Abstract
Objective
Intraductal papillary mucinous neoplasm (IPMN) consists of four epithelial subtypes that correlate with histological grades and risks for malignant transformation. mAb Das-1 is a monoclonal antibody against a colonic epithelial phenotype that is reactive to premalignant conditions of the upper GI tract. We sought to assess the ability of mAb Das-1 to identify IPMN with high risk of malignant transformation.
Design
mAb Das-1 reactivity was evaluated in 94 patients with IPMNs by immunohistochemistry. Lesional fluid from 38 separate patients with IPMN (n=27), low-grade non-mucinous cystic neoplasms (n=7) and pseudocysts (n=4) was analysed by ELISA and western blot.
Results
Immunohistochemistry
Normal pancreatic ducts were non-reactive and low-grade gastric-type IPMN (IPMN-G) (1/17) and intermediate-grade IPMN-G (1/23) were minimally reactive with mAb Das-1. In contrast, mAb Das-1 reactivity was significantly higher in high-risk/malignant lesions (p<0.0001) including: intestinal-type IPMN with intermediate-grade dysplasia (9/10); high-grade dysplasia of gastric (4/7), intestinal (12/12), oncocytic (2/2) and pancreatobiliary types (2/2); and invasive tubular (8/12), colloid (7/7) and oncocytic (2/2) carcinoma. The sensitivity and specificity of mAb Das-1 for high-risk/malignantIPMNs were 85% and 95%, respectively.
Lesional fluid
Samples from low- and intermediate-grade IPMN-G (n=9), and other low-grade/benign non-mucinous lesions demonstrated little reactivity with mAb Das-1. Conversely, cyst fluid from high-risk/malignant IPMNs (n=18) expressed significantly higher reactivity (p<0.0001). The sensitivity and specificity of mAbDas-1 in detecting high-risk/malignant IPMNs were 89% and 100%, respectively.
Conclusions
mAb Das-1 reacts with high specificity to tissue and cyst fluid from high-risk/malignant IPMNs and thus may help in preoperative clinical risk stratification.
INTRODUCTION
Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas are characterised by intraductal proliferation of neoplastic mucinous cells with various degrees of cytological atypia, which usually form papillae and lead to cystic dilatation of pancreatic ducts, forming clinically detectable masses.1 Since the first description of IPMNs,2 these lesions have been recognised with increasing frequency, accounting for up to 20% of all resected pancreatic specimens in large referral centres.3 Similarly, a recent study of 2832 consecutive abdominal CT scans undertaken for indications unrelated to pancreatic disease found a prevalence of asymptomatic pancreatic cysts to be 2.6% among all patients and 8.7% among those above the age of 80.4
Macroscopically, IPMN is classified into main-duct, branch-duct, and mixed types based on the differential involvement of the pancreatic duct system. We have shown that main-duct and mixed-type IPMNs are more likely to have invasive carcinoma compared with branch-duct type (48% and 42% vs 11%) and, subsequently, 5-year disease specific survival rates of main-duct and mixed-type IPMNs are significantly lower than that of branch-duct type (65% and 77% vs 91%).5 Histologically, IPMN is thought to progress from low-grade dysplasia (adenoma) to intermediate- and high-grade dysplasia (carcinoma in situ) and invasive carcinoma.3,6 While the 5-year survival of patients with resected non-invasive IPMN is as high as 77%–94%, invasive IPMN carries a poorer survival of 33%–43%.3,7-10 Given the significant difference in survival between invasive and non-invasive IPMNs, as well as between main-duct and branch-duct IPMNs, clinical guidelines have been adopted to assist clinicians in determining when a lesion should be surgically resected.11 However, while sensitive (97%–100%), these guidelines have proven to be highly non-specific (23%–30%), especially among branch-duct IPMN.12-14 The guidelines have been recently modified in order to improve the specificity, but their performance is yet unknown.15 Given the prevalence of asymptomatic cysts in an elderly population who tend to have substantial clinical comorbidities, more specific tools that can segregate high-risk/malignant from low-risk lesions are needed. Evaluation of cyst fluid cytologically for high-grade atypical epithelial cells appears to improve specificity; however, interpretation requires expertise and not all fluid from high-risk cysts contain epithelial cells that can be evaluated.16 In an effort to improve diagnostic accuracy, analyses of cyst fluid for genetic changes have been used and several biomarkers including Plectin-1 have been investigated.17,18 However, more specific markers of clinically high-risk lesions are needed to aid in the preoperative diagnosis and risk stratification of patients with IPMN.
