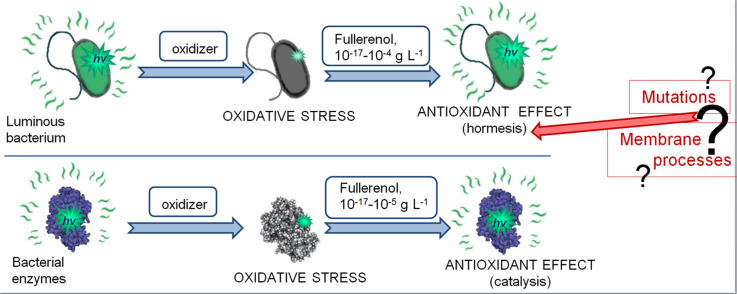
Abstract
Fullerenols are nanosized water-soluble polyhydroxylated derivatives of fullerenes, specific allotropic form of carbon, bioactive compounds and perspective pharmaceutical agents. Antioxidant activity of fullerenols was studied in model solutions of organic and inorganic toxicants of oxidative type – 1,4-benzoquinone and potassium ferricyanide. Two fullerenol preparations were tested: С60О2–4(ОН)20–24 and mixture of two types of fullerenols С60О2–4(ОН)20–24+С70О2–4(ОН)20–24. Bacteria-based and enzyme-based bioluminescent assays were used to evaluate a decrease in cellular and biochemical toxicities, respectively. Additionally, the enzyme-based assay was used for the direct monitoring of efficiency of the oxidative enzymatic processes. The bacteria-based and enzyme-based assays showed similar peculiarities of the detoxification processes: (1) ultralow concentrations of fullerenols were active (ca 10–17–10−4 and 10–17–10−5 g/L, respectively), (2) no monotonic dependence of detoxification efficiency on fullerenol concentrations was observed, and (3) detoxification of organic oxidizer solutions was more effective than that of the inorganic oxidizer. The antioxidant effect of highly diluted fullerenol solutions on bacterial cells was attributed to hormesis phenomenon; the detoxification was concerned with stimulation of adaptive cellular response under low-dose exposures. Sequence analysis of 16S ribosomal RNA was carried out; it did not reveal mutations in bacterial DNA. The suggestion was made that hydrophobic membrane-dependent processes are involved to the detoxifying mechanism. Catalytic activity of fullerenol (10−8 g/L) in NADH-dependent enzymatic reactions was demonstrated and supposed to contribute to adaptive bacterial response.
Abbreviations: NADH, nicotinamide adenine dinucleotide disodium salt reduced; FMN, flavinmononucleotide; GT, general toxicity; OxT, oxidative toxicity; F-60, fullerenol С60О2–4(ОН)20–24;; F-60, 70, mixture of fullerenols С60О2–4(ОН)20–24 and С70О2–4(ОН)20–24
Keywords: Fullerenol, Antioxidant activity, Luminous marine bacteria, Bacterial enzymes, Hormesis, Ultralow concentrations
Graphical abstract
Antioxidant effects of fullerenols in oxidizer solutions with luminous bacteria and their enzymes as biological test systems. The effects on bacterial cells are attributed to hormesis phenomenon, while the intensification of biochemical reactions is explained in terms of fullerenol catalytic activity.
Highlights
-
•
Cellular and enzymatic luminescent assays evaluate fullerenol’ antioxidant activity.
-
•
Ultra-dilute solutions of fullerenols exhibit antioxidant activity.
-
•
Antioxidant fullerenol’ effect on bacterial cells is assigned to hormesis phenomenon.
-
•
Fullerenol catalytic activity is responsible for acceleration of biochemical reactions.
-
•
Organic oxidizer solutions are detoxified efficiently.
1. Introduction
Fullerenols are bioactive compounds, polyhydroxylated water-soluble derivatives of fullerenes, specific allotropic form of carbon [1]. They are promising for application in different fields of physics, chemistry, nanobiotechnology, pharmacology, and medicine [2], [3], [4], [5]. Chemical structure of a representative of fullerenol group, C60(OH)x, is presented in Fig. 1.
Fig. 1.
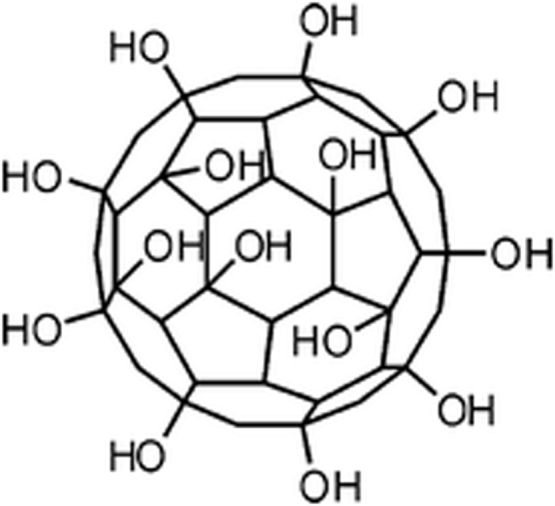
Hypothetical structure of fullerenol C60(OH)x[1].
Similar to fullerene C60, fullerenols behave like electron deficient agents and readily react with electron rich species. This feature makes fullerenols effective catalysts in chemical and biochemical processes. Fullerenols are amphiphilic structures: hydroxyl groups provide them with aqueous solubility while the fragments of fullerene skeleton – with affinity to lipid structures of cellular membranes [6], [7]. The aqueous solubility of fullerenols depends on the amount of hydroxyl groups [8].
Due to hydrophilic properties and ability to scavenge free radicals, fullerenols could provide a serious alternative to the conventional pharmacological agents in chemotherapy, treatment of neurodegenerative diseases, and radiobiology [4], [9]. A range of fullerenol biological effects is wide: from cell protection [4], [6] to drug transport [10] and neutralization [4]. Biological activity of a series of fullerenols С60(ОН)12–14, С60(ОН)18–24, С60(ОН)30–38, was studied by Eropkin and co-workers [8]. Fullerenols С60(ОН)18–24 revealed maximal biological activity [3], [4], [11]. The fullerenols demonstrated antioxidant activity, neutralizing reactive oxygen and nitrogen species [5], [11], [12], [13]. The antioxidant property endows fullerenols with ability to treat neurological diseases [4], [5], [12], [14], to function as radioprotectors [12] or antitumor agents [15].
Luminous marine bacteria are proper candidates for study the antioxidant activity of fullerenols. The bacteria have been used as a toxicity bioassay for several decades [16], [17], [18], [19], [20]. The tested parameter here is luminescence intensity; it can be easily measured instrumentally with simple physical devices. High rates of bioluminescence registration and simplicity of the test organism pave the way for simultaneous analyses of a lot of test-samples under comparable external conditions resulting in a proper statistical treatment.
Bacterial bioluminescent assays can be based on biological systems of different complexity – bacteria or their enzymes [17], [21], [22]. Along with water-soluble bacterial preparations, the solid preparations of immobilized bacteria and their enzymes have been developed [23], [24], [25], [26], [27]. The bacteria-based and enzyme-based assays allow studying mechanisms of toxic effects at cellular and molecular levels, respectively. First classification of toxic effects in the bioluminescent enzyme system, based on physicochemical, chemical, and biochemical processes, was suggested in [28] and developed in [29], [30], [31], [32].
