Abstract
Evidence indicates that after brain injury, neurogenesis is enhanced in regions such as hippocampus, striatum, and cortex. To study the role of hypoxia-inducible factor-1 (HIF−1α) and Wnt signaling in cerebral ischemia/hypoxia-induced proliferation of neural stem cells (NSCs), we investigated the proliferation of NSCs, expression of HIF−1α, and activation of Wnt signaling under conditions of pathologic hypoxia in vitro. NSCs were isolated from 30-day-old Sprague–Dawley rats and subjected to 0.3% oxygen in a microaerophilic incubation system. Cell proliferation was evaluated by measuring the diameter of neurospheres and by bromodeoxyuridine incorporation assays. Real-time quantitative PCR and Western blotting were used to detect mRNA and protein levels of HIF-1α, β-catenin, and cyclin D1 in the NSCs. The results showed that hypoxia increased NSC proliferation and the levels of HIF-1α, β−catenin, and cyclin D1 (p < 0.05). Blockade of the Wnt signaling pathway decreased hypoxia-induced NSC proliferation, whereas activation of this pathway increased hypoxia-induced NSC proliferation (p < 0.05). Knockdown of HIF-1α with HIF-1α siRNA decreased β−catenin nuclear translocation and cyclin D1 expression, and inhibited proliferation of NSCs (p < 0.05). These findings indicate that pathologic hypoxia stimulates NSC proliferation by increasing expression of HIF-1α and activating the Wnt/β-catenin signaling pathway. The data suggest that Wnt/β-catenin signaling may play a key role in NSC proliferation under conditions of pathologic hypoxia.
Keywords: Wnt, Hypoxia inducible factor-1 alpha, Neurogenesis, Neural stem cells
Introduction
Data have shown that after brain injury, neurogenesis is enhanced in various regions, such as hippocampus, striatum, and cortex(1). Experimental evidence indicates that endogenous neural stem cells (NSCs) are activated in response to cerebral ischemia in both rodents and humans (2–5) and that activated NSCs may participate in neurologic recovery after an ischemic/hypoxic insult (6–11). However, the mechanisms responsible for NSC proliferation under conditions of pathologic hypoxia are unclear.
One particular proliferation pathway that has been well described is the Wingless-type (Wnt) pathway. The Wnt pathway regulates embryonic NSC patterning, cell fate determination, and cell proliferation (12). Additionally, Wnt signaling was observed to regulate adult rat hippocampal neurogenesis. Indeed, Wnt3a mutant mice exhibit underdevelopment of the hippocampus because of lack of proliferation (13). Wnt family members are expressed in hippocampal astrocytes, whereas hippocampal stem/progenitor cells express receptors and signaling components for Wnt proteins (14). Exogenous expression of Wnt3a enhances neurogenesis in the hippocampus and retina (15). Furthermore, Wnt signaling was observed in adult mouse subventricular zone (SVZ) NSCs (16) and in adult dentate gyrus. The Wnt receptor FZD1 is mainly expressed in NSCs and serves to amplifyprogenitors and immature neurons (17). These findings provide evidence that the Wnt pathway is involved in adult stem cell maintenance and neurogenesis.
Oxygen (O2) is an important energy source for cell metabolism, and its concentration is tightly regulated in the central nervous system. Studies have implicated O2 and its signal transduction pathways in controlling cell proliferation, fate, and morphogenesis during brain development. At low O2 tensions, hypoxia-inducible factor-1α (HIF-1α) facilitates signal transduction pathways that promote self-renewal (e.g., Notch), and inhibits pathways that promote NSC differentiation or apoptosis (e.g., bone morphogenetic proteins) (18). One study indicated that O2 availability has a direct role in stem cell regulation through HIF−1α modulation of Wnt/β-catenin signaling during brain development (19). Hypoxia-inducible factor-1α acts upstream of the Wnt/β-catenin pathway and also may contribute to the production of VEGF (20).
Cerebral ischemia leads to hypoxia and activates HIF-1α (21–23). Cerebral ischemia/hypoxia also enhances endogenous neurogenesis (7, 24). However, the role of HIF-1α and Wnt signaling in cerebral ischemia/hypoxia-induced neurogenesis remains unclear. Previously, we reported that hypoxia stimulates proliferation of NSCs that were cultured from cortex of fetal Sprague-Dawley (SD) rats on embryonic day 5.5 and that hypoxia increases cyclin D1 expression through activation of the JNK signaling pathway (25). In the present study, we used cultured NSCs isolated from the SVZ of 30-day-old SD rats and mimicked pathologic hypoxia in a microaerophilic incubation system to determine the proliferation of NSCs and expression of HIF−1α after hypoxia, and to explore the relationship of Wnt signaling activation by the HIF−1α protein.
Materials and Methods
NSC culture
SD rats were housed in the Experimental Animal Center of Xi’an Jiaotong University School of Medicine (Certificate No. 22-9601018). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23). Experimental protocols were approved by the Animal Care and Use Regulations of Xi’an Jiaotong University School of Medicine. All efforts were made to minimize the number of animals used and their suffering. NSCs were isolated from the SVZ of 30-day-old SD rats and propagated with the neurosphere method as described previously (26). In brief, fresh SVZ formation was microdissected, cut into small pieces, and incubated in a digestion solution (trypsin 0.01%, EDTA 200 mM, glucose 0.6%, MgCl2 1 mM in PBS [all from Sigma, St. Louis, CA, USA]) at 37 °C for 10 min. Then, the tissues were mechanically dissociated into single-cell suspensions. The single-cell suspension was resuspended in serum-free DMEM/F12 (Dulbecco’s modified Eagle medium and Ham’s F12 [1:1], 10 ng/ml basic fibroblast growth factor, 20 ng/ml epidermal growth factor, 1% penicillin, 1% streptomycin, 1% N2, 2% B27 supplement [all from Invitrogen, Carlsbad, CA, USA], and 2.5 μg/ml heparin [Sigma]). Cells were plated at an initial concentration of 100,000 cells/ml in 50 ml cell culture flasks at 37°C with 5% CO2. After 5–7 days in vitro, the primary neurospheres were passaged. The neurospheres were dissociated with 0.05% trypsin and 200 μM EDTA for 10 min at 37 °C and mechanically triturated into a single-cell suspension. The single cells were cultured at a density of 50,000 cells/ml for 5 days, when neurospheres of 90–120 μm in diameter had been propagated (passage 1 neurospheres). Passage 1 neurospheres were processed for experiments as described below, and at least three independent experiments were performed for each assay.
