Abstract
Mesenchymal stem cells (MSCs) inhibit the proliferation or activation of lymphocytes, and their inhibitory effects do not require human leukocyte antigen (HLA)-matching because MSCs express low levels of HLA molecules. Therefore, MSCs may be able to regulate immune responses. In this study, we determined whether MSCs could inhibit psoriasis-like skin inflammation in mice. After induction of psoriasis-like skin inflammation using intradermal injection of IL-23 or topical application of imiquimod with or without treatment with MSC, mouse skins were collected, and H&E staining and real-time PCR were performed. IL-23-induced skin inflammation was inhibited when MSCs were injected on day −1 and day 7. The expression of proinflammatory cytokines such as IL-6, IL-17, and TNF-α was inhibited by MSC injection, and the expression of chemokines such as CCL17, CCL20, and CCL27 was also decreased in mouse skin. We also determined whether MSCs could not only prevent but also treat psoriasis-like skin inflammation in mice. Furthermore, in vitro experiments also showed anti-inflammatory effects of MSCs. Dendritic cells which are co-cultured with MSCs suppressed CD4+ T cell activation and differentiation, which are important for the pathogenesis of psoriasis. These results suggest that MSCs could be useful for treating psoriasis.
Abbreviations: hUCB-MSC, human umbilical cord blood-derived mesenchymal stem cell; IL, interleukin; BMDC, bone marrow-derived dendritic cell; IDO, indoleamine 2,3-dioxygenase
Keywords: Mesenchymal stem cells, Psoriasis, Skin inflammation, Anti-inflammatory effects
Highlights
-
•
Mesenchymal stem cells inhibit psoriasis-like skin inflammation in mice.
-
•
Mesenchymal stem cells modulate dendritic cell function.
-
•
Dendritic cells that co-cultured with mesenchymal stem cells regulate CD4+ T cell differentiation.
1. Introduction
Mesenchymal stem cells (MSCs) have inhibitory effects on innate and adaptive immune cells. It has been shown that MSCs inhibit CD4+ T cell proliferation and differentiation and dendritic cell (DC) maturation and induce regulatory T (Treg) cell differentiation [1], [2], [3], [4]. Therefore, MSCs could be used for the treatment of many immune cell-mediated diseases because of their regulatory effects on immune cells. Indeed, some experimental results show that MSCs can prevent or treat autoimmune diseases, such as experimental autoimmune encephalomyelitis (EAE) [5] and collagen-induced arthritis [6]. However, the mechanisms of immune suppression by MSCs are not well understood. Even though many immuno-suppressive molecules such as IL-10 [7], transforming growth factor (TGF)-β [8], nitric oxide [9], indoleamide 2,3-deoxygenase [10], and prostaglandin (PG) E2 [11] are involved in MSC-mediated immune suppression, it has been reported that human umbilical cord blood-derived MSC produces PGE2 and PGE2 might be important factor to inhibit colitis in mice [12]. However, further experiments are necessary to determine whether there are other mediators are required to inhibit colitis by hUCB-MSCs.
MSCs can be isolated from bone marrow, umbilical cord blood, and adipose tissue. Although many researchers have used bone marrow-derived (BM)-MSC to determine their immuno-suppressive effects and their possible use for the treatment of diseases, human umbilical cord blood-derived (hUCB)-MSCs have recently been regarded as an another source for MSCs [13], [14].
Similar to BM-MSCs, hUCB-MSCs do not express Major Histocompatibility Complex class II (MHCII), CD40, CD80, and CD86, which are involved in T cell activation for transplant rejection. Thus, it was suggested that hUCB-MSCs could be used for stem cell therapy because of their low immunogenicity and it was demonstrated that hUCB-MSCs are effective in modulating immune cells and treating diseases [12], [15]. Furthermore, hUCB-MSCs do not raise ethical issue for clinical applications. Thus, hUCB-MSCs have many advantages for the treatment of immune cell-mediated diseases.
Psoriasis is a chronic skin inflammatory disorder, and its histological features are characterized by epidermal hyperplasia, increased angiogenesis and immune cell infiltration [16]. Although the pathogenesis of psoriasis is not fully understood, numerous evidences suggest that Th17 cell is a major player in the pathogenesis of psoriasis [17], [18]. Therefore, it has been proposed that targeting IL-17 or its related cytokines may be an effective therapy for the psoriasis. Indeed, anti-IL-12/23p40 antibody down-regulates psoriasis-related cytokine and chemokine gene expressions in psoriasis patients [19]. It has also been reported that human anti-IL-17A antibody can effectively treat psoriasis, confirming that the IL-17/IL-23 axis is a good target for psoriasis treatment [20].
Th17 cells are involved not only in psoriasis but also in other autoimmune diseases, such as EAE, collagen-induced arthritis, inflammatory bowel disease, and uveitis [21], [22], [23], [24]. Therefore, the pathogenesis of psoriasis is similar to that of other autoimmune diseases and treatment methods for psoriasis might be applied to other autoimmune diseases.
