Abstract
Traumatic brain injury (TBI) is thought to be a risk factor for dementia, including dementia due to Alzheimer’s disease (AD). However, the influence of TBI history on the neuropsychological course of AD is unknown and, more broadly, the effect of TBI history on age-related cognitive change is poorly understood. We examined the relationship between history of TBI with loss of consciousness (LOC) history and cognitive change in participants with normal cognition and probable AD, stratified by APOE ε4 allele status. The sample included 706 participants (432 with normal cognition; 274 probable AD) from the National Alzheimer’s Coordinating Center (NACC) dataset that completed the Uniform Data Set evaluation between 2005 and 2014. Normal and probable AD participants with a history of TBI were matched to an equal number of demographically and clinically similar participants without a TBI history. In this dataset, TBI with LOC was defined as brain trauma with brief or extended unconsciousness. For the normal and probable AD cohorts, there was an average of 3.2 ± 1.9 and 1.8 ± 1.1 years of follow-up, respectively. 30.8% of the normal cohort were APOE ε4 carriers, whereas 70.8% of probable AD participants were carriers. Mixed effects regressions showed TBI with LOC history did not affect rates of cognitive change in APOE ε4 carriers and non-carriers. Findings from this study suggest that TBI with LOC may not alter the course of cognitive function in older adults with and without probable AD. Future studies that better characterize TBI (e.g., severity, number of TBIs, history of subconconcussive exposure) are needed to clarify the association between TBI and long-term neurocognitive outcomes.
Keywords: Alzheimer’s disease, APOE, cognitive decline, dementia, loss of consciousness, normal cognition, risk factor, traumatic brain injury
INTRODUCTION
More than 10 million individuals worldwide are affected annually by traumatic brain injury (TBI), and the annual incidence of TBI in the United States is over 1.7 million [1, 2]. The true prevalence of TBI is likely even greater given that a majority of TBIs are mild in severity and may not be recognized or reported [3, 4]. TBI is indeed a major public health and socioeconomic concern. In 2010, TBI resulted in $11.5 billion in direct medical costs and $64.8 billion in indirect costs to the US health system [5, 6]. In 2009, TBI accounted for at least 2.4 million emergency department visits, hospitalizations, or deaths in the US alone [5].
TBI ranges in severity from mild to moderate to severe, categories that are differentiated by the immediate clinical symptoms following the blow to the head (e.g., loss of consciousness [LOC] duration). Post-TBI clinical sequelae are heterogeneous and include a range of acute and chronic neurological, psychiatric, and physical symptoms. Moderate to severe TBIs are generally associated with unfavorable outcomes, including death, vegetative state, severe disability [7], and long-term clinical impairment, with the greatest recovery occurring during the first 6- to 18-months post-injury [8]. In contrast, clinical sequelae following a single mild TBI are often short-lived, lasting from several days to a few weeks [9], but in some instances symptoms can be prolonged to over several months and, in rare cases, longer than one year [10, 11].
The chronic and progressive effects of TBI have received increasing investigation, with several studies examining TBI as a possible risk factor for dementia from neurodegenerative diseases, such as Alzheimer’s disease (AD). TBI (mild to severe) has been reported to elevate risk for dementia, but the literature on its association with AD, in particular, is not entirely consistent and may be dependent on TBI severity [12, 13]. Moderate to severe TBIs are generally believed to confer increased risk for dementia, though the specific risk for dementia due to AD remains unclear because a majority of studies rely on clinical diagnoses, without biomarker or neuropathological confirmation of etiology. The evidence for mild TBI as a risk factor for dementia (due to AD or not) is less conclusive [14–17]. It is plausible that the association between TBI and AD risk may be moderated by factors such as the Apolipoprotein E (APOE) ε4 allele [18–20] that were not considered in negative studies. Moreover, there is an overlap of symptoms between i) combat personnel with a history of TBI and co-occurring post-traumatic stress disorder and ii) contact-sport athletes with chronic traumatic encephalopathy (CTE) diagnosed at autopsy, which raises the possibility of clinical misdiagnosis [21]. In fact, because CTE is often clinically similar to AD and believed to be partially caused by recurrent head trauma, it is possible that CTE is misdiagnosed as AD in the setting of head trauma [22–24]. A further complication of any study investigating the effects of TBI on AD is the possibility of reverse causation, where undiagnosed AD results in TBI [25].
Even though the effect of severe TBI is associated with increased risk of dementia, its association with accelerated age-related cognitive decline has received less attention. The extant longitudinal studies show TBI, across the severity spectrum, is associated with persistent impairments on speeded neuropsychological tasks [8, 26, 27]. One study [28] found mild cognitive decline over a 30-year period among people with varying TBI severity, with age at injury and gender influencing the effect, suggesting that increases in the plasticity capacity of the brain and the presence of estrogen and progesterone may be neuroprotective. The relationship between TBI and long-term cognitive trajectories remains poorly understood due to study methodological limitations of the literature, including small sample sizes, short follow-up periods, biased samples, high attrition rates, limited or no reports of exposure to repetitive head impacts, and very brief cognitive test batteries. Moreover, to our knowledge, no study has examined whether TBI influences the cognitive trajectory of AD.
The purpose of the current study was to examine the effect of previous TBI on the rate of cognitive change in healthy older adult controls and participants with probable AD from the National Alzheimer’s Coordinating Center (NACC) database [29–31]. We hypothesized that the presence of previous TBI with LOC will have a negative effect on the rate of cognitive change among older adults with normal cognition or diagnosed with AD dementia. We assessed the effect of TBI on specific cognitive domain scores derived by dynamic factor analysis. We minimized the possibility of reverse causality by carefully designing a case-controlled study comparing participants with a history of TBI versus demographically and clinically matched participants without a TBI history. All participants were stratified by APOE ε4 allele presence/absence status in order to examine whether this genetic factor modified the association between TBI and cognitive change. Although NACC has a large sample size, follows participants annually over several years, and administers a standardized cognitive test battery, TBI in the NACC dataset is only defined as brain trauma with brief LOC (<5 min) or with extended LOC (≥5 min). There is no variable in the NACC UDS 2.0 related to TBI without LOC. Our exclusion criteria did not preclude participants with TBI without LOC to be included in the study as controls. Additional data about the severity of the TBI (e.g., Glasgow Coma Scale), the type and duration of residual symptoms, the age of the subject at time of TBI, or additional exposure to previous TBIs or to repetitive head impacts through contact sports or other activities, are not ascertained. Consequently, the present study concentrated in investigating retrospective TBI with a history of LOC.
