Abstract
Peroxisome proliferator-activated receptor β/δ (PPARβ/δ) is a member of the nuclear receptor superfamily and a ligand-activated transcription factor that is involved in the regulation of the inflammatory response via activation of anti-inflammatory target genes and ligand-induced disassociation with the transcriptional repressor B-cell lymphoma 6 (BCL6). Chronic pancreatitis is considered to be a significant etiological factor for pancreatic cancer development, and a better understanding of the underlying mechanisms of the transition between inflammation and carcinogenesis would help further elucidate chemopreventative options. The aim of this study was to determine the role of PPARβ/δ and BCL6 in human pancreatic cancer of ductal origin, as well as the therapeutic potential of PPARβ/δ agonist, GW501516. Over-expression of PPARβ/δ inhibited basal and TNFα-induced Nfkb luciferase activity. GW501516-activated PPARβ/δ suppressed TNFα-induced Nfkb reporter activity. RNAi knockdown of Pparb attenuated the GW501516 effect on Nfkb luciferase, while knockdown of Bcl6 enhanced TNFα-induced Nfkb activity. PPARβ/δ activation induced expression of several anti-inflammatory genes in a dose-dependent manner, and GW501516 inhibited Mcp1 promoter-driven luciferase in a BCL6-dependent manner. Several pro-inflammatory genes were suppressed in a BCL6-dependent manner. Conditioned media from GW501516-treated pancreatic cancer cells suppressed pro-inflammatory expression in THP-1 macrophages as well as reduced invasiveness across a basement membrane. These results demonstrate that PPARβ/δ and BCL6 regulate anti-inflammatory signaling in human pancreatic cancer cells by inhibiting NFκB and pro-inflammatory gene expression, and via induction of anti-inflammatory target genes. Activation of PPARβ/δ may be a useful target in pancreatic cancer therapeutics.
Keywords: Pparbeta, Bcl6, Pancreatic cancer, Inflammation, Macrophage
Highlights
-
•
PPARβ/δ and BCL6 regulate anti-inflammatory signaling in human pancreatic cancer cells by inhibiting NFκB and pro-inflammatory gene expression.
-
•
GW501516-activated PPARβ/δ abrogates TNFα-induced pro-inflammatory signaling in a BCL6-dependent manner.
-
•
Media conditioned by GW501516-treated pancreatic cancer cells reduced pro-inflammatory signaling in macrophages.
-
•
Targeting PPARβ/δ with specific activators may be useful strategy in pancreatic cancer therapeutics.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease, ranking as the fourth leading cause of cancer death with a 5-year survival rate of 6% [1]. Behavioral risk factors associated with PDAC include smoking and alcoholism, as well as metabolic disorders obesity and diabetes mellitus. Treatment with gemcitabine and fluorouracil yielded 20% overall survival of PDAC to 6 months, to more current regimes of nab-paclitaxel/gemcitabine and FOLFIRINOX yielding to 35–48% overall survival to 9–11 months [2]. Despite these regimes, substantial improvements in therapeutics over the last 20 years have been lacking, identification of predictive biomarkers and therapeutic targets are sorely needed.
There is a strong correlation between chronic pancreatitis and pancreatic cancer development [3]. Molecular changes have been observed during the transition from pancreatitis to pancreatic cancer including mutations in inflammatory signaling molecules kirsten rat sarcoma viral oncogene homolog (Kras) [4], serine protease inhibitor kazal type 1 (Spink1) [5], cyclooxygenase-2 (Cox2), and nitric oxide (No) [6], [7]. Nuclear factor κ-light chain enhancer of activated B cells (Nfkb1) is master regulator of inflammatory response and genes involved in cell cycle, angiogenesis, and apoptosis [3]. Several pancreatic cancer cell lines and tissue have shown constitutive activation of Nfkb1 [8], [9]. Autocrine secretion of interleukin 1α (IL1α) activates Nfkb1 in pancreatic cancer, concurrently, NFκB1 enhanced expression of IL1α resulting in a positive feedback loop for this constitutive activation [10]. NFκB1 signaling dysregulates downstream targets involved in angiogenesis and metastasis such as vascular endothelial growth factor (Vegf) and interleukin 8 (Il8) [11]. Clearly, NFκB1 plays an essential role in cancer progression, and with the complexity of KRAS pathways making targeting therapeutics difficult, NFκB1 becomes increasingly attractive candidate for treatment.
Evidence over the past 5-years has indicated Pparb is a feasible target for chemoprevention [12], albeit not without controversy [13]. PPARβ/δ is a ubiquitously expressed ligand-activated transcription factor that controls a number of cellular functions, and involved in several metabolic disorders such as diabetes, obesity, and atherosclerosis [14], [15], [16]. It resides in the nucleus where it associated with transcriptional suppressor BCL6 [17]. Through ligand activation of PPARβ/δ, BCL6 dissociates from the complex and decreases inflammatory signaling by binding NFκB1 and STAT1 [18]. In addition, PPARβ/δ dimerizes with retinoid x receptor (RXR) and directly regulates expression of certain anti-inflammatory genes, such as interleukin-1 receptor antagonist (Il1ra). The biological ramifications of complex formation between PPARβ/δ and BCL6 are still being elucidated, but both appear to be effective repressors of inflammatory markers in cell and animal models [19].
Little is known about the function of PPARβ/δ and BCL6 in the pancreas or their roles in the etiology of PDAC. GW501516, a PPARβ/δ agonist, attenuates inflammatory response in two different pancreatitis mouse models [20]. We sought to determine the role of PPARβ/δ and BCL6 in the human ductal pancreas in regards to inflammation, and the therapeutic potential of GW501516. Our observations show that GW501516-mediated anti-inflammatory signaling via PPARβ/δ is present in two pancreatic cancer cell lines- Mia PaCa-2 and BxPc-3. PPARβ/δ suppresses Nfkb1 activity. Several pro-inflammatory markers are inhibited by PPARβ/δ ligands in a BCL6-dependent manner. Conditioned media experiments using RNAi to reduce expression of PPARβ/δ and BCL6 in pancreatic cancer cells implicated both proteins as regulators of inflammatory gene expression in a human macrophage cell line, THP-1 cells, as well as affect macrophage recruitment.