MSC can be used to treat Th17-mediated autoimmune diseases, and psoriasis is an autoimmune disease with similar pathogenesis to that of other autoimmune diseases. Therefore, we hypothesized that hUCB-MSCs could be used to effectively treat psoriasis. In this study, we demonstrated that hUCB-MSCs ameliorate psoriasis-like skin inflammation in mice and have regulatory effects on immune cells, including CD4+ T cells and DCs.
2. Materials and methods
2.1. Mice
C57/BL6 male mice were housed in an environmentally controlled room with a 12:12-h light-dark cycle and free access to laboratory chow and water. Mice between 8 and 12 weeks of age were used. The protocol for mouse use was approved by the Catholic Research Institute of the Medical Science Committee.
2.2. Isolation and culture of hUCB-MSCs
hUCB-MSCs were isolated and maintained as previously described [12]. The stem cell characteristics of hUCB-MSCs were verified by determination of their differentiation, proliferation, and immunological phenotypes as previously described [25]. The study was conducted according to the principles of the Declaration of Helsinki and IRB was approved from Seoul National University (approved number; 1109/001-006).
2.3. IL-23-mediated psoriasis-like skin inflammation in mice
IL-23-mediated psoriasis-like skin inflammation was induced as previously described [26]. Phosphate buffered saline (20 μl) or recombinant mouse IL-23 (500 ng/20 μl) was injected intradermally into the ears of wild type mice every other day for 15 days. On day 15, mouse ears were collected and stored at −80 °C until use for real-time PCR experiments or fixed in 4% paraformaldehyde for paraffin section.
2.4. Imiquimod-induced psoriasis-like skin inflammation in mice
Imiquimod-induced psoriasis-like skin inflammation was induced as described previously [27]. Briefly, 62.5 mg of imiquimod cream (5%; 3.125 mg of the active compound) was applied on the shaved back skin of C57BL/6 mice every day for 5 days with or without subcutaneous injection of hUCB-MSCs on day −1. The mice were euthanized on day 6 for analysis.
2.5. Flow cytometry
For the single cell suspensions, the ears or back skins were collected and single cell suspensions were prepared as previously described [26]. Spleens and draining lymph nodes were collected and minced through 70 µm mesh for single cell suspensions. The cells were collected, washed and stimulated with plate-bound anti-CD3 (BD Pharmingen) and soluble anti-CD28 (eBioscience) antibodies in the presence of GolgiStop (BD Biosciences) for 5 h. Cells were harvested and intracellular staining was performed according to the manufacturer's instructions (BD Pharmingen). The cells were acquired on a flow cytometer (FACS Calibur, BD Bioscience) and analyzed using FlowJo software (Ashland, OR).
2.6. Generation of bone marrow-derived DC (BMDC) cells
CD11c+ cells were generated from bone marrow using a lineage negative cell isolation kit (Miltenyi Biotec) as previously described [28]. The lineage negative cells were grown with Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum, granulocyte-macrophage colony-stimulating factor (10 ng/ml), and IL-4 (10 ng/ml) for 5 days. For activation of DCs, TNF-α (20 ng/ml) or lipopolysaccharide (1 μg/ml) was treated for 16 h.
2.7. Co-culture of DC and CD4+ T cells
A transwell plate (Corning, Acton, MA) was used to prevent MSCs from DC contact. MSCs and immature DCs were placed in the upper and lower chambers of the transwell plate, respectively, at a 1:10 ratio. Each well in a 6-well transwell co-culture plate contained 1×106 DCs in RPMI 1640 medium, supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin, recombinant mouse GM-CSF (20 ng/ml), and IL-4 (10 ng/ml). lipopolysaccharide (1 μg/ml) was added to the DC layer for 1 d to stimulate immature DCs. After stimulation, DCs were co-cultured with MSC in a transwell plate for 16 h and MSCs were removed. Naïve CD4+ T cells were added for contact co-culture of DCs and cultured under Th17 or Treg cell conditions for 4 days. The CD4+ T cells were collected, and total RNA was isolated.
2.8. RNA isolation, cDNA synthesis, and quantitative real-time PCR
Total RNA was isolated from CD4+ T cells or mouse skin with the TRIzol reagent (Invitrogen) as previously described [29]. The primer sets for IL-6, IL-10, IL-17, TNF-α, IL-22, IL-20, CXCL1, CCL17, CCL20, and CCL27 were purchased (Qiagen, Hilden, Germany). PCR was performed using Rotor-Gene 6000 (Corbett, AUS) and the QuantiTect SYBR Green PCR Kit (Qiagen). The amplification program consisted of 1 cycle of 95 °C for 10 min, followed by 35 cycles of 95 °C for 20 s, 55 °C for 20 s, and 72 °C for 20 s. For relative quantification of gene expressions, delta-delta Ct method was used and GAPDH mRNA level was used as an endogenous normalization control.
2.9. Carboxyfluorescein succinimidyl ester (CFSE) dilution assay
CFSE dilution assay was performed using CellTrace CFSE Cell Proliferation kit (Molecular Probes, Inc., Eugene OR) according to the manufacturer's instruction. Briefly, purified naïve CD4+ T cells were incubated with CFSE at 37 °C for 10 min. After quenching the staining by the addition of ice-cold culture media, the cells were incubated for 5 min on ice followed by the collection and washing of cells. The cells were co-cultured with DCs and anti-CD3 antibody for 4 days. Graphical displays showing each cell division were obtained using Flow Jo software.