MATERIALS AND METHODS
Study sample
We designed a case-control study using data from the NACC dataset using Uniform Data Set version 1.0–2.0 (UDS) visits between September 2005 and September 2014. For the design of the case-control study, we followed the STROBE guidelines for observational studies [32]. The current sample included 706 participants (432 with normal cognition; 274 diagnosed with probable AD). The NACC was established by the National Institute on Aging in 1999 to promote collaborative AD research. It is a publicly accessible database of standardized clinical data gathered from 34 past and present Alzheimer’s Disease Centers (ADCs) across the U.S. The regional ADCs are composed of universities and other institutions, and participants come from clinic samples, public recruitment efforts, other ongoing studies, and occasionally population-based samples. Beginning in 2005, the regional ADCs have contributed participant cognitive, behavioral, and functional data to form the NACC-UDS database. The NACC-UDS database has been previously described [29–31, 33].
The current study sample was restricted to participants 50 years or older, with English as their primary language, available APOE genotype data, no history of alcohol or substance abuse, and at least two UDS visits. To minimize the possibility of reverse causality where cognitive decline causes a TBI, we only included participants with no reported TBI after their first visit. All participants were evaluated according to standardized protocols that included a trained clinician interviewing the participant and an informant. Data queried included the UDS test battery, demographic variables, Clinical Dementia Rating – Sum of Boxes (CDR-SB) score, APOE genotype, and diagnosis. At the time of data collection for this study, AD was diagnosed via the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria [34]. MCI diagnosis was based on criteria defined by Petersen [35].
Standard protocol approvals registrations and patients’ consent
The Institutional Review Boards at the individual ADCs approved this study in accord with the Helsinki Declaration of 1975. Informed consent was obtained from all participants and informants at the individual ADCs.
Traumatic brain injury history
There are two variables in NACC UDS 1.0–2.0 that are related to TBI: a) brain trauma with brief LOC (<5 min) and b) brain trauma with extended LOC (≥5 min). These variables were combined into a single dichotomous TBI exposure variable (history of TBI with LOC versus no history of TBI with LOC). We excluded participants with unknown history or recent/active history of TBI to minimize effects of reverse causality. We identified two matched cohorts: a) a “normal” cohort included 432 participants with normal cognition at all visits; and b) an “AD cohort” included 274 participants diagnosed with probable AD diagnosis at all visits. In the NACC dataset, 216 participants with normal cognition and a history of TBI with LOC were randomly matched to an equal number of TBI-absent NACC participants with normal cognition who satisfied all the inclusion criteria by age, sex, and education. When participants in the “normal cohort” converted to MCI or dementia, their observations after conversion were not considered in analyzing their cognitive trajectory, in order to estimate the effect of TBI with LOC during a period with no objective evidence of cognitive decline (i.e., preclinical). Similarly for the “AD cohort”, 137 participants who had a history of TBI with LOC were randomly matched to an equal number of TBI-absent NACC participants with probable AD by age, sex, education, number of UDS visits, and dementia severity at baseline (measured by CDR-SB). All participants had no other neurological conditions.
Measures
The main outcomes were demographically-corrected normative standardized scores of neuropsychological tests that form the UDS battery (see Weintraub et al. [33] for a full description of the battery). All neuropsychological tests are widely used in clinical and research settings and have established psychometric data. The neuropsychological tests used and their respective theoretical cognitive domains that they operationalize included: 1) episodic memory (Wechsler Memory Scale [WMS] Logical Memory subtest: Immediate and Delayed recall); 2) attention and psychomotor speed (WMS Digit Span Forward and Backward: total correct and longest span; Trail Making Test part A [time]; Wechsler Adult Intelligence Scale-Revised [WAIS-R] Digit Symbol subtest); 3) executive function (Trail Making Test part B [time]); and 4) language (Animal and Vegetable fluency; Boston Naming Test). Lastly, the Mini-Mental State Examination (MMSE) as a measure of general cognitive function (score out of 30), and the CDR-SB as a measure of dementia severity were examined separately in the AD cohort.
Statistical analysis
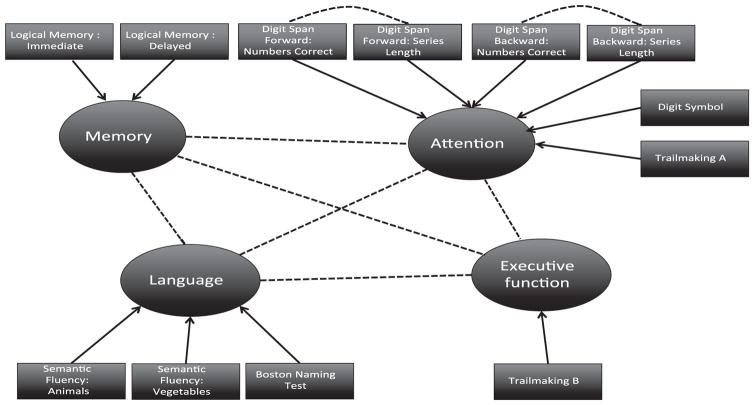
Aggregate cognitive domain scores were first estimated using dynamic factor analysis given a fixed structure shown in Fig. 1 that follows the structure described in Morris et al. [31]. Dynamic Factor analysis is a statistical technique that attempts to explain co-movement among trajectories of observed test scores by introducing latent common trends that are presumed to drive the change of the test scores. Conditional on the unobserved variables and their past history, the observed scores are assumed to be uncorrelated. Similar to simple factor analysis, factor loadings show the relationship between test scores and their common trends. Similar to simple factor analysis, we assume that the estimated factors follow a standard normal distribution. Consequently, all factors are standardized and effects are directly comparable across domains. To estimate the parameters in the dynamic factor model, we used maximum likelihood estimated with a 2-step modified Expectation/Conditional Maximization Either (ECME) algorithm [36] implemented in Ox Programming Language [37].