Recently, morphological variations of IPMN have been recognised and criteria established for distinguishing IPMN into four distinct epithelial subtypes: gastric, intestinal, pancreatobiliary and oncocytic.19 Similarly, invasive carcinoma arising in IPMN (invasive IPMN) has also been morphologically classified into colloid, tubular and oncocytic carcinomas.20,21 Of those, the gastric type (IPMN-G) comprises the majority of branch-duct IPMN, and rarely exhibits high-grade dysplasia (carcinoma in situ). Invasion is uncommon, but when it occurs, it is usually of the tubular type. The intestinal type (IPMN-I) that makes up the majority of the main-duct IPMN often exhibits intermediate- to high-grade dysplasia and is prone to developing invasive carcinoma. Given its propensity to involve the main duct and to develop invasive carcinoma, IPMN-I, even of intermediate-grade, may warrant surgical intervention. Both pancreatobiliary (IPMN-PB) and oncocytic (IPMN-O) types are rare, but typically demonstrate high-grade dysplasia and often contain invasive or minimally invasive carcinoma.22
Using a colon epithelial protein (CEP), we have previously developed a novel murine monoclonal antibody, mAb Das-1 (formerly known as 7E12H12, IgM isotype), which reacts specifically with normal colonic epithelium.23 Both via immunoper-oxidase and immunofluorescence assays, we and others, have independently demonstrated that mAb Das-1 specifically reacts with both non-goblet and goblet cell colonic epithelium, but not with normal small intestinal enterocytes from the duodenum, jejunum or ileum.23,24 mAb Das-1 reactivity is similarly absent from normal pancreatic, gastric and oesophageal mucosa as well as benign oesophagitis and gastritis. However, it is strongly expressed in preneoplastic and intestinal phenotypic changes in these organs as it is highly sensitive and specific for the detection of Barrett’s oesophagus, incomplete type gastric intestinal metaplasia and cancer developed therefrom.25-29
In the present study, we explore the ability of mAb Das-1 to distinguish intestinal-type IPMNs and high-grade dysplasia of other types with or without associated invasive carcinoma from low-risk IPMNs (IPMN-G with low- or intermediate-grade dysplasia). We examined the immunoreactivity of the mAb against tissue with all epithelial subtypes of IPMN of varying grades of dysplasia as well as invasive carcinomas associated with IPMN. Finally, we analysed lesional fluid aspirates from an additional cohort of IPMNs, benign/low-grade cystic neoplasms and pseudocysts of the pancreas for the quantitative expression of CEP, the target protein of mAb Das-1, in order to examine the potential of Das-1 expression to assist in the preoperative diagnosis and risk stratification of patients with IPMN.
MATERIALS AND METHODS
Study subjects
The Massachusetts General Hospital and Rutgers-Robert Wood Johnson Medical School institutional review boards approved this study. A total of 306 patients with surgically resected, pathologically confirmed IPMN between January 1990 and December 2010 were identified from a prospectively collected database of Massachusetts General Hospital. Of those, material from 94 patients was used on the basis of availability of tissue blocks in an attempt to encompass all main epithelial subtypes and all histological grades (see online supplementary figure 1). Normal pancreatic ducts adjacent to the relevant IPMN lesion were identified in the majority of the cases and served as an internal control. Comparison of the examined (n=94) and unexamined (n=212) patients in the histology cohort demonstrated no significant difference in histological grade, or macroscopic or epithelial subtype (see online supplementary table 1).
Lesional fluid was collected from a separate cohort of 38 patients with IPMN, non-mucinous cystic neoplasms and pancreatic pseudocysts between July 2010 and February 2012 (see online supplementary figure 1). Fluid was aspirated peri-operatively from IPMNs with (n=6) or without (n=21) invasive carcinoma, serous cystadenomas (n=4), solid pseudopapillary neoplasms (n=2), and cystic, low-grade neuroendocrine tumour (n=1). In addition, fluid from benign pancreatic pseudocysts (n=4) was obtained by routine endoscopic ultrasound and cyst aspiration. Final diagnosis was verified with the pathological assessment of the resected specimens. The 27 IPMN cases included in this study were selected from 80 IPMNs surgically resected during the study period depending on availability of cyst fluid (see online supplementary figure 1). Among lesional fluid from IPMN, there was no significant difference in the distribution of dysplastic grades and epithelial and macroscopic subtypes of examined cases (n=27) and unexamined cases (n=53) (see online supplementary table 2).
Pathological evaluation of tissue specimens
For the purpose of this study, all the IPMN cases were examined independently of the original diagnosis and reactivity with mAb Das-1 by one of the authors specialised in the field (MM-K), and classified into 4-tiered dysplastic grades (low-, intermediate- and high-grade and invasive carcinoma). Epithelial subtypes were determined on the basis of their epithelial morphology on routine H&E staining and, when available, immunoreactivity against mucin glycoproteins according to previously established criteria.19 There was discordant interpretation of dysplastic grades and/or epithelial subtypes between the original diagnosis and re-evaluation in 25% cases, and consensus interpretation among the pathologists was captured in those cases.
IPMN lesions were evaluated on a per-patient basis, based on the most predominant epithelial subtype, and the highest grade lesion demonstrated. There were 73 non-invasive and 21 invasive IPMNs in the cohort. The non-invasive IPMNs consisted of 47 gastric-type, 22 intestinal-type, two oncocytic-type, and two pancreatobiliary-type lesions, encompassing all epithelial subtypes of IPMN. The invasive IPMNs were classified into: tubular adenocarcinoma (n=12), colloid carcinoma (n=7) and oncocytic carcinoma (n=2). The type of duct involvement by IPMN was determined by macroscopic and microscopic examinations as well as by imaging studies, and was classified into main-duct, branch-duct and mixed-type IPMNs (table 1).11 For the purpose of this study, we classified each IPMN lesion into two categories (low-risk (IPMN-G with low- or intermediate-grade dysplasia) versus high-risk and malignant (IPMN-G with high-grade dysplasia, IPMN-I, IPMN-O, IPMN-PB and invasive IPMN)) based on the reported clinicopathological characteristics and prognosis of each epithelial subtype (table 2).