2.10. Statistical analysis
Data are presented as mean ± standard error of mean (SEM), and statistical comparisons between groups were performed using unpaired 2-tailed t-tests. All experiments were performed at least three times. P-values <0.05 were considered statistically significant.
3. Results
3.1. hUCB-MSCs prevent IL-23-induced psoriasis-like skin inflammation in mice
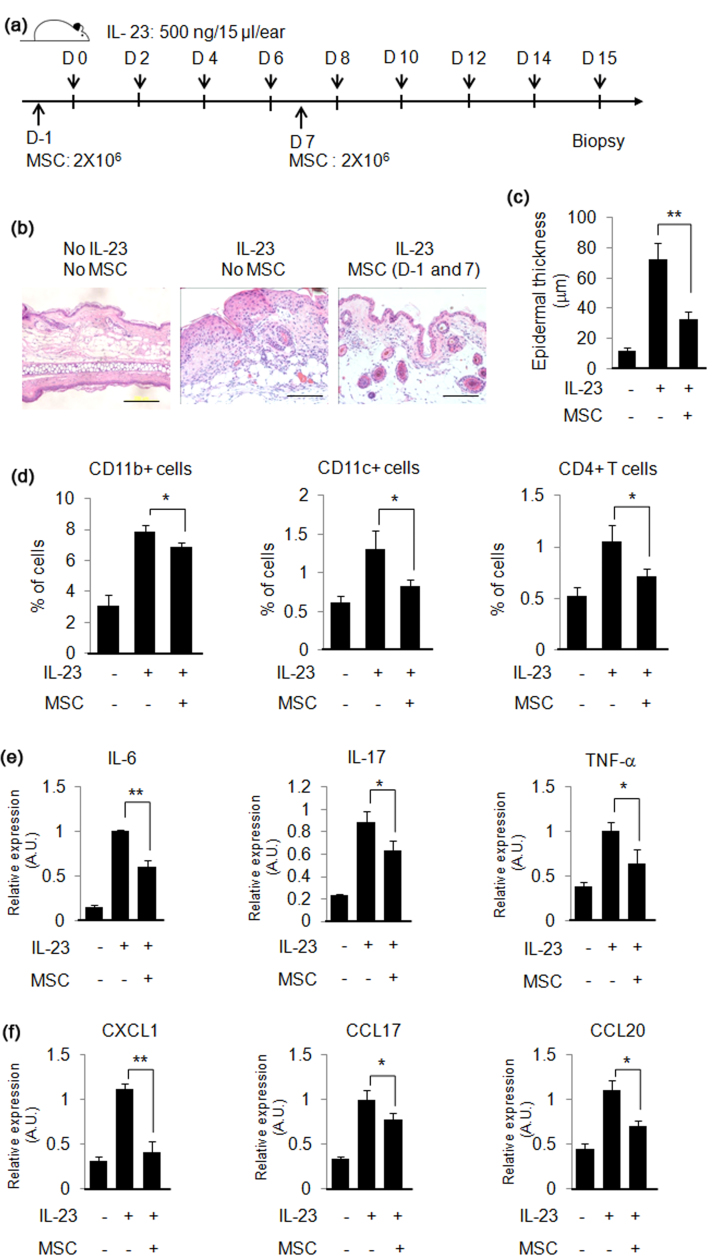
In order to determine whether hUCB-MSCs can prevent psoriasis-like skin inflammation, MSCs were injected subcutaneously near the ear of mouse on day −1 and 7. Psoriasis-like skin inflammation was induced by intradermal injection of IL-23 into the ears eight times for 2 weeks, and the mice were euthanized on day 15 (Fig. 1a). Histological analysis by hematoxylin and eosin (H&E) staining showed that intradermal injection of IL-23 increased epidermal thickness and immune cell infiltration, which are characteristics of psoriasis. On the other hand, MSC injection (2×106 cells per injection) inhibited the IL-23-mediated increase of epidermal thickness and immune cell infiltration (Fig. 1b and c). The results of flow cytometry analysis showed that the infiltration of CD4+ T cells, CD11b+ cells and CD11c+ cells was induced by IL-23 injection, and MSCs prevented the immune cell infiltration into the skin (Fig. 1d). Increased gene expression of pro-inflammatory cytokines and chemokines are also features of psoriasis. IL-23 increased gene expression of IL-1β, IL-6, IL-17, IL-22, IL-20 and TNF-α, and MSCs inhibited IL-23-induced pro-inflammatory gene expression in the skin (Fig. 1e). Increased chemokine gene expression by IL-23 in mouse skin was also inhibited by MSCs (Fig. 1f). These results suggest that pre-treatment of MSCs could prevent IL-23-mediated psoriasis-like skin inflammation in mice.