Evaluation of antioxidant activity of bioactive compounds is a novel and prospective field in biosensor application [33], [34]. The assay systems based on luminous marine bacteria and/or their enzyme reactions are proper candidates in this field. The both bioassays, cellular and enzymatic, can be used to evaluate general toxicity of test samples under conditions of oxidative stress. Additionally, the enzymatic assay is specific to oxidizers [32]; therefore it can be used for direct monitoring of oxidative toxicity of solutions. The oxidative toxicity is attributed to redox activity of toxic compounds, while the general toxicity considers, in a nonadditive way, all interactions of exogenous compounds with the components of the bioluminescent enzymatic assay system – redox reactions, hydrophobic and polar interactions. Previously [35], [36] the general and oxidative toxicities of solutions of organic and inorganic oxidizers, quinones and polyvalent metals, were studied using the bioluminescent enzymatic assay. Decrease of both general and oxidative toxicities under addition of humic substances (products of natural decomposition of organic matter in soil and bottom sediments) was studied in [37], [38], [39]. Description of the enzyme bioluminescent technique to evaluate antioxidant activity and toxicity of bioactive compounds is presented in [40]. It should be noted that, similar to reactive oxygen species, active chlorine species can also produce toxic effects [41].
Current work uses fullerenols as bioactive compound models; the oxidative stress is simulated in solutions of model inorganic and organic oxidizers – complex salt potassium ferricyanide K3[Fe(СN)6] and 1,4-benzoquinone, respectively. Potassium ferricyanide was chosen because of its stability in water solutions (in contrast to unclustered iron salts) and monoelectron oxidative transition Fe3+/Fe2+. The 1,4-benzoquinone was chosen being a typical representative of the organic oxidizer group. In nature, quinones can appear as a result of oxidative transformation of various phenols [42], [43], i.e. numerous group of hydroxylated aromatic compounds, third in the top list of widespread pollutants (after metal salts and oil products), and frequent components of industrial wastewaters.
Current study evaluates changes of general and oxidative toxicities under addition of fullerenols as detoxifying agents. Cellular and enzymatic assays are used for the evaluation the toxicities. A wide range of the fullerenol concentrations is tested, including ultralow concentrations. Detoxifying activities of two types of fullerenols (С60О2–4(ОН)20–24 and a mixture of fullerenols С60О2–4(ОН)20–24+С70О2–4(ОН)20–24) are under investigation. Additionally, our work focuses on chemical and biochemical processes taking place in the course of detoxification of the oxidizer solutions; the rates of chemical and biochemical processes in oxidizer +fullerenol solutions are analyzed.
The paper describes the results in the following sequence: Section 3.1 presents the evaluation of proper conditions for toxicity measurements focusing on fullerenol and oxidizer concentrations; mutagenicity of the oxidizer solutions is tested; Section 3.2 studies a response of bacterial cells to the fullerenol exposure in the oxidizer solutions; changes of general toxicity are determined; Section 3.3 studies the effects of the fullerenols on the bioluminescent enzymatic system; changes of general and oxidative toxicities are evaluated; rates of chemical and biochemical reactions are determined. Section Discussion considers peculiarities of fullerenol detoxifying effect (4.1) and presents speculations on mechanism of biological activity of highly diluted fullerenol solutions (4.2).
2. Materials and methods
2.1. Reagents and equipment
Toxicity of water solutions of model oxidizers K3[Fe(СN)6] (potassium ferricyanide) and 1,4-benzoquinone was assessed using two bioluminescent assay systems: (1) bacterial assay, i.e. Microbiosensor 677F, based on the lyophilized luminous bacteria Photobacterium phosphoreum, and (2) enzymatic assay, i.e. the preparation based on the coupled enzyme system NADH: FMN-oxidoreductase from Vibrio fischeri (0.15 a.u.) and luciferase from Photobacterium leiognathi, 0.5 mg/ml [44]. All the biological preparations were produced at the Institute of Biophysics SB RAS (Krasnoyarsk, Russia). The chemicals used were: NADH from ICN, USA; FMN, and tetradecanal from SERVA, Germany; potassium ferricyanide of analytical grade, Khimreactiv, Russia; 1,4-benzoquinone, Aldrich, USA.
The enzymatic system includes two coupled reactions:
| (1) |
| (2) |
To construct the enzyme system, 0.1 mg/ml enzyme preparation, 5∙10-4 M FMN, 4∙10−4 M NADH, and 0.002% tetradecanal solutions were used. The assay was performed in a 0.05 M phosphate buffer (pH 6.8) at room temperature.
Fullerenes were synthesized by carbon helium high-frequency arc plasma at atmospheric pressure [45], [46]. The fullerene content in carbon soot was about 12.6%. Fullerene mixture was extracted with toluene, and the individual C60 fullerene was separated by liquid chromatography with turbostratic graphite (with interplanar distance 3.42 Å) as a stationary phase and toluene/hexane (4:6) mixture as a mobile phase. Fullerenol С60О2–4(ОН)20–24 (F-60) and mixture of fullerenols С60О2–4(ОН)20–24 and С70О2–4(ОН)20–24 (with 60% of F-60) (F-60,70) were produced by fullerene hydroxylation in nitric acid followed by the hydrolysis of the polynitrofullerenes [3], [46], [47]. The fullerenol preparations were characterized with IR and photoelectron spectroscopies [48].
Measurements of bioluminescent intensity were carried out with bioluminometers BLM-3606 (Nauka Special Design Bureau, Russia) and TriStar LB 941 (Berthold Technologies, Germany). Optical density of solutions was measured by double-beam spectrophotometer UVIKON-943 (KONTRON Instruments, Italy).
2.2. Experimental data processing
Effects of fullerenols on bioluminescence of bacterial and enzyme assay systems were evaluated by relative bioluminescent intensity, :
| (3) |
Here, Icontr and IF are maximal bioluminescent intensities in the absence and presence of fullerenols, respectively.
General Toxicity (GT) of the oxidizer solutions was evaluated by a similar way (Eq. (3a)), i.e. with relative bioluminescent intensity, :
| (3a) |
Here, and are maximal bioluminescent intensities in the absence and presence of oxidizers, respectively.
Effective concentrations of oxidizers decreasing bioluminescent intensity by 50% (=0.5), EC50, were determined.
To characterize the changes of General Toxicity (GT) under the exposure to fullerenols, both bioluminescent assays, bacterial and enzymatic, were applied. The detoxification coefficients, DGT, were calculated as:
| (4) |
where and are relative bioluminescent intensities in oxidizer solutions at EC50, in the presence and absence of fullerenols, respectively. Values of DGT were determined at different fullerenol concentrations.
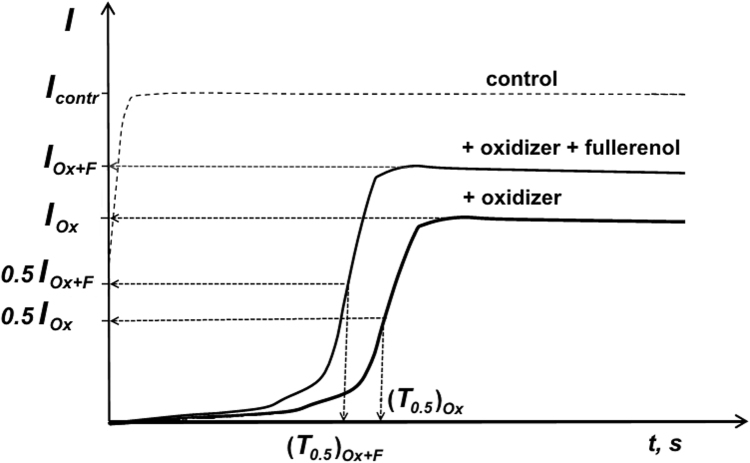
To characterize the Oxidative Toxicity (OxT) of oxidizer solutions, the bioluminescent enzymatic assay was used; an example of the bioluminescent kinetics is schematically presented in Fig. 2. Bioluminescence delay period in oxidizer solutions, (T0.5)ox, is indicated in this Figure. Changes of Oxidative Toxicity (OxT) under fullerenol exposure were characterized with detoxification coefficients, DOxT:
| (5) |
Fig. 2.