NSC hypoxia exposure
Passage 1 NSCs were placed into a microaerophilic incubation system (BUGBOX-M; Ruskinn, Bridgend, UK) in a humidified environment with 0.3% O2/94.7% N2/5% CO2 (27). The cells were cultured in the incubator at 37 °C for different durations (3, 6, 12, 24, and 48 h). We chose 12 h of hypoxia for experimental conditions based on our preliminary findings and reports from others (28, 29). After 12 h of hypoxia, the cells were returned to normoxic conditions consisting of humidified 95% air/5% CO2 for 1–3 days. The control groups were continuously maintained under normoxic conditions for the full duration.
siRNA synthesis and transfection
For HIF-1α gene silencing, HIF-1α siRNA (sense: 5′-GGG CCG UUC AAU UUA UGA ATT-3′; antisense: 5′-UUC AUA AAU UGA ACG GCC CTT-3′) and negative control siRNA (NC-siRNA, sense: 5′-UUC UCC GAA CGU GUC ACG UTT-3′; antisense: 5′-ACG UGA CAC GUU CGG AGA ATT-3′) were chemically synthesized by GenePharma Corporation (Shanghai, China). Lipofectamine 2000 (Invitrogen) was used to optimize siRNA transfection. All siRNA transfections were performed in serum-free DMEM/F12. Lipofectamine-siRNA complexes were initially formed with 500 nM siRNA and diluted to the desired concentrations. Lipofectamine and siRNA were diluted in serum-free DMEM/F12 and incubated for 5 min at room temperature. After the two solutions had been gently mixed, the mixture was incubated for 15 min at room temperature. Finally, the complexes were diluted to the desired transfection concentrations and added to the plated cells. Transfection efficiency of siRNA was analyzed by flow cytometry.
Measurement of neurosphere diameter
Neurosphere diameter was measured by the methods previously described with some modifications (30). While being monitored under an inverted phase contrast microscope, passage 1 neurospheres (60–90 μm diameter) were individually transferred with a sterile capillary tube into the wells (10 neurospheres/well) of non-adherent 96-well plates with 200 μl of serum-free medium and incubated for 2 days. After the NSCs were incubated with Lipofectamine-siRNA complexes (50 nM) for 4 h, Wnt inhibitor DKK-1 (200 ng/ml; R&D Systems, Inc., USA) DKK-1 was first identified as an inhibitor of Wnt signalling pathway in 1999 (31) or Wnt pathway activator lithium chloride (32) (LiCl, 6 mM; Sigma) for 4 h, they were placed in the microaerophilic incubation system for 12 h, followed by 1, 2, or 3 days of normoxic culture. At the end of the culture period, we measured the neurosphere diameter using a BX-51 fluorescence microscope and DP71 camera with Image-Pro Express software (version 5.1; Olympus, Tokyo, Japan). Results were collected as the average of more than three independent experiments. The percentage increase in diameter was calculated as follows: (diameter at harvest – diameter on day 1 before treatment) × 100/diameter on day 1 before treatment. Data were analyzed and the growth rates of neurospheres under different conditions were compared (normoxia vs. hypoxia; LiCl treatment vs. control; DKK-1 treatment vs. control).
BrdU incorporation, immunofluorescence, and immunocytochemistry staining
We investigated the effect of hypoxia on the proliferative activity of NSCs by bromodeoxyuridine (BrdU, Sigma) incorporation. Passage 1 NSCs were cultured for 2 days before being exposed to hypoxic conditions for 6, 12, or 24 h. Next, the NSCs were treated with Lipofectamine-siRNA complexes (50 nM siRNA) for 4 h, cultured under hypoxic conditions for 12 h, and allowed to recover in normoxia for 1, 2, or 3 days. The neurospheres were labeled with 10 μM BrdU for 4 h and then dissociated into single cells and plated onto poly-L-lysine–coated coverslips at a concentration of 50,000/ml per well in 24-well plates. After 6 h of attachment, the cells were fixed in 4% paraformaldehyde in PBS and processed for immunofluorescence staining. In brief, the cells were incubated in 2 N hydrochloric acid for 30 min at 37 °C and 0.1 M sodium borate (pH 8.5) for 10 min. Cells were incubated overnight with mouse monoclonal anti-BrdU antibody (1:200; Chemicon, Billerica, MA, USA) in PBS containing 0.1% Triton X-100 and 2% bovine serum albumin at 4 °C. The cells were reacted with FITC-conjugated anti-mouse IgG (1:200) for 1 h at room temperature. Labeled cells were counterstained with 50 μg/ml propidium iodide (PI, Sigma) and mounted. BrdU-labeled cells were counted by using fluorescence microscopy and normalized to the total number of propidium iodide-stained cells. For HIF-1α and β-catenin immunofluorescence staining, we used neurospheres or dissociated single cells prepared as described above. Cells were incubated overnight with mouse monoclonal anti-HIF-1α (1:100; Neomarker, Fremont, CA, USA) or rabbit polyclonal anti-β-catenin (1:1000; Cell Signaling Technology, Inc., Danvers, MA, USA) in PBS containing 0.1% Triton X-100 and 2% bovine serum albumin at 4 °C. The cells were reacted with Cy3-conjugated anti-mouse IgG (1:200) for 1 h at room temperature. Labeled cells were counterstained with 20 μg/ml 4′6-diamidino-2-phenylindole (DAPI, Sigma) and mounted, and images were collected on a BX-51 fluorescence microscope (Olympus, Tokyo, Japan). It has been shown that cyclin D1 lies downstream of β-catenin proteins and is important for the regulation of NSC proliferation during embryonic life (33–35). Therefore we also performed cyclin D1 immunocytochemistry staining of neurospheres and dissociated single cells. Cells were incubated overnight with mouse monoclonal anti-cyclin D1 (1:500; Neomarker) and then with anti-mouse biotin-conjugated IgG secondary antibody for 2 h. Avidin-biotin enzyme reagent (Vector Labs, Burlingame, CA, USA) and DAB (Sigma) were used to visualize the positive signal.
Real-time quantitative PCR analysis
At the time point of interest, we collected the cells and extracted total RNA using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. RNA was quantified by spectrophotometry (Nanodrop, ThermoFisher, Grand Island, NY, USA). The RNA was reverse transcribed to cDNA with a reverse transcriptase kit (PrimeScript RT Reagent Kit; TaKaRa, Japan). Relative abundance of each mRNA sample was quantified by performing quantitative PCR with specific primers and the SYBR Premix Ex Taq (TaKaRa). Primers for HIF-1α (forward: 5′-CCA GAT TCA AGA TCA GCC AGC A-3′; reverse: 5′-GCT GTC CAC ATC AAA GCA GTA CTC A-3′), β-catenin (forward: 5′-AAG TTC TTG GCT ATT ACG ACA-3′; reverse: 5′-ACA GCA CCT TCA GCA CTC T-3′), cyclin D1 (forward: 5′-CTA ATG TAA AGC CAG CCG CAA TG-3′; reverse: 5′-TGG ACA CAG CAG CCC TCA AG-3′), and β-actin (forward: 5′-GGA GAT TAC TGC CCT GGC TCC TA-3′; reverse: 5′-GAC TCA TCG TAC TCC TGC TTG CTG-3′) were designed and synthesized by TaKaRa Biotechnology. Real-time PCR was carried out by using an iQ Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Cycle threshold values were obtained from the Bio-Rad iQ5 2.0 Standard Edition Optical System software (Bio-Rad). Data were analyzed with the ΔΔCt method, and β-actin served as an internal control (36, 37). Data are presented as the mean ± SD of three separate experiments carried out in duplicate.