2. Materials and methods
2.1. Cells and reagents
Human pancreatic cancer cells, Mia PaCa-2 (COX2 negative, CRL-1420) and BxPc-3 (COX2 positive, CRL-1687) were purchased from ATCC (Manassas, VA) and cultured in high glucose DMEM containing 10% FBS. Human embryonic kidney 293 cells were cultured in DMEM containing 10% FBS. THP-1 cells were cultured in RPMI 1640 media supplemented with 10% FBS. All cell media contained 100U penicillin and streptomycin, and cells were cultured in a humidified atmosphere at 37 °C containing 5% CO2. All media components and FBS were purchased from Gibco BRL/Life Technologies (Carlsbad, CA). GW501516 used as a positive control for PPARβ/δ, and phorbol 12-myristate 13-acetate (PMA), used to differentiate THP-1 cells, was purchased from Sigma Chemical Company. The 2.8 kb mMcp1 (accession #U12470) promoter fragment cloned into the luciferase reporter vector pGL3-basic (Promega) was provided by Dr. Ronald Evans (Salk Institute for Biological Studies, La Jolla, CA). Transfection control plasmids pRL-TK and pRLCMV were purchased from Promega (Madison, WI). Recombinant htnfa was purchased from Invitrogen (Carlsbad, CA) and reconstituted in nanopure water. MISSION© Pparb, Bcl6, Il1ra, and scrambled non-targeting glycerol stocks were purchased from Sigma-Aldrich. High Capacity cDNA Archive Kit and ABI 7300 real-time PCR system were purchased from Applied Biosystems (Foster City, CA). The pPACKH1 packaging plasmids and the pCDNA3.1/Pparb-FLAG plasmid were provided by Dr. Curtis Omiecinski (Penn State University). CytoSelect TM 96-well Invasion Assay (basement membrane, fluormetric format) was purchased from Cell Biolabs, Inc. (San Diego, CA) and used according to manufacturer's instructions.
2.2. NF-κB1 reporter assays
Mia PaCa-2 cells were seeded at 7.5×105 cells in 10 cm tissue culture dishes. Cells were transiently transfected with 9 μg pNkfb1-luciferase and 1 μg pRLCMV using LipofectAMINE (Invitrogen) reagent for 6 h and allowed to recover overnight. Cells were challenged with the indicated treatments 24 h post-transfection, and Nfkb1-luciferase activity was measured using Dual Luciferase Reporter Assay (Promega) and normalized using control luciferase activity). Cells were transfected with 5 μg Pparb-FLAG and 4 μg pNfkb1-luciferase and 1 μg pRLCMV.
2.3. Isolation of total RNA and quantitative PCR
Total RNA was isolated from Mia PaCa-2 and BxPc-3 cells using Tri-Reagent and the manufacturer's recommended protocol (Sigma). Reverse transcription of 1 μg mRNA was done using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Primers for quantitative Polymerase Chain Reaction (PCR) were designed based on published sequences in GenBank and are shown in Table 1. The housekeeping gene βactin was used to normalize all the tested genes. The data shown are representative of three independent experiments with triplicate samples.
Table 1.
List of qPCR primers.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| bactin | AACAAGAGGCCACACAAATAGG | CAGATGTACAGGAATAGCCTCCG |
| Il1ra | GGGAACTTTGCACCCAACAT | TTGGCAGGTACTCAGCGAATG |
| Tgfb | AGGTCCTTGCGGAAGTCAATG | CTATTGCTTCAGCTCCACGGA |
| Sod1 | TGCTTCCCCACACCTTCACTGGT | ATGGCGACGAAGGCCGTGTG |
| Fgf21 | CGCTGGCACAGGAACCTGGA | ACCAGAGCCCCGAAAGTCTCCT |
| Mcp1 | GGACGCATTTCCCCAGTACA | CCGAGAACGAGATGTGGACA |
| Mcp3 | ATGAGGTAGAGAAGGGAGGAGCAT | CAAACTGGACAAGGAGATCTGTGC |
| Tnfa | TGGATGTTCGTCCTCCTCACA | ATCAATCGGCCCGACTATCTC |
| Il1b | TCCTTAGTCCTCGGCCAAGAC | GTGCCATGGTTTCTTGTGACC |
| Il6 | CCGTCGAGGATGTACCGAATT | GCCACTCACCTCTTCAGAACG |
| Cox2 | CGGTGTTGAGCAGTTTTCTCC | AAGTGCGATTGTACCCGGAC |
2.4. Mcp1 reporter assay
Mia PaCa-2 cells transiently expressing non-targeting control, Bcl6 or Pparb shRNA were plated in 10 cm tissue culture dishes as described. Cells were transiently transfected with 9 μg Mcp1-luciferase and 1 μg pRLCMV for 6 h and allowed to recover overnight. Cells were then challenged with the indicated treatments 24 h post-transfection and Mcp1 promoter driven luciferase was assayed and corrected using the internal transfection control pRLCMV.
2.5. Differentiation of THP-1 cells with PMA
Differentiation was achieved by resuspending THP-1 cells at a density of 2×105 cells/mL in serum-free RPMI 1640 media supplemented with 100 nM PMA for 24 h. Cells were then allowed to recover in media containing 10% FBS for a further 24 h before use in experiments.
2.6. Lentiviral RNAi
HEK-293 cells were grown to confluency in 10 cm tissue culture dishes under the conditions described above. The cells were then transiently transfected with 4.6 μg of scrambled non-targeting control, or Pparb, Bcl6, or Il1ra shRNA, as well as 2.4 μg each of pPACKH1 packaging plasmids, using LipofectAMINE 2000. Cells were transfected for 6 h and allowed to recover overnight in normal media. Fresh media was added the following morning, and pseudoviral supernatant was generated for 72 h. Supernatant was then harvested and passed through a 0.4 µm filter under sterile conditions. Polybrene (Millipore, Billerica, MA) was then added to a final concentration of 5 μg/mL and the pseudoviral supernatant was then added directly to target cells for 6 h. Infected cells were allowed to recover overnight following the addition of 6 mL complete media and knockdown of target genes was assessed by qPCR 48 h post-infection. Knock down of indicated genes were quantitated as described in a previous report [21].
2.7. Conditioned media experiments
Control or knockdown Mia PaCa-2 cells were plated at a density of 7.5×105 cells in 10 cm tissue culture plates. Following overnight recovery the cells were challenged with 1 ng/mL TNFα with or without 500 nM GW501516 for 24 h. Conditioned media was collected and centrifuged at 200×g at 20 °C for 10 min and any unused media was stored at −80 °C. The same volume of normal media containing TNFα with or without 500 nM GW501516 was prepared at the start of the experiment and was used as control media. For gene expression assays, undiluted conditioned media from control or knockdown Mia PaCa-2 cells were added directly to THP-1 cells and the cells were incubated for 24 h. Conditioned media was removed the following day and total RNA was isolated and reverse transcribed as described above.