Fig. 1.
hUCB-MSCs prevent IL-23-induced psoriasis-like skin inflammation in mice. (a) Experimental scheme for the induction of IL-23-induced psoriasis-like skin inflammation and treatment with hUCB-MSCs in mice. (b) H&E staining of mouse ear skin. Bar=200 µm (c) Intradermal injection of IL-23 increased epidermal thickness, and treatment with hUCB-MSCs inhibited the IL-23-mediated increase in epidermal thickness. (d) Percentages of CD11b+, CD11c+, and CD4+ T cells in the skin were determined through flow cytometry. Psoriasis-related pro-inflammatory cytokine gene (e) and chemokine gene (f) expression was measured by real-time PCR. The results shown are representative of three independent experiments. A.U.: Arbitrary Unit. Data are shown as mean±SEM. *P<0.05, **P<0.005.
3.2. hUCB-MSCs treat IL-23-induced psoriasis-like skin inflammation in mice
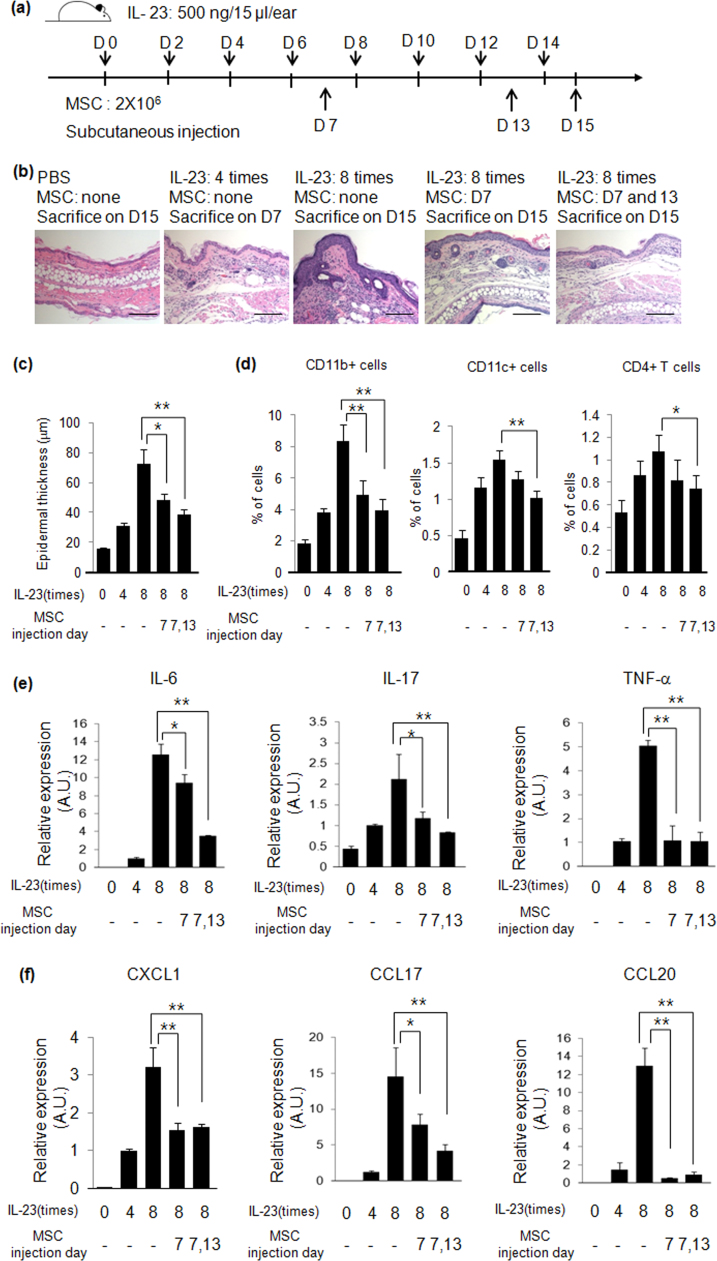
Psoriasis-like skin inflammation was induced by intradermal injection of IL-23 every other day for 2 weeks, and hUCB-MSCs were injected subcutaneously on day 7 and 13 to determine whether on-going psoriasis can be treated by hUCB-MSCs (Fig. 2a). Four intradermal injections of IL-23 increased epidermal thickness and immune cell infiltration, and eight injections of IL-23 induced a greater increase in epidermal thickness and immune cell infiltration. By contrast, subcutaneous injection of hUCB-MSCs on day 7 inhibited the IL-23-mediated increase in epidermal thickness and immune cell infiltration, and two injections of hUCB-MSCs (on day 7 and 13) induced a greater inhibition of epidermal thickness and immune cell (CD11b+, CD11c+ and CD4+ T cells) infiltration (Fig. 2b - d). Pro-inflammatory cytokine and chemokine gene expression also increased on day 7 and were further enhanced on day 15. However, subcutaneous injection of hUCB-MSCs inhibited this gene expression (Fig. 2e and f). Along with our histological data, these results suggest that hUCB-MSCs can treat developing psoriasis.