Western Blot analysis
Cells were collected at the time point of interest and then lysed in RIPA lysis buffer. Using the ProteoJET™ Cytoplasmic and Nuclear Protein Extraction Kit (Fermentas, Grand Island, NY, USA), we extracted cytoplasmic and nuclear proteins separately, subjected them to electrophoresis on 10% SDS-polyacrylamide gels, and transferred them to a nitrocellulose membrane (38, 39). After blocking the membrane for 2 h with 5% nonfat dry milk in TBST (10 mM Tris-HCl and 0.05% Tween 20), we incubated it with primary antibodies overnight at 4 °C and then with secondary antibody for 2 h at room temperature. The primary antibodies included mouse monoclonal anti-HIF-1α (1:200; Neomarker), rabbit polyclonal anti-β-catenin (1:2000; Cell Signaling Technology, Inc.), mouse monoclonal anti-cyclin D1 (1:1000; Neomarker), mouse monoclonal anti-β-tubulin (1:5000; Neomarker), and rabbit polyclonal anti-β-actin (1:5000; Santa Cruz Biotechnology, Dallas, TX, USA). The membranes were incubated in the dark with ECL (Amersham, Piscataway, NJ, USA). The luminescent signal was recorded and quantified with the Syngene G Box. The data were sent to the computer for analysis and documentation.
Statistical analysis
Data are expressed as means ± SD. Statistical analyses were carried out with SPSS 13.0 software. The Student’s t-test was used to compare values between two groups, and one-way ANOVA followed by Tukey’s post hoc test was used to compare values among more than two groups. A value of p < 0.05 was considered statistically significant.
Results
Hypoxia increases the expression of HIF-1α and Wnt signaling components
To explore the effects of hypoxia-induced NSC proliferation on signaling pathways, we used immunofluorescence staining, immunohistochemical staining, real-time PCR, and Western blot analysis to evaluate the expression of HIF-1α, β-catenin, and cyclin D1 at both mRNA and protein levels. Immunofluorescence staining showed HIF-1α localization in the cytoplasm of normoxic cells. Nuclear expression of HIF-1α was enhanced after hypoxia exposure and was greater at 12 h than at 6 h (Fig. 1A). We found that the levels of HIF-1α mRNA (Fig. 1B) and protein (Fig. 1C, D) increased significantly after 6, 12, and 24 h of hypoxia compared with levels in normoxic cells (p < 0.05) and reached a peak at 12 h (p < 0.01; Fig. 1B, D). Furthermore, the mRNA levels of both β-catenin (Fig. 2C) and cyclin D1 (Fig. 2F) were significantly greater after 6, 12, and 24 h of hypoxia than in cells that remained normoxic. The immunofluorescence staining showed both cytoplasmic and nuclear expression of β-catenin in neurospheres and dissociated into single cells (Fig. 2A, D). Staining showed that β-catenin expression was localized in the cytoplasm under normoxic conditions but increased in the nucleus after hypoxia. Nuclear expression was greater after 12 h of hypoxia than after 6 h of hypoxia. Western blotting results showed that total protein expression of β-catenin (cytoplasmic and nuclear) increased after hypoxia, but quantitative analysis showed that total protein did not differ between the different hypoxia groups. Interestingly, the nuclear expression of β-catenin increased significantly with increased duration of hypoxia (p < 0.05 at 6 and 12 h; p < 0.01 at 24 h; Fig. 2B, E). Immunohistochemical staining showed nuclear expression of cyclin D1 in NSCs (Fig. 2H), and quantitative analysis showed that cyclin D1 expression increased in NSCs after hypoxia exposure (Fig. 2G). The changes in protein level of β-catenin in nucleus and of cyclin D1 were consistent with their mRNA changes.
Figure 1.
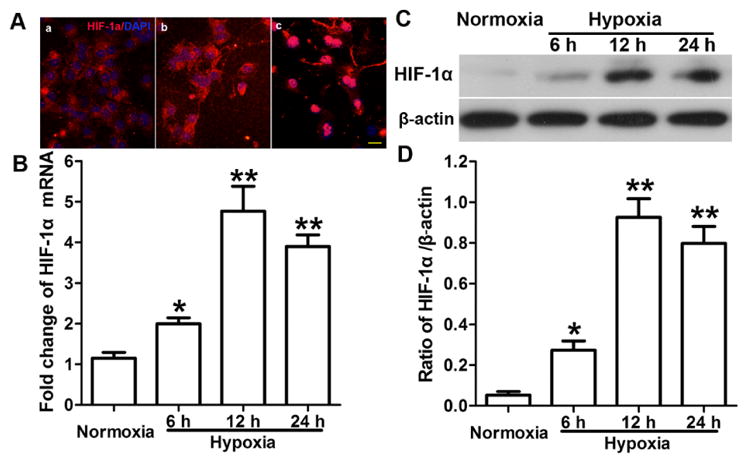
Hypoxia increases HIF-1α expression. (A) Immunofluorescence staining showed expression of HIF-1α (red) and DAPI labeled nucleus (blue) in conditions of normoxia (a), 6 h hypoxia (b), and 12 h hypoxia (c). HIF-1α was expressed in the cytoplasm of normoxic cells, but nucleus expression increased with hypoxia exposure and was greater at 12 h than at 6 h of hypoxia. Scale bar = 20 μm. (B) Real-time q-RT PCR results show that the levels of HIF-1α mRNA increased after 6 to 24 h of hypoxia compared with levels in normoxic cells and peaked at 12 h. Data is represented as mean ± SD, *p < 0.05, **p < 0.01 vs. normoxic group, n = 3, one way ANOVA followed by Tukey post-hoc test was used. (C, D) Western blot results showed HIF-1α protein expression increased after 6 to 24 h of hypoxia compared with expression in the normoxic group and peaked at 12 h. Data is represented as mean ± SD, *p < 0.05, **p < 0.01 vs. normoxic group, n = 3, one way ANOVA followed by Tukey post-hoc test was used.
Figure 2.