Table 1.
Clinicopathological features of histology study cohort (n=94)
| Macroscopic subtype | Number (%) | Histological grade | Number (%) | Epithelial subtype | Number (%) |
|---|---|---|---|---|---|
| Main duct | 10 (10.6) | Low | 17 (18.1) | Gastric | 47 (50.0) |
| Mixed type | 48 (51.1) | Intermediate | 33 (35.1) | Intestinal | 22 (23.4) |
| Branch duct | 36 (38.3) | High | 23 (24.5) | Oncocytic | 2 (2.1) |
| Invasive | 21 (22.3) | Pancreatobiliary | 2 (2.1) | ||
| Tubular adenocarcinoma | 12 (12.8) | ||||
| Colloid carcinoma | 7 (7.5) | ||||
| Oncocytic carcinoma | 2 (2.1) |
Table 2.
mAb Das-1 reactivity in patients with intraductal papillary mucinous neoplasm (IPMN) or invasive IPMN by epithelial subtype and grade of dysplasia
| Total* | Low-risk
|
High-risk and malignant
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IPMN-G
|
IPMN-I
|
IPMN-O | IPMN-PB | Invasive IPMN
|
||||||
| LGD | IGD | HGD | IGD | HGD | HGD | HGD | Tubular | Colloid | Onc | |
| 17 | 23 | 7 | 10 | 12 | 2 | 2 | 12 | 7 | 2 | |
| mAb Das-1 expression | ||||||||||
| Grade 1 | 1 | 0 | 1 | 2 | 2 | 0 | 1 | 1 | 0 | 0 |
| Grade 2 | 0 | 1 | 1 | 3 | 0 | 1 | 0 | 1 | 4 | 2 |
| Grade 3 | 0 | 0 | 2 | 4 | 10 | 1 | 1 | 6 | 3 | 0 |
| All | 1 | 1 | 4 | 9 | 12 | 2 | 2 | 8 | 7 | 2 |
| 5.9% | 4.3% | 57% | 90% | 100% | 100% | 100% | 67% | 100% | 100% | |
| p Value† | 0.003 | <0.0001 | <0.0001 | 0.007 | 0.007 | <0.0001 | <0.0001 | 0.007 | ||
Performance for high-risk and malignant IPMN cases: sensitivity 85%, specificity 95%.
Each patient was classified into the 10 categories based on the most prevalent epithelial subtype of the highest dysplastic grade.
Each category was compared with low- and intermediate-grade IPMN-G.
Colloid, colloid carcinoma; HGD, high-grade dysplasia; IGD, intermediate-grade dysplasia; IPMN-G, gastric-type IPMN; IPMN-I, intestinal-type IPMN; IPMN-O, oncocytic-type IPMN; IPMN-PB, pancreatobiliary-type IPMN; LGD, low-grade dysplasia; Onc, oncocytic carcinoma; Tubular, tubular adenocarcinoma.
Immunohistochemistry using mAb Das-1
Serial 5 μm sections were obtained from all examined tissue blocks and H&E stained sections were reviewed to ensure the presence of representative lesions in the same block. Tissue sections from each lesion were examined with mAb Das-1 using a sensitive immunoperoxidase assay, as described previously.25-28 Each experiment also included at least two slides of normal colon and duodenal tissue sections as positive and negative controls, respectively (figure 1A, B). Non-neoplasic pancreatic tissue with or without IPMN-related pancreatitis and normal pancreatic ducts served as internal controls (figure 1C, starred). Reactivity to mAb Das-1 was considered positive if a crisp golden brown staining of cells was present. Two investigators (KKD and MM-K) reviewed each slide together. Samples were graded based on the percentage of affected cells that were positive in each relevant IPMN lesion: grade 0 (negative) <5%, grade 1 5%–25%, grade 2 25%–50% and grade 3 >50%.
Figure 1.
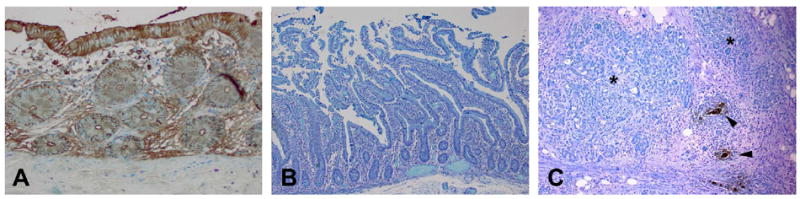
Immunoperoxidase staining of mAb Das-1 with normal colon (A), normal duodenum (B) and invasive intraductal papillary mucinous neoplasm (IPMN) with associated pancreatitis (C). Strong reactivity is evident against the nests of invasive IPMN (arrowhead), whereas adjacent chronic pancreatitis (*) and normal pancreatic ducts did not show any reactivity. Colonic epithelium reacts strongly with mAb Das-1 both in the cytoplasm and also along the periphery of the cells (membrane domain). Duodenal epithelium did not show any reactivity.