Bioluminescent kinetics in solution of a model oxidizer (Ox) and fullerenol (F).
here, (T0.5)Оx+F and (T0.5)Оx are bioluminescence delay periods in oxidizer solutions in the presence and absence of fullerenols, respectively. Values of DOxT were determined at different fullerenol concentrations.
Values of DGT>1 and DOxT >1 showed a decrease of GT and OxT in oxidizer solutions under the exposure to fullerenols, i.e. detoxification of the oxidizer solutions. Values of DGT ≈1 and DOxT ≈1 showed the absence of the fullerenol effects.
Values of SD for DGT and DOxT did not exceed 0.1. The data for the calculations of DGT or DOxT were obtained in three parallel experiments with five samplings for all fullerenol and control solutions. Values of SD for NADH oxidation rates were 10–8 М/min; the data for the rate calculations were obtained in three experiments with five samplings in each of them.
2.3. Sequence analysis
Mutagenic effect of the oxidizer solutions was examined using sequence analysis of the 16S ribosomal RNA gene of P.Phosphoreum. This gene was chosen for genetic analysis as a model for evaluating nonspecific DNA damage. The analysis was performed on the samples of bacterial suspensions exposed to 1,4-benzoquinone (2 10−7M and 10−6M); the results were compared to those obtained on the control bacterial suspensions.
3. Results
3.1. Evaluation of fullerenol and oxidizer concentrations for toxicity measurements
3.1.1. Influence of fullerenols on bioluminescent intensity
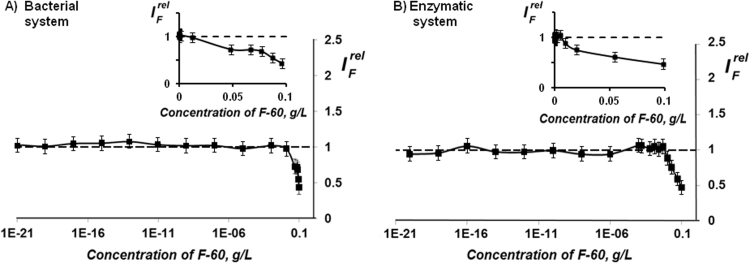
Fig. 3 presents a dependence of bioluminescent intensity (, Eq. (3)) of luminous bacterial and enzyme assay system on concentrations of fullerenol F-60. As is evident from this Figure, the fullerenol suppresses bioluminescence of the bacterial and enzymatic systems at concentrations >10-2 g/L and >5∙10-3 g/L, respectively. Similar results were obtained for the other fullerenol preparation, F-60,70.
Fig. 3.
Bioluminescent intensity at different concentrations of F-60 in bacterial (A) and enzymatic (B) systems.
The suppression of bioluminescent intensity is an evidence of fullerenol toxic effect; it is concerned with inhibition of membrane and intracellular processes (for bacterial cells) or chemical and biochemical reactions (for enzyme system). Additional reasons for the bioluminescence suppression can be: the effect of “optic filter” as a result of bioluminescence absorption/reabsorption [17] and “concentration quenching” resulted from collisional intermolecular interactions. The latter processes are concerned with peculiarities of the luminescence registration in solutions and do not contribute to toxic effects.
Basing on the experiments described, we chose the range of fullerenol concentrations for further experiments (<10−4 g/L) providing the absence of the fullerenol inhibiting effect. The fullerenol concentrations varied in this range to decrease General Toxicity (GT) and Oxidative Toxicity (OxT) in solutions of oxidizers (organic and inorganic) as described below in 3.2, 3.3.
3.1.2. Oxidizers: effective concentrations (EC50) and mutagenicity
Effective concentrations of oxidizers decreasing bioluminescent intensity by 50% (=0.5), EC50, were determined with bacterial and enzymatic bioluminescent assays. The EC50 values of 1,4-benzoquinone were 2.5∙10-7 M and 10−4 M for bacterial and enzymatic assays, respectively, while the EC50 values of potassium ferricyanide were 4∙10−2 M and 2·10−4 M. Lower EC50 values of 1,4-benzoquinone reveal higher toxicity of this oxidizer. This result is supported by the differences in standard redox potentials (0.7 V and 0.36 V at neutral pH for 1,4-benzoquinone and potassium ferricyanide, respectively) [49] and hydrophobic characteristics of the oxidizers.
The ЕС50 values were applied in further experiments to monitor changes of General Toxicity (GT) in oxidizer solutions under addition of fullerenols, as described in Sections 3.2 and 3.3.1 for bacterial and enzymatic assays, respectively.
Mutagenic effect of organic oxidizer solutions (1,4-benzoquinone, 2.5 10−7M and 10−6 M) was examined using sequence analysis of the 16S ribosomal RNA gene; the results were compared to those obtained on the control bacterial suspension. No changes in the analyzed gene sequence were found under the conditions of the experiment. Hence, the analysis did not reveal any mutations in DNA of the bacteria exposed to the oxidizer.
3.2. Bacterial response to the fullerenol exposure in solutions of oxidizers
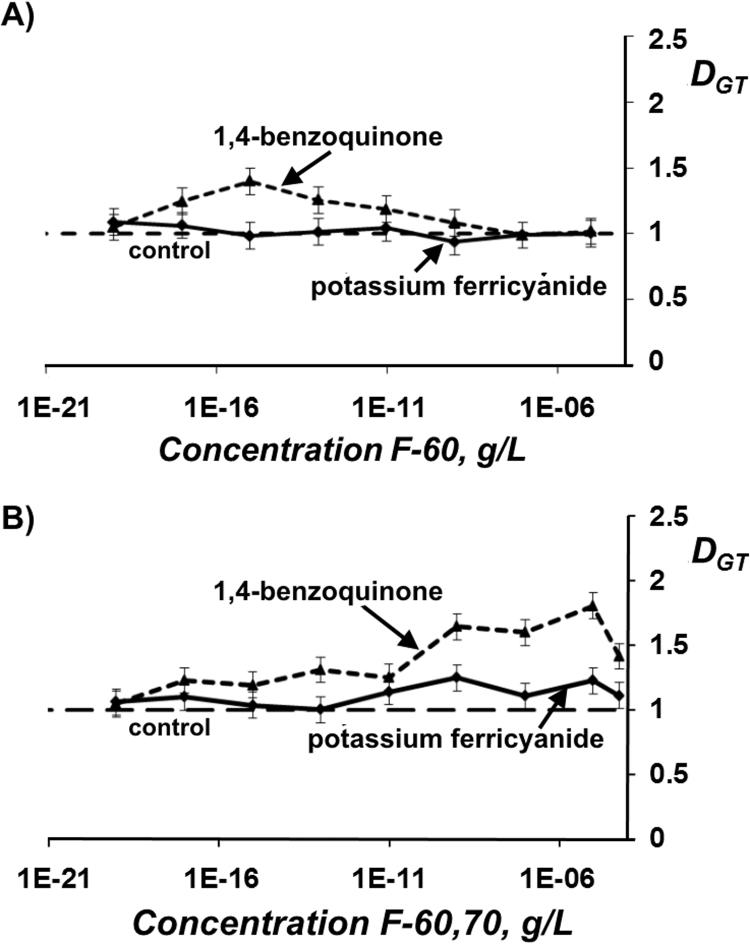
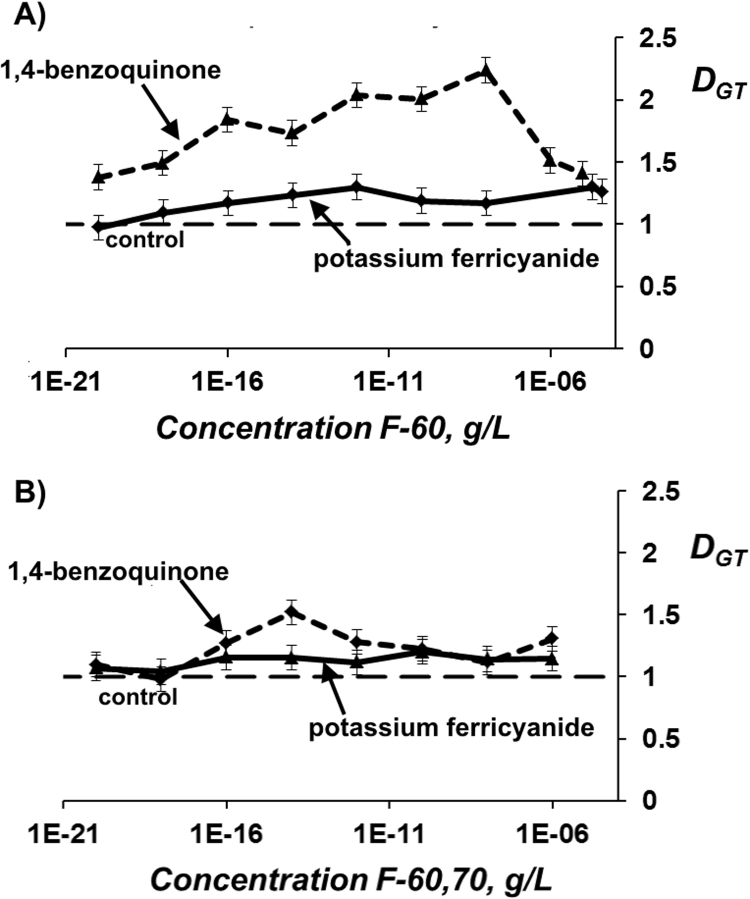
Detoxifying ability of fullerenols was studied using the bacteria-based assay. Bioluminescent intensity of the bacteria was measured in solutions of model oxidizers (1,4-benzoquinone and potassium ferricyanide) at ЕС50 in the absence and presence of fullerenols F-60 and F-60,70. Concentrations of the fullerenols varied in a wide range as shown in Fig. 4. Detoxification coefficients DGT were calculated according to Eq. (4).