Fig. 2.
hUCB-MSCs treat IL-23-mediated psoriasis-like skin inflammation in mice. (a) Experimental scheme for induction of IL-23-mediated skin inflammation and treatment with hUCB-MSCs in mice. (b) H&E staining of mouse ear skin. Bar=100 µm (c) Epidermal thickness of ear skin was measured at several regions throughout the epidermis of skin section. (d) Psoriasis-related pro-inflammatory cytokine (e) and chemokine (f) gene expression was determined by real-time PCR. A.U.: Arbitrary Unit. Data are shown as mean±SEM. *P<0.05, **P<0.005.
3.3. hUCB-MSCs ameliorate imiquimod-induced psoriasis-like skin inflammation in mice
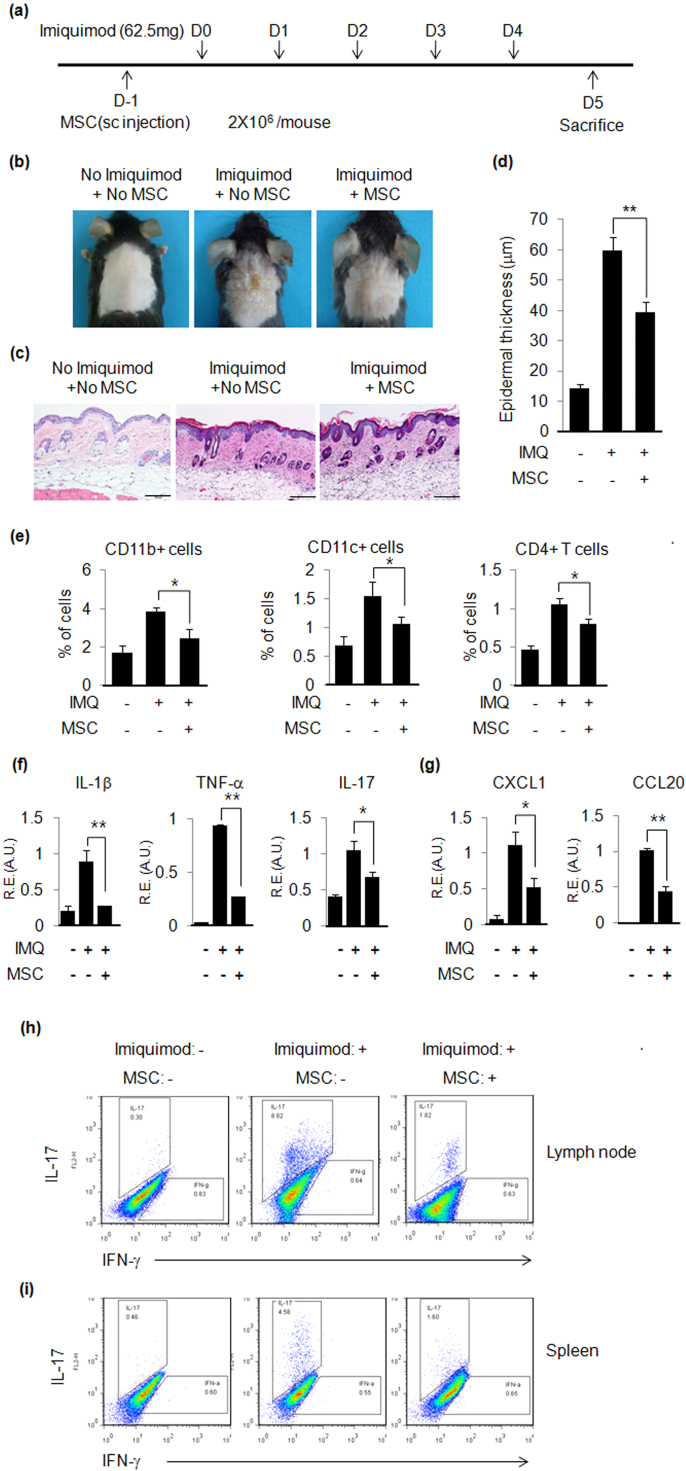
In order to determine the inhibitory effects of hUCB-MSCs on psoriasis development, hUCB-MSCs were injected subcutaneously on day −1, and psoriasis was induced by five applications of imiquimod (Fig. 3a). hUCB-MSCs inhibited psoriasis-like skin inflammation (Fig. 3b). Subcutaneous injection of hUCB-MSCs decreased epidermal thickness by 33% compared with imiquimod-applied positive control mice (Fig. 3c and d), and immune cell infiltration was also inhibited by hUCB-MSCs (Fig. 3e). A real-time PCR experiment showed that hUCB-MSCs inhibited pro-inflammatory cytokine and chemokine gene expression, which is consistent with our IL-23-mediated psoriasis data (Fig. 3f and g). Furthermore, we performed flow cytometry to determine whether hUCB-MSCs can inhibit Th17 cell differentiation, which is critical for psoriasis pathogenesis, in draining lymph nodes and spleens. Application of imiquimod increased Th17 cell populations (8.82% in draining lymph nodes and 4.58% in spleens), but subcutaneous injection of hUCB-MSCs decreased Th17 cell populations to 1.82% and 1.60% in draining lymph nodes and spleens, respectively (Fig. 3h and i). These results imply that hUCB-MSCs inhibit psoriasis-like skin inflammation through suppression of Th17 cell differentiation.