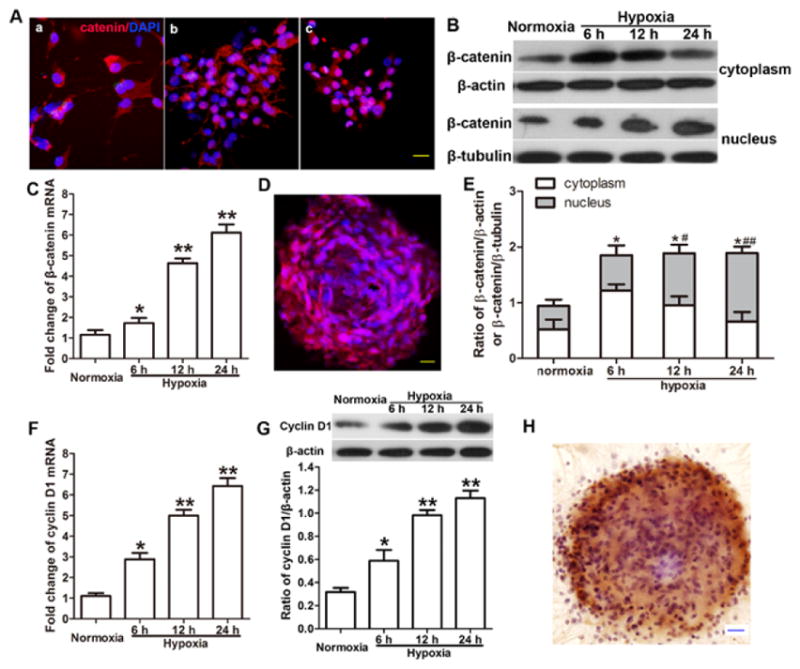
Hypoxia increases the expression of Wnt signaling components in neural stem cells (NSCs). (A) Representative image of immunofluorescence staining shows expression of β-catenin (red) and DAPI labeled nucleus (blue) in conditions of normoxia (a), 6 h hypoxia (b), and 12 h hypoxia (c). β-catenin was localized in cytoplasm of normoxic NSCs, but nucleus expression increased with hypoxia exposure and was significantly increased at 12 h than at 6 h of hypoxia. Scale bar = 20 μm. (B) Representative image of Western blotting shows β-catenin expression in cytoplasm and nucleus. (C) Real time q-RT PCR results showed mRNA level of β-catenin was elevated after 6 to 24 h of hypoxia compared with that in normoxia-treated cells. Data is represented as mean ± SD, *p < 0.05, **p < 0.01 vs. normoxic group, n = 3, one way ANOVA followed by Tukey post-hoc test was used. (D) Representative image shows immunofluorescence staining of neurospheres that express β-catenin (red) and DAPI-labeled nucleus (blue). Scale bar = 20 μm. (E) Quantitative results of Western blot analysis showed that hypoxia increased the total protein level of β-catenin (cytoplasm and nucleus) compared with that in the normoxic group. *p < 0.05 vs. normoxic group. Hypoxia also increased the nuclear protein level of β-catenin compared with that in the normoxic group. # p < 0.05, ## p < 0.01 vs. normoxic group. Three independent repeated experiment, Data is represented as mean ± SD, one way ANOVA followed by Tukey post-hoc test was used. (F) Real time q-RT PCR results showed hypoxia increased the mRNA level of cyclin D1 compared with that in the normoxic group. Data is represented as mean ± SD, *p < 0.05, **p < 0.01 vs. normoxic group, n = 3, one way ANOVA followed by Tukey post-hoc test was used. (G) Quantitative results of Western blot analysis showed that hypoxia increased the protein level of cyclin D1 compared with that in the normoxic group. Data is represented as mean ± SD, *p < 0.05, **p < 0.01 vs. normoxic group, n = 3, one way ANOVA followed by Tukey post-hoc test was used. (H) Representative image of a neurosphere shows immunohistochemical staining of cyclin D1 (brown) and Nissl-labeled nucleus (blue). Scale bar = 20 μm.
Wnt signaling pathway regulates hypoxia-induced NSC proliferation
To explore whether the Wnt signaling pathway is involved in hypoxia-induced NSC proliferation, we activated or blocked the pathway with LiCl and DKK-1, respectively. Western blot analysis showed that hypoxia increased β-catenin expression (p < 0.05; Fig. 3A, B). Pretreatment with LiCl further increased β-catenin expression under both normoxic and hypoxic conditions (p < 0.05; Fig. 3A). However, pretreatment with DKK-1 decreased β-catenin expression under hypoxia (p < 0.05; Fig. 3B). Furthermore, pretreatment with LiCl enhanced neurosphere diameter under conditions of both normoxia and hypoxia followed by reoxygenation for 1 day (p < 0.05; Fig. 3C). Interestingly, pretreatment with LiCl enhanced neurosphere diameter not only under hypoxic conditions but also under normoxic conditions. In contrast, pretreatment with DKK-1 significantly stunted the increase in neurosphere diameter after hypoxia and reoxygenation for 1, 2, and 3 days (p < 0.05; Fig. 3D). Pretreatment with DKK-1 under normoxic conditions reduced the growth rate of neurospheres compared with that in the control group. Interestingly, pretreatment with DKK-1 under hypoxic conditions significantly reduced the growth rate of neurospheres compared with that in the control group (p < 0.05; Fig. 3D).
Figure 3.
