Abstract
Objectives
To describe the characteristics of intraductal papillary mucinous neoplasms (IPMNs) with predominant involvement of the main pancreatic duct (MPD), analyzing predictors for survival and recurrence.
Background
IPMNs involving the MPD harbor a high likelihood of malignancy and different biological features. The appropriateness of including cases with minimal noncircumferential MPD involvement has been challenged because these show clinicopathological features that are similar to branch duct IPMN. Accordingly, their exclusion has led to a redefinition of MPD IPMN (MD-IPMN).
Methods
Retrospective review of resected MD-IPMN from 1990 to 2013. All slides were reviewed by a single pancreatic pathologist and classified on the basis of epithelial type and invasive component.
Results
A total of 223 patients underwent resection for IPMN involving the MPD. Of these, 50 were excluded because of minimal MPD involvement. Among the 173 patients analyzed, median age was 68 years and 55% were males. Predominant epithelial phenotype was intestinal (50%). Forty-eight patients (28%) had low- or intermediate-grade dysplasia, whereas 125 (72%) had either high-grade dysplasia (33%) or invasive carcinoma (39%). Of the 67 invasive IPMNs, 39 were tubular carcinomas (58%) and invasion was minimal (<5 mm) in 28 (42%). The 5-year overall survival rate was 69% and the disease-specific survival rate was 83%. The estimated recurrence rate at 10 years was 25%. Size and type of the invasive component, lymph node positivity, and a positive resection margin were predictors for both survival and recurrence (P < 0.05).
Conclusions
MD-IPMN is mainly intestinal-type and malignant. After resection, it has a very favorable prognosis, especially in the absence of macroscopic invasive carcinoma.
Keywords: cystic neoplasm, IPMN, intraductal papillary mucinous neoplasm, MD-IPMN, pancreas
As our understanding of intraductal papillary mucinous neoplasms of the pancreas (IPMNs) has evolved, their heterogeneity has become apparent. One of the first distinctions made was the site of involvement. Thirty years ago, when IPMNs were first described, the majority of reported cases involved the main pancreatic duct (MPD).1–3 With time, clinicians and pathologists became aware that the disease could also originate in the branches of the pancreatic ductal system.4–6 The distinction is important because main duct (MD) IPMNs, whether symptomatic or not, have a high rate of malignancy, whereas branch duct (BD) IPMNs, which very often are incidentally discovered, have a much lower rate.7–10 These observations eventually led to management algorithms, like the Sendai guidelines,11 recommending resection for all MD-IPMNs, but observation for asymptomatic BD-IPMNs that have a low risk of malignancy, assessed by size and other morphologic features.11–13
Historically, IPMNs that involve both the MPD and its branches (referred to as either combined or “mixed” IPMNs) have been categorized together with MD-IPMN, based on the fact that their epidemiology and risk of malignancy were believed to be similar.14 However, not infrequently the classification of the IPMN type was done after resection, and the presence of histological involvement, often minute, led to placement in the mixed category of BD-IPMNs that otherwise had no radiologic or gross involvement of the main duct.15 Review of these cases has shown that this minimal involvement does not carry the same malignant potential as MD-IPMN, and has prompted revision of the definitions of mixed type IPMN.16 The exclusion of cases of mixed type IPMN with only minimal involvement of the main duct has thus led to a redefinition of MD cases.
The aim of this study is to describe the clinical and pathological features of a large cohort of IPMNs that predominantly affect the MPD, and to identify predictors for recurrence and survival after resection.
MATERIAL AND METHODS
Study Population
After approval by the institutional review board of the Massachusetts General Hospital, the prospectively collected database of patients operated for IPMN was queried. From March 1990 to January 2013, 430 patients underwent surgical resection for IPMN.
Demographic information, symptoms at presentation, radiological workup, surgical procedures, pathology report, and postoperative course data were retrieved. For survival and recurrence analysis, both retrospective review of electronic medical records and follow-up phone calls to patients or referring physicians, according to our institutional protocol, were carried out.
Pathologic Examination
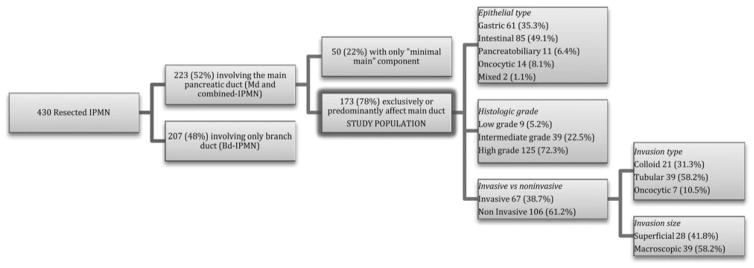
All slides from resected specimens were rereviewed by a single gastrointestinal pathologist with expertise in the field (MM-K). Of the 430 IPMN specimens, 223 (52%) had involvement of the MPD on microscopic examination (Fig. 1). However, 50 (22%) of those cases did not show gross abnormalities of the MPD (except for dilation) and only exhibited noncircumferential involvement of the MPD by mucinous epithelium limited to few sections (“minimal main” mixed type IPMN), and were therefore excluded from the analysis. The remaining 173 patients with IPMN involving the MPD almost exclusively or extensively formed the study cohort.
FIGURE 1.
Flow chart of study population.