Fig. 3.
hUCB-MSCs inhibit imiquimod-induced psoriasis-like skin inflammation in mice. (a) Experimental scheme for imiquimod-induced psoriasis-like skin inflammation and hUCB-MSC treatment. (b) Picture of imiquimod-induced psoriasis-like skin inflammation in mice. hUCB-MSCs ameliorated imiquimod-induced psoriasis-like skin inflammation. (c) H&E staining of mouse back skin. Bar=100 µm (d) Epidermal thickness was increased by imiquimod and subcutaneous injection of hUCB-MSCs reduced the increased epidermal thickness. Epidermal thickness was measured at several regions throughout the skin section. (e) Percentages of immune cells in the skin. (f) Psoriasis-related pro-inflammatory cytokine and (g) chemokine gene expression was determined by real-time PCR. R.E: Relative Expression. A.U.: Arbitrary Unit. Intracellular staining of (h) lymph node cells and (i) spleen cells was performed as described in Materials and Methods section. Data are shown as mean±SEM. *P<0.05, **P<0.005.
3.4. hUCB-MSCs regulate CD4+ T cell proliferation and differentiation through modulation of DC function
MSCs are known to modulate CD4+ T cell proliferation and differentiation. Therefore, we confirmed that hUCB-MSCs also have regulatory effects on CD4+ T cells. Co-culture of hUCB-MSCs with CD4+ T or CD8+ T cells in trans-well plates inhibited the proliferation of T cells when the T cell-to-MSC ratio was 50:1 or 10:1 (Fig. S1). We also determined whether hUCB-MSCd affect Th17 cell and Treg cell differentiation, as these two CD4+ T cell types are critical for psoriasis pathogenesis. The results showed that hUCB-MSCs inhibit CD4+ T cell differentiation when they are co-cultured with CD4+ T cells under Th17 cell conditions (Fig. S2a). On the other hand, hUCB-MSCs induced Treg cell differentiation (Fig. S2b), that is consistent with previous results [2], [4]. It was also reported that MSCs can inhibit DC maturation [30], and our experiments produced similar results, showing that hUCB-MSCs inhibited DC maturation (Fig. S3). Furthermore, DCs which is co-cultured with hUCB-MSCs showed increased IL-10 and IDO expression, suggesting that anti-proliferative effect of the MSC-treated DC might be from the increased IL-10 and IDO expression (Fig. S4).
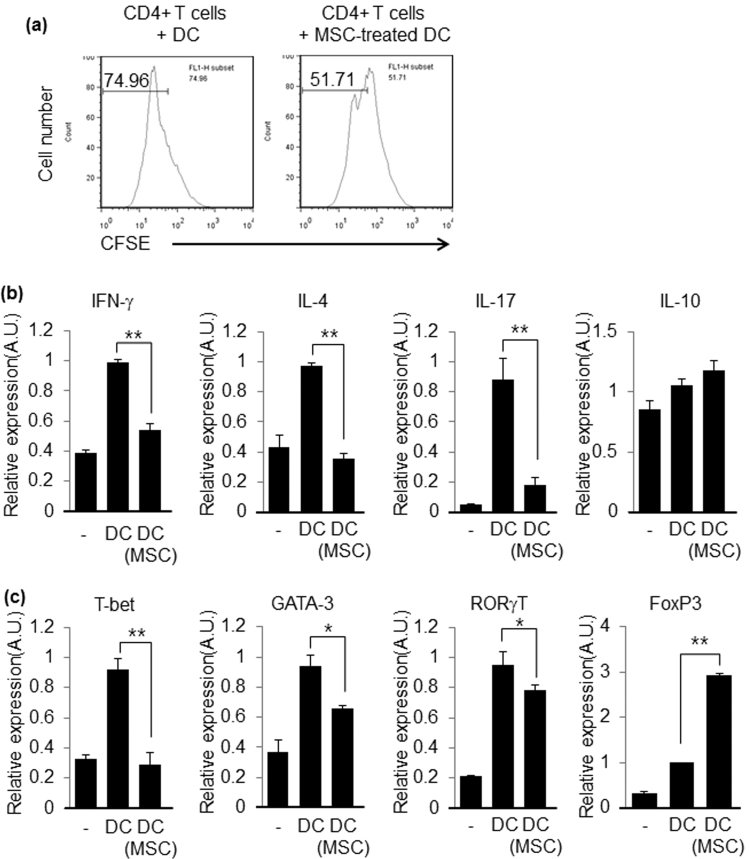
Even though several studies suggest that MSCs can modulate DC maturation, there are no data showing that MSC-pretreated DCs can modulate CD4+ T cell activation and differentiation. Thus, we determined whether DCs co-cultured with hUCB-MSCs during stimulation with TNF-α regulate CD4+ T cell proliferation and differentiation. We found that DCs co-cultured with hUCB-MSCs inhibited CD4+ T cell proliferation (Fig. 4a). In addition, hUCB-MSC-pretreated DCs inhibited Th1, Th2, and Th17 cell differentiation when they were co-cultured with naïve CD4+ T cells under Th1, Th2, and Th17 cell differentiation conditions (Fig. 4b and c). Furthermore, hUCB-MSC-pretreated DCs enhanced Treg cell differentiation (Fig. 4b and c). These results imply that MSCs modulate CD4+ T cells activation and differentiation not only directly but also indirectly through the modulation of DC function.