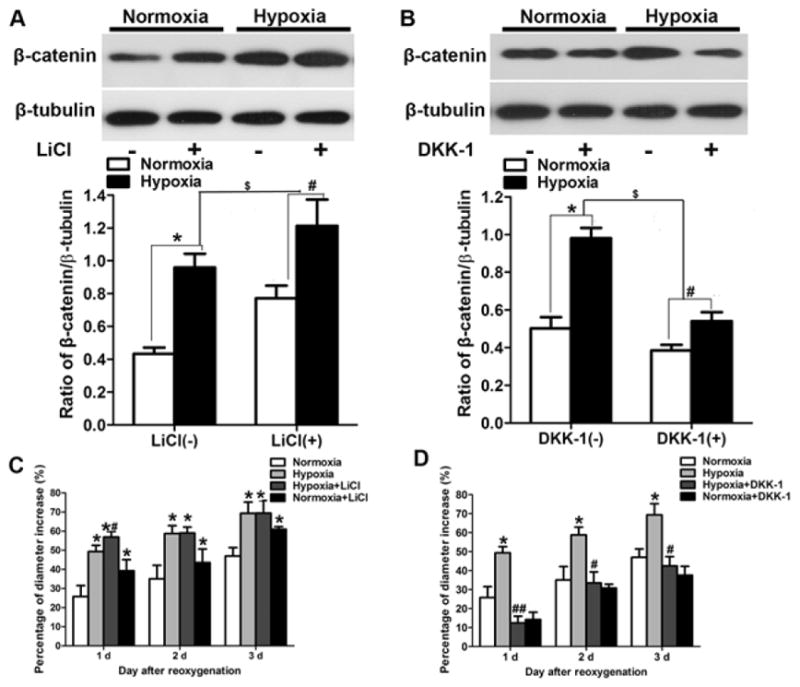
The Wnt signaling pathway regulates hypoxia-induced neural stem cell (NSC) proliferation. (A, B) Western blot analysis of nucleus β-catenin. (A) Hypoxia increased protein expression in the presence (+) and absence (−) of LiCl. *p < 0.05, #p < 0.05 vs. the respective normoxia group. Pretreatment with LiCl (6 mM) increased β-catenin expression under normoxic and hypoxic conditions, $p < 0.05 LiCl (+) vs. LiCl (−). (B) Hypoxia also increased β-catenin protein expression in the presence and absence of DKK-1. *p < 0.05, # p < 0.05 vs. the respective normoxia group. However, β-catenin expression was lower in NSCs pretreated with DKK-1 (200 ng/ml) than in NSCs not exposed to DKK-1. $p < 0.05 DKK-1 (+) vs. DKK-1 (−). (C) Pretreatment with LiCl enhanced neurosphere diameter growth rate under conditions of normoxia and hypoxia followed by reoxygenation for 1, 2, and 3 days. *p < 0.05 vs. normoxic group; #p < 0.05 vs. normoxia LiCl (+). (D) Hypoxia increased neurosphere diameter; *p < 0.05 vs. normoxic group. Pretreatment with DKK-1 reduced neurosphere diameter growth rate after hypoxia followed by reoxygenation for 1, 2, and 3 days; #p < 0.05 hypoxia vs. hypoxia DKK-1 (+). All data are represented as mean ± SD, from three independent repeated experiment, one way ANOVA followed by Tukey post-hoc test was used.
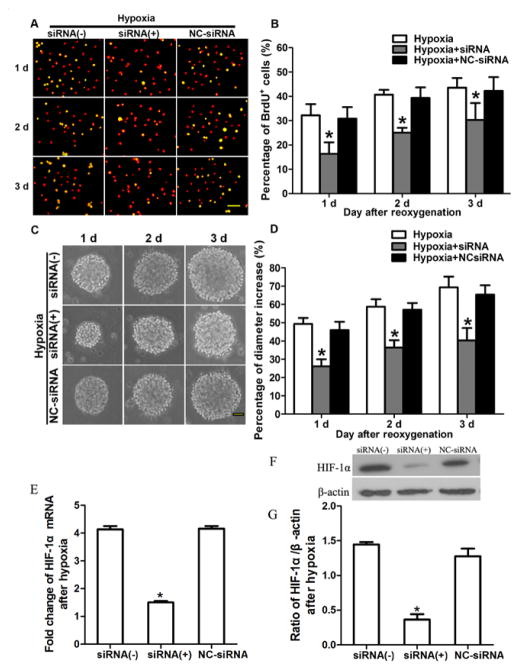
Inhibition of HIF-1α depresses Wnt/β-catenin signaling and hypoxia-induced NSC proliferation
To determine whether hypoxia-induced NSC proliferation is related to the activation of Wnt signaling, which is initiated by HIF-1α, we treated passage 1 neurospheres with HIF-1α gene-silencing siRNA under hypoxic and normoxic conditions. HIF-1α siRNA significantly decreased the percentages of BrdU-positive cells (Fig. 4A, B) and reduced neurosphere diameter (Fig. 4C, D) after hypoxia followed by 1, 2, and 3 days of reoxygenation (p < 0.05; Fig. 4B, D). There was no significant difference in the percentage of BrdU-positive cells (Fig. 4B) or neurosphere diameter growth rate (Fig. 4D) between the group treated with NC-siRNA and the group treated with hypoxia alone (p > 0.05). Furthermore, HIF-1α siRNA significantly decreased the mRNA levels and protein expression of HIF-1α (p < 0.05; Fig. 4E, F, G). Western blot analysis showed that the total protein expression of β-catenin (cytoplasmic and nuclear) increased after hypoxia, but quantitative analysis showed no difference in total protein among the hypoxic groups treated without HIF-1α siRNA, with HIF-1α siRNA, and with NC-siRNA (Fig. 5A, B). However, HIF-1α siRNA treatment significantly reduced the protein expression of β-catenin in the nucleus (p < 0.05; Fig. 5A, B). HIF-1α siRNA also significantly reduced the cyclin D1 expression (p < 0.01; Fig. 5C, D). However, there was no significant difference in the mRNA and protein levels of β-catenin and cyclin D1 between the group treated with NC-siRNA and the control group (no siRNA) after hypoxia.
Figure 4.
Inhibition of HIF-1α depresses hypoxia-induced neural stem cell (NSC) proliferation and expression of HIF-1α. (A) Representative images of BrdU incorporation in NSCs pretreated with HIF-1α siRNA [siRNA(+)], with negative control siRNA (NC-siRNA), or without siRNA [siRNA(−)] after hypoxia and reoxygenation for 1, 2, and 3 days. BrdU is green, propidium iodide is red, and merged positive cells are yellow. Scale bar = 50 μm. (B) HIF-1α siRNA decreased the percentage of BrdU-positive NSCs after exposure to hypoxia and reoxygenation for 1, 2, and 3 days. Data is represented as mean ± SD, *p < 0.05 vs. negative control group (NC-siRNA), n = 3, one way ANOVA followed by Tukey post-hoc test was used. (C) HIF-1α siRNA slowed neurosphere enlargement when NSCs were exposed to hypoxia followed by reoxygenation for 1, 2, and 3 days. Scale bar = 50 μm. (D) HIF-1α siRNA reduced the growth rate of NSC neurospheres. Data is represented as mean ± SD, *p < 0.05 vs. negative control group (NC-siRNA), n = 3, one way ANOVA followed by Tukey post-hoc test was used. (E) Real-time q-RT PCR showed that HIF-1α siRNA reduced the mRNA levels of HIF-1α. *p < 0.05 vs. negative control group (NC-siRNA). (F and G) Western blot analysis showed that treatment with siRNA hypoxia inhibition the protein expressed of HIF-1α compared with control group both with siRNA (−), and NC-siRNA groups. *p < 0.05.
Figure 5.