Fig. 4.
Detoxification coefficients DGT vs. concentration of fullerenols F-60 (A) and F-60,70 (B) in solutions of 1,4-benzoquinone (2.5∙10-7 M) and potassium ferricyanide (4·10-2 М). Bacteria-based assay.
Fig. 4 shows that 1,4-benzoquinone solutions were detoxified (DGT>1) in the concentration ranges of 10–17–10−8 and 10–17−10−4 g/L for F-60 and F-60,70, respectively. Maximal values of DGT were about 1.5 and 1.8, respectively. In solutions of potassium ferricyanide, the values of DGT were lower: they are not more than 1.3 for F-60,70 and close to “1” at all F-60 concentrations. No monotonic dependencies of DGT on fullerenol concentrations were observed in all cases.
3.3. Effects of fullerenols on enzymatic processes
This section presents fullerenol effects on the bioluminescent system of coupled enzymatic reactions (reactions (1), (2)). It studies: (1) enzymatic activity characterized by bioluminescent intensity of the coupled enzymatic system (I, Figs. 2), (2) redox properties of the enzyme system evaluated by the bioluminescence induction period (T0.5, Figs. 2), and (3) rates of oxidation of NADH, endogenous reducer, a low-molecular component of the bioluminescent enzyme system. Changes of these characteristics are presented in Sections 3.3.1, 3.3.2, and 3.3.3, respectively.
3.3.1. Changes of enzymatic activity under the action of fullerenols in oxidizer solutions
Bioluminescent intensity was measured in solutions of oxidizers in the absence and presence of fullerenols: Iox and Iox+F, respectively, Fig. 2. Detoxification coefficients DGT calculated with Eq. (4), are presented in Fig. 5. Fig. 5A demonstrates detoxifying effect of F-60 (DGT >1) in oxidizer solutions in at different fullerenol concentrations. Lower detoxification coefficients DGT were found under exposure to F-60,70, Fig. 5B. It is evident that DGT values in solutions of organic oxidizer, 1,4-benzoquinone, are higher than those of inorganic oxidizer, potassium ferricyanide. Probably, hydrophobic properties of the solution components (fullerenol+oxidizer) are critical for the enzymatic process, similar to the cellular process.
Fig. 5.
Detoxification coefficients DGT vs. concentration of fullerenols F-60 (A) and F-60,70 (B) in solutions of 1,4-benzoquinone (10−4 M) and potassium ferricyanide (2·10−4 М). Enzyme-based assay.
3.3.2. Changes of redox properties of the bioluminescent enzyme system in oxidizer+fullerenol solutions
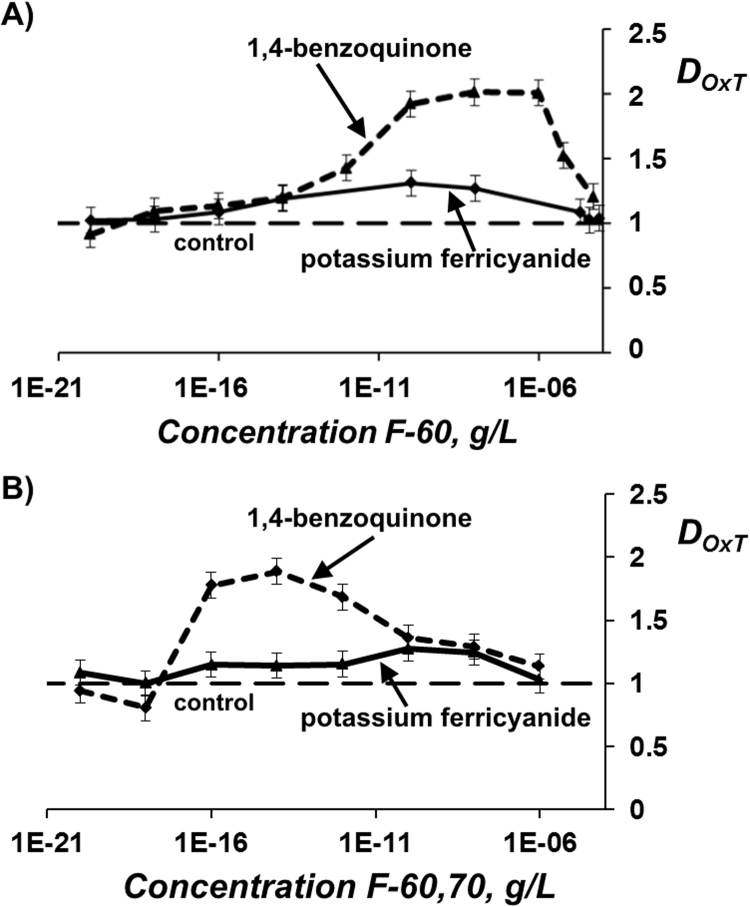
Bioluminescent kinetics of the enzymatic system was studied in solutions of model organic and inorganic oxidizers. Induction periods were measured in the absence and presence of fullerenols: (T0.5)ox and (T0.5)ox+F, respectively, Fig. 2. Detoxification coefficients DOxT were calculated with Eq. (5).
Fig. 6 demonstrates the dependences of DOxT on fullerenol concentrations. Detoxification coefficients DOxT in the solutions of organic oxidizer 1,4-benzoquinone (Fig. 6A,B) were found to be higher than these in ferricyanide solutions; they reached up to “2” in the cases of both F-60 and F-60,70. These data demonstrate an effective decrease of oxidative toxicity in the range of ca 10–12–10-6 g/L for F-60, and ca 10–16–10-4 g/L for F-60,70.
Fig. 6.
Detoxification coefficients DOxT vs. concentration of fullerenols F-60 (A) and F-60,70 (B) in solutions of 1,4-benzoquinone (10−4 M) and potassium ferricyanide (2·10−4 М). Enzyme-based assay.