Fig. 1.
Structure of the dynamic factor analysis model. Arrows show how observed neuropsychological scores load into specific domains and dotted lines show imposed correlation between scores or domains.
For analyses of baseline characteristics, the Chi-Square test was used to compare proportions for binary and categorical variables. Continuous variables are presented in the results in Table 1 as mean ± SD and compared using T-tests. The domain scores calculated from dynamic factor analysis were used as outcomes in mixed effects regression models. These models included the following as covariates: age at first visit, sex, education, the presence of any APOE ε4 allele, the presence of diagnostic history of depression in the last 2 years and conversion status at the last NACC visit (for the normal cohort) as main effects. Moreover, a two-way interaction with time in the study was created of each of the following covariates: age at first visit, sex, education, conversion status at the last NACC visit (for the normal cohort). This was performed in order to adjust for the effect of these covariates on the rate of cognitive decline. Rates of change by exposure and by the presence of any APOE ε4 allele were estimated by including a three-way interaction term of time in the study, TBI with LOC exposure, and APOE ε4 allele presence/absence status. Missing data were handled through the mixed effect model framework, which has been shown superior to other method due to the use of maximum likelihood estimation procedures [38]. Mixed effects regression models were performed using SAS (version 9.3). The dynamic factor model results in higher power for a given sample size compared to non-dynamic factor models and to individual observed scores [36]. In order to assess statistical power, we followed a bootstrapping scheme using already collected NACC data. For a given effect size, error level, and sample size, we simulate 1000 subsamples with replacement. We then run mixed effect regressions and calculate the percentage of statistically significant effects among all subsamples. For a sample size of 274 with similar distribution of length of follow-up visits to the current study, we calculated through simulations that we have 80% power to detect small differences in the rate of change in all domains. For episodic memory, we can detect differences in the rates of changes of observed scores for this domain (i.e., Logical Memory: Immediate and Delayed recall) as small as 0.08 SDs, with age, sex, and education adjustments. Similarly, for attention and psychomotor speed, language, and executive function, we can detect differences in the adjusted observed scores for each domain as small as 0.05, 0.06, and 0.03 SDs respectively.
Table 1.
Group comparisons
| Variable | History of TBI with LOC | No history of TBI with LOC | test statistic | p-value |
|---|---|---|---|---|
| Normal cohort (N = 432) | n = 216 | n = 216 | ||
| Age at baseline-mean (st.dev.) | 72.2 (8.6) | 72.3 (8.4) | 0.14 | 0.8875 |
| Education Years-mean (st.dev.) | 16.3 (2.5) | 16.3 (2.4) | −0.08 | 0.9372 |
| Sex (n (%) women) | 115 (53.2%) | 115 (53.2%) | 0.00 | 1.000 |
| Years of follow-up-mean (st.dev.) | 3.1 (1.9) | 3.2 (2.0) | 0.39 | 0.6982 |
| APOE ε4 + n (%) | 64 (33.3%) | 53 (28.3%) | 1.1 | 0.2930 |
| Conversion to MCI or Dementia n (%) | 27 (12.5%) | 32 (14.8%) | 0.49 | 0.4836 |
| Thyroid disease n (%) | 38 (17.7%) | 39 (18.1%) | 0.02 | 0.8999 |
| Diabetes n (%) | 19 (8.8%) | 20 (9.3%) | 0.02 | 0.8666 |
| Stroke n (%) | 5 (2.3%) | 2 (0.9%) | 1.32 | 0.2504 |
| Transient ischemic attack n (%) | 12 (5.7%) | 11 (5.1%) | 0.07 | 0.7855 |
| Congestive heart failure n (%) | 4 (1.9%) | 6 (2.8%) | 0.41 | 0.5222 |
| Hypertension n (%) | 102 (47.4%) | 112 (51.9%) | 0.84 | 0.3599 |
| AD cohort (N = 274) | n = 137 | n = 137 | ||
| Age at baseline-mean (st.dev.) | 74.8 (7.9) | 75.0 (7.7) | 0.20 | 0.8403 |
| Education Years-mean (st.dev.) | 15.3 (2.8) | 15.4 (2.8) | 0.16 | 0.8735 |
| Sex (n (%) women) | 53 (38.7%) | 53 (38.7%) | 0.00 | 1.00 |
| Years of follow-up-mean (st.dev.) | 1.8 (1.1) | 1.8 (1.0) | 0.00 | 0.9973 |
| APOE ε4 + n (%) | 96 (70.1%) | 98 (71.5%) | 0.07 | 0.7904 |
| CDR-SB at baseline-mean (st.dev.) | 4.9 (1.9) | 4.8 (1.9) | −0.02 | 0.9837 |
| Thyroid disease n (%) | 26 (19.0%) | 23 (17.0%) | 0.17 | 0.6771 |
| Diabetes n (%) | 16 (11.7%) | 16 (11.6%) | 0.00 | 0.9824 |
| Stroke n (%) | 4 (2.9%) | 7 (5.2%) | 0.85 | 0.3558 |
| Transient ischemic attack n (%) | 5 (3.7%) | 8 (5.9%) | 0.75 | 0.3863 |
| Congestive heart failure n (%) | 4 (2.9) | 1 (0.7%) | 1.79 | 0.1810 |
| Hypertension n (%) | 66 (48.5%) | 77 (57.5%) | 2.16 | 0.1414 |
RESULTS
For the normal cohort (N = 432), the average age was 72.2 ± 8.5 years with 3.2 ± 1.9 (range: 0.8–7.4) years of follow-up with normal cognition. The majority were not APOE ε4 allele carriers (72.9%) and did not convert to MCI or dementia (86.3%). For the AD cohort (N = 274), the average age was 74.9 ± 7.8 years with 1.8 ± 1.1 (range: 0.7–7.5) years of follow-up with probable AD. The majority were men (61.3%) and were APOE ε4 allele carriers (70.8%). On average, participants in the AD cohort had mild dementia with a mean CDR-SB of 4.9 ± 1.9. Table 1 shows comparisons of the main demographic variables by exposure category (TBI versus no TBI). There were no significant differences between the exposure groups within cohorts for all demographic variables.