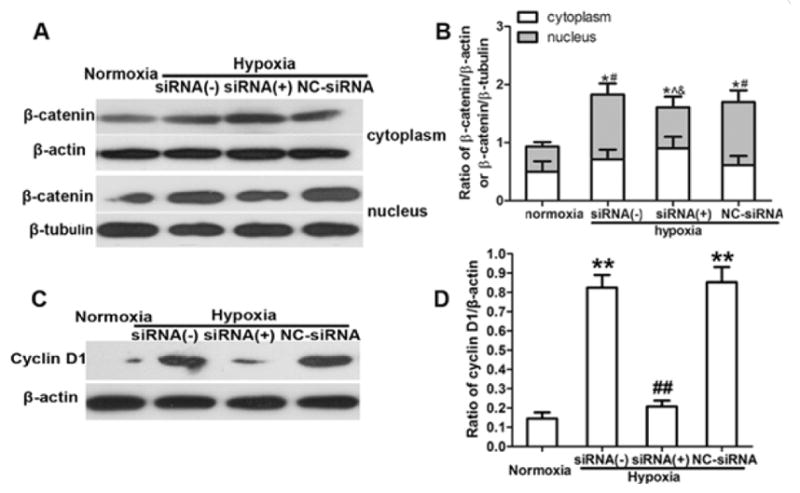
HIF-1α siRNA depresses the expression of Wnt signaling components in neural stem cells (NSCs). (A) Representative image of Western blot shows β-catenin expression in the cytoplasm and nucleus. (B) Quantification of Western blot analysis showed that hypoxia increased the total protein level of β-catenin (cytoplasm and nucleus) compared with that in the normoxic group. *p < 0.05 vs. normoxic group. However, the total protein expression in the siRNA (+), siRNA (−), and NC-siRNA groups did not differ. Hypoxia increased the nucleus β-catenin protein level compared with that in the normoxic group. # p < 0.05 vs. normoxic group. HIF-1α siRNA reduced the protein expression of nuclear β-catenin compared with levels in the control group [siRNA (−)] and negative control group (NC-siRNA). ^ p < 0.05 vs. siRNA (−); & p < 0.05 vs. NC-siRNA. (C) Representative image of Western blot shows cyclin D1 expression in NSCs pretreated with HIF-1α siRNA. (D) HIF-1α siRNA reduced the protein expression of cyclin D1. ## p < 0.01 vs. NC-siRNA group. However, NC-siRNA and siRNA (−) control groups maintained high expression of cyclin D1 after hypoxia. ** p < 0.01 vs. normoxic group. All data are represented as mean ± SD, from three independent repeated experiment, one way ANOVA followed by Tukey post-hoc test was used.
Discussion
In the present study, we investigated the regulation of NSC proliferation by analyzing the expression of HIF-1α and activation of Wnt signaling in cell cultures exposed to pathologic hypoxia. The results showed that hypoxia induced increases in NSC proliferation and elevations in the mRNA and protein levels of HIF-1α, β-catenin, and cyclin D1. Blockade of the Wnt signaling pathway decreased hypoxia-induced NSC proliferation, whereas activation of the Wnt signaling pathway increased hypoxia-induced NSC proliferation. Using siRNA to inhibit HIF-1α signaling in hypoxia-exposed NSCs, we observed that repression of HIF-1α decreased β-catenin nuclear translocation, cyclin D1 expression, and NSC proliferation. Taken together, the results suggest that pathologic hypoxia stimulates NSC proliferation through HIF-1α modulation of the Wnt/β-catenin signaling pathway.
It is known that the proliferation of many types of cultured stem cells, including NSCs (40) is enhanced after prolonged exposure to low, but physiologic O2 concentrations of about 1.5–3% (41). After focal cerebral ischemia, tissue oxygen concentration approaches 0% in the ischemic core (42, 43). On this basis, one of our main questions was to clarify whether this pathologic anoxia or hypoxia favors NSC proliferation. By microscopic observation and neurosphere diameter measurement, we found that mimicking pathologic hypoxia enhanced self-renewal and proliferation of cultured NSCs in vitro. HIF-1 is a key transcription factor that responds to hypoxia. The increase in HIF-1 activity is primarily due to the hypoxia-induced stabilization and activation of HIF-1α (44). In our study, the expression of HIF-1α mRNA in NSCs increased from 6 h to 12 h during exposure to 24-h hypoxia. It has been shown that overexpression of HIF-1α induces proliferation of NSCs under normal oxygen conditions and under hypoxia. Zhang et al. (45) found that when NSCs were cultured in a 3% O2 environment for 3 days, the levels of HIF-1á mRNA expression did not change as compared with those in cells grown under normal conditions. However, HIF-1α protein expression was greater after 3 to 72 h of hypoxia than it was under normoxic conditions. We found that when siRNA was used to downregulate HIF-1α genes, proliferation of NSCs was inhibited under both hypoxic and normoxic conditions, indicating that HIF-1α is involved in hypoxia-induced NSC proliferation.
The Wnt signaling pathway controls many events during embryonic development and regulates proliferation, morphology, motility, and cell fate at the cellular level. Activation of the Wnt/β-catenin pathway is characterized by 1) stabilization of cytoplasmic β-catenin after receptor engagement by Wnt ligands; 2) β-catenin nuclear translocation, 3) β-catenin interaction with lymphoid enhancer-binding factor-1/T-cell factor-1 (LEF/TCF) transcription factors; and 4) stimulation of target genes (46–48). A study also has shown Wnt family gene mRNA expression in NSCs that were isolated from rat SVZ (49). In non-neuronal cell types, Wnt/β-catenin signaling is downregulated after hypoxia, leading to enhanced growth and impaired differentiation (50, 51). In immunofluorescence staining assays, we observed β-catenin localization and expression in cytoplasm and nucleus, and enhanced nuclear expression after hypoxia. We also demonstrated increased expression of β-catenin protein and cyclin D1. As one of the target genes of the canonical Wnt/β-catenin pathway(52), cyclin D1 functions to regulate the cell cycle and is associated with hypoxia-induced NSC proliferation. Furthermore, treatment with DKK-1, a Wnt signaling pathway blocker, decreased β-catenin nuclear translocation and inhibited hypoxia-induced NSC proliferation. Conversely, activation of Wnt signaling with LiCl increased hypoxia-induced NSC proliferation, though it did not alter β-catenin nuclear translocation. Although we observed the NSCs from 30-day-old rats and mimicked pathologic hypoxia with 0.3% O2, our results are consistent with the findings that Wnt/β-catenin activity is closely associated with low O2 regions in the subgranular zone of the hippocampus and that hypoxia (1.5% O2) activates cell proliferation and Wnt/β-catenin signaling in mouse embryonic cells. These findings suggest that the Wnt signaling pathway activated by hypoxia is important for regulating NSC proliferation during brain development and during hypoxia that mimics ischemic stroke.
Although previous studies have indicated that HIF-1α potentiates Wnt signaling during brain development (19), this pathway has not been well studied under pathologic conditions such as cerebral ischemic stroke. Using siRNA to inhibit HIF-1α signaling in NSCs exposed to pathologic hypoxia, we found that suppression of HIF-1α signaling reduced β-catenin nuclear translocation, reduced cyclin D1 expression, and retarded NSC proliferation. It has been reported that β-catenin induces hippocampal NSC proliferation under hypoxic conditions partly via cyclin D1 (35). Recently, studies (19, 53) have reported that in embryonic stem cells and isolated embryonic NSCs, fibroblast growth factor 2 and Wnt signals promote NSC self-renewal via β-catenin accumulation, which leads to the promotion of proliferation by LEF/TCF-mediated cyclin D1 expression. Our data support the idea that HIF-1α activates the Wnt/β-catenin signaling pathway in adult NSCs maintained under conditions of pathologic hypoxia (0.3% oxygen), and that this pathway leads to increased expression of cyclin D1 and proliferation of NSCs.