The epithelial dysplasia was graded as low (mild), intermediate (moderate), and high (severe) according to the 2010 World Health Organization classification system.17 If different grades coexisted in one lesion, the highest degree of dysplasia was recorded. Epithelial subtypes of the intraductal component were classified as gastric, intestinal, pancreatobiliary, and oncocytic types based on cytomorphological features of the papillae and, when available, immunohistochemical demonstration of MUC1, MUC2, and MUC5A glycoproteins.18–20 IPMN lesions not infrequently exhibit multiple epithelial types; in such cases, the most prevalent epithelium associated with the highest degree of dysplasia was captured for analysis. If present, an invasive carcinoma arising in the background of IPMN (invasive IPMN) was classified into the colloid, tubular, or oncocytic type.19,21,22 Invasive components were also classified into superficial (minimally invasive) and macroscopic using a 5-mm size cutoff on microscopic evaluation.23 A concomitant pancreatic ductal adenocarcinoma (concomitant PDAC) was defined as an invasive carcinoma that developed away from the corresponding IPMN lesion with an uninvolved segment of the pancreas present between the two.24–26
Standard pathological examination of resected specimens included TNM staging according to the 2010 classification of the American Joint Committee on Cancer,27 the status of the pancreatic transection margin (classified as negative, positive for low-grade, intermediate-grade, high-grade dysplasia, or invasive carcinoma), retroperitoneal margin and uncinate margin, and the presence or absence of lymphatic, vascular, and perineural tumor invasion. Both at univariate and multivariate analysis, the pancreatic resection margin was referred as “positive” for the presence of either high-grade dysplasia and invasive carcinoma, and “negative” for the absence of IPMN or for the presence of either low- or intermediate-grade of dysplasia.
Surgical Approach to MD-IPMN
The surgical policy of the Department of Surgery of the Massachusetts General Hospital for the treatment of IPMN is in concordance with the International Consensus Guidelines for management of IPMNs and mucinous cystic neoplasms of the pancreas and its following revised version.11,12 In particular, the presumptive involvement of the MPD in an IPMN is considered as an indication for surgical resection, as long as the patient is a good surgical candidate and has a reasonable life expectancy. Because of the high likelihood of harboring malignancy, all MD-IPMN are treated through typical pancreatectomies with lymph node dissection. Intraoperatively, the frozen section analysis of the resection margin is performed, and if invasive carcinoma or high-grade dysplasia is found, the resection is extended up to a total pancreatectomy. The decision to perform a total pancreatectomy, however, is individualized and takes into consideration the patient’s age and comorbidities.
Statistical Analysis
Statistical analyses were performed with Stata software (version 12.0; StataCorp LP, College Station, TX). Continuous data were expressed as mean ± standard deviation. Univariate analyses for categorical variables were done with the χ2 test and Fisher exact test and continuous data were analyzed by using the Student t test and analysis of variance method. Corrections for multiple comparisons were performed using the Bonferroni method. Multivariate analyses for the assessment of predictors of clinical-pathological features were done using multinomial and binary logistic regression stepwise methods with backward elimination. A 2-tailed P value of less 0.05 was considered statistically significant in the model.
For overall survival, disease-specific survival, and recurrence, the Kaplan-Meier method was used. The differences in survival and recurrence were tested with log-rank tests. To define predictors of survival and recurrence in a multivariate fashion, Cox proportional hazards regression models with stepwise backward elimination were used.
RESULTS
Demographics, Clinical, and Pathologic Data
Demographics and clinical data are shown in Table 1. The median age of patients with MD-IPMN was 68 years (range, 37–87 years), and 55% were males. At diagnosis, 74 patients (43%) were asymptomatic. Among patients with symptoms, the most common was weight loss (32%), followed by pancreatitis (26%) and obstructive jaundice (14%). The majority of MD-IPMN occurred in the pancreatic head (109 patients, 63%), followed by the body and the tail of the pancreas (12% and 11%, respectively). In 14% of patients, the tumor involved the entire gland.
TABLE 1.
Demographics and Clinical Characteristics of the Study Population
| Overall | Low-intermediate Grade | High Grade | P | Noninvasive | Invasive | P | |
|---|---|---|---|---|---|---|---|
| No. patients | 173 | 48 (27.7%) | 125 (72.5%) | 106 (61.2%) | 67 (38.7%) | ||
| Males | 95 (54.9%) | 22 (45.8%) | 73 (58.4%) | N. S. | 54 (50.9%) | 41 (61%) | 0.187 |
| Age, mean ± SD (range) (yr) | 67.4 ± 10.3 (37–87) | 67.6 ± 10.2 (40–87) | 67.3 ± 10.4 (37–87) | N. S. | 66.8 ± 10.9 (37–87) | 68.4 ± 9.3 (47–85) | N. S. |
| CA 19.9 >37 at diagnosis, (U/mL) | 46 (26.5%) | 6 (12.5%) | 40 (32%) | 0.009 | 15 (14.1%) | 31 (46.0%) | <0.001 |
| No symptoms | 74 (42%) | 25 (52.0%) | 49 (39.2%) | N. S. | 53 (50.0%) | 21 (31.3%) | 0.016 |
| Pancreatitis | 45 (26.0%) | 14 (29.1%) | 31 (24.8%) | N. S. | 33 (31.1%) | 12 (17.9%) | 0.053 |
| Obstructive jaundice | 24 (13.8%) | 2 (4.1%) | 22 (17.6%) | 0.022 | 6 (5.6%) | 18 (26.8%) | <0.001 |
| Weight loss > 7 kg | 56 (32.3%) | 14 (29.1%) | 42 (33.6%) | N. S | 27 (25.4%) | 29 (43.2%) | 0.015 |
| Preoperative diabetes | 47 (27.1%) | 7 (14.5%) | 40 (32.0%) | 0.021 | 23 (21.7%) | 24 (35.8%) | 0.042 |
| History of smoking | 102 (58.9%) | 27 (56.2%) | 74 (59.2%) | N. S. | 65 (61.3%) | 36 (53.7%) | N. S. |
| Family history of PDAC | 23 (13.2%) | 7 (14.5%) | 16 (12.8%) | N. S. | 15 (14.1%) | 8 (11.9%) | N. S. |
| Surgical procedure | 0.361 | 0.025 | |||||
| Whipple | 109 (63.0%) | 31 (64.5%) | 78 (62.4%) | 72 (67.9%) | 37 (55.2%) | ||
| Distal | 40 (23.1%) | 14 (29.2%) | 26 (20.8%) | 24 (22.6%) | 16 (23.9%) | ||
| Middle | 4 (2.3%) | 1 (2.1%) | 3 (2.4%) | 3 (2.8%) | 1 (1.5%) | ||
| Total | 18 (10.4%) | 2 (4.2%) | 16 (12.8%) | 5 (4.8%) | 13 (19.4%) | ||
| Dorsal | 2 (1.1%) | 0 | 2 (1.6%) | 2 (1.9%) | 0 | ||
| Epithelial type | <0.001 | <0.001 | |||||
| Gastric | 61 (35.2%) | 32 (66.7%) | 29 (23.2%) | 46 (43.4%) | 15 (22.4%) | ||
| Intestinal | 85 (49.13%) | 16 (33.3%) | 69 (55.2%) | 51 (48.1%) | 34 (50.7%) | ||
| Pancreatobiliary | 11 (6.3%) | 0 | 11 (8.8%) | 5 (4.7%) | 6 (9.0%) | ||
| Oncocytic | 14 (8.0%) | 0 | 14 (11.2%) | 3 (2.8%) | 11 (16.4%) | ||
| Mixed | 2 (1.1%) | 0 | 2 (1.6%) | 1 (0.9%) | 1 (1.5%) |
Boldface values indicate statistically significant.