Fig. 4.
MSC-treated DCs inhibit CD4+ T cell proliferation and differentiation. (a) Proliferation of CD4+ T cells was analyzed with CFSE dilution assay. The results shown are representative of three independent experiments. (b and c) Expressions of signature cytokine and transcription factors were determined by real-time PCR from co-cultured CD4+ T cells with DCs, which were or were not pre-treated with hUCB-MSCs, under Th1, Th2, Th17, or Treg cell conditions. A.U.: Arbitrary Unit. Data are shown as mean ± SEM. *P<0.05, **P<0.005.
4. Discussion
Psoriasis is a common chronic skin disease. Even though 2–3% of the worldwide population is affected, effective therapies for psoriasis have not been developed [31]. To develop better therapeutics for the treatment of psoriasis, many studies have focused on the pathogenesis of psoriasis. Historically, psoriasis was considered a Th1 cell-mediated disease. However, recent experimental and clinical evidence suggests that Th17 cells and their related cytokines and chemokines are critical for the pathogenesis of psoriasis. Therefore, many researchers have targeted Th17 cells or its signature cytokine IL-17 to treat psoriasis. Indeed, human anti-IL-17A antibody effectively treats psoriasis, confirming that the IL-17/IL-23 axis is a good target for psoriasis [20].
Recently, MSCs have been shown to have immunosuppressive effects [3]. Furthermore, it has been proposed that MSCs could inhibit Th17 cell differentiation [2]. These discoveries of immunosuppressive function and the ability to inhibit CD4+ T cell differentiation have driven scientists to test the possibility that MSCs can be used for the treatment of autoimmune diseases, because Th17 cells are major targets of autoimmune diseases. So far, it has been demonstrated that MSCs can inhibit collagen-induced arthritis and EAE, which are mouse model of rheumatoid arthritis and multiple sclerosis, respectively [5], [6]. These results imply that MSC could be an attractive candidate for application in the treatment of autoimmune diseases.
Psoriasis is also an autoimmune disease, in which Th17 cells and IL-17 are important for pathogenesis. However, it has not been investigated whether MSCs can be used for the prevention and/or treatment of psoriasis. In this study, we demonstrated that hUCB-MSCs can prevent and treat psoriasis in mouse models of psoriasis. We demonstrated that subcutaneous injection of MSCs prevents and treat IL-23-mediated psoriasis-like skin inflammation (Figs. 1and2). Similar results were obtained in the imiquimod-induced psoriasis-like skin inflammation experiments.
Although MSCs are known to inhibit DC maturation, it has not been shown that MSC-treated DCs can regulate CD4+ T cell responses. In this study, we demonstrated that hUCB-MSC-treated DCs can inhibit CD4+ T cell proliferation and Th17 cell differentiation and induce Treg cell differentiation. These results suggest that DCs that are contacted with soluble factors from hUCB-MSCs can regulate CD4+ T cell immune responses. The direct contact of MSCs to CD4+ T cells may be necessary for the induction of Treg cells differentiation and the inhibition of Th17 cell differentiation [4]. We also confirmed that co-culture of hUCB-MSCs with CD4+ T cells inhibited CD4+ T cell proliferation and Th17 cell differentiation but induced Treg cell differentiation (Fig. S1). However, it seems that there are low chances for MSCs to contact CD4+ T cells directly when they are administrated in vivo. In this study, we demonstrated that hUCB-MSC-treated DCs inhibited Th17 cell differentiation and induced Treg cell differentiation (Fig. 4). Therefore, the inhibitory effects of MSCs on autoimmune diseases, such as psoriasis, might result from the inhibition of Th17 cells and induction of Treg cells through the interaction between CD4+ T cells and the DCs encountering secreted factors from MSCs.
Previous studies suggest that MSCs inhibit DC maturation by soluble factors secreted from MSCs, such as IL-10 and PGE2 [30], [32]. We also confirmed that DC maturation is inhibited when DCs are co-cultured with hUCB-MSCs in a transwell plate (Fig. S3). hUCB-MSCs inhibited not only DC maturation but also gene expression in DCs. Thus, it is also possible that DCs themselves may have anti-inflammatory effects without contact with CD4+ T cells. More interestingly, we demonstrated that hUCB-MSC-treated DCs inhibit Th17 cell differentiation and induce Treg cell differentiation. These results imply that the immunosuppressive effect of MSCs might result from complex interactions between immune cells and MSCs both directly and indirectly, even though more experiments are required to elucidate the immunosuppressive mechanisms of MSCs. Finally, we determined how long the injected MSC existed using real-time PCR analysis. Fig. S5 shows that human GAPDH expression in the MSC-injected mouse skins was detected until day 3 after injection while the transcript was bare detectable on day 7. In addition, we could not detect human GAPDH expression in other tissues, such as the spleen, lung and liver, implying that the injected MSC may not travel and only work at the injected site locally.