Table 2 shows all factor loading estimates for both the normal and AD cohorts. All factor loadings were statistically significant. The model had excellent fit with = 0.996 for both the normal and AD cohorts. Table 3 shows the rates of change and their differences between exposure groups (TBI with LOC present versus absent) stratified by APOE ε4 allele presence/absence group for the main cognitive factors as well as for the MMSE and CDR-SB. Figures 2 and 3 show spaghetti plots of individual linear trajectories and average unadjusted rates of change by TBI exposure history and APOE ε4 allele presence/absence group from a simple linear regression model. Additional results are provided in Supplementary Tables 1–3, showing rates of change and their differences for each individual neuropsychological score used as an input variable in the dynamic factor analysis
Table 2.
Factor loadings estimates (p-value)
| Normal cohort | AD cohort | |
|---|---|---|
| Memory | ||
| Logical Memory: Immediate | 1 (constrained) | 1 (constrained) |
| Logical Memory: Delayed | 0.52 (<0.0001) | 0.45 (<0.0001) |
| Attention | ||
| Digits Span Forward: Number Correct | 1 (constrained) | 1 (constrained) |
| Digits Span Forward: Series Length | 0.20 (<0.0001) | 0.17 (<0.0001) |
| Digits Span Backward: Number Correct | 0.13 (<0.0001) | 0.18 (<0.0001) |
| Digits Span Backward: Series Length | 0.15 (<0.0001) | 0.18 (<0.0001) |
| Trailmaking A Time | 0.04 (<0.0001) | 0.09 (<0.0001) |
| Digit Symbol | 0.05 (<0.0001) | 0.10 (<0.0001) |
| Executive Function | ||
| Trailmaking B Time | 1 (constrained) | 1 (constrained) |
| Language | ||
| Semantic Fluency: Animals | 1 (constrained) | 1 (constrained) |
| Semantic Fluency: Vegetables | 0.21 (<0.0001) | 0.31 (<0.0001) |
| Boston Naming Test | 0.19 (<0.0001) | 0.29 (<0.0001) |
Table 3.
Rates of temporal change (S.E) and their differences (S.E.) between TBI exposure groups by presence(+)/absence(−) of APOE ε4. All outcomes – except CDR-SB– are in standardized units. Positive (negative) values for the difference in the rate of change indicate that the TBI present group changes slower (faster) over time compared to the TBI absent group
| ε4 +
|
ε4-
|
|||||||
|---|---|---|---|---|---|---|---|---|
| TBI history Estimate (S.E.) | No TBI history Estimate (S.E.) | Difference | TBI history Estimate (S.E.) | No TBI history Estimate (S.E.) | Difference | |||
| Estimate (S.E.) | t-test (p-value) | Estimate (S.E.) | t-test (p-value) | |||||
| Normal cohort | ||||||||