2.8. Cell migration assay
Experiments were performed using the CytoSelect TM 96-Well Invasion Assay (Basement Membrane, Fluormetric Format) according to the manufacturer's instructions. The basement membrane was allowed to reach room temperature for 30 min, and rehydrated using warm, serum-free DMEM. THP-1 cells were then seeded into each well at a density of 2×106 cells/mL in serum-free media. Conditioned media, as well as control media (DMEM containing 10% FBS, along with TNFα with or without GW501516) was added to the feeder tray to act as a chemoattractant and the entire apparatus was placed in an incubator at 37 °C containing 5% CO2 for 24 h. CyQuant® GR dye/lysis buffer solution was added to the invading cells following completion of the assay and the resulting mixture was incubated at room temperature for 20 mins. Invading cells were quantified by reading the fluorescence at 480 nm/520 nm. All measurements were performed in triplicate.
2.9. Statistical analysis
Quantitative data are presented as mean±SEM. ANOVA with p-value<0.05 was used to determine whether differences among variables were significant. Normality was checked using Anderson-Darling test, and the general linear model, followed by the Tukey post hoc test to analyze differences between treatments. All data analyses were performed by MiniTAB Ver.14 (MiniTAB, State College, PA) or JMP (SAS Institute, Cary, NC) and data were plotted by Prism 5.01 (GraphPad Software, San Diego, CA).
3. Results
3.1. Over-expression of Pparb inhibits Nfkb1 activity in Mia PaCa-2 cells
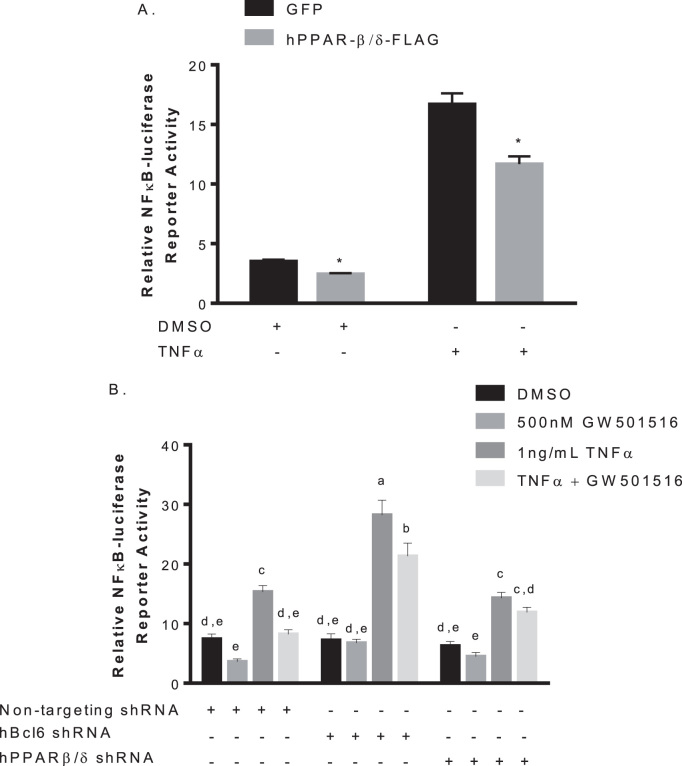
NFκB1 is a key regulator of inflammation [22] and is affected by PPARβ/δ via the p65 subunit [19]. To substantiate the anti-inflammatory effect of PPARβ/δ in human pancreatic cancer cells we sought to evaluate the expression of the nuclear receptor on NFκB1 modulation. Mia PaCa-2 cells were transfected with an Nfkb1 response element-luciferase along with pcDNA3.1-Pparb-FLAG or empty vector. Over-expression of Pparb reduced basal Nfkb1 activity. Treatment with 1 ng/mL tumor necrosis factor α (TNFα) induced Nfkb1 reporter activity almost three-fold in control, this effect was diminished in cells over-expressing Pparb, even in the absence of ligand (Fig. 1A). This indicates PPARβ/δ associates with and suppresses Nfkb1 activity in human pancreatic cancer cells, suggesting an anti-inflammatory role for PPARβ/δ in the pancreas.
Fig. 1.
Effects of Pparb expression and activation on Nfkb1 activity. A. Pparb expression decreases basal and stimulated Nfkb1 activity. Mia PaCa-2 cells were seeded at 7.5×105/mL in 10 cm culture dishes and transfected with 9 µg Nfkb1-luciferase and 1 µg pRLCMV with or without pcDNA3.1-Pparb-FLAG for 24 h before challenge with TNFα or DMSO control. Luciferase activity was assayed and corrected for transfection efficiency. *p<0.05 B. BCL6 also plays a role in suppression of Nfkb1 activity. Mia PaCa-2 cells were seeded at 7.5×105/mL in 10 cm dishes, infected with 4.6 µg of siRNA targeted against Pparb, Bcl6, 2.4 µg pPACKH1 packaging plasmid for 6 h, and were transfected with Nfkb1-luciferase. Cells were then challenged with the indicated treatments for 24 h and luciferase activity was assayed and corrected for transfection efficiency. Different letters indicate a statistical difference at p<0.05 using Tukey's multicomparison test.
3.2. Activation of PPARβ/δ reduces Nfkb1 activity in Mia PaCa-2 cells
To determine if Nfkb1 activity could be influenced by GW501516-induced interaction with PPARβ/δ in human pancreatic cancer cells, Mia PaCa-2 cells with Pparb or Bcl6 knocked down were transfected with Nfkb1 response element-luciferase and treated with 1 ng/mL TNFα in the presence or absence of 500 nM GW501516. In control cells, GW501516 reduced both basal and TNFα-stimulated Nfkb1 luciferase activity, as well as slightly elevating basal activity though not significant. TNFα-induced reporter activity was significantly increased when Bcl6 was knocked down, and GW501516 slightly lowered this effect. GW501516 treatment did not alter basal activity. Both basal and TNFα-stimulated Nfkb1 activity were unaffected by GW501516 treatment when Pparb was knocked down (Fig. 1B). These results indicate the observed reduction in Nfkb1 activity by GW501516 may require both PPARβ/δ and its associated tumor suppressor BCL6.