Recently, it has been reported that skin MSCs from psoriasis patients inhibit T cell proliferation less effectively compared to normal MSC [33]. These results indicated that the injection of normal MSC might suppress T cell proliferation in skin and imply that MSC could be a good therapeutic. As a conclusion, our experimental results imply that stem cell therapy could be applied to prevent and treat psoriasis.
Acknowledgements
This research was supported by a grant of Korea Health Technology R&D Project through Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI12C17990100).
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.10.002.
Appendix A. Transparency document
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Rasmusson I., Ringden O., Sundberg B. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp. Cell Res. 2005;302:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Ghannam S., Pene J., Torcy-Moquet G. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomew A., Sturgeon C., Siatskas M. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 4.English K., Ryan J.M., Tobin L. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play -redundant roles in human mesenchymal stem cell induction of CD4+ CD25+ High forkhead box P3+ regulatory T cells. Exp. Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zappia E., Casazza S., Pedemonte E. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 6.Augello A., Tasso R., Negrini S.M. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 7.Batten P., Sarathchandra P., Antoniw J.W. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng. 2006;12:2263–2273. doi: 10.1089/ten.2006.12.2263. [DOI] [PubMed] [Google Scholar]
- 8.Groh M.E., Maitra B., Szekely E. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 2005;33:928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Sato K., Ozaki K., Oh I. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 10.Meisel R., Zibert A., Laryea M. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogenic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 12.Kim H.S., Shin T.H., Lee B.C. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX-2. Gastroentorol. 2013;145:1392–1403. doi: 10.1053/j.gastro.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Flynn A., Barry F., O’Brien T. UC blood-derived mesenchymal stromal cells: an overview. Cytotherapy. 2007;9:717–726. doi: 10.1080/14653240701584578. [DOI] [PubMed] [Google Scholar]
- 14.Secco M., Zucconi E., Vieira N.M. Multipotent stem cells from umbilical cord: cord is richer than blood. Stem Cells. 2008;26:146–150. doi: 10.1634/stemcells.2007-0381. [DOI] [PubMed] [Google Scholar]
- 15.Wang M., Yang Y., Yang D. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220–232. doi: 10.1111/j.1365-2567.2008.02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowes M.A., Bowcock A.M., Krueger J.G. Pathogenesis and therapy of psoriasis. Krueger. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 17.Lowes M.A., Kikuchi T., Fuentes-Duculan J. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Invest. Dermatol. 2008;128:207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 18.Di Cesare A., Di Meglio P., Nestle FO F.O. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J. Invest. Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 19.Toichi E., Torres G., McCormick T.S. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J. Immunol. 2006;177:4917–4926. doi: 10.4049/jimmunol.177.7.4917. [DOI] [PubMed] [Google Scholar]
- 20.Hueber W., Patel D.D., Dryja T. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl. Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 21.Reboldi A., Coisne C., Baumjohann D. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 22.Nakae S., Nambu A., Sudo K. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 23.Ito R., Ita M., Shin-Ya M. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem. Biophys. Res. Commun. 2008;377:12–16. doi: 10.1016/j.bbrc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Amadi-Obi A., Yu C.R., Liu X. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 25.Seo Y., Yang S.R., Jee M.K. Human umbilical cord blood-derived mesenchymal stem cells protect against neuronal cell death and ameliorate motor deficits in Niemann Pick type C1 mice. Cell Transplant. 2011;20:1033–1047. doi: 10.3727/096368910X545086. [DOI] [PubMed] [Google Scholar]
- 26.Hedrick M.N., Lonsdorf A.S., Shirakawa A.K. CCR6 is required for IL-23-induced prosiasis-like inflammation in mice. J. Clin. Invest. 2009;119:2317–2329. doi: 10.1172/JCI37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Fits L., Mourits S., Voerman J.S. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y.S., Ahjoku A.-O., Yu C.-R. Retinal cells suppress intraocular inflammation (uveitis) through production of interleukin-27 and interleukin-10. Immunology. 2011;132:492–502. doi: 10.1111/j.1365-2567.2010.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y.S., Cheon I.S., Kim B.-K. Loss of extracellular superoxide dismutase induces severe IL-23-mediated skin inflammation in mice. J. Inves. Dermatol. 2013;133:732–741. doi: 10.1038/jid.2012.406. [DOI] [PubMed] [Google Scholar]
- 30.Liu W.H., Liu J.J., Wu J. Novel mechanism of inhibition of dendritic cells maturation by mesenchymal stem cells via interleukin-10 and the JAK1/STAT3 signaling pathway. PLoS One. 2013;8:e55487. doi: 10.1371/journal.pone.0055487. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Schon M.P., Boehncke W.H. Psoriasis. N. Engl. J. Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 32.Spaggiari G.M., Abdelrazik H., Becchetti F. MSCs inhibit monocytederived DC maturation and function by selectively interfering with generation of immature DCs: central role of MSC-derived PGE2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 33.Liu R., Wang Y., Zhao X. Lymphocyte inhibition is compromised in mesenchymal stem cells from psoriatic skin. Eur. J. Dermatol. 2014;24:560–567. doi: 10.1684/ejd.2014.2394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material