3.3.3. Reaction rates in enzymatic assay system in solutions of oxidizers and fullerenols
The rates of NADH oxidation were studied in the presence and absence of fullerenol F-60, in enzymatic and nonenzymatic processes, Table 1.
Table 1.
Rates of NADH oxidation in the absence (V) and presence (VF) of fullerenol – F-60, СNADH=1.6∙10–4 М, СF-60=10–8 g/L. CFMN=5.4∙10–5 М, E – enzyme preparation. Registration wavelength 340 nm. SD for V and VF was 10–8 М/min.
| No | Components of solution |
V·108, М/min |
||
|---|---|---|---|---|
| V | VF | VF- V | ||
| Nonenzymatic processes | ||||
| 1 | NADH (auto-oxidation) | 6 | 10 | 4 |
| 2 | NADH+FMN | 32 | 32 | 0 |
| Enzymatic processes | ||||
| 1Е | NADH+Е | 4 | 9 | 5 |
| 2Е | NADH+FMN+Е | 317 | 374 | 57 |
The rates of NADH auto-oxidation in the absence (No. 1) and presence (No. 1E) of the enzymes are presented in the Table 1. These values are close to those determined previously [36], [39]. The data show that the addition of F-60 to NADH solutions increases the auto-oxidation rates by 4∙10−8 and 5∙10−8 М/min, respectively.
Reactions No. (2) and 2E (Table 1) present endogenous processes in the bioluminescent assay system, i.e. nonenzymatic and enzymatic redox reactions of the endogenous reducer and oxidizer (NADH and FMN, respectively, reaction (1), Section 2.1). Addition of F-60 increased the rate of the enzymatic reaction by 57∙10−8 М/min, Table 1. Similar results were obtained with fullerenol F-60,70.
Light-absorption spectra of oxidizer solutions (EC50, Section 3.1.2) were studied under exposure to fullerenols of different concentrations: 10–17−10−4 g/L. No changes in oxidizer concentrations were observed during the 4-h exposure.
4. Discussion
4.1. Peculiarities of fullerenol detoxifying effect
Fig. 4, Fig. 5, Fig. 6 demonstrate that DGT and DOxT exceed “1” in a wide range of fullerenol concentrations, revealing detoxification ability of fullerenols in oxidizer solutions. Both cellular and enzymatic assays demonstrate the detoxifying activity of the fullerenols. Three peculiarities of the fullerenol detoxifying effect are evident from these experiments: (1) highly diluted fullerenol solutions were active, (2) no monotonic dependencies of DGT or DOxT on fullerenol concentrations were observed, and (3) detoxification of organic oxidizer solutions was more effective.
The NADH, organic reducer, is a component of a lot of biochemical reactions, including reaction (1) in the bioluminescent system of coupled enzymatic reactions (Section 2.1). The change of rate of NADH oxidation serves as an indicator of intensification (or slowdown) of redox processes in the assay system. Section 3.3.3 studied rates of NADH oxidation in the absence and presence of fullerenol (10−8 g/L), in enzymatic and nonenzymatic processes, Table 1. Acceleration of auto-oxidation processes (No. 1 and 1E, Table 1) by the fullerenol was found. This result shows that the fullerenol stimulates an oxidation of the endogenous reducer, NADH, withdrawing it from the biochemical process, decreasing the bioluminescent intensity in accordance with reactions (1), (2) (Section 2.1), and contributing to toxicity increase (DGT<1). On the other hand, fullerenol increased the rate of the enzymatic redox reaction of endogenous reducer and endogenous oxidizer (NADH and FMN, respectively, reaction (1), Section 2.1) by 57∙10-8 М/min (No. 2E, Table 1). So, the experiments demonstrate that fullerenols are able to increase toxicity of aqueous solutions by acceleration of NADH auto-oxidation, or decrease the toxicity by acceleration of endogenous NADH-dependent biochemical processes. In both cases, fullerenol in highly diluted solutions (10–8 g/L, corresponding to ca 10–11 M) can be considered as a catalyzer of the redox reactions.
Light-absorption measurements in oxidizer solutions showed independence of 1,4-benzoquinone or ferricyanide concentrations on the exposure to fullerenol and, hence, did not confirm the neutralization of the oxidizers in the solutions, outside the bioassay systems.
All the peculiar experimental results suggest a specific mechanism of the fullerenol biological influence.
4.2. On mechanism of antioxidant effects in highly diluted solutions of fullerenols
Ultralow bioactive concentrations of fullerenols are a subject of a special interest. Previous sections showed that antioxidant effects of the fullerenols take place within ca 10–17–10−4 and 10–17–10−5 g/L for bacteria-based and enzyme-based assays, respectively. The active concentrations may correspond to several tens of fullerenol molecules per liter.
Activation of vital functions of various organisms by low-intensive exposures is a well-known effect, common to all living organisms. It is attributed to triggering of cell defense response under the influence of low concentrations of toxins, low dose radiation, and other stressors. It is known that low doses of bioactive substances can serve as effective drugs [50], [51], [52].
Generally, low-intensive exposures are described in terms of hormesis phenomenon. “Hormesis” is a term for “a generally favorable biological response to low exposures to toxins and other stressors.” “It is characterized by low-dose stimulation and high-dose inhibition, resulting in either a J-shaped or an inverted U-shaped dose response” [51], [53], [54], [55], [56]. The rapid and extensive exponential growth of hormesis citations in the biomedical community takes place for the last two decades [55]. Evidence emerged that hormesis is highly generalizable, independent on biological model or endpoint measured, inducing agent and level of biological organization (e.g. cell, organ, organism). The hormesis mechanism is not understood yet and still lacks strong experimental support, which leads to uncertainty as to the exact underlying causes of hormesis [57]. There exist two models explaining mechanism of hormesis; they consider the adaptive response as related with DNA damage or cell membrane processes [50], [58], [59], [60], [61].
In our previous studies, we demonstrated a positive response of luminous bacteria to low-dose radiation exposure. The results of a series of investigations were summarized in a review [62]. Independency of the bacterial luminescent response on active concentration of beta-emitting radionuclide tritium was demonstrated for intact and lyophilized bacteria in a wide radioactivity interval: 10-4-200 MBq/L [22]. Similar low-dose radiation effects, current study showed (a) positive response of the luminous bacteria to fullerenol exposure and (b) absence of linear dose-effect dependencies: the bacterial and enzyme luminescent responses did not depend on fullerenol concentrations in a wide concentration interval: ca 10–17–10−4 and 10–17–10−5 g/L, respectively.
Biological efficiency of ultralow concentrations of hydrated fullerenes was determined and discussed earlier in [63], [64]. This effect was attributed to fullerene ability to adjust dynamic structure of aqueous media and to “regulate redox processes (especially those involving oxygen) in aqueous systems”. Earlier [65], a role of aqueous medium in antiradical activity of fullerenols was discussed. According to [62], [66], reactive oxygen species in aqueous media might contribute to bacterial bioluminescence activation under low-dose exposures.
Sequence analysis did not reveal any mutagenic effect of the oxidizer solutions under the conditions of the experiments. Therefore, the low-concentration fullerenol effect might be due to “nongenetic” mechanism of the cellular response. The results of this study might be interpreted using the novel “exposome” concept, that complements the genome and encompasses the totality of environmental (i.e. non-genetic) exposures [67].
The difference in effects of fullerenols in solutions of organic and inorganic oxidizers might be concerned with membrane processes in the luminous bacteria. Probably, the combination of amphiphilic compounds (1,4-benzoquinon+fullerenol) in water solutions promotes membrane processes resulting in the intensification of protecting metabolic response of the bacterial cells. Changes in structural organization and fluidity of lipid bilayers in hydrophobic parts of a membrane by F-60 were previously reported in [68]. In our resent experiments under conditions excluding penetration of radionuclide tritium to the bacterial cells [69], a preference of membrane process for the bacterial bioluminescence activation was proved.