3.3. GW501516 induces expression of anti-inflammatory genes in Pparb-dependent manner
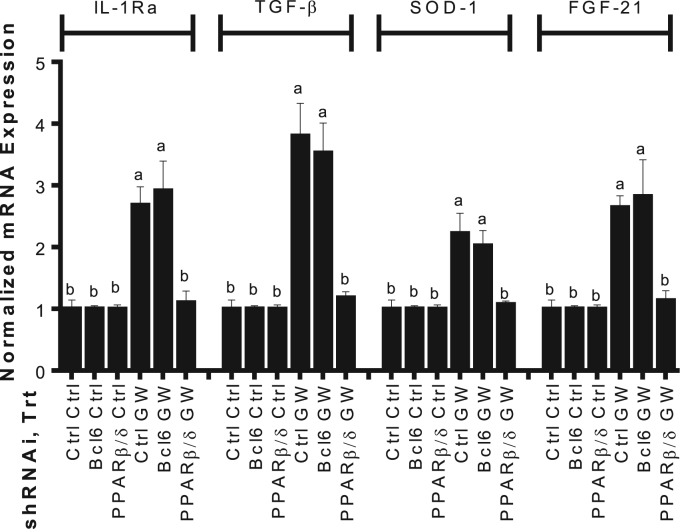
Activation of PPARβ/δ affects anti-inflammatory genes in many cell lines [22], [23], [24]. Mia PaCa-2 cells with Pparb or Bcl6 knocked down were treated with 500 nM GW501516 or DMSO control, and mRNA expression was assessed via qPCR. GW501516 increased expression of interleukin-1 receptor agonist (Il1ra), transforming growth factor β (Tgfb), superoxide dismutase-1 (Sod1), and fibroblast growth factor 21 (Fgf21) in control and cells with Bcl6 knocked down. When Pparb was knocked down, GW501516 treatment did not increase expression of these target genes (Fig. 2). These results indicate the anti-inflammatory effects of GW501516 are mediated in part by the direct induction by PPARβ/δ.
Fig. 2.
GW501516 activated PPARβ/δ exerts anti-inflammatory effects by increasing expression of target genes. Mia PaCa-2 cells were seeded 7.5×105/mL in 10 cm dishes and transiently infected with 4.6 µg of lentiviral-mediated shRNAs targeted against Pparb or Bcl6 for 6 h, and 2.4 µg of pPACKH1 packaging plasmid. Cells were then treated with 500 nM GW501516 or vehicle for 24 h. Gene expression was determined by qPCR and expressed as fold induction following normalization to bactin. Different letters indicate a statistical difference at p<0.05 using ANOVA and Tukey's post hoc multicomparison test.
3.4. GW501516 inhibits expression of TNFα-induced pro-inflammatory genes in Bcl6-dependent manner
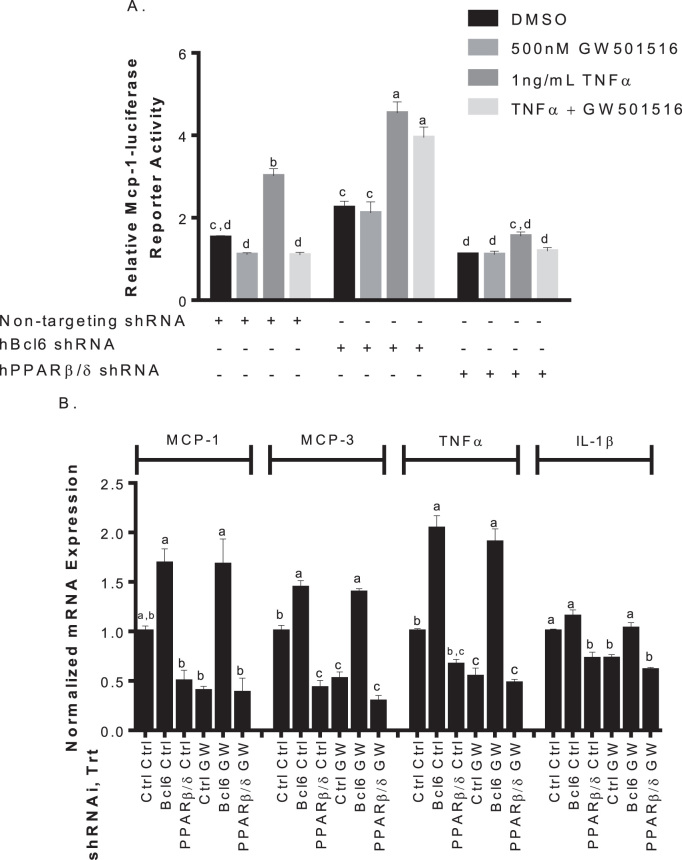
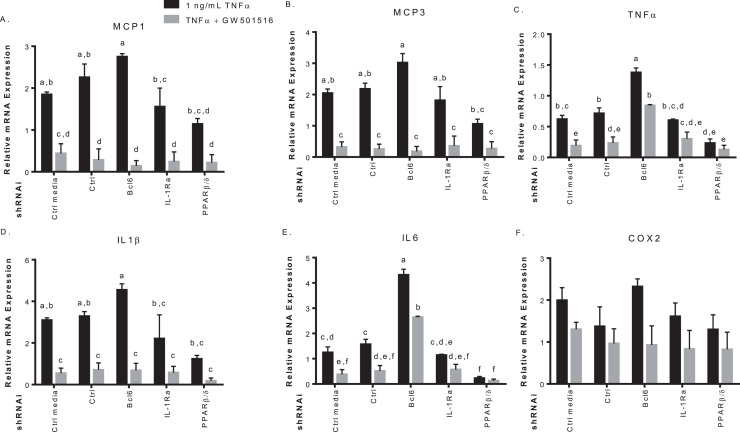
The anti-inflammatory role of PPARβ/δ is mediated in part by BCL6 [16], [25], and involves several pro-inflammatory markers [17], [23], [25]. Mia PaCa-2 cells expressing shRNA targeting Pparb or Bcl6 along with monocyte chemoattractant protein-1 (Mcp1) promoter-luciferase, were treated with 1 ng/mL TNFα to induce an inflammatory response. Mcp1 promoter-luciferase activity was increased in control cells, and treatment with GW501516 reduced TNFα-induced reporter activity to basal level. GW501516 reduced basal levels as well, however, not to a significant degree. TNFα-induced Mcp1 promoter-luciferase activity was significantly higher when Bcl6 was knocked down, and GW501516 had no effect. When Pparb was knocked down, TNFα-stimulated Mcp1 promoter activity was reduced to approximately basal level with GW501516 having no effect (Fig. 3A). Reduction in Bcl6 appears to increase TNFα-induced activity of pro-inflammatory Mcp1, whereas its release (via reduction in Pparb) decreases reporter activity.
Fig. 3.