N. S. indicates nonsignificant.
Of the 173 patients, 125 (72.2%) had either high-grade dysplasia (58 patients, 33.5%) or invasive cancer (67 patients, 38.7%). The remaining 48 patients were classified as intermediate-grade (39 patients, 22.5%) or low-grade dysplasia (9 patients, 5.3%). Invasive neoplasms were found to be tubular in 39 cases (58.2%), colloid in 21 (31.3%), and oncocytic in 7 (10.5%) (Table 2). The degree of invasion was superficial in 28 specimens (42%) and macroscopic in 39 (58%). In 5 patients (2.9%), a concurrent PDAC was found. The associated IPMN in those cases was of intermediate grade in 3, and high-grade dysplasia and invasive tubular carcinoma in 1 each. The distinct PDAC was located in the head of the pancreas, in close proximity to the IPMN, in 4 cases, whereas in 1 case, the PDAC was in the pancreatic body and the IPMN in the tail; it was stage IIB in 4 cases and in the remaining it was stage IIA.
TABLE 2.
Demographics, Clinical, and Pathologic Features, and Postoperative Outcomes of Resected Invasive MD-IPMN
| All Invasive All Invasive Carcinomas | Minimally Invasive (<5 mm) | Macroscopic Invasive (>5 mm) | P | |
|---|---|---|---|---|
| No. patients | 67 | 28 (41.8%) | 39 (58.2%) | |
| Males | 41 (61.1%) | 17 (60.7%) | 24 (61.5%) | N. S. |
| Age, mean ± SD (yr) | 68.4 ± 9.3 | 67.7 ± 9.2 | 68.8 ± 9.5 | N. S. |
| CA 19.9 >37 U/mL at diagnosis | 31 (46.2%) | 6 (21.4%) | 25 (64.1%) | 0.001 |
| No symptoms | 21 (31.3%) | 10 (35.7%) | 11 (28.2%) | 0.513 |
| Pancreatitis | 12 (17.9%) | 7 (25%) | 5 (12.8%) | 0.200 |
| Obstructive jaundice | 18 (26.8%) | 4 (14.2%) | 14 (35.9%) | 0.049 |
| Weight loss > 7 lb | 29 (43.2%) | 12 (42.8%) | 17 (43.5%) | N. S |
| Preoperative diabetes | 24 (35.8%) | 8 (28.5%) | 16 (41%) | 0.021 |
| History of smoking | 36 (53.7%) | 14 (50%) | 22 (56.4%) | N. S. |
| Family history of PDAC | 8 (11.9%) | 5 (17.8%) | 3 (7.6%) | N. S. |
| Epithelial type | 0.196 | |||
| Gastric | 15 (22.3%) | 3 (10.7%) | 12 (30.8%) | |
| Intestinal | 34 (50.7%) | 15 (53.6%) | 19 (48.7%) | |
| Pancreatobiliary | 6 (8.9%) | 3 (10.7%) | 3 (7.7%) | |
| Oncocytic | 11 (16.4%) | 7 (25%) | 4 (10.3%) | |
| Mixed | 1 (1.4%) | 0 | 1 (2.5) | |
| Invasive type | 0.288 | |||
| Colloid | 21 (31.3%) | 6 (21.4%) | 15 (38.5%) | |
| Tubular | 39 (58.2%) | 18 (64.3%) | 21 (53.8%) | |
| Oncocytic | 7 (10.5%) | 4 (14.3%) | 3 (7.7%) | |
| N1 | 22 (32.8%) | 4 (14.2%) | 18 (46.1%) | 0.006 |
| 5-year overall survival rate | 50% | 68% | 35% | 0.031 |
| 5-year disease-specific survival rate | 62% | 85% | 48% | 0.009 |
| 5-year recurrence rate | 41% | 28% | 51% | 0.047 |
N. S. indicates nonsignificant.
Other concomitant tumors found in the specimen were: pancreatic neuroendocrine tumor in 5 cases (2.9%), adenocarcinoma of the ampulla in 2 cases, and mucinous cystic neoplasm and gastrointestinal stromal tumor of the duodenum in 1 case each.
TNM staging of the 67 invasive cancers was T3 in 43.3%, T2 in 26.9%, and T1 in 29.8%. The median number of nodes resected in patients with invasive tumors was 16, but only 32.8% of patients had involvement of one or more nodes.