Western blot and ELISA analyses of cyst fluid aspirates for mAb Das-1
Pancreatic cyst fluid aspirates were aliquoted and frozen at −80°C. The samples were de-identified and the researchers conducting the assays were blinded to their clinical pathological diagnosis. Fluid was assayed for total protein concentration and all samples were normalised to equal protein amount. When necessary, mucinous cyst material was dissolved by alkalinisation.
Western blot analysis of cyst fluid was completed by standard method, as previously described with the mAb Das-1 IgM antibody.30,31 Pancreatic cyst fluid was normalised by protein amount and 25 μg was used for each sample. Some of the samples showed very high concentration of CEP protein on initial western blot experiments and so they were sequentially diluted to allow for improved visualisation of the band. Enriched CEP (2 μg) was run in parallel as a positive control and the specific MOPC-IgM was used in parallel as a negative control. Reactivity was detected by chemiluminescence method with mAb Das-1 (ascites 1:1000 dilution).
Sandwich ELISA was performed with mAb Das-1 IgM and mAb Das-1 IgG isotypes. We have previously described the isotype switching of the mAb Das-1 IgM antibody to an IgG isotype.32 The ELISA plate was coated with 1 μg of mAb Das-1 IgM in each well overnight at 4°C. The plate was sequentially incubated with blocking buffer (1% normal goat serum, 1% BSA in PBS), 100 μg of cyst fluid samples or 0.5 μg of enriched CEP protein for positive control, 0.5 μg of mAb Das-1 IgG (anti-CEP) antibody, and AP-conjugated anti-mouse IgG antibody (1:20 000) at 37°C. Each incubation was for 60 min except for the primary antibody (90 min), and optical density (OD) was detected at 405 nm. All experiments were conducted in triplicate and normalised with respect to reactivity of the positive control.
Statistical analysis
Das-1 expression was compared between low-risk IPMN (IPMN-G with low- or intermediate-grade) and other individual epithelial subtypes using Fisher’s exact test. Das-1 reactivity was also correlated with the other clinicopathological parameters by Fisher’s exact test. Statistical significance among fluid specimens was calculated by Mann–Whitney test. All mean values are displayed with error bars indicating SD and p<0.05 was considered statistically significant. The analyses were performed with GraphPad Prism 6.0 statistical software (GraphPad Software San Diego, California, USA). In accordance with guidelines for reporting cancer biomarkers, STARD and REMARK guidelines were observed (see online supplementary table 3).
RESULTS
Study population
Of the 94 patients, 48 (51%) were men and age ranged from 37 to 90 years (mean 68 years). The main pancreatic duct was involved by IPMN in 58 patients (10 main-duct type and 48 mixed type) and the lesion was confined to branch ducts in 36 patients (table 1). There was no significant difference in Das-1 expression in the other clinicopathological parameters including age (p=0.811), sex (p=0.216) and type of duct involvement (branch-duct type vs main-duct and mixed type, p=0.198).
mAb Das-1 is specific for high-risk and malignant IPMN lesions
All normal pancreatic duct controls were negative for Das-1. Pancreatic parenchyma including IPMN associated pancreatitis (figure 1C, starred) was also non-reactive in all the samples examined. When reactive to mAb Das-1, the lesional epithelium of IPMNs exhibited intense staining in cytoplasmic and/or membranous patterns. Overall, lesions from 48 (51%) patients were considered positive using the cut-off of 5% (of the lesional cells positive for Das-1).
The results of the mAb Das-1 reactivity are summarised in table 2. Among IPMN-G, only 1 (5.9%) of 17 with low-grade dysplasia and 1 (4.3%) of 23 with intermediate-grade dysplasia were reactive to mAb Das-1. Conversely, IPMN-G with high-grade dysplasia reacted in 4 (57%) of seven cases (figure 2). Compared with IPMN-G with low-or intermediate-grade dysplasia, mAb Das-1 expression was significantly higher in IPMN-G with high-grade dysplasia (p=0.003). Among IPMN-I, 9 (90%) of 10 patients with intermediate-grade dysplasia (p<0.0001) were positive for Das-1 as were 12 (100%) of 12 with high-grade dysplasia (p<0.0001) (figure 3A). IPMN-O demonstrated reactivity in 2 (100%) of two cases (p=0.007) (figure 3B). Similarly, IPMN-PB was positive in 2 (100%) of two cases (p=0.007).
Figure 2.
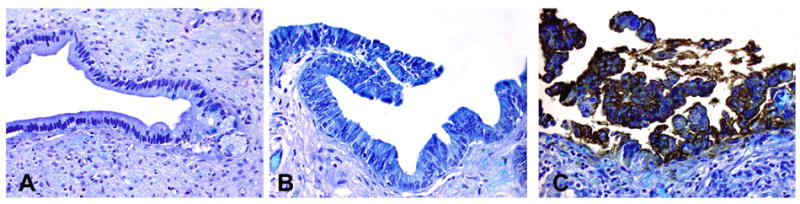
Immunoperoxidase staining of mAb Das-1 against gastric-type intraductal papillary mucinous neoplasm (IPMN-G) of varying dysplasia. IPMN-G with low- (A) and intermediate-grade (B) dysplasia did not react. However, IPMN-G with high-grade (C) dysplasia showed intense membranous and cytoplasmic staining.