| MMSE (Standardized Units) | 0.06 (0.15) | 0.06 (0.15) | −0.00 (0.04) | −0.07 (0.9421) | 0.08 (0.15) | 0.10 (0.15) | −0.02 (0.03) | −0.62 (0.5341) |
| CDR-SB (Sum of Boxes) | 0.01 (0.06) | 0.00 (0.06) | 0.01 (0.02) | 0.41 (0.6814) | 0.00 (0.06) | −0.01 (0.06) | 0.01 (0.01) | 0.53 (0.5928) |
| Memory (Standardized Units) | 0.30 (0.15) | 0.28 (0.16) | 0.02 (0.05) | 0.35 (0.7272) | 0.39 (0.16) | 0.31 (0.16) | 0.08 (0.03) | 2.42 (0.0100) |
| Attention (Standardized Units) | −0.03 (0.09) | −0.06 (0.09) | 0.04 (0.02) | 1.63 (0.1045) | −0.07 (0.09) | −0.05 (0.09) | −0.01 (0.02) | −0.84 (0.4021) |
| Executive Function (Standardized Units) | 0.15 (0.10) | 0.13 (0.10) | 0.02 (0.03) | 0.54 (0.5907) | 0.14 (0.10) | 0.14 (0.10) | −0.00 (0.02) | −0.15 (0.8788) |
| Language (Standardized Units) | 0.14 (0.13) | 0.20 (0.14) | −0.06 (0.04) | −1.45 (0.1472) | 0.20 (0.14) | 0.20 (0.14) | −0.00 (0.03) | −0.01 (0.9896) |
| AD cohort | ||||||||
| MMSE (Standardized Units) | −4.24 (1.47) | −4.07 (1.48) | −0.17 (0.30) | −0.55 (0.5797) | −4.05 (1.52) | −4.52 (1.52) | 0.47 (0.45) | 1.05 (0.2940) |
| CDR-SB (Sum of Boxes) | 0.75 (1.01) | 0.83 (1.01) | −0.08 (0.19) | −0.40 (0.6919) | 1.06 (1.04) | 0.81 (1.04) | 0.26 (0.27) | 0.95 (0.3429) |
| Memory (Standardized Units) | −0.22 (0.24) | −0.19 (0.24) | −0.03 (0.05) | −0.72 (0.4722) | −0.21 (0.25) | −0.15 (0.25) | −0.06 (0.07) | −0.84 (0.4036) |
| Attention (Standardized Units) | −0.49 (0.20) | −0.53 (0.20) | 0.04 (0.04) | 1.11 (0.2702) | −0.47 (0.20) | −0.49 (0.20) | 0.02 (0.06) | 0.47 (0.6380) |
| Executive Function (Standardized Units) | −0.36 (0.17) | −0.34 (0.18) | −0.02 (0.03) | −0.47 (0.6391) | −0.33 (0.18) | −0.33 (0.18) | 0.00 (0.05) | 0.09 (0.9312) |
| Language (Standardized Units) | −0.94 (0.32) | −0.83 (0.32) | −0.11 (0.06) | −1.83 (0.0685) | −0.92 (0.33) | −0.95 (0.33) | 0.04 (0.10) | 0.38 (0.7016) |
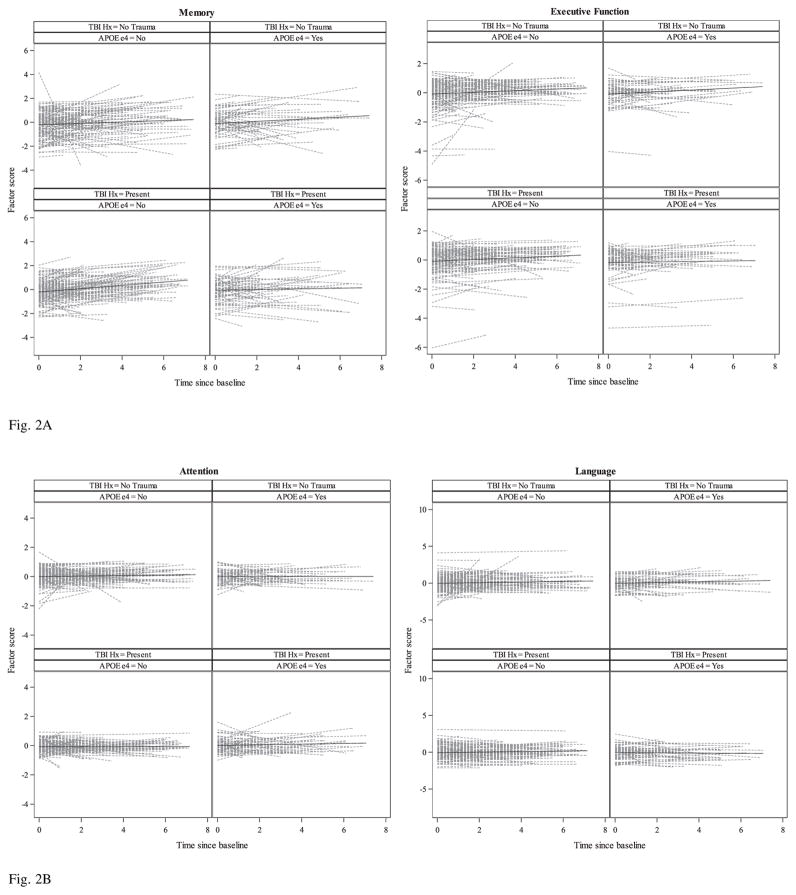
Fig. 2.
Fig. 2A. Spaghetti plots of individual linear trajectories and unadjusted mean trajectory lines (SD per year) of standardized factor scores (Memory and Executive Function) from simple linear regressions for the “Normal cohort” by TBI exposure and APOE ε4 groups.
Fig. 2B. Spaghetti plots of individual linear trajectories and unadjusted mean trajectory lines (SD per year) of standardized factor scores (Attention and Language) from simple linear regressions for the “Normal cohort” by TBI exposure and APOE ε4 groups.
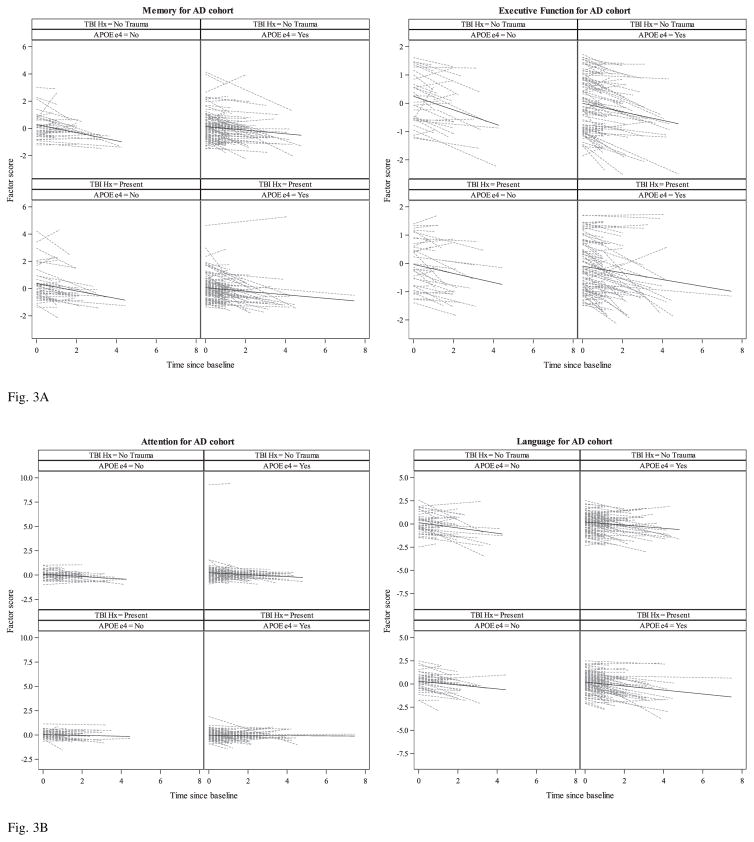
Fig. 3.