Hence, the low-concentration antioxidant effects of fullerenols on bacterial cells can be attributed to hormesis phenomenon, while the intensification of biochemical reactions is explained in terms of fullerenol catalytic activity. Cellular and enzymatic bioluminescent assays showed that solutions of organic oxidizer are detoxified more effectively than those of inorganic oxidizer, indicating the importance of hydrophobic interactions in the detoxification mechanism.
5. Conclusions
The study considers antioxidant properties of bioactive compounds, fullerenols – nanosized water-soluble derivatives of fullerenes, specific allotropic form of carbon. Two fullerenol preparations were tested: С60О2–4(ОН)20–24 and mixture of two types of fullerenols С60О2–4(ОН)20–24+С70О2–4(ОН)20–24. Fundamental and applied aspects of the fullerenol antioxidant activity were under consideration.
The study promotes application of bioluminescent assays to evaluate detoxifying activity of bioactive compounds, with fullerenols taken as an example. Bacteria-based assay was used to demonstrate a decrease in cellular toxicity, while the enzyme-based assay – in biochemical toxicity. Additionally, the enzyme-based assay was used for the direct monitoring of the efficiency of oxidative enzymatic processes.
Bacteria-based and enzyme-based assays have demonstrated similar peculiarities of the detoxification processes: (1) ultralow concentrations of fullerenols were active (ca 10–17–10−4 and 10–17–10−5 g/L, respectively), (2) no monotonic dependencies of detoxification efficiency on fullerenol concentrations were observed, and (3) detoxification of organic oxidizer solutions was more effective than that of the inorganic oxidizer. First and second peculiarities attribute the antioxidant effects of fullerenols to hormesis phenomenon, which is generally considered as a basis for biological adaptive response. Since the sequence analysis of 16S ribosomal RNA gene did not reveal any mutagenic effects of oxidizer solutions, the results support the concept of a “nongenetic” mechanism of the cellular response to the low-concentration fullerenol exposure.
Higher detoxification efficiency in solutions of the organic oxidizer supports a suggestion on involving hydrophobic (probably membrane-dependent) processes to the detoxification mechanism.
Catalytic activity of fullerenol (10−8 g/L) in NADH-dependent enzymatic reactions was demonstrated and supposed to contribute to the adaptive bacterial response.
Current work elaborates physicochemical, biochemical and cellular basis for bioluminescence-based sensors aimed at evaluation of antioxidant activity of bioactive compounds. The study develops application of luminous marine bacteria and their enzyme reactions for the antioxidant activity monitoring. High potential of the bioluminescence systems for studying the biological activity in ultra-diluted solutions is demonstrated. The bioluminescent bacteria- and enzyme-based assays are suitable for low-dose effect evaluation due to simplicity and high rates of bioluminescence measurements. These properties ensure higher reliability of the biological measurements.
Apart from the quantitative evaluation, molecular mechanisms of adaptive response in the bacterial cells should be studied in further experiments using a number of methods applicable for intracellular processes: from membrane penetrability to gene regulation, enzyme activity, ATP consumption and crystallinity of intracellular macrocomponents.
Acknowledgements
The work was supported by the Russian Foundation for Basic Research, Grants No 15-03-06786 and 15–43-04377-sibir; the state budget allocated to the fundamental research at the Russian Academy of Sciences (project No 01201351504). Adaptation of the bioluminescent enzymatic technique to assess the antioxidant activity of bioactive compounds (humates and fullerenols) was partially supported by the Russian Science Foundation, Grant N 16-06-14-10115.
Footnotes
Transparency data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.10.011.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Taroni P., D'Andrea C., Valentini G., Cubeddu R., Hu D.-N., Roberts J.E. Fullerol in human lens and retinal pigment epithelial cells: time domain fluorescence spectroscopy and imaging. Photochem. Photobiol. Sci. 2011;10:904–910. doi: 10.1039/c0pp00312c. [DOI] [PubMed] [Google Scholar]
- 2.Chiang L.Y., Swirczewski J.W., Hsu C.S., Chowdhury S.K., Cameron S., Creegan K. Multi-hydroxy additions onto C60 fullerene molecules. J. Chem. Soc. Chem. Commun. 1992;24:1791–1793. [Google Scholar]
- 3.Goncharova E.A., Isakova V.G., Tomashevich E.V., Churilov G.N. Obtaining of water-soluble polyhydroxylated fullerenols with iron nanoparticles as catalyzers. Vestn. SibGAU. 2009;22:90–93. (In Russian) [Google Scholar]
- 4.Grebowski J., Kazmierska P., Krokosz A. Fullerenols as a new therapeutic approach in nanomedicine. Biomed. Res. Int. 2013;2013:1–9. doi: 10.1155/2013/751913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z., Wang S., Lu Z., Gao X. Syntheses, structures and antioxidant activities of fullerenols: knowledge learned at the atomistic level. J. Clust. Sci. 2015;26:375–388. [Google Scholar]
- 6.Foley S., Crowley C., Smaihi M., Bonfils C., Erlanger B.F., Seta P., Larroque C. Cellular localization of a water-soluble fullerene derivative. Biochem. Biophys. Res. Commun. 2002;294:116–119. doi: 10.1016/S0006-291X(02)00445-X. [DOI] [PubMed] [Google Scholar]
- 7.Grebowski J., Krokosz A., Puchala M. Fullerenol C60(OH)36 could associate to band 3 protein of human erythrocyte membranes. Biochim. Biophys. Acta. 1828;2013:2007–2014. doi: 10.1016/j.bbamem.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Eropkin M.Yu, Melenevskaya E.Yu, Nasonova K.V., Bryazzhikova T.S., Eropkina E.M., Danilenko D.M., Kiselev O.I. Synthesis and biological activity of fullerenols with various contents of hydroxyl groups. Pharm. Chem. J. 2013;47:87–91. [Google Scholar]
- 9.Cai X., Hao J., Zhang X., Yu B., Ren J., Luo C., Li Q., Huang Q., Shi X., Li W., Liu J. The polyhydroxylated fullerene derivative C60(OH)24 protects mice from ionizingradiation-induced immune and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2010;243:27–34. doi: 10.1016/j.taap.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Slavic M., Djordjevic A., Radojicic R., Milovanovic S., Orescanin-Dusic Z., Rakocevic Z., Spasic M.B., Blagojevic D. FullerenolC60(OH)24 nanoparticles decrease relaxing effects of dimethyl sulfoxide on rat uterus spontaneous contraction. J. Nanopart. Res. 2013;15:1–10. [Google Scholar]