The transcriptional repressor BCL6 contributes to the anti-inflammatory actions of GW501516 by suppressing target gene expression. A. Effects of Pparb and Bcl6 knock-down on TNFα-induced Mcp1 promoter driven luciferase. Mia PaCa-2 cells transiently expressing 4.6 μg of the indicated shRNAs for 6 h and were transfected with 9 μg of pGL3-Mcp1 promoter luciferase and treated with vehicle, 500 nM GW501516 or 1 ng/mL TNFα with or without GW501516 for 24 h before luciferase activity was assayed. B. Repression of TNFα-induced pro-inflammatory target genes by BCL6. Mia PaCa-2 cells expressing the indicated shRNAs were treated with 1 ng/mL TNFα with or without 500 nM GW501516 for 24 h. Gene expression was determined by qPCR and expressed as fold induction following normalization to bactin. Different letters indicate a statistical difference at p<0.05 using ANOVA and Tukey's post hoc multicomparison test.
To see if this effect was observed at the mRNA level, qPCR was performed on Mcp1 and other known BCL6 target genes in cells under the same conditions. GW501516 activation reduced mRNA in Mcp3, Tnfa, and interleukin-1β (Il1b) in control infected cells, with minor effect on Mcp1. This affect was not present when Bcl6 was knocked down, and mRNA levels were increased with or without GW501516. When Pparb was knocked down, mRNA levels were reduced despite TNFα treatment (Fig. 3B). Taken together, these results confirm the anti-inflammatory effect of GW501516 is mediated in part by BCL6.
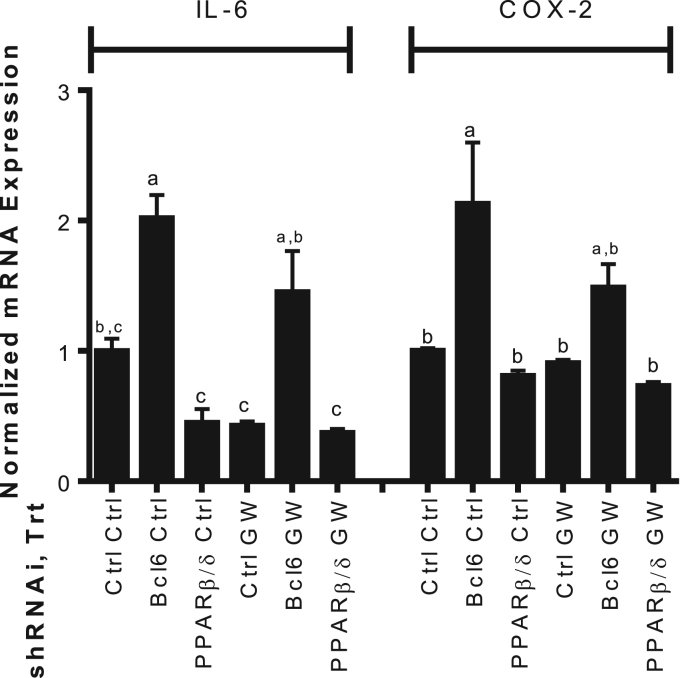
3.5. GW501516 is anti-inflammatory in Cox2-positive human pancreatic cancer cells
Since Mia PaCa-2 cells are reported to not express prostaglandin-endoperoxide synthase 2 (Cox2) or interleukin 6 (Il6), it is important to substantiate these results in a Cox2-positive cell line such as the pancreatic cancer cells, BxPc-3. Cox2 plays a substantial role in angiogenesis by the metabolism of arachidonic acid to factors that contribute to angiogenesis signaling propagation [26]. This cell line was transiently infected with Pparb, Bcl6, or scrambled control shRNA and treated with TNFα with or without GW501516. GW501516 reduced the TNFα-induced expression of the Pparb target Il6 [27] though not significant, and there was no significant change in Cox2 mRNA. Knock down of Bcl6 significantly increased inflammatory response (Il6 and Cox2 expression) and was not significantly affected by PPARβ/δ activation. Pparb knock down resulted in lower expression of Il6 mRNA despite GW501516 (Fig. 4). These results substantiate in BxPc-3 cells, GW501516 exerts its affects via release of BCL6 from the PPARβ/δ complex. Additionally, in our view, the therapeutic potential of this pathway is not negatively altered by the absence of this mechanism in Mia PaCa-2 cells.
Fig. 4.
Effects of Pparb and Bcl6 knock down in Cox2-positive human pancreatic cancer cells. BxPc-3 cells transiently expressing 4.6 μg of the indicated shRNAs were challenged with 1 ng/mL TNFα with or without 500 nM GW501516 for 24 h. RNA was extracted by standard tri-reagent protocol. Gene expression was determined by qPCR and expressed as fold induction following normalization to βactin. Different letters indicate a statistical difference at p<0.05 using ANOVA and Tukey's post hoc multicomparison test.
3.6. GW501516 affects crosstalk between human pancreatic cancer cells and THP-1 macrophages
Tumor associated macrophages secrete molecules that aid in the immunosuppressive microenvironment allowing malignancy and cancer progression by promoting angiogenesis and metastasis [28]. To determine if conditioned media from Mia PaCa-2 cells could influence gene expression in macrophages, Mia PaCa-2 cells were treated with 1 ng/mL TNFα with or without 500 nM of GW501516 after RNAi knockdown of Pparb, Bcl6, Il1ra, and scrambled control for 48 h. Conditioned media was added for 24 h to THP-1 cells differentiated to macrophages. Accumulation of mRNA was examined via qPCR. Media in absence of cells and media from cells not infected with the pseudovirus were also used as controls along with media conditioned by cells infected with scrambled non-targeting shRNA. Our results show significant GW501516-induced reduction of pro-inflammatory markers within the THP-1 cells including Mcp1, Mcp3, Tnfa, Il1b, and Il6, but not Cox2 in cells treated with control media and media from the scrambled control shRNA group (Fig. 5). Conditioned media taken from Bcl6-knock down cells treated with TNFα increased expression of inflammatory markers in THP-1 cells, this inflammatory response was decreased upon treating Mia PaCa-2 cells with GW501516. When Pparb was knocked down in Mia PaCa-2 cells, Tnfa and Il6 production in condition-media treated THP-1 cells was repressed (Fig. 5C&E). Il1ra knock downs in Mia PaCa-2 did not significantly alter gene expression in THP-1 cells compared to control. Mcp1 and Mcp3 were repressed in THP-1 macrophages following conditioned media from GW501516-treated Il1ra knock downs suggesting Il1ra does not play a significant role in the crosstalk between pancreatic cancer cells and macrophages.
Fig. 5.