This study provides evidence that pathologic hypoxia stimulates NSC proliferation through increases in expression of HIF-1α and activation of the Wnt/β-catenin signaling pathway. Hypoxia activates HIF-1 signaling, which induces the cytoplasmic accumulation of β-catenin and its translocation to the nucleus, where it regulates the expression of Wnt target genes like cyclin D1, ultimately resulting in proliferation of NSCs. These results reveal a potential mechanism of neural repair that should be further explored in conditions of pathologic hypoxia, like cerebral ischemia in vivo.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30960107, 81070998), the Natural Science Foundation of Qinghai province of China (No.2013-Z-727), and NIH R01NS078026 and R01AT007317. The authors thanks China Scholarship Council supported to visiting Johns Hopkins University (201308635039). The authors thank Jiarui Wang and Claire Levine for assistance with manuscript preparation.
References
- 1.Ohira K. Injury-induced neurogenesis in the mammalian forebrain. CELL MOL LIFE SCI. 2011;68(10):1645–1656. doi: 10.1007/s00018-010-0552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota NL, Binda E, et al. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLOS ONE. 2010;5(1):e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103(35):13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang P, Liu Y, Li J, Kang Q, Tian Y, Chen X, et al. Cell proliferation in ependymal/subventricular zone and nNOS expression following focal cerebral ischemia in adult rats. NEUROL RES. 2006;28(1):91–96. doi: 10.1179/016164106X91942. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Liu Y, Li J, Kang Q, Tian Y, Chen X, et al. Decreased neuronal nitric oxide synthase expression and cell migration in the peri-infarction after focal cerebral ischemia in rats. NEUROPATHOLOGY. 2007;27(4):347–354. doi: 10.1111/j.1440-1789.2007.00791.x. [DOI] [PubMed] [Google Scholar]
- 6.Hou SW, Wang YQ, Xu M, Shen DH, Wang JJ, Huang F, et al. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. STROKE. 2008;39(10):2837–2844. doi: 10.1161/STROKEAHA.107.510982. [DOI] [PubMed] [Google Scholar]
- 7.Miles DK, Kernie SG. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. HIPPOCAMPUS. 2008;18(8):793–806. doi: 10.1002/hipo.20439. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama D, Matsuyama T, Ishibashi-Ueda H, Nakagomi T, Kasahara Y, Hirose H, et al. Injury-induced neural stem/progenitor cells in post-stroke human cerebral cortex. EUR J NEUROSCI. 2010;31(1):90–98. doi: 10.1111/j.1460-9568.2009.07043.x. [DOI] [PubMed] [Google Scholar]
- 9.Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, et al. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. NAT NEUROSCI. 2010;13(2):173–179. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- 10.Shen CC, Yang YC, Chiao MT, Cheng WY, Tsuei YS, Ko JL. Characterization of endogenous neural progenitor cells after experimental ischemic stroke. CURR NEUROVASC RES. 2010;7(1):6–14. doi: 10.2174/156720210790820208. [DOI] [PubMed] [Google Scholar]
- 11.Wang XL, Zhao YS, Hu MY, Sun YQ, Chen YX, Bi XH. Umbilical cord blood cells regulate endogenous neural stem cell proliferation via hedgehog signaling in hypoxic ischemic neonatal rats. BRAIN RES. 2013;1518:26–35. doi: 10.1016/j.brainres.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. NAT REV NEUROSCI. 2005;6(5):351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 13.Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. DEVELOPMENT. 2000;127(3):457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 14.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, et al. Wnt signalling regulates adult hippocampal neurogenesis. NATURE. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 15.Yang XT, Bi YY, Chen ET, Feng DF. Overexpression of Wnt3a facilitates the proliferation and neural differentiation of neural stem cells in vitro and after transplantation into an injured rat retina. J NEUROSCI RES. 2014;92(2):148–161. doi: 10.1002/jnr.23314. [DOI] [PubMed] [Google Scholar]
- 16.Bonnert TP, Bilsland JG, Guest PC, Heavens R, McLaren D, Dale C, et al. Molecular characterization of adult mouse subventricular zone progenitor cells during the onset of differentiation. EUR J NEUROSCI. 2006;24(3):661–675. doi: 10.1111/j.1460-9568.2006.04912.x. [DOI] [PubMed] [Google Scholar]
- 17.Mardones MD, Andaur GA, Varas-Godoy M, Henriquez JF, Salech F, Behrens MI, et al. Frizzled-1 receptor regulates adult hippocampal neurogenesis. MOL BRAIN. 2016;9:29. doi: 10.1186/s13041-016-0209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J CELL PHYSIOL. 2009;220(3):562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 19.Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, et al. O2 regulates stem cells through Wnt/beta-catenin signalling. NAT CELL BIOL. 2010;12(10):1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SH, Kim MH, Han HJ. Arachidonic acid potentiates hypoxia-induced VEGF expression in mouse embryonic stem cells: involvement of Notch, Wnt, and HIF-1. AJP: Cell Physiology. 2009;297(1):C207–C216. doi: 10.1152/ajpcell.00579.2008. [DOI] [PubMed] [Google Scholar]
- 21.Liu BN, Han BX, Liu F. Neuroprotective effect of pAkt and HIF-1 alpha on ischemia rats. ASIAN PAC J TROP MED. 2014;7(3):221–225. doi: 10.1016/S1995-7645(14)60025-0. [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Sharma G, Mishra V. Hypoxia inducible factor-1: its potential role in cerebral ischemia. CELL MOL NEUROBIOL. 2012;32(4):491–507. doi: 10.1007/s10571-012-9803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Qian Z, Pan L, Li H, Zhu H. Hypoxia-inducible factor 1 mediates the anti-apoptosis of berberine in neurons during hypoxia/ischemia. ACTA PHYSIOL HUNG. 2012;99(3):311–323. doi: 10.1556/APhysiol.99.2012.3.8. [DOI] [PubMed] [Google Scholar]