Fig. 3A. Spaghetti plots of individual linear trajectories and unadjusted mean trajectory lines (SD per year) of standardized factor scores (Memory and Executive Function) from simple linear regressions for the “AD cohort” by TBI exposure and APOE ε4 groups.
Fig. 3B. Spaghetti plots of individual linear trajectories and unadjusted mean trajectory lines (SD per year) of standardized factor scores (Attention and Language) from simple linear regressions for the “AD cohort” by TBI exposure and APOE ε4 groups.
Normal cognition group
For both APOE ε4 carriers and non-carriers, there was significant improvement in the change of episodic memory over time with or without history of TBI with LOC. Furthermore, among APOE ε4 non-carriers, those with a TBI history exhibited a faster improvement in episodic memory over time compared to those without TBI history. For the language domain, all groups exhibited improvement over time, but a statistically significant slope was only present for APOE ε4 non-carriers without TBI history. No group showed a significant improvement over time in attention or executive function, or for the MMSE and CDR-SB. Sex did not significantly affect the rate of change in any cognitive domain. Education had a significant effect in executive function (p = 0.0286), with more years of schooling resulting in slower improvement. Age at baseline was negatively associated with the rate of change for memory (p = 0.0001), executive function (p = 0.0001), and language (p = 0.0078), with older participants improving at a slower rate. There were no significant differences at baseline by TBI group or APOE ε4 allele presence/absence status. There were no significant differences in the effects of sex, education, APOE ε4 allele presence/absence status, and age by TBI group.
AD cohort
There were no significant differences in the rate of decline in any domain between those with and those without TBI history, irrespective of the presence of APOE ε4 allele. Attention and language significantly declined for both APOE ε4 carriers and non-carriers irrespective of exposure to TBI with LOC. Both APOE ε4 groups declined over time in executive function, but it was only statistically significant for APOE ε4 carriers with TBI history. Although both APOE ε4 groups with and without TBI history showed decline in episodic memory performance, it was not statistically significant. Rates of change between APOE ε4 carrier groups were compared, but no difference in slope were statistically significant, for subjects with and without TBI history across all domains. Sex and education did not significantly affect the rate of decline in any cognitive domain. When stratified by TBI group, education was significant in language, only among those with TBI history (p = 0.0062), with more years of schooling resulting in faster decline. No other significant differences in the effects of sex and education by exposure group were found. Age at baseline was significant for attention (p = 0.0013), executive function (p = 0.0047) and language (p = 0.0003) with younger participants declining faster. There were no significant differences at baseline by TBI group or by the presence of APOE ε4 allele for all domains except for attention, where those with no TBI with LOC history had significantly higher scores at baseline (difference = 0.17 std. units, p = 0.0424). Moreover, when stratified by TBI group, the effect of age at baseline on the rate of change for attention was significant only among those with no TBI history (p = 0.0184), but not among those with history of TBI with LOC (p = 0.5174). The effects of age at baseline on the changes of memory, executive function and language were similar in the two exposure groups.
MMSE scores declined over time for APOE ε4 carriers with TBI history (change = −4.2 points per years, p = 0.0044) and without TBI history (change = −4.1 points per years, p = 0.0064). Similarly, there was significant decline in the MMSE scores for non-carriers with TBI history (change = −4.1 points per years, p = 0.0086) and without TBI history (change = −4.5 points per years, p = 0.0033). For CDR-SB, there was no significant decline over time in any APOE ε4 allele presence/absence group for subjects with and without TBI history.
DISCUSSION
Although much research has examined TBI as a possible risk factor for AD [12], little is known regarding how TBI influences the rate of age-related cognitive change. We conducted a retrospective matched case-control study using participants with normal cognition and probable AD from the large, longitudinal, NACC database to examine the relationship between TBI with LOC history and the rate of cognitive change. Our findings show that history of TBI with LOC did not affect the rate of cognitive change over time for participants with normal cognition or probable AD. Despite the large NACC sample, our findings should still be interpreted cautiously due to the crude and limited assessment of TBI history available through the NACC database. The operationalization of TBI in this study was assessed via self-report, which can be accompanied by subjective recall bias, or confounded by poor memory recall, particularly in this sample. More importantly, details regarding TBI exposure of this sample are unknown, including when the injury occurred, severity, or whether participants had a single TBI or repeated TBIs with LOC. Current evidence shows that there may be a dose-response relationship between TBI and risk of neurodegenerative disease [39]. Repetitive mild TBI is common among athletes participating in amateur contact sports such as American football [40], as well as in military veterans [41]. Moreover, a history of participating in contact sports, even without a reported TBI history, may be a risk factor for long-term neurological consequences [42]. NACC does not collect information on participants’ athletic or military history
The current results are inconsistent with some longitudinal structural and functional imaging studies in participants with a history of TBI (with or without LOC). Specifically, multiple studies have found an association between TBI and acute structural and functional brain alterations, which persist and possibly progress over time [43–50]. TBI history has been theorized to decrease an individuals’ ability to cope with age- and/or AD-related pathology and to accelerate cognitive decline [51]. Indeed, this hypothesis is supported by research that links TBI with increased AD-related pathology such as amyloid-β[50, 52–54], even though it has not been confirmed in the NACC dataset previously [55]. TBI may indeed increase the risk for probable AD [17, 45–47], even if, TBI with LOC may not affect its course after onset.
The presence of the APOE ε4 allele has been shown to increase the risk of AD [48, 49], but it may not have an effect on disease course after dementia diagnosis [56]. In our study, among APOE ε4 negative normal controls, those with a history of TBI with LOC showed a significant improvement in the memory domain over time compared to those with no history of TBI. It is possible that those without an ε4 allele may have a better ability to return to their corresponding baseline after injury, which may be reflected in this finding. No other significant differences were found in any domain between those with a history and no history of TBI stratified by the presence of APOE ε4 allele. Although a meta-analysis of 14 observational studies, Zhou et al. [34] show that the presence of APOE ε4 increased the risk of unfavorable outcome at 6-months after injury, our results suggest that the long-term effects of APOE ε4 on cognitive change may be minimal.