Conditioned media from Mia PaCa-2 cells influences gene expression in differentiated THP-1 cells. Mia PaCa-2 cells were infected with 4.6 μg of the indicated shRNAs for 6 h, recovered overnight, and treated with 1 ng/mL of TNFα with or without 500 nM GW501516 to condition the media for 48 h. Equal aliquots of conditioned media or control media (media placed in a 10 cm dish without cells) were then added to PMA-differentiated THP-1 cells. Gene expression of the pro-inflammatory Mcp1 (A), Mcp3 (B), Tnfa (C), IL1ra (D), Il6 (E) and Cox2 (F) was determined by qPCR and expressed as fold induction following normalization to bactin. Different letters indicate a statistical difference at p<0.05 using Tukey's multicomparison test.
3.7. GW501516 reduces THP-1 cell invasion in BCL6-dependent manner
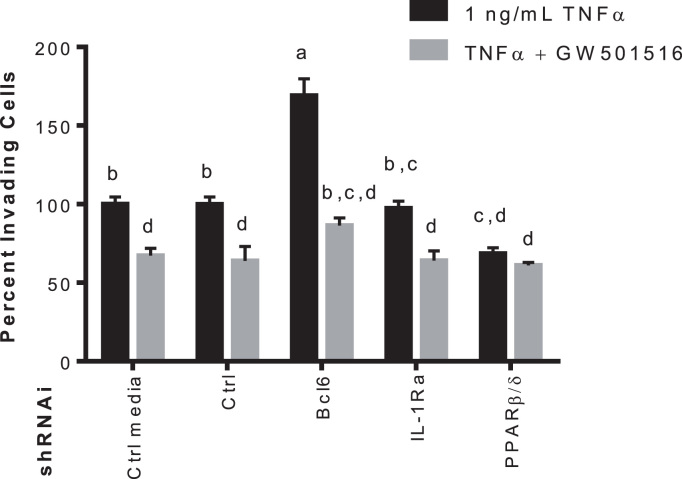
The BCL6 dissociation from PPARβ/δ by GW501516 activation suppresses production of genes involved in macrophages chemotaxis, therefore it was necessary to see if media conditioned by pancreatic cancer cells under the same conditions would influence macrophage migration across a basement membrane. THP-1 cells were seeded into the upper chamber of an invasion plate in serum-free media, and conditioned media from Mia PaCa-2 cells expressing the indicated shRNAs treated with TNFα with or without GW501516 was used as the chemoattractant. GW501516-treated Mia PaCa-2 conditioned media reduced the amount of invading THP-1 cells, this was not seen when Pparb was knocked down in the pancreatic cell line. Interestingly, TNFα-induced invasion was increased 70% when Bcl6 was knocked down in Mia PaCa-2. THP-1 migration was decreased 30% when Pparb was knocked down in pancreatic cancer cells regardless of TNFα or GW501516 (Fig. 6). BCL6 plays an integral part in the anti-inflammatory effects of GW501516, and seems to be the case for its anti-migratory effects as well. Though, PPARβ/δ is clearly anti-inflammatory when activated by GW501516, it appears that when in the inactive state, PPARβ/δ holds association with BCL6 preventing it from exerting its effects.
Fig. 6.
Mia PaCa-2 GW501516-conditioned media reduces the percentage of invading THP-1 cells across a basement membrane. Media was conditioned by Mia PaCa-2 cells expressing the indicated shRNAs and treated with TNFα with or without 500 nM GW501516 for 48 h. Conditioned media was used as a chemoattractant for THP-1 cells. THP-1 monocytes were allowed to migrate across the membrane for 24 h and relative invasion was quantified using the CytoSelect 96-well cell invasion assay with fluormetric readings at 480 nm/520 nm. Different letters indicate a statistical difference at p<0.05 using Tukey's multicomparison test.
3.8. PPARβ/δ expression increases Mia PaCa-2 cell growth
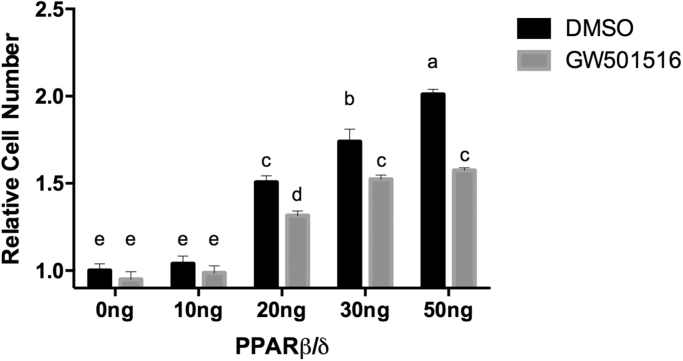
Next, we sought to determine if increasing levels of PPARβ/δ expression affected cancer cell growth in a cell culture model. Mia PaCa-2 cells transfected with pCDNA3.1- PPARβ/δ -FLAG or empty vector control were treated with GW501516 or DMSO control and allowed to grow for 72 h (Fig. 7). There was a 50% increase in cell proliferation following transfection with 20 ng PPARβ/δ and a doubling of cell number with 50 ng PPARβ/δ. This effect was reversed by the addition of GW501516 in cells transfected with 20–50 ng PPARβ/δ plasmid. This further supports that sequestration of BCL6 by overexpression of PPARβ/δ results in outcomes consistent with enhanced growth and that agonist treatment partially restores BCL6 function.
Fig. 7.
PPARβ/δ expression increases Mia PaCa-2 cell growth and is ameliorated by GW501516. Mia PaCa-2 cells were seeded in 96-well plates at 2×103 per well, treated with 250 nM GW501516 or DMSO control, and allowed to grow for approximately 72 h. Plates were analyzed for luminescence using a GloMax microplate luminometer set at 750 nm. Different letters indicate a statistical difference at P<0.05 using Tukey's multicomparison test.
4. Discussion
There is a growing amount of evidence [12] in support of the health benefits of PPARβ/δ agonists in particular in regard to protecting cells from inflammation. Several studies have shown inflammation to be correlated with pancreatic cancer progression [3], [22], [29], [30], [31]. This study provides further evidence that GW501516-activated PPARβ/δ reduces TNFα-induced Nfkb1 activity and inflammatory signaling via direct induction of anti-inflammatory genes, as well as indirect inhibition of pro-inflammatory genes by BCL6 dissociation in human pancreatic cancer cells. Additionally, GW501516 treatment of Mia PaCa-2 cells affected gene expression in macrophages in manner consistent with decreased invasive potential. This may indicate PPARβ/δ agonists affect crosstalk between cancer cells and tumor-associated macrophages. This is imperative because neoplastic response to therapy is not solely based on the genomic anomalies the cancer cell contains, but also the numerous factors within the tumor microenvironment. Macrophages are key regulators of the tumor microenvironment, angiogenesis, and metastasis [32]. Re-polarization of these immune cells, in response to extracellular cues may provide an effective therapeutic option, although many intracellular signals that control polarization are still being elucidated. Whether macrophage re-polarization or reduction in macrophage population would be a more effective strategy based on tissue type and disease progression is being investigated [33]. Macrophage deletion in breast [34], and Ewing's sarcoma animal models have resulted in decrease tumor incidence [35].
