K/Akt signaling. Int J. Oncol. 2006;28:723–730. [PubMed] [Google Scholar]
- 31.Hsieh A.C., Liu Y., Edlind M.P., Ingolia N.T., Janes M.R., Sher A., Shi E.Y., Stumpf C.R., Christensen C., Bonham M.J., Wang S., Ren P., Martin M., Jessen K., Feldman M.E., Weissman J.S., Shokat K.M., Rommel C., Ruggero D. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vecchione A., Marchese A., Henry P., Rotin D., Morrione A. The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol. Cell Biol. 2003;23:3363–3372. doi: 10.1128/MCB.23.9.3363-3372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material