Figure 3.
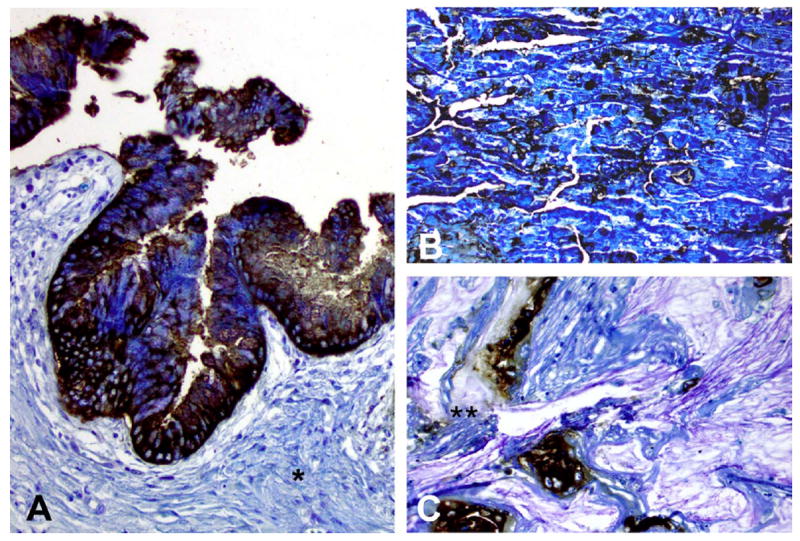
Immunoperoxidase staining of mAb Das-1 against intestinal-type intraductal papillary mucinous neoplasm (IPMN) (A), oncocytic-type IPMN (B) and invasive IPMN with colloid carcinoma (C). mAb Das-1 reacted strongly with both cytoplasmic and membranous staining in high-grade lesions of both intestinal and oncocytic subtypes of IPMN (A and B). Of note, the stroma adjacent to the IPMN lesion (*) is not reactive to mAb Das-1. Among invasive IPMN with colloid carcinoma, staining was evident both in the carcinomatous cells as well as in secreted mucinous material (**).
Among invasive IPMNs, mAb Das-1 was positive in 8 (67%) of 12 tubular adenocarcinomas (p<0.0001), 7 (100%) of seven colloid carcinomas (p<0.0001) and 2 (100%) of two oncocytic carcinomas (p=0.007). Das-1 staining among invasive IPMN samples was intense, often diffuse and mostly cytoplasmic. Interestingly, Das-1 expression was seen both in carcinomatous cells as well as in secreted mucinous material in colloid carcinomas (figure 3C, double starred).
Overall, the sensitivity and specificity of Das-1 in segregating high-risk and malignant IPMNs (IPMN-G with high-grade dysplasia, IPMN-I, IPMN-O, IPMN-PB and invasive IPMN) from low-risk lesions (IPMN-G with low- or intermediate-grade dysplasia) was 85% (95% CI 0.73 to 0.93) and 95% (95% CI 0.83 to 0.99), respectively, with a likelihood ratio of 17.04 (table 2).
mAb Das-1 is significantly overexpressed in pancreatic cyst fluid from high-risk and malignant IPMNs in comparison with low-risk lesions
The lesional fluid from pseudocysts (n=4) and low-grade non-mucinous cystic neoplasms (n=7) demonstrated very little reactivity with mAb Das-1 by sandwich ELISA assay (OD 0.005 ±0.005 and 0.062±0.071, respectively). Low- and intermediate-grade IPMN-G similarly showed little reactivity (n=9, OD 0.097±0.086). Conversely, high-risk and malignant IPMN lesions (n=18) expressed a significantly higher amount of reactivity (OD 1.082±0.752) when compared with low- and intermediate-grade IPMN-G (p<0.0001) and all benign pancreatic lesions (p<0.0001) (figure 4). When stratified by subtype, high-grade IPMN-O (n=2, OD 0.402±0.354), IPMN-G with high-grade dysplasia or invasive carcinoma (n=4, OD 0.691±0.374), IPMN-I with intermediate-grade dysplasia (n=4, OD 0.810±0.386), IPMN-I with high-grade dysplasia (n=3, OD 1.215±0.676) and invasive carcinomas arising in IPMN-I (n=5, OD 1.806±0.893) demonstrated a progressive increase in reactivity to mAb Das-1 among each subtype. When the cut-off OD value was set at 0.270 (the mean+2 SD of low-risk IPMN-G), two high-risk IPMN lesions were interpreted as negative: a case with intermediate-grade IPMN-I exhibiting focal intestinal-type epithelium in a broad background of gastric-type epithelium, and a case with IPMN-O (that clinically presented with a mural nodule). Overall, the sensitivity and specificity of mAb Das-1 for segregating high-risk IPMNs from low-risk IPMNs were 89% (95% CI 0.65 to 0.99) and 100% (95% CI 0.66 to 1.0), respectively, and for segregating high-risk IPMNs from all the low-risk/benign lesions were 89% (95% CI 0.65 to 0.99) and 100% (95% CI 0.83 to 1.0), respectively.