Excluding the 18 total pancreatectomies of the cohort (Table 1), the final pancreatic transection margin was evaluated in 155 cases. Of these, 119 were negative (76.8%), 19 (12.2%) had low- to intermediate-grade dysplasia, 10 (6.4%) had high-grade dysplasia, and 7 (4.6%) had invasive carcinoma. The 17 margins with either high-grade dysplasia or invasive carcinoma were referred as positive in the analysis.
Surgical Procedures and Perioperative Outcomes
Table 1 summarizes the 173 operations that were carried out. In 132 cases (76.3%), the frozen section of the pancreatic transection margin was sent for the intraoperative examination. Frozen section of the margin was negative for IPMN in 94 cases (71.2%), revealed the presence of low- to intermediate-grade dysplasia in 17 cases (12.8%), high-grade dysplasia in 11 (8.4%), and of invasive carcinoma in 10 cases (7.6%). Of these 21 cases with either high-grade dysplasia or invasive carcinoma, 15 (71.4%) underwent extension of the resection. Overall, the frozen section diagnosis did not change the preoperatively planned surgical management in 101 cases, but did result in extension of the resection in 31 (23.4%). Of these, 20 eventually ended up with extended partial resections and a total pancreatectomy in 11 (8.3%). Final pathologic examination confirmed the frozen section diagnosis in the great majority of cases (95.4%), but in 6 cases (4.6%) did not. In 5 of them, the diagnosis on the resection margin changed from negative to positive (3 for high-grade dysplasia and 2 for invasive carcinoma), but none underwent reoperation. In the remaining case, the final diagnosis was negative as opposed to a frozen section positive for high-grade dysplasia.
Twenty-seven patients (15.6%) experienced major surgical complications during their postoperative course. In particular, the rate for clinically relevant pancreatic fistula (grade B or C)28 was 8%, whereas 6.3% patients had delayed gastric emptying, and 1.7% developed an intraabdominal abscess. No patients required reoperation. One patient died within the 90-day postoperative period from heart failure, but in-hospital and 30-day mortality were nil.
Among the 67 patients with invasive carcinoma, 25 (37.3%) received adjuvant therapy. This consisted of chemotherapy and radiation in 12 cases (48%) and of chemotherapy alone in 13 (52%).
Of the 155 patients, whose pancreatic remnant was evaluated at follow-up through computed tomographic scanning and/or magnetic resonance imaging, 36 (23%) had dilatation of the MPD (>5 mm). If only patients undergoing Whipple resection were considered, the rate of pancreatic remnants with a dilated MPD was 27% (29/108) after a median of 62 months. Among these 29 patients, only 3 had definite recurrence of the MD-IPMN (10%).
Epithelial Subtypes and Predictors of Malignancy
Almost half (49.7%) of the MD-IPMN arose from an intestinal epithelial type (85 cases), followed by gastric (35.7%, 61 cases), oncocytic (8.2%, 14 cases), and pancreatobiliary type (6.4%, 11 cases). High-grade dysplasia was present only in 29 (47.5%) cases with gastric phenotype, whereas this was present in 69 (81.1%) of those with intestinal type. All the cases with pancreatobiliary or oncocytic type were classified as high-grade dysplasia. The epithelial types did not show any correlation with sex, age, or clinical presentation except for pancreatitis, which was present in 31 (36.4%) cases of the intestinal type (P = 0.04).
The presence of high-grade dysplasia was significantly associated with a serum CA 19.9 more than 37 U/mL [P = 0.002; OR, 4.9; 95% confidence interval (CI), 1.7–13.7] and inversely associated with a gastric epithelial type (P < 0.001; OR, 0.17; 95% CI, 0.07–0.37) at binary logistic regression.
The occurrence of invasive carcinoma was also independently associated with CA 19.9 more than 37 U/mL and in addition with jaundice, weight loss more than 7 lb and oncocytic epithelial type at multivariate analysis (P < 0.05). Of note, 21 of the 67 patients with invasive carcinoma (31.3%) were asymptomatic at diagnosis. No differences in terms of smoking and a family history of PDAC were found between patients with invasive and noninvasive tumors.
Invasive Size and Type
A total of 42% of invasive carcinomas (28 patients) had an invasive component of less than 5 mm and were therefore classified as minimally invasive. As shown in Table 2, macroscopic invasion was more likely to be associated with an elevated CA 19.9 (64% vs 21%, P = 0.001), jaundice (36% vs 14%, P = 0.049), and diabetes (41% vs 28%, P = 0.021) at clinical presentation. Macroscopic invasive tumors also had a higher likelihood of harboring positive nodes (46% vs 14% in the minimally invasive tumors, P = 0.006).
Regarding the histologic types of invasive carcinoma, colloid carcinomas had macroscopic invasion in 71.4% of cases, whereas the proportion in tubular carcinomas was 53.8% and 42.8% in the oncocytic ones (P = 0.288). Nodal involvement, however, was more prevalent in tubular carcinoma (41%) than colloid (23.8%) and oncocytic (14.2%) carcinomas (P = 0.217).
As for the background epithelium, all the 21 colloid carcinomas arose from an intestinal epithelial subtype and all the 7 oncocytic from the homonym epithelial subtype, whereas the 39 tubular adenocarcinomas showed gastric epithelium in 39% of cases, intestinal in 34%, pancreatobiliary in 16%, and oncocytic in 11%.
Survival and Recurrence Analysis
Overall Survival
None of the patients died from postoperative complications and all the patients were followed up after surgery through scheduled outpatient clinics and phone calls at regular time points. One patient was lost to follow-up at 3 months after surgery. Fifty-one patients (29.5%) died during follow-up after a median of 21 months (range, 2–171 months). The other 122 were followed for a median of 56 months (range, 3–236 months). The 5-year overall survival rate was 69% (95% CI = 0.60–0.76) with the estimated median overall survival period of 14 years.