- 24.Xiao X, Liu Y, Qi C, Qiu F, Chen X, Zhang J, et al. Neuroprotection and enhanced neurogenesis by tetramethylpyrazine in adult rat brain after focal ischemia. NEUROL RES. 2010;32(5):547–555. doi: 10.1179/174313209X414533. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Tian Y, Yao L, Zhang J, Liu Y. Hypoxia stimulates proliferation of rat neural stem cells with influence on the expression of cyclin D1 and c-Jun N-terminal protein kinase signaling pathway in vitro. NEUROSCIENCE. 2010;165(3):705–714. doi: 10.1016/j.neuroscience.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Jiao Q, Chen X, Yang P, Zhao B, Zheng P, et al. mGluR5 is involved in proliferation of rat neural progenitor cells exposed to hypoxia with activation of mitogen-activated protein kinase signaling pathway. J NEUROSCI RES. 2012;90(2):447–460. doi: 10.1002/jnr.22751. [DOI] [PubMed] [Google Scholar]
- 27.Tian Y, Liu Y, Chen X, Kang Q, Zhang J, Shi Q, et al. AMN082 promotes the proliferation and differentiation of neural progenitor cells with influence on phosphorylation of MAPK signaling pathways. NEUROCHEM INT. 2010;57(1):8–15. doi: 10.1016/j.neuint.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Burgers HF, Schelshorn DW, Wagner W, Kuschinsky W, Maurer MH. Acute anoxia stimulates proliferation in adult neural stem cells from the rat brain. EXP BRAIN RES. 2008;188(1):33–43. doi: 10.1007/s00221-008-1336-6. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki M, Nelson AD, Eickstaedt JB, Wallace K, Wright LS, Svendsen CN. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. EUR J NEUROSCI. 2006;24(3):645–653. doi: 10.1111/j.1460-9568.2006.04957.x. [DOI] [PubMed] [Google Scholar]
- 30.Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85(2):141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 31.Fedi P, Bafico A, Nieto SA, Burgess WH, Miki T, Bottaro DP, et al. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J BIOL CHEM. 1999;274(27):19465–19472. doi: 10.1074/jbc.274.27.19465. [DOI] [PubMed] [Google Scholar]
- 32.Silva AK, Yi H, Hayes SH, Seigel GM, Hackam AS. Lithium chloride regulates the proliferation of stem-like cells in retinoblastoma cell lines: a potential role for the canonical Wnt signaling pathway. MOL VIS. 2010;16:36–45. [PMC free article] [PubMed] [Google Scholar]
- 33.Cui XP, Xing Y, Chen JM, Dong SW, Ying DJ, Yew DT. Wnt/beta-catenin is involved in the proliferation of hippocampal neural stem cells induced by hypoxia. Ir J Med Sci. 2011;180(2):387–393. doi: 10.1007/s11845-010-0566-3. [DOI] [PubMed] [Google Scholar]
- 34.Sundberg M, Savola S, Hienola A, Korhonen L, Lindholm D. Glucocorticoid hormones decrease proliferation of embryonic neural stem cells through ubiquitin-mediated degradation of cyclin D1. J NEUROSCI. 2006;26(20):5402–5410. doi: 10.1523/JNEUROSCI.4906-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowalczyk A, Filipkowski RK, Rylski M, Wilczynski GM, Konopacki FA, Jaworski J, et al. The critical role of cyclin D2 in adult neurogenesis. J CELL BIOL. 2004;167(2):209–213. doi: 10.1083/jcb.200404181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zan L, Zhang X, Xi Y, Wu H, Song Y, Teng G, et al. Src regulates angiogenic factors and vascular permeability after focal cerebral ischemia-reperfusion. NEUROSCIENCE. 2014;262:118–128. doi: 10.1016/j.neuroscience.2013.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao XC, Zhang LM, Tong DY, An P, Jiang C, Zhao P, et al. Propofol increases expression of basic fibroblast growth factor after transient cerebral ischemia in rats. NEUROCHEM RES. 2013;38(3):530–537. doi: 10.1007/s11064-012-0945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng T, Wang W, Li Q, Han X, Xing J, Qi C, et al. Cerebroprotection of flavanol (−)-epicatechin after traumatic brain injury via Nrf2-dependent and -independent pathways. Free Radic Biol Med. 2016;92:15–28. doi: 10.1016/j.freeradbiomed.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang CF, Cho S, Wang J. (−)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann Clin Transl Neurol. 2014;1(4):258–271. doi: 10.1002/acn3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J NEUROSCI. 2000;20(19):7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csete M. Oxygen in the cultivation of stem cells. Ann N Y Acad Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 42.Goda F, O’Hara JA, Liu KJ, Rhodes ES, Dunn JF, Swartz HM. Comparisons of measurements of pO2 in tissue in vivo by EPR oximetry and microelectrodes. ADV EXP MED BIOL. 1997;411:543–549. doi: 10.1007/978-1-4615-5865-1_67. [DOI] [PubMed] [Google Scholar]
- 43.Silver I, Erecinska M. Oxygen and ion concentrations in normoxic and hypoxic brain cells. ADV EXP MED BIOL. 1998;454:7–16. doi: 10.1007/978-1-4615-4863-8_2. [DOI] [PubMed] [Google Scholar]
- 44.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J BIOL CHEM. 1995;270(3):1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 45.Zhang CP, Zhu LL, Zhao T, Zhao H, Huang X, Ma X, et al. Characteristics of neural stem cells expanded in lowered oxygen and the potential role of hypoxia-inducible factor-1Alpha. NEUROSIGNALS. 2006;15(5):259–265. doi: 10.1159/000103385. [DOI] [PubMed] [Google Scholar]
- 46.Reya T, Clevers H. Wnt signalling in stem cells and cancer. NATURE. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 47.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. CELL. 1997;88(6):789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 48.Willert K, Nusse R. Beta-catenin: a key mediator of Wnt signaling. CURR OPIN GENET DEV. 1998;8(1):95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- 49.Morris DC, Zhang ZG, Wang Y, Zhang RL, Gregg S, Liu XS, et al. Wnt expression in the adult rat subventricular zone after stroke. NEUROSCI LETT. 2007;418(2):170–174. doi: 10.1016/j.neulet.2007.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. NAT IMMUNOL. 2005;6(3):314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 51.Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. NAT CELL BIOL. 2007;9(2):210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 52.Schmitt-Graeff A, Ertelt-Heitzmann V, Allgaier HP, Olschewski M, Nitschke R, Haxelmans S, et al. Coordinated expression of cyclin D1 and LEF-1/TCF transcription factor is restricted to a subset of hepatocellular carcinoma. LIVER INT. 2005;25(4):839–847. doi: 10.1111/j.1478-3231.2005.01069.x. [DOI] [PubMed] [Google Scholar]
- 53.Bizen N, Inoue T, Shimizu T, Tabu K, Kagawa T, Taga T. A growth-promoting signaling component cyclin D1 in neural stem cells has antiastrogliogenic function to execute self-renewal. STEM CELLS. 2014;32(6):1602–1615. doi: 10.1002/stem.1613. [DOI] [PubMed] [Google Scholar]