Figure 4.
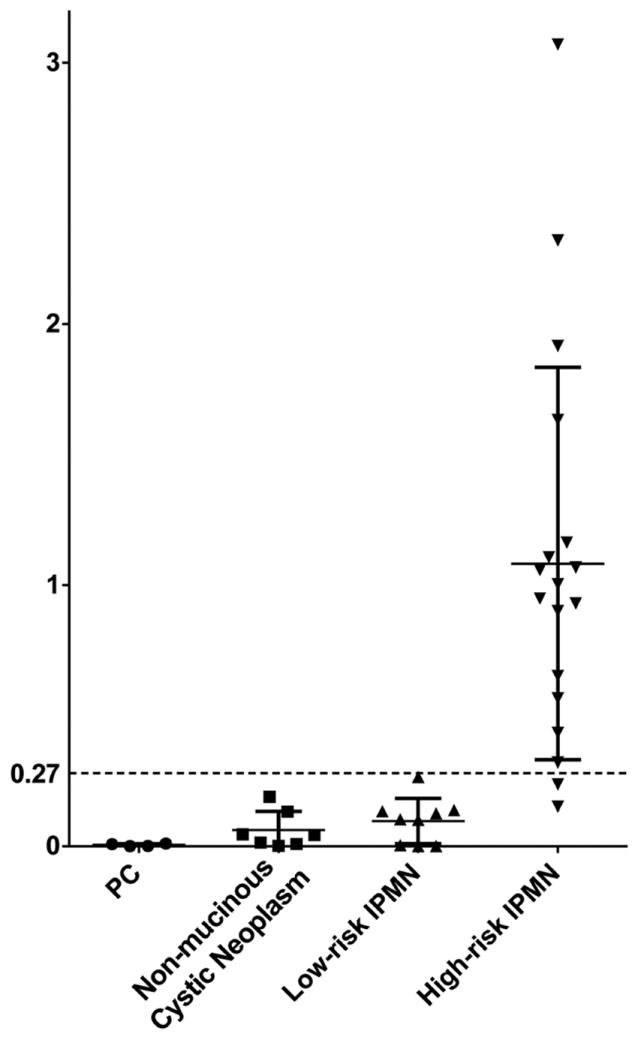
Lesional fluid immunoreactivity against mAb Das-1 by ELISA. Optical density values as determined by ELISA in high-risk intraductal papillary mucinous neoplasm (IPMN) (high-grade gastric-type IPMN (IPMN-G), intermediate- and high-grade intestinal-type IPMN, oncocytic-type IPMN, and invasive IPMN) (n=18), low-risk IPMN (low- and intermediate-grade gastric-type IPMN) (n=9), non-mucinous cystic neoplasm (serous cyst adenoma, solid pseudopapillary neoplasm, and cystic, low-grade neuroendocrine tumour) (n=7) and pancreatic pseudocysts (PC, n=4). Bars indicate the mean and SD in each column. Reactivity of mAb Das-1 from lesional fluid from high-risk IPMN was significantly higher than that from low-risk IPMN (p<0.0001) and all low-grade/benign lesions (p<0.0001). Using a cut-off of 0.27 (the mean+2SD of low-risk IPMN-G), the sensitivity and specificity of Das-1 for segregating high-risk IPMNs from low-risk IPMNs were 89% and 100%, respectively, and for segregating high-risk IPMNs from all the low-risk/benign lesions were 89% and 100%, respectively.
All ELISA results were confirmed by western blot analysis as well. Specifically, examination of representative samples of cyst fluid by western blot analysis demonstrates a progressive increase in mAb Das-1 reactivity from low-grade IPMN-G to high-grade IPMN-G and to minimally invasive IPMN-I lesions (figure 5A, Lanes 1, 2, 3 respectively). Whereas 25 μg of total protein was required for IPMN-G samples to demonstrate a clear band, IPMN-I samples were sequentially diluted to 1 μg of total protein to achieve a similar result because of the high concentration of CEP in these samples (figure 5A). All cyst fluid was correlated histopathologically to the corresponding resected surgical specimen (figure 5B).
Figure 5.