Of the 51 deaths, the majority (29 cases, 57%) were not related to IPMN. The causes were cardiopulmonary in 24% and other primary malignant tumors in 21%. Patients who died of causes other than IPMN were more frequently females (52% vs 27%) and older (mean, 71.1 ± 7 years vs 68.8 ± 10 years) than the ones who died of IPMN-related causes, but those differences did not reach statistical significance.
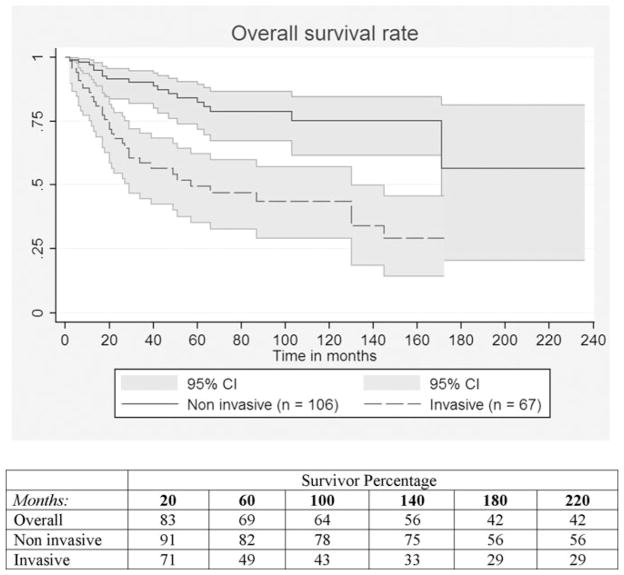
At univariate analysis, the presence of invasive component was the strongest predictor of overall survival (P < 0.001) (Fig. 2). Other factors negatively associated with overall survival were: macroscopic invasion (P = 0.03), a resection margin positive for high-grade dysplasia or invasive carcinoma (P = 0.005), N 1 status (P = 0.001), and stage II tumor over stage I (P = 0.005). Of note, for the entire group of invasive tumors (67 patients) the 5-year overall survival rate was 50%, whereas it was 68% for the minimally invasive tumors and only 35% for those with macroscopic invasion (P = 0.03).
FIGURE 2.
Overall survival curves for invasive versus noninvasive MD-IPMNs (P < 0.001).
Using Cox regression model, the presence of an invasive component (P < 0.001) was predicted for overall survival in the entire study population. For patients with cancer, invasive type and a positive N status were independent predictors of survival (P = 0.029 and P = 0.001, respectively).
Disease-specific Survival
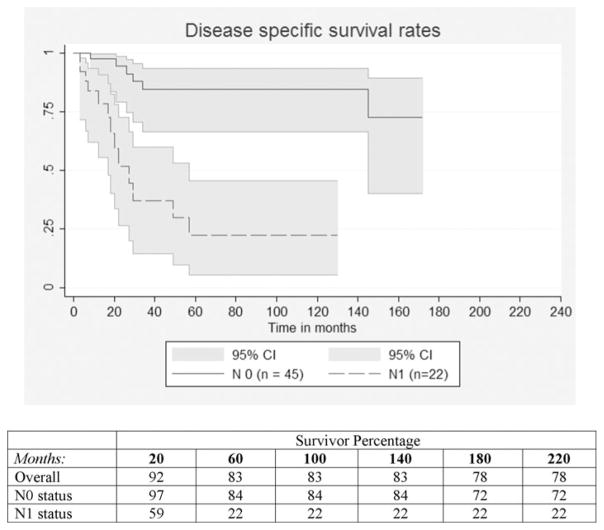
Twenty-two patients experienced IPMN-related death (12.7%) after a median of 20.5 months. The estimate of disease-specific survival rate was 83% (95% CI = 0.75–0.88) at 5 years. Invasive component, macroscopic size of invasion, positive resection margin and positive N status (Fig. 3) were again negatively associated with survival at univariate analysis (P < 0.05). Of note, none of the 14 patients with oncocytic epithelial type experienced an IPMN-related death.
FIGURE 3.
Disease-specific survival curves for N0 versus N1 invasive MD-IPMN (P < 0.001).
Among patients with invasive carcinoma: invasive type, T status, N status and a positive pancreatic resection margin were all predictors of disease-specific survival (Cox regression model, P < 0.05). In particular, tubular carcinoma had a significantly higher risk of disease-specific death over the colloid type (P = 0.012, hazards ratio = 6.7).
Recurrence
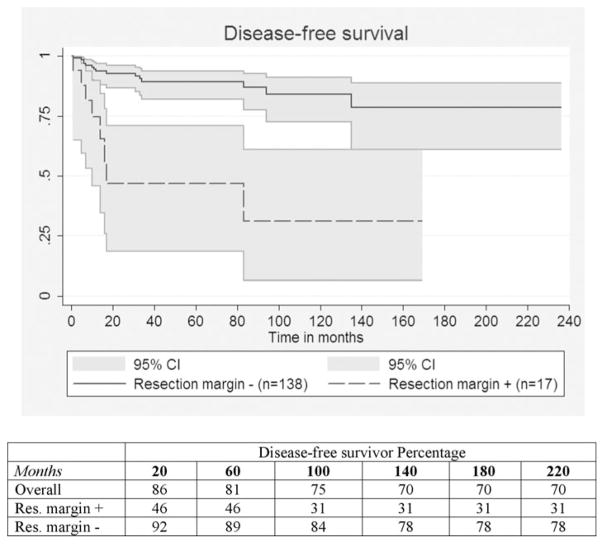
Thirty-two patients (18.5%) experienced recurrence of the IPMN at a median of 12 months (range, 1–135 months). The overall 5-year recurrence rate was 19% and the estimated 10-year recurrence rate 25%. Recurrence site was in the pancreatic remnant in 14 patients (44%), liver in 11 (34%), and peripancreatic lymph nodes in 7 (22%). Of the 14 patients who had recurrence in the pancreatic remnant, 5 (36%) underwent completion pancreatectomy. The pathological examination on second resection specimen revealed 2 IPMNs with high-grade dysplasia of the oncocytic subtype and 3 invasive IPMNs, 2 tubular and 1 oncocytic, respectively. None of them experienced a new recurrence after reoperation. A positive resection margin was the most significant predictor of recurrence at univariate analysis (P < 0.001) (Fig. 4). Other factors associated with recurrence (P < 0.05) were high-grade dysplasia, invasive component, macroscopic size of invasion, N1 status and T2 status. Among the entire study population, independent predictors of recurrence were the presence of invasive tumor (P = 0.004, hazards ratio 5.9) and a positive pancreatic resection margin (P = 0.046, hazards ratio = 2.6) with the Cox regression model.