Pparb over-expression significantly reduced basal and TNFα-induced Nfkb1 reporter activity in a process likely mediated, in part, by direct interaction with the p65 subunit as described previously [19]. GW501516 reduced Nfkb1 activity in control cells, but not when Pparb or Bcl6 were knocked down, indicating the potential repressive mechanism is dependent on both proteins. The p65 subunit of NFκB1 is constitutively active in human pancreatic cancer cells [36], and is implicated in the progression of pancreatic tumorigenesis [37]. Inhibition of Nfkb1 via PPARβ/δ has already been established in endothelial cells and cardiomyocytes [38], but GW501516-mediated inhibition in the pancreas occurred directly or indirectly via ERK1/2 map kinase phosphorylation as in adipocytes [27] has yet to be determined. TNFα-induced Nfkb1 activity was significantly increased when Bcl6 was knocked down indicating Bcl6 does participate in regulation of Nfkb1 activity. GW501516 may provide a useful therapeutic strategy by attenuation of Nfkb1 by both PPARβ/δ and BCL6.
GW501516 also increased mRNA expression of anti-inflammatory markers- IL1Ra, TGFβ, SOD1, and FGF21. IL1Ra, an IL1β antagonist [39], and TGFβ are both PPARβ/δ targets that contribute to the inhibition of IL1β-induced cell migration. SOD1 was also induced, a gene involved in oxidative stress and contains a functional Peroxisome Proliferator Response Element (PPRE) in its 5′-flanking region [23]. FGF21 is regulated by PPAR-dependent pathways in mice, and protects pancreatic acini from damage via inflammation [40]. We demonstrate here the anti-inflammatory properties of GW501516 in human pancreatic cancer is in part through activation of PPARβ/δ and that the reduction in inflammatory response is in part through direct induction of anti-inflammatory genes by PPARβ/δ.
GW501516 also increased mRNA expression of anti-inflammatory markers- Il1ra, Tgfb, Sod1, and Fgf21. IL1Ra, an IL1β antagonist [39], and TGFβ are both PPARβ/δ targets that contribute to the inhibition of Il1β-induced cell migration in vascular smooth muscle cells [41]. Sod1 was also induced, a gene involved in oxidative stress and contains a functional peroxisome proliferator response element (PPRE) in its 5′ flanking region [23]. Fgf21 is regulated by PPAR-dependent pathways in mice, and protects pancreatic acini from damage via inflammation [40]. We demonstrate here the anti-inflammatory properties of GW501516 in human pancreatic cancer is in part through activation of PPARβ/δ and that the reduction in inflammatory response is in part through direct induction of anti-inflammatory genes by PPARβ/δ.
Unlike PPARβ/δ, the closely related subtypes PPARα and PPARγ lack affinity for BCL6. Since dissociation of BCL6 from the PPARβ/δ complex is largely responsible for the anti-inflammatory properties of PPARβ/δ ligands in mouse macrophages [25], and in animal models [16], this is a unique mode of action available to this receptor. Our data shows this Bcl6 pathway is active in the human pancreas and is dependent on activation of PPARβ/δ. Conversely, PPARβ/δ in the inactive state sequesters BCL6, preventing its anti-inflammatory properties. This may indicate increased expression of Pparb, despite being anti-inflammatory when activated, may play a pro-inflammatory role in the unliganded state as well. L-165041, a PPARβ/δ activator, has been found in cardiomyocytes to increase free BCL6 by not only dissociating BCL6 from PPARβ/δ, but also increasing expression by JNK/p38/Akt activation [42].
Inhibition of Vegf reduced macrophage infiltration and cancer growth in an orthotopic mouse model of pancreatic cancer [43]. Conditioned media from TNFα-treated Mia PaCa-2 cells effected gene expression in THP-1 cells, inducing pro-inflammatory gene expression including Vegf. Conditioned media from cells treated with GW501516 significantly lowered mRNA expression of pro-inflammatory markers in macrophages. Utilizing RNAi in this study has shown PPARβ/δ and BCL6 may play a role in this pancreatic cancer-macrophage crosstalk. GW501516 reduced macrophage infiltration by 30% in control cells, and a reduction in BCL6 protein showed a 70% increase in invasion clarifying the importance of this transcriptional suppressor in macrophage migration. More importantly, however, was the reduction in PPARβ/δ protein lowered invading macrophages compared to control. Further experimentation is required to fully elucidate the role PPARβ/δ and BCL6 play in the exocrine pancreas, but this data is in support of the anti-inflammatory properties of this pathway and potential for ameliorating cancer:macrophage crosstalk in the human pancreas.
Footnotes
Transparency document associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2016.10.014.
Contributor Information
Russell W. Smith, Email: rws197@psu.edu.
Jeffrey D. Coleman, Email: colemanjd1@gmail.com.
Jerry T. Thompson, Email: jt@mdbiosystems.com.
John P. Vanden Heuvel, Email: jpv2@psu.edu, jpv2@psu.edu.
Appendix A. Transparency document
Supplementary material
.