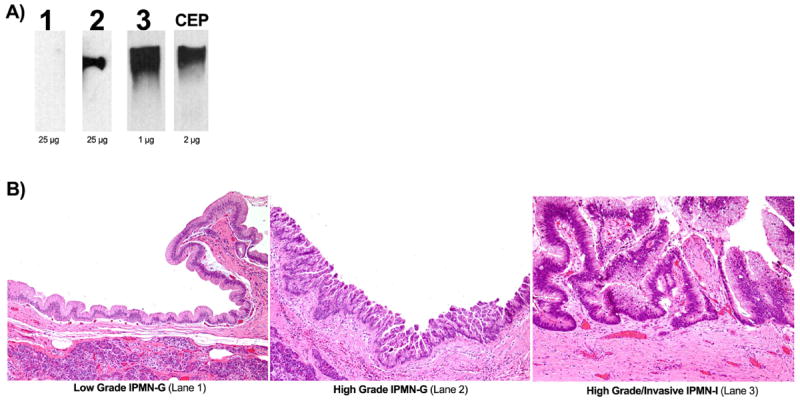
Lesional cyst fluid immunoreactivity against mAb Das-1 by western blot analysis. (A) Lane 1 contains cyst fluid from a patient with low-grade gastric-type intraductal papillary mucinous neoplasm (IPMN); Lane 2 contains cyst fluid from high-grade gastric-type IPMN; Lane 3 contains cyst fluid from high-grade intestinal-type IPMN with minimal invasion; Lane 4 contains enriched CEP, the mAb Das-1 antigen. Cyst fluid in Lanes 1 and 2 were run with 25 μg of total protein, whereas the fluid in Lane 3 had to be diluted to 1 μg of total protein to visualise the band clearly. This suggests a significantly higher amount of CEP in high-grade lesions. (B) H&E staining of the surgically resected specimens corresponding to the pancreatic cyst fluid analysed in A—Lanes 1, 2 and 3— are shown here.
DISCUSSION
Although the frequency of diagnosis of IPMN continues to increase, there remain few biomarkers available to distinguish low-risk from high-risk and malignant IPMNs in the preoperative setting. Here we demonstrate that mAb Das-1, a monoclonal antibody reactive to a colon epithelial specific antigen, is a potential novel biomarker to identify both high-risk and malignant IPMN lesions. Indeed, in our tissue cohort encompassing all epithelial and invasive subtypes and histological grades of IPMN, mAb Das-1 was able to distinguish high-risk and malignant lesions with 85% sensitivity and 95% specificity compared with low-risk lesions. In addition, we were able to demonstrate the specific detection of the mAb Das-1 antigen, CEP,30 in cyst fluid from IPMN by both ELISA and western blot analyses with 89% sensitivity and 100% specificity for high-risk/malignant IPMN.
There are several limitations of our study. As our patient cohort was entirely from a single tertiary referral centre, there may be a referral or treatment access bias. In our tissue cohort, our 94 cases were chosen non-randomly on the basis of availability and a desire to encompass all epithelial subtypes and histological grades. However, we have evaluated the distribution of epithelial subtypes, macroscopic subtypes, and dysplastic grades between the examined patients and the unexamined members of this cohort and found no statistical difference in our patient selection (see online supplementary table 1). We have also performed similar statistical analysis on our pancreatic fluid cohort which similarly showed no sign of bias (see online supplementary table 2). In our histological analysis there was discordant interpretation of dysplastic grades and/or epithelial subtypes between the original diagnosis and re-evaluation in 25% cases, possibly reflecting generally moderate interobserver concordance in areas of pathology in which dysplastic lesions on a spectrum are assigned into distinct categories. Preoperative carcinoembryonic antigen (CEA) measurements in the lesional fluid were not available in the majority of our cohort. As a result, we were unable to correlate mAb Das-1 expression to CEA in this study. However, recent evidence has suggested that CEA is not effective in discerning the malignant potential of cystic pancreatic lesions. Rather, its primary clinical utility seems to be in distinguishing mucinous lesions (ie, IPMN and mucinous cyst neoplasms) from non-mucinous lesions (ie, pseudocyst and serous cyst adenomas).33 What we have demonstrated histopathologically as well as in cyst fluid is that expression of mAb Das-1 segregates clinically high-risk IPMNs from low-risk lesions by immunohistochemistry, ELISA and western blot analysis.
Examination of mAb Das-1 reactivity in foetal tissues by Badve et al demonstrated expression of the Das-1 antigen in organs arising from the primitive gut (oropharynx, lung, oesophagus, stomach, biliary tree, pancreas, liver, intestine) and ureteric bud.34 Interestingly, studies by ourselves and others have demonstrated that while Das-1 is expressed in the foetal oesophagus, stomach, small bowel and bladder, it is lost in the respective adult organs, and reappears in precancerous and cancerous conditions like Barrett’s oesophagus/oesophageal adenocarcinoma,25,28 incomplete-type gastric intestinal metaplasia/gastric adenocarcinoma,26,29 small intestinal adenomas/adenocarcinoma35 and cystitis profunda/bladder adenocarcinoma.36 These findings suggest that mAb Das-1 has potential in serving as a novel oncofetal marker. While expression of Das-1 is present in fetal pancreatic tissue and absent in adult pancreata,34 in this paper we demonstrate that expression returns in IPMN increasing based on the degree of dysplasia and associated adenocarcinoma. Further analysis of mAb Das-1 reactivity in sporadic pancreatic ductal adenocarcinomas, and malignant conditions arising from other organs in which Das-1 is expressed in the foetal stages, may both advance our knowledge of pathophysiological mechanisms of malignant transformation and have diagnostic or prognostic potential.