- 11.Mirkov S.M., Djordjevic A.N., Andric N.L., Andric S.A., Kostic T.S., Bogdanovic G.M., Vojinovic-Miloradov M.B., Kovacevic R.Z. Nitric oxide-scavenging activity of polyhydroxylated fullerenol, C60(OH)24. Nitric Oxide. 2004;11:201–207. doi: 10.1016/j.niox.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Djordjevic A.B., Srdjenovic M., Seke D., Petrovic R., Injac R., Mrdjanovic J. Review of synthesis and antioxidant potential of fullerenol nanoparticles. J. Nanomater. 2015;2015:1–15. [Google Scholar]
- 13.Injac R., Prijatelj M., Strukelj B. Fullerenol nanoparticles: toxicity and antioxidant activity. Methods Mol. Biol. 2013;1028:75–100. doi: 10.1007/978-1-62703-475-3_5. [DOI] [PubMed] [Google Scholar]
- 14.Djordjevic A., Canadanovic-Brunet J.M., Vojinovic-Miloradov M., Bogdanovic G. Antioxidant properties and hypothetic radical mechanism of fullerenol C60(OH)24. Oxid. Commun. 2004;27:806–812. [Google Scholar]
- 15.Jiao F., Liu Y., Qu Y., Li W., Zhou G.Q., Ge C.C., Li Y.F., Sun B.Y., Chen C.Y. Studies on antitumor and antimetastatic activities of fullerenol in a mouse breast cancer model. Carbon. 2010;48:2231–2243. [Google Scholar]
- 16.Bulich A.A., Isenberg D.L. Use of the luminescent bacterial system for rapid assessment of aquatic toxicity. ISA Trans. 1981;20:29–33. [PubMed] [Google Scholar]
- 17.Fedorova E., Kudryasheva N., Kuznetsov A., Mogil’naya O., Stom D. Bioluminescent monitoring of detoxification processes: activity of humic substances in quinone solutions. J. Photochem. Photobiol. B. 2007;88:131–136. doi: 10.1016/j.jphotobiol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Girotti S., Ferri E.N., Fumo M.G., Maiolini E. Monitoring of environmental pollutants by bioluminescent bacteria. Anal. Chim. Acta. 2008;608:2–29. doi: 10.1016/j.aca.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Kudryasheva N.S., Kratasyuk V.A., Esimbekova E.N., Vetrova E.V., Nemtseva E.V., Kudinova I.Y. Development of the bioluminescent bioindicators for analyses of pollution. Field Anal. Chem. Tech. 1998;2:277–280. [Google Scholar]
- 20.Roda A., Pasini P., Mirasoni M., Michchelini E., Guardigli M. Biotechnological application of bioluminescence and chemiluminescence. Trends Biotech. 2004;22:295–303. doi: 10.1016/j.tibtech.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Rozhko T.V., Kudryasheva N.S., Kuznetsov A.M., Vydryakova G.A., Bondareva L.G., Bolsunovsky A.Ya. Effect of low-level α-radiation on bioluminescent assay systems of various complexity. Photochem. Photobiol. Sci. 2007;6:67–70. doi: 10.1039/b614162p. [DOI] [PubMed] [Google Scholar]
- 22.Selivanova M.A., Mogilnaya O.A., Badun G.A., Vydryakova G.A., Kuznetsov A.M., Kudryasheva N.S. Effect of tritium on luminous marine bacteria and enzyme reactions. J. Environ. Radioact. 2013;120:19–25. doi: 10.1016/j.jenvrad.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Esimbekova E.N., Kondik A.M., Kratasyuk V.A. Bioluminescent enzymatic rapid assay of water integral toxicity. Environ. Monit. Assess. 2013;185:5909–5916. doi: 10.1007/s10661-012-2994-1. [DOI] [PubMed] [Google Scholar]
- 24.Kratasyuk V.A., Esimbekova E.N. Applications of luminous bacteria enzymes in toxicology. Comb. Chem. High Throughput Screen. 2015;18:952–959.. doi: 10.2174/1386207318666150917100257. [DOI] [PubMed] [Google Scholar]
- 25.Efremenko E.N., Maslova O.V., Kholstov A.V., Senko O.V., Ismailov A.D. Biosensitive element in the form of immobilized luminescent photobacteria for detecting ecotoxicants in aqueous flow-through systems. Luminescence. 2016 doi: 10.1002/bio.3104. [DOI] [PubMed] [Google Scholar]
- 26.Ismailov A.D., Aleskerova L.E. Photobiosensors containing luminescent bacteria. Biochemistry. 2015;80:733–744. doi: 10.1134/S0006297915060085. [DOI] [PubMed] [Google Scholar]
- 27.Ranjan R., Rastogi N.K., Thakur M.S. Development of immobilized biophotonic beads consisting of Photobacteriumleiognathi for the detection of heavy metals and pesticide. J. Hazard. Mater. 2012;225–226:114–123. doi: 10.1016/j.jhazmat.2012.04.076. [DOI] [PubMed] [Google Scholar]
- 28.Kudryasheva N. Bioluminescence and exogenous compounds: physicochemical basis for bioluminescence assay. J. Photochem. Photobiol. B. 2006;83:77–86. doi: 10.1016/j.jphotobiol.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Kirillova T.N., Kudryasheva N.S. Effect of heavy atom in bioluminescent reactions. Anal. Bioanal. Chem. 2007;387:2009–2016. doi: 10.1007/s00216-006-1085-y. [DOI] [PubMed] [Google Scholar]
- 30.Kirillova T.N., Gerasimova M.A., Nemtseva E.V., Kudryasheva N.S. Effect of halogenated fluorescent compounds on bioluminescent reactions. Anal. Bioanal. Chem. 2011;400:343–351. doi: 10.1007/s00216-011-4716-x. [DOI] [PubMed] [Google Scholar]
- 31.Nemtseva E.V., Kudryasheva N.S. The mechanism of electronic excitation in bacterial bioluminescent reaction. Russ. Chem. Rev. 2007;76:91–100. [Google Scholar]
- 32.Vetrova E.V., Kudryasheva N.S., Kratasyuk V.A. Redox compounds influence on the NAD(P)H:FMN-oxidoreductase-luciferase bioluminescent system. Photochem. Photobiol. Sci. 2007;6:35–40. doi: 10.1039/b608152e. [DOI] [PubMed] [Google Scholar]
- 33.Slavova-Kazakova A.K., Angelova S.E., Veprintsev T.L., Denev P., Fabbri D., Dettori M.A., Kratchanova M., Naumov V.V., Trofimov A.V., Vasil'ev R.F., Delogu G., Kancheva V.D. Antioxidant potential of curcumin-related compounds studied by chemiluminescence kinetics, chain-breaking efficiencies, scavenging activity (ORAC) and DFT calculations. Beilstein J. Org. Chem. 2015;11:1398–1411. doi: 10.3762/bjoc.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fedorova G.F., Kancheva V.D., Menshov V.A., Naumov V.V., Vasil'ev R.F., Veprintsev T.L., Trofimov A.V., Tsaplev Yu.B., Yablonskaya O.I. Exogenous and endogenous mediators of oxygen metabolism: alternatives for chemical and biological activity. Stud. Nat. Prod. Chem. 2016;47:357–385. [Google Scholar]
- 35.Kudryasheva N., Vetrova E., Kuznetsov A., Kratasyuk V., Stom D. Bioluminescent assays: effects of quinones and phenols. Ecotoxicol. Environ. Saf. 2002;53:221–225. doi: 10.1006/eesa.2002.2214. [DOI] [PubMed] [Google Scholar]
- 36.Tarasova A.S., Stom D.I., Kudryasheva N.S. Effect of humic substances on toxicity of inorganic oxidizer bioluminescent monitoring. Environ. Toxicol. Chem. 2011;30:1013–1017. doi: 10.1002/etc.472. [DOI] [PubMed] [Google Scholar]
- 37.Kudryasheva N.S., Tarasova A.S. Pollutant toxicity and detoxification by humic substances: mechanisms and quantitative assessment via luminescent biomonitoring. Environ. Sci. Pollut. Res. Int. 2015;22:155–167. doi: 10.1007/s11356-014-3459-6. [DOI] [PubMed] [Google Scholar]
- 38.Tarasova A.S., Stom D.I., Kudryasheva N.S. Antioxidant activity of humic substances via bioluminescent monitoring in vitro. Environ. Monit. Assess. 2015;187:89. doi: 10.1007/s10661-015-4304-1. [DOI] [PubMed] [Google Scholar]