Although the study was adequately powered to detect clinically significant differences, there are methodological limitations, which may have contributed to the observed null findings. Although participants had a remote history of TBI with LOC, the time since TBI was unknown. The role of time-since-TBI on cognitive decline has not been investigated in the literature and should be the target of future work, as it is possible that individuals who sustain a TBI at a younger age may be more resilient to the long-term effects of TBI. Furthermore, we did not test the effect on the rate of cognitive change immediately after diagnosis since our AD matched sample included incident (participants who were cognitively normal when they entered the NACC study and converted to AD dementia) and prevalent cases (participants who entered the NACC study while already being diagnosed with AD dementia). If cognitive changes are non-linear after diagnosis, then some participants may already have experienced the majority of cognitive decline before enrollment in NACC. Additionally, despite our case-control matched design, exposure to TBI remains non-randomized, which may limit the generalizability of the results. Lastly, a major limitation in the study is the lack of neuropathological confirmation of AD diagnosis; only a subset of participants from NACC agree to brain donation to form the Neuropathology Data Set and inclusion of this subset would significantly reduce the sample size. Similarly, inclusion of in vivo biomarkers of AD (e.g., hippocampal atrophy, PET amyloid imaging) would have provided key insight into the present findings, however, only a subset of NACC ADCs have contributed imaging data and thus inclusion of any imaging variables would also significantly reduce the sample size of the study and limit statistical power. Despite these limitations, our results are consistent with recently published clinical and neuropathological studies [24] that indicate that TBI with LOC is not associated with AD dementia.
In this study, we investigated the effect of exposure to TBI with LOC on the rates of cognitive change among normal controls and participants diagnosed with probable AD. Although we expected the rates of cognitive change to differ significantly between those with a history of TBI compared to those with no history of TBI, we found no significant difference between the groups, even when we stratified by the presence of APOE ε4. Future studies should collect information on the number of past TBIs (including mild TBIs, as well as exposure to subconcussive trauma through contact sports and other activities) along with time since TBI, which may play a significant role in cognitive change.
Supplementary Material
Acknowledgments
The study is funded by NIA/NIH grant P30 AG013846 (PI Neil Kowall, MD). The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI Marie-Francoise Chesselet, MD, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda VanEldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), andP50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0585r3).
Footnotes
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-160585.
References
- 1.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation. 2007;22:341–353. [PubMed] [Google Scholar]
- 2.Faul M, Xu L, Wald M, Coronado V. Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- 3.Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: Chronic traumatic encephalopathy. PM R. 2011;3:S460–S467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Daneshvar DH, Nowinski CJ, McKee AC, Cantu RC. The epidemiology of sport-related concussion. Clin Sports Med. 2011;30:1–17. vii. doi: 10.1016/j.csm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronado VG, McGuire LC, Sarmiento K, Bell J, Lionbarger MR, Jones CD, Geller AI, Khoury N, Xu L. Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J Safety Res. 2012;43:299–307. doi: 10.1016/j.jsr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein EA, Corso PS, Miller TR. Incidence and Economic Burden of Injuries in the United States. Oxford University Press; Oxford: 2006. [Google Scholar]
- 7.Maas AIR, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 8.Ruttan L, Martin K, Liu A, Colella B, Green RE. Long-term cognitive outcome in moderate to severe traumatic brain injury: A meta-analysis examining timed and untimed tests at 1 and 4.5 or more years after injury. Arch Phys Med Rehabil. 2008;89:S69–S76. doi: 10.1016/j.apmr.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: A meta-analysis. J Int Neuropsychol Soc. 2005;11:345–357. doi: 10.1017/s1355617705050411. [DOI] [PubMed] [Google Scholar]
- 10.Ponsford J, Willmott C, Rothwell A, Cameron P, Kelly AM, Nelms R, Curran C, Ng K. Factors influencing outcome following mild traumatic brain injury in adults. J Int Neuropsychol Soc. 2000;6:568–579. doi: 10.1017/s1355617700655066. [DOI] [PubMed] [Google Scholar]
- 11.Bigler ED. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc. 2008;14:1–22. doi: 10.1017/S135561770808017X. [DOI] [PubMed] [Google Scholar]
- 12.Faden AI, Loane DJ. Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurother J Am Soc Exp Neurother. 2015;12:143–150. doi: 10.1007/s13311-014-0319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci. 2015;66:75–80. doi: 10.1016/j.mcn.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordström P, Michaëlsson K, Gustafson Y, Nordström A. Traumatic brain injury and young onset dementia: A nationwide cohort study. Ann Neurol. 2014;75:374–381. doi: 10.1002/ana.24101. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y-K, Hou S-W, Lee C-C, Hsu C-Y, Huang Y-S, Su Y-C. Increased risk of dementia in patients with mild traumatic brain injury: A nationwide cohort study. PloS One. 2013;8:e62422. doi: 10.1371/journal.pone.0062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: The role of age and severity. JAMA Neurol. 2014;71:1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta KM, Ott A, Kalmijn S, Slooter AJ, van Duijn CM, Hofman A, Breteler MM. Head trauma and risk of dementia and Alzheimer’s disease: The Rotterdam Study. Neurology. 1999;53:1959–1962. doi: 10.1212/wnl.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 18.Mayeux R, Ottman R, Maestre G, Ngai C, Tang MX, Ginsberg H, Chun M, Tycko B, Shelanski M. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 19.Koponen S, Taiminen T, Kairisto V, Portin R, Isoniemi H, Hinkka S, Tenovuo O. APOE-epsilon4 predicts dementia but not other psychiatric disorders after traumatic brain injury. Neurology. 2004;63:749–750. doi: 10.1212/01.wnl.0000134603.57107.2f. [DOI] [PubMed] [Google Scholar]