FIGURE 4.
Disease-free survival curves for positive for high-grade dysplasia or invasive carcinoma versus negative resection margins (P < 0.001).
Distinct PDAC
Of the 5 patients with IPMN and concurrent PDAC (2.9% of total cohort), 1 died of a cerebral vascular accident 6 months after surgery. The other 4 patients had recurrence of pancreatic cancer after a median of 3 months (range, 1–4 months). Three had evidence of metastatic adenocarcinoma in the liver and 1 in both liver and lungs. Two of them eventually died of recurrent PDAC 5 and 13 months after surgery, whereas the other 2 are still alive after 11 and 16 months, respectively. Resulting median overall survival for patients with IPMN and concurrent PDAC was 11 months. None of the patients showed evidence of recurrent IPMN during follow-up.
DISCUSSION
IPMNs represent a heterogeneous group of neoplasms, whose classification remains challenging as we attempt to identify subgroups with distinct clinical, pathological, and biological features. Our pathological reassessment of mixed IPMNs allowed for exclusion of lesions with minimal MD involvement, and as a result we have a more homogeneous subgroup of IPMNs that have extensive involvement of the MPD. This cluster of tumors has very demarcated characteristics, which are distinct from BD-IPMNs or mixed forms with only minimal involvement of the MPD. IPMNs with extensive involvement of the MPD occur most commonly in the seventh decade and in males (55%), and more than ¾ of the cases involve the head of the pancreas. They present in asymptomatic individuals in 42% of cases, a rate that is higher than previously reported.8,29,30 This is particularly concerning because malignancy had developed by the time of diagnosis in 72% of patients. Unlike branch-duct IPMNs, which are mostly benign and of the gastric epithelial subtype,10,12,13,17,19 MD-IPMNs are invasive in 39% of patients, have positive lymph nodes in 33%, and show a background of intestinal epithelial subtype in 49% of cases. These features are consistent with the recent report by Tamura et al,29 who described 56 cases of resected MD-IPMN, of which 34% were invasive and 38% of the intestinal subtype. The exclusion in our cohort of the mixed form with a minimal involvement of MPD may explain our higher proportions of malignancy and intestinal subtype. Because of this high frequency of malignancy, we continue to support the indication for resection of all IPMNs with an exclusive or extensive involvement of the MPD, as recommended by the International Consensus Guidelines.11,12 The surgical treatment of MD-IPMN provided a 5-year overall survival rate of 69% and a disease-specific survival rates of 83%, with 57% of deaths being caused by other age-related conditions such as cardiovascular problems or different malignancies.
Almost 10 years after our previously reported experience on MD-IPMN,8 knowledge regarding the pathological features of IPMNs has increased, and we are now aware of the role played by histological subtypes in determining biological behavior.19,21,22,31 A search for clinical predictors of epithelial subtypes and invasive components did not provide any significant result other than the known association between pancreatitis and intestinal type IPMN (P = 0.015). The abundant and thick mucus produced by this neoplasm likely causes obstruction and the consequent acute pancreatic inflammatory process. We also confirm that gastric type IPMN invariably evolves into tubular carcinoma and that colloid carcinomas all derive from intestinal type IPMN, as previously reported by our group and others.19,21,32 We found a significant association between elevated serum CA 19.9 at diagnosis, jaundice, and weight loss and the presence of high-grade dysplasia or invasive cancer. These are previously reported predictive factors with high specificity for malignant MD-IPMNs.8,29,30,33,34 However, almost 70% of patients with high-grade dysplasia presented with a level of CA 19.9 under the normal threshold of 37 U/mL, demonstrating the low sensitivity of the biomarker in this setting.
Several recent articles have pointed out the prognostic value of the histologic type of invasion, with colloid carcinoma having a better prognosis than the non–intestinal-derived cancers, in particular the tubular type.22,35–37 We found that tubular carcinoma arising in IPMNs with predominant involvement of the MPD has a worse overall and disease-specific survival than the colloid. Our data on the higher rates of N1 tubular carcinomas over the colloid are consistent with that published by Yopp et al36 and might partially explain the different survival. We also found that none of the 7 oncocytic carcinomas of our series died of disease recurrence. Although it could be explained by the superficial nature of invasion in the majority of oncocytic carcinomas (4 over 7), an actual benefit in disease-specific survival for oncocytic invasive IPMNs has never been reported to date, and needs to be validated in other series.
Minimally invasive carcinoma represents a unique feature of IPMNs; 42% of the invasive cancers in our cohort fell in this category. We found that these minimally invasive carcinomas had a 14% rate of associated lymph node positivity, which is similar to that reported by others35 and that their 5-year survival rate was twice that of carcinoma with macroscopic invasion. Thus, we reinforce that the size of the invasive component is a predictor of survival after resection for MD-IPMN.23 We also found a 2.9% prevalence of distinct synchronous PDAC. This is less than the 4% to 9% that has been described in all types of resected IPMNs,26,38 and closer to the 3.5% incidence described by Tamura et al29 in his series involving only MD-IPMN. All 5 patients with distinct PDAC in our series had a very poor prognosis.