- 39.Tarasova A.S., Kislan S.L., Fedorova E.S., Kuznetsov A.M., Mogilnaya O.A., Stom D.I., Kudryasheva N.S. Bioluminescence as a tool for studying detoxification processes in metal salt solutions involving humic substances. J. Photochem. Photobiol. В. 2012;117:164–170. doi: 10.1016/j.jphotobiol.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Kudryasheva N.S., Kovel E.S., Sachkova A.S., Vorobeva A.A., Isakova V.G., Churilov G.N. Bioluminescent enzymatic assay as a tool for study antioxidant activity and toxicity of bioactive compounds. Photochem. Photobiol. 2016 doi: 10.1111/php.12639. [DOI] [PubMed] [Google Scholar]
- 41.Mishra O.P., Popov A.V., Pietrofesa R.A., Christofidou-Solomidou M. Gamma-irradiation produces active chlorine species (ACS) in physiological solutions: secoisolariciresinol diglucoside (SDG) scavenges ACS - a novel mechanism of DNA. Radioprot. Biochim. Biophys. Acta (BBA) Gen. Sub. 2016;1860:1884–1897. doi: 10.1016/j.bbagen.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esguerra K.V.N., Fall Y., Petitjean L., Lumb J.P. Controlling the catalytic aerobic oxidation of phenols. J. Am. Chem. Soc. 2014;136:7662–7668. doi: 10.1021/ja501789x. [DOI] [PubMed] [Google Scholar]
- 43.Kamnev A.A., Dykman R.L., Kovács K., Pankratov A.N., Tugarova A.V., Homonnay Z., Kuzmann E. Redox interactions between structurally different alkylresorcinols and iron(III) in aqueous media: frozen-solution 57Fe Mössbauer spectroscopic studies, redox kinetics and quantum chemical evaluation of the alkylresorcinol reactivities. Struct. Chem. 2014;25:649–657. [Google Scholar]
- 44.Kuznetsov A.M., Rodicheva E.K., Shilova E.V. Bioassay based on lyophilized bacteria. Biotekhnologiya. 1996;9:57–61. (In Russian) [Google Scholar]
- 45.Churilov G.N. Synthesis of fullerenes and other nanomaterials in arc discharge. Fuller. Nanotub. Car. N. 2008;16:395–403. [Google Scholar]
- 46.Churilov G.N., Kratschmer W., Osipova I.V., Glushenko G.A., Vnukova N.G., Kolonenko A.L., Dudnik A.I. Synthesis of fullerenes in a high-frequency arc plasma under elevated helium pressure. Carbon. 2013;62:389–392. [Google Scholar]
- 47.Isakova V.G., Goncharova E.A., Bayukov O.A., Churilov G.N. Hydroxylation of fullerenes modified with iron nanoparticles. Russ. J. Appl. Chem. 2011;84:1165–1169. [Google Scholar]
- 48.Juan Li, Zhang M., Sun B., Xing G., Song Yan, Guo HaiLi, Chang Ya, Ge Yo, Zhao Yu. Separation and purification of fullerenols for improved biocompatibility. Carbon. 2012;50:460–469. [Google Scholar]
- 49.Vanýsek P. Standard Electrochemical Potentials. CRC Handb. Chem. Phys. 1983;64:156–163. [Google Scholar]
- 50.Burlakova E.B., Konradov A.A., Maltseva E.X. Effect of extremely weak chemical and physical stimuli on biological systems. Biophysics. 2004;49:522–534. [Google Scholar]
- 51.Calabrese E.J. Hormesis: a fundamental concept in biology, Microb. Cell. 2014;1:145–149. doi: 10.15698/mic2014.05.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang C.R., Tian Y., Wang X.R., Yu H.X., Lu X.W., Wang C., Wang H. Hormesis effects and implicative application in assessment of leadcontaminated soils in roots of Vicia faba seedlings. Chemosphere. 2010;80:965–971. doi: 10.1016/j.chemosphere.2010.05.049. [DOI] [PubMed] [Google Scholar]
- 53.Baldwin J., Grantham V. Radiation hormesis: historical and current perspectives. J. Nucl. Med. Technol. 2015;43:242–246. doi: 10.2967/jnmt.115.166074. [DOI] [PubMed] [Google Scholar]
- 54.Calabrese E.J., Baldwin L.A. The frequency of U-shaped dose responses in the toxicological literature. Toxicol. Sci. 2001;62:330–338. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- 55.Calabrese E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013;43:580–606. doi: 10.3109/10408444.2013.808172. [DOI] [PubMed] [Google Scholar]
- 56.Kaiser J. Hormesis: sipping from a poisoned chalice. Science. 2003;302:376–379. doi: 10.1126/science.302.5644.376. [DOI] [PubMed] [Google Scholar]
- 57.Shi J., Huber M., Wang T., Dali W., Lin Z., Chun-Sheng Y. Progress in the studies on hormesis of low-dose pollutants. Environ. Dis. 2016;1:58–64. [Google Scholar]
- 58.Albers R.W. Biochemical aspects of active transport. Annu. Rev. Biochem. 1967;36:727–756. doi: 10.1146/annurev.bi.36.070167.003455. [DOI] [PubMed] [Google Scholar]
- 59.Mothersill C., Seymour C. Implications for human and environmental health of low doses of ionising radiation. J. Environ. Radioact. 2014;133:5–9. doi: 10.1016/j.jenvrad.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Rana D., Matsuura T., Kassim M.A., Ismail A.F. Radioactive decontamination of water by membrane processes. Desalination. 2013;321:77–92. [Google Scholar]
- 61.Serment-Guerrero J., Breña-Valle M., Aguilar-Moreno M., Balcázar M. Evidence of DNA double strand breaks formation in Escherichia coli bacteria exposed to alpha particles of different LET assessed by the SOS response. Appl. Radiat. Isot. 2012;71:66–70. doi: 10.1016/j.apradiso.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Kudryasheva N.S., Rozhko T.V. Effect of low-dose ionizing radiation on luminous marine bacteria: radiation hormesis and toxicity. J. Environ. Radioact. 2015;142:68–77. doi: 10.1016/j.jenvrad.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Voeikov V.L., Yablonskaya O.I. Stabilizing effects of hydrated fullerenes C-60 in a wide range of concentrations on luciferase, alkaline phosphatase, and peroxidase in vitro. Electromagn. Biol. Med. 2015;34:160–166. doi: 10.3109/15368378.2015.1036077. [DOI] [PubMed] [Google Scholar]
- 64.Yablonskaya O.I., Ryndina T.S., Voeikov V.L., Khokhlov A.N. A paradoxal effect of hydrated C60-fullerene in an ultralow concentration on the viability and aging of cultivated chinese hamster cells. Mosc. Univ. Biol. Sci. Bull. 2013;68:63–68. [Google Scholar]
- 65.Bensasson R.V., Bretteich M., Frederiksen J., Gottinger H., Hirsch A., Land E.J., Leach S., McGarvey D.J., Schonberger H. Reactions of (aq), CO2−, HO−, O2− and O2(1Dg) with a dendro[60]fullerene and C60[C(COOH)2]n (n =52−6) Free Radic. Biol. Med. 2000;29:26–33. doi: 10.1016/s0891-5849(00)00287-2. [DOI] [PubMed] [Google Scholar]
- 66.Alexandrova M., Rozhko T., Vydryakova G., Kudryasheva N. Effect of americium-241 on luminous bacteria. Role of peroxides. J. Environ. Radioact. 2011;102:407–411. doi: 10.1016/j.jenvrad.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 67.Wild C.P. The exposome: from concept to utility. Int. J. Epidemiol. 2012;41:24–32. doi: 10.1093/ije/dyr236. [DOI] [PubMed] [Google Scholar]
- 68.Brisebois P.P., Arnold A.A., Chabre Y.M., Roy R., Marcotte I. Comparative study of the interaction of fullerenol nanoparticles with eukaryotic and bacterial model membranes using solid-state NMR and FTIR spectroscopy. Eur. Biophys. J. 2012;41:535–544. doi: 10.1007/s00249-012-0809-5. [DOI] [PubMed] [Google Scholar]
- 69.Rozhko T.V., Badun G.A., Razzhivina I.A., Guseynov O.A., Guseynova V.E., Kudryasheva N.S. On mechanism of biological activation by tritium. J. Environ. Radioact. 2016;157:131–135. doi: 10.1016/j.jenvrad.2016.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material