References
- 1.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA. Cancer J. Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Vaccaro V. Metastatic pancreatic cancer: is there a light at the end of the tunnel? World J. Gastroenterol. 2015;21:4788. doi: 10.3748/wjg.v21.i16.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ling S., Feng T., Jia K., Tian Y., Li Y. Inflammation to cancer: the molecular biology in the pancreas (review) Oncol. Lett. 2014;7:1747–1754. doi: 10.3892/ol.2014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z. Activated K-Ras and INK4a/Arf deficiency promote aggressiveness of pancreatic cancer by induction of EMT consistent with cancer stem cell phenotype. J. Cell. Physiol. 2013;228:556–562. doi: 10.1002/jcp.24162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Tremblay K., Dubois-Bouchard C., Brisson D., Gaudet D. Association of CTRC and SPINK1 gene variants with recurrent hospitalizations for pancreatitis or acute abdominal pain in lipoprotein lipase deficiency. Front. Genet. 2014;5:90. doi: 10.3389/fgene.2014.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlosser W. Cyclooxygenase-2 is overexpressed in chronic pancreatitis. Pancreas. 2002;25:26–30. doi: 10.1097/00006676-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Pitocco D. Oxidative stress, nitric oxide, and diabetes. Rev. Diabet. Stud. 2010;7:15–25. doi: 10.1900/RDS.2010.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liptay S. Mitogenic and antiapoptotic role of constitutive NF-κB/Rel activity in pancreatic cancer. Int. J. Cancer. 2003;105:735–746. doi: 10.1002/ijc.11081. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann A., Baltimore D. Circuitry of nuclear factor κB signaling. Immunol. Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 10.Niu J., Li Z., Peng B., Chiao P.J. Identification of an autoregulatory feedback pathway involving interleukin-1α in induction of constitutive NF-κB activation in pancreatic cancer cells. J. Biol. Chem. 2004;279:16452–16462. doi: 10.1074/jbc.M309789200. [DOI] [PubMed] [Google Scholar]
- 11.Melisi D. Secreted interleukin-1alpha induces a metastatic phenotype in pancreatic cancer by sustaining a constitutive activation of nuclear factor-kappaB. Mol. Cancer Res. 2009;7:624–633. doi: 10.1158/1541-7786.MCR-08-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters J.M., Yao P.-L., Gonzalez F.J. Targeting peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) for cancer chemoprevention. Curr. Pharmacol. Rep. 2015;1:121–128. doi: 10.1007/s40495-015-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuo X. Potentiation of colon cancer susceptibility in mice by colonic epithelial PPAR-δ/β overexpression. J. Natl. Cancer Inst. 106, dju052. 2014 doi: 10.1093/jnci/dju052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredenrich a., Grimaldi P. a. PPAR delta: an uncompletely known nuclear receptor. Diabetes Metab. 2005;31:23–27. doi: 10.1016/s1262-3636(07)70162-3. [DOI] [PubMed] [Google Scholar]
- 15.Bastie C. PPARdelta and PPARgamma: roles in fatty acids signalling, implication in tumorigenesis. Bull. Cancer. 2002;89:23–28. [PubMed] [Google Scholar]
- 16.Takata Y. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc. Natl. Acad. Sci. USA. 2008;105:4277–4282. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barish G.D. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc. Natl. Acad. Sci. USA. 2008;105:4271–4276. doi: 10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim H.J. PPARβ/δ ligand L-165041 ameliorates Western diet-induced hepatic lipid accumulation and inflammation in LDLR-/- mice. Eur. J. Pharmacol. 2009;622:45–51. doi: 10.1016/j.ejphar.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Bishop-Bailey D., Bystrom J. Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation. Pharmacol. Ther. 2009;124:141–150. doi: 10.1016/j.pharmthera.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Paterniti I. Peroxisome proliferator-activated receptor β/δ agonist GW0742 ameliorates cerulein- and taurocholate-induced acute pancreatitis in mice. Surg. (United States) 2012;152:90–106. doi: 10.1016/j.surg.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Coleman J.D., Thompson J.T., Smith R.W., Prokopczyk B., Vanden Heuvel J.P. Role of peroxisome proliferator-activated receptor β/δ and B-cell lymphoma-6 in regulation of genes involved in metastasis and migration in pancreatic cancer cells. PPAR Res. 2013;2013 doi: 10.1155/2013/121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantovani A. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 23.Fan Y. Suppression of pro-inflammatory adhesion molecules by PPAR-δ in human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:315–321. doi: 10.1161/ATVBAHA.107.149815. [DOI] [PubMed] [Google Scholar]
- 24.Kim H.J. Transforming growth factor-beta1 is a molecular target for the peroxisome proliferator-activated receptor delta. Circ. Res. 2008;102:193–200. doi: 10.1161/CIRCRESAHA.107.158477. [DOI] [PubMed] [Google Scholar]
- 25.Lee C.-H. Transcriptional repression of atherogenic inflammation: modulation by PPAR. Science. 2003;302:453–457. doi: 10.1126/science.1087344. (80-.) [DOI] [PubMed] [Google Scholar]
- 26.Masferrer J.L. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 27.Rodríguez-Calvo R. Activation of peroxisome proliferator-activated receptor β/δ inhibits lipopolysaccharide-induced cytokine production in adipocytes by lowering nuclear factor-κB activity via extracellular signal-related kinase 1/2. Diabetes. 2008;57:2149–2157. doi: 10.2337/db08-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morse M. a., Hall J.R., Plate J.M.D. Countering tumor-induced immunosuppression during immunotherapy for pancreatic cancer. Expert Opin. Biol. Ther. 2009;9:331–339. [Google Scholar]
- 29.Sica A., Allavena P., Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 30.Greer J.B., Whitcomb D.C. Inflammation and pancreatic cancer: an evidence-based review. Curr. Opin. Pharmacol. 2009;9:411–418. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Demaria S. Cancer and inflammation: promise for biologic therapy. J. Immunother. 2010;33:335–351. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruffell B., Coussens L.M. Macrophages and Therapeutic Resistance in Cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai X. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J. Mol. Cell Biol. 2012;4:341–343. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- 34.Lin E.Y., Pollard J.W. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 35.Zeisberger S.M. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br. J. Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Z., Vocadlo D.J., Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-kB activity in pancreatic cancer cells. J. Biol. Chem. 2013;288:15121–15130. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yip-Schneider M.T. Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Mol. Cancer Ther. 2005;(4):587–594. doi: 10.1158/1535-7163.MCT-04-0215. [DOI] [PubMed] [Google Scholar]
- 38.Smeets P.J.H. Inflammatory pathways are activated during cardiomyocyte hypertrophy and attenuated by peroxisome proliferator-activated receptors PPARa and PPARb. J. Biol. Chem. 2008;283:29109–29118. doi: 10.1074/jbc.M802143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bresnihan B. Interleukin-1 receptor antagonist treatment in rheumatoid arthritis. Mod. Ther. Rheum. Dis. 2001:109–120. doi: 10.1136/ard.58.2008.i96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson C.L. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137:1795–1804. doi: 10.1053/j.gastro.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 41.Kim H.J. PPARδ inhibits IL-1β-stimulated proliferation and migration of vascular smooth muscle cells via up-regulation of IL-1Ra. Cell. Mol. Life Sci. 2010;67:2119–2130. doi: 10.1007/s00018-010-0328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altieri P. Inhibition of doxorubicin-induced senescence by PPARβ/δ activation agonists in cardiac muscle cells: cooperation between PPARβ/δ and Bcl6. PLoS One. 2012;7:e46126. doi: 10.1371/journal.pone.0046126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dineen S.P. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 2008;68:4340–4346. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material