Our analysis confirms that the strongest predictor for diminished survival after resection of MD-IPMN is the presence of invasive carcinoma. After surgical resection for invasive IPMNs arising in the MPD, recurrence occurs in 39% of cases and can be predicted by the magnitude of invasion, the N status and the pancreatic resection margin, whereas adjuvant treatment did not seem to significantly affect the outcome. Our findings on the role of pancreatic resection margin are of particular importance in light of their high statistical significance and of the paucity of data analyzing resected MD-IPMN in which the transection margin had either high-grade dysplasia or invasive carcinoma. To decrease recurrence risk, we strongly believe that R0 resections should be achieved, avoiding margin positivity for both high-grade dysplasia and invasive carcinoma, and because of this there is an important role for the frozen section analysis of the pancreatic transection margin.39–41 Intraoperative diagnosis in our series demonstrated a very high accuracy and indicated extension of the resection in 1 of 4 cases, highlighting the need to have a pathologist familiar with this disease available at the time of the operation. Because total pancreatectomy is a possibility (in this series 10.4% of patients had a total pancreatectomy; in 7 of them it was the original plan, but in 11 it was done prompted by the findings on frozen section), it is critical that the surgeon discuss this beforehand with the patient and his or her family, including the risk/benefit of brittle diabetes versus complete resection of malignant disease.
Finally, regardless of the specific resected invasive type, disease-free survival of MD-IPMN carcinomas of 79% and 62% at 2 and 5 years, respectively, suggest that the same surveillance protocol of conventional PDAC should be followed for these patients. However, better outcomes are expected.
The presence of dilation of the MPD alone during follow-up is not indicative for recurrence of MD-IPMN, in particular after a Whipple because, in these cases, prominence of the MPD is frequently due to stricture of the pancreatic anastomosis.12,40 We found that more than 1 of 4 patients will develop radiologic evidence of MPD dilation at a median of 5 years after pancreatoduodenectomy, but that only 1 of 10 will actually harbor a recurrence of the disease.
CONCLUSIONS
We report the largest single-institution experience with IPMN predominantly involving the MPD. Our analysis shows that these lesions occur mostly in the elderly, are frequently asymptomatic, and very commonly harbor either high-grade dysplasia or invasive cancer. Surgery can be accomplished with very low morbidity and mortality. After resection, the 5-year survival rate is 69%, with death most frequently from reasons unrelated to the IPMN. The estimated 10-year recurrence rate in the pancreas and other sites was 25%. The size and type of the invasive component, lymph node positivity, and a positive resection margin were all important determinants of recurrence and survival.
Acknowledgments
The authors acknowledge Douglas Hayden, PhD, from the Harvard Catalyst, for providing consultation in the statistical analysis and interpretation of data.
Footnotes
Disclosure: Supported by National Institutes of Health/National Cancer Institute grants R01CA169086 and P01CA117969 (to S.T.). The other authors declare no conflict of interest.
References
- 1.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–321. doi: 10.1097/00000658-200109000-00005. discussion 321–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloppel G, Solcia E, Longnecker DS. Histological typing of tumors of the exocrine pancreas: World Health Organization International Histological Classification of Tumors. 2. New York, NY: Springer Verlag; 1996. [Google Scholar]
- 3.Ohhashi K, Murakami Y, Murayama M, et al. Four cases of mucin-producing cancer of the pancreas on specific findings of the papilla of Vater. Prog Dig Endosc. 1982;20:348–351. [Google Scholar]
- 4.Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–1136. doi: 10.1001/archsurg.134.10.1131. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2010;139:708e1–713.e2. doi: 10.1053/j.gastro.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79. doi: 10.1053/j.gastro.2007.05.010. quiz 309–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrell JJ, Fernandez-del Castillo C. Pancreatic cystic neoplasms: management and unanswered questions. Gastroenterology. 2013;144:1303–1315. doi: 10.1053/j.gastro.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 8.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152(Suppl 1):S4–S12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas. 2011;40:364–370. doi: 10.1097/MPA.0b013e31820a5975. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Sahora K, Mino-Kenudson M, Brugge W, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013;258:466–475. doi: 10.1097/SLA.0b013e3182a18f48. [DOI] [PubMed] [Google Scholar]
- 14.Crippa S, Fernandez-Del Castillo C, Salvia R, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213–219. doi: 10.1016/j.cgh.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvia R, Malleo G, Marchegiani G, et al. Pancreatic resections for cystic neoplasms: from the surgeon’s presumption to the pathologist’s reality. Surgery. 2012;152:S135–S142. doi: 10.1016/j.surg.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Sahora K, Fernandez-del Castillo C, Dong F. Not all combined intraductal papillary mucinous neoplasms behave like main-duct lesions: implications of minimal involvement of the main pancreatic duct. Surgery. 2014;156:611–621. doi: 10.1016/j.surg.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adsay NV, Kloppel G, Fukushima N, et al. Intraductal neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. World Health Organization Classification of tumors, pathology, and genetics of tumors of the digestive system. Lyon, France: IARC Press; 2010. pp. 304–313. [Google Scholar]