- 20.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JC. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 21.Shively S, Scher AI, Perl DP, Diaz-Arrastia R. Dementia resulting from traumatic brain injury. Arch Neurol. 2012;69:1245–1251. doi: 10.1001/archneurol.2011.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay J, Johnson VE, Smith DH, Stewart W. Chronic traumatic encephalopathy: The neuropathological legacy of traumatic brain injury. Annu Rev Pathol. 2016;11:21–45. doi: 10.1146/annurev-pathol-012615-044116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeKosky ST, Blennow K, Ikonomovic MD, Gandy S. Acute and chronic traumatic encephalopathies: Pathogenesis and biomarkers. Nat Rev Neurol. 2013;9:192–200. doi: 10.1038/nrneurol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, Keene CD, Sonnen J, Montine TJ, Bennett DA, Leurgans S, Schneider JA, Larson EB. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 2016;73:1062–1069. doi: 10.1001/jamaneurol.2016.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: A review of the literature. Maturitas. 2013;75:51–61. doi: 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Millis SR, Rosenthal M, Novack TA, Sherer M, Nick TG, Kreutzer JS, High WM, Ricker JH. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil. 2001;16:343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Dikmen SS, Machamer JE, Powell JM, Temkin NR. Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2003;84:1449–1457. doi: 10.1016/s0003-9993(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 28.Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Longitudinal cognitive changes in traumatic brain injury A 30-year follow-up study. Neurology. 2006;66:187–192. doi: 10.1212/01.wnl.0000194264.60150.d3. [DOI] [PubMed] [Google Scholar]
- 29.Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA NIA-Alzheimer’s Disease Centers. The National Alzheimer’s Coordinating Center (NACC) Database: An Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 30.Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA NIA Alzheimer’s Disease Centers. The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 31.Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int J Surg Lond Engl. 2014;12:1495–1499. [Google Scholar]
- 33.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou W, Xu D, Peng X, Zhang Q, Jia J, Crutcher KA. Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma. 2008;25:279–290. doi: 10.1089/neu.2007.0489. [DOI] [PubMed] [Google Scholar]
- 35.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 36.Tripodis Y, Zirogiannis N. Dynamic factor analysis for multivariate time series: An application to cognitive trajectories. Int J Clin Biostat Biom. 2015;1 doi: 10.23937/2469-5831/1510001. pii: 001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doornik JA. An Object-Oriented Matrix Programming Language Ox 6. Timberlake Consultants Ltd; London: 2009. [Google Scholar]
- 38.Mallinckrodt CH, Clark WS, David SR. Accounting for dropout bias using mixed-effects models. J Biopharm Stat. 2001;11:9–21. doi: 10.1081/BIP-100104194. [DOI] [PubMed] [Google Scholar]
- 39.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: Substrates of dementia? Nat Rev Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langlois J, Rutland-Brown W, Thomas K. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2006. [Google Scholar]
- 41.Bryan CJ, Clemans TA. REpetitive traumatic brain injury, psychological symptoms, and suicide risk in a clinical sample of deployed military personnel. JAMA Psychiatry. 2013;70:686–6691. doi: 10.1001/jamapsychiatry.2013.1093. [DOI] [PubMed] [Google Scholar]
- 42.Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto-Ortolaza A, Johnston AE, DeSaro P, Boylan KB, Graff-Radford NR, Wszolek ZK, Rademakers R, Boeve BF, McKee AC, Dickson DW. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol (Berl) 2015;130:877–889. doi: 10.1007/s00401-015-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aungst SL, Kabadi SV, Thompson SM, Stoica BA, Faden AI. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab. 2014;34:1223–1232. doi: 10.1038/jcbfm.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: Association with chronic microglial activation. J Neuropathol Exp Neurol. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mouzon BC, Bachmeier C, Ferro A, Ojo J-O, Crynen G, Acker CM, Davies P, Mullan M, Stewart W, Crawford F. Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann Neurol. 2014;75:241–254. doi: 10.1002/ana.24064. [DOI] [PubMed] [Google Scholar]
- 46.Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, Sherman JE, Johnson SC. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42:503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farbota KDM, Sodhi A, Bendlin BB, McLaren DG, Xu G, Rowley HA, Johnson SC. Longitudinal volumetric changes following traumatic brain injury: A tensor-based morphometry study. J Int Neuropsychol Soc. 2012;18:1006–1018. doi: 10.1017/S1355617712000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, Pandey CM, Narayana PA. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neurocognitive function. J Neurotrauma. 2009;26:481–495. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- 49.Ng K, Mikulis DJ, Glazer J, Kabani N, Till C, Greenberg G, Thompson A, Lazinski D, Agid R, Colella B, Green RE. Magnetic resonance imaging evidence of progression of subacute brain atrophy in moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2008;89:S35–S44. doi: 10.1016/j.apmr.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain J Neurol. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker KR, Tesco G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front Aging Neurosci. 2013;5:29. doi: 10.3389/fnagi.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM-Y, Clark RS, Marion DW, Wisniewski SR, DeKosky ST. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 53.Hong YT, Veenith T, Dewar D, Outtrim JG, Mani V, Williams C, Pimlott S, Hutchinson PJA, Tavares A, Canales R, Mathis CA, Klunk WE, Aigbirhio FI, Coles JP, Baron J-C, Pickard JD, Fryer TD, Stewart W, Menon DK. Amyloid imaging with carbon 11–labeled Pittsburgh compound B for traumatic brain injury. JAMA Neurol. 2014;71:23–31. doi: 10.1001/jamaneurol.2013.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-β pathology: A link to Alzheimer’s disease? Nat Rev Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sayed N, Culver C, Dams-O’Connor K, Hammond F, Diaz-Arrastia R. Clinical phenotype of dementia after traumatic brain injury. J Neurotrauma. 2013;30:1117–1122. doi: 10.1089/neu.2012.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verghese PB, Castellano JM, Holtzman DM. Roles of apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.