- 18.Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–799. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa T, Hatori T, Fujita I, et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509–516. doi: 10.1136/gut.2010.210567. [DOI] [PubMed] [Google Scholar]
- 20.Ban S, Naitoh Y, Mino-Kenudson M, et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol. 2006;30:1561–1569. doi: 10.1097/01.pas.0000213305.98187.d4. [DOI] [PubMed] [Google Scholar]
- 21.Mino-Kenudson M, Fernandez-del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011;60:1712–1720. doi: 10.1136/gut.2010.232272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Distler M, Kersting S, Niedergethmann M, et al. Pathohistological subtype predicts survival in patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg. 2013;258:324–330. doi: 10.1097/SLA.0b013e318287ab73. [DOI] [PubMed] [Google Scholar]
- 23.Nara S, Shimada K, Kosuge T, et al. Minimally invasive intraductal papillary-mucinous carcinoma of the pancreas: clinicopathologic study of 104 intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2008;32:243–255. doi: 10.1097/PAS.0b013e3181484f1e. [DOI] [PubMed] [Google Scholar]
- 24.Biankin AV, Kench JG, Biankin SA, et al. Pancreatic intraepithelial neoplasia in association with intraductal papillary mucinous neoplasms of the pancreas: implications for disease progression and recurrence. Am J Surg Pathol. 2004;28:1184–1192. doi: 10.1097/01.pas.0000131556.22382.3c. [DOI] [PubMed] [Google Scholar]
- 25.Ideno N, Ohtsuka T, Kono H, et al. Intraductal papillary mucinous neoplasms of the pancreas with distinct pancreatic ductal adenocarcinomas are frequently of gastric subtype. Ann Surg. 2013;258:141–151. doi: 10.1097/SLA.0b013e31828cd008. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi K, Kanemitsu S, Hatori T, et al. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571–580. doi: 10.1097/MPA.0b013e318215010c. [DOI] [PubMed] [Google Scholar]
- 27.TNM Staging. 7. Adams County, PA: American Joint Committee on Cancer; 2010. [Accessed June 10, 2014]. database online. Available at: http://www.cancerstaging.org. [Google Scholar]
- 28.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Tamura K, Ohtsuka T, Ideno N, et al. Treatment strategy for main duct intraductal papillary mucinous neoplasms of the pancreas based on the assessment of recurrence in the remnant pancreas after resection: a retrospective review. Ann Surg. 2014;259:360–368. doi: 10.1097/SLA.0b013e3182a690ff. [DOI] [PubMed] [Google Scholar]
- 30.Takuma K, Kamisawa T, Anjiki H, et al. Predictors of malignancy and natural history of main-duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2011;40:371–375. doi: 10.1097/MPA.0b013e3182056a83. [DOI] [PubMed] [Google Scholar]
- 31.Mohri D, Asaoka Y, Ijichi H, et al. Different subtypes of intraductal papillary mucinous neoplasm in the pancreas have distinct pathways to pancreatic cancer progression. J Gastroenterol. 2012;47:203–213. doi: 10.1007/s00535-011-0482-y. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Jang KT, Mo Park S, et al. Prognostic relevance of pathologic subtypes and minimal invasion in intraductal papillary mucinous neoplasms of the pancreas. Tumour Biol. 2011;32:535–542. doi: 10.1007/s13277-010-0148-z. [DOI] [PubMed] [Google Scholar]
- 33.Correa-Gallego C, Do R, Lafemina J, et al. Predicting dysplasia and invasive carcinoma in intraductal papillary mucinous neoplasms of the pancreas: development of a preoperative nomogram. Ann Surg Oncol. 2013;20:4348–4355. doi: 10.1245/s10434-013-3207-z. [DOI] [PubMed] [Google Scholar]
- 34.Abdeljawad K, Vemulapalli KC, Schmidt CM, et al. Prevalence of malignancy in patients with pure main duct intraductal papillary mucinous neoplasms. Gastrointest Endosc. 2014;79:623–629. doi: 10.1016/j.gie.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Nakata K, Ohuchida K, Aishima S, et al. Invasive carcinoma derived from intestinal-type intraductal papillary mucinous neoplasm is associated with minimal invasion, colloid carcinoma, and less invasive behavior, leading to a better prognosis. Pancreas. 2011;40:581–587. doi: 10.1097/MPA.0b013e318214fa86. [DOI] [PubMed] [Google Scholar]
- 36.Yopp AC, Katabi N, Janakos M, et al. Invasive carcinoma arising in intraductal papillary mucinous neoplasms of the pancreas: a matched control study with conventional pancreatic ductal adenocarcinoma. Ann Surg. 2011;253:968–974. doi: 10.1097/SLA.0b013e318214bcb4. [DOI] [PubMed] [Google Scholar]
- 37.Kang MJ, Lee KB, Jang JY, et al. Evaluation of clinical meaning of histological subtypes of intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2013;42:959–966. doi: 10.1097/MPA.0b013e31827cddbc. [DOI] [PubMed] [Google Scholar]
- 38.Ingkakul T, Sadakari Y, Ienaga J, et al. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg. 2010;251:70–75. doi: 10.1097/SLA.0b013e3181c5ddc3. [DOI] [PubMed] [Google Scholar]
- 39.Couvelard A, Sauvanet A, Kianmanesh R, et al. Frozen sectioning of the pancreatic cut surface during resection of intraductal papillary mucinous neoplasms of the pancreas is useful and reliable: a prospective evaluation. Ann Surg. 2005;242:774–778. doi: 10.1097/01.sla.0000188459.99624.a2. discussion 778–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White R, D’Angelica M, Katabi N, et al. Fate of the remnant pancreas after resection of noninvasive intraductal papillary mucinous neoplasm. J Am Coll Surg. 2007;204:987–993. doi: 10.1016/j.jamcollsurg.2006.12.040. discussion 993–995. [DOI] [PubMed] [Google Scholar]
- 41.Fujii T, Kato K, Kodera Y, et al. Prognostic impact of pancreatic margin status in the intraductal papillary mucinous neoplasms of the pancreas. Surgery. 2010;148:285–290. doi: 10.1016/j.surg.2010.03.013. [DOI] [PubMed] [Google Scholar]