IPMN follow a classical adenoma-carcinoma sequence, progressing from low-grade and intermediate-grade dysplasia to carcinoma in situ and invasive carcinoma. We found that mAb Das-1 expression was preferentially expressed in higher-grade lesions. While mAb Das-1 reacted with low- and intermediate-grade IPMN-G only among 5% of samples respectively, the antibody reacted with 57% of IPMN-G with high-grade dysplasia. Similarly, the antibody reacted with 100% of IPMN-I with high-grade dysplasia in comparison with 90% of IPMN-I with intermediate-grade dysplasia. Among invasive IPMNs, staining with mAb Das-1 significantly reacted with 67% of tubular carcinomas and 100% of colloid and oncocytic carcinomas, an interesting finding given the recent evidence we have demonstrated that IPMN-I predominantly progresses to colloid carcinoma as opposed to IPMN-G which often progresses to tubular carcinoma.37 With regard to the performance of the immunohistochemistry assay, approximately 56% of positive samples stained at a grade 3 level (>50% of affected glands stained positive) and the vast majority (83%) of samples stained at a grade 2 level or higher (>25% of affected glands).
With a specificity of detecting high-risk and malignant IPMNs of 95% in tissue samples, mAb Das-1 offers significantly improved specificity over current clinical guidelines. While highly sensitive (97%–100%), these guidelines have proven to be highly non-specific (23%–30%) in validation cohorts.12-14 Further studies need to be performed to evaluate if combining mAb Das-1 with highly sensitive clinical guidelines or other preoperative biomarkers may enhance the overall performance characteristics. Clinical difficulty particularly arises in the management of branch-duct IPMN lesions, which are often small in size, IPMN-G in epithelial subtype, and frequently associated with little invasion and fair survival.38 However, some studies have suggested a close to 20% 10-year risk of developing malignant transformation in branch-duct IPMN39 and their prognosis can be dismal once invasive cancer has developed.37 A few small studies have demonstrated that panels of microRNAs may be useful in differentiating invasive from non-invasive IPMNs.40 A recent publication has evaluated pancreatic juice for GNAS, demonstrating high specificity for detection of IPMN even in small lesions, and in several cases without clinically apparent IPMN.41 However, while GNAS may be associated with even preclinical manifestations of cystic transformation of the pancreatic duct, it does not appear to correlate to epithelial subtype, histological grade and ultimately disease prognosis.41 Mutant TP53 has also been recently reported in microdissected pancreatic intraepithelial neoplasias (PanINs), IPMN and pancreatic ductal adenocarcinomas; however, its presence in IPMN was found in only 2/22 (9.1%) cases with intermediate-grade dysplasia and 8/21 (38.1%) cases with high-grade dysplasia.42 Similar rates of detection were noted in secretin-stimulated pancreatic juice in patients with intermediate-grade PanIN/IPMN and high-grade PanIN/IPMN (combined cohorts) (1/15 (6.7%) vs 4/8 (50%)).42 In order to capture high-risk or high-grade IPMN lesions before developing invasion to help guide clinical decision-making, markers that correlate more closely with degree of dysplasia may be warranted. mAb Das-1 may be of particular utility in distinguishing those low- and intermediate-grade branch-duct IPMN-G of limited clinical significance from the high-grade IPMN-G/IPMN-I/IPMN-O/IPMN-PB that carry a poorer prognosis and would thus benefit from definitive surgical management.
Among cyst fluid samples, we similarly observed a trend of progressively increasing mAb Das-1 reactivity among IPMNs with progressively increasing dysplasia mirroring our findings in tissue specimens. There appears to be a distinct cut-off between low- and intermediate-grade IPMN-G and higher-grade/risk lesions. Using this threshold, we demonstrated 89% sensitivity and 100% specificity of mAb Das-1 for segregating high-risk IPMNs from low-risk/benign lesions. The prevalence of high-grade lesions may be lower in larger, population-based prospective cohorts, causing diagnostic accuracy to be lower than reported here. Thus, a larger validation cohort is necessary to obtain appropriate receiver operating characteristics for mAb Das-1 in evaluating pancreatic cyst fluid and is currently underway.
FNA with cytology is currently the standard practice in the analysis of presurgical cyst fluid samples. Unfortunately, pre-operative evaluation of cytology in lesional fluid was not available in the majority of our cohort. While highly specific in the hands of an experienced GI cytopathologist,43 studies have brought into question its utility when acellular material is aspirated and expert interpretation is not available.44 In our study, cyst fluid samples from IPMN were assayed with high reliability for mAb Das-1 using standard techniques. In fact, among IPMN-I, given the large amount of CEP protein present in the samples, fluid needed to be diluted to 1 μg of total protein to achieve an acceptable result in western blot analysis. To put this in perspective, 2 μg of enriched CEP was required to demonstrate a similar response as a positive control. Similarly, in the ELISA assay, enriched CEP protein yielded a result that was less than that of some invasive IPMN-I derived cyst fluid. From in vitro experiments using the colon cancer cell line LS180, CEP was found to be released from the cells into the culture medium.30 This suggests that CEP is a secretory protein, which explains the intense staining of extracellular mucin in colloid carcinoma as well as the presence of CEP in cyst fluid. Taken together, it is promising that even in acellular cyst fluid aspirates or limited sample materials, mAb Das-1 may still be able to be analysed. Furthermore, in contrast to cytology, it is technically easy to perform these standardised techniques and they are not operator dependent. Validation in a larger cohort of benign and malignant cystic pancreatic lesions with direct comparison with cytology will help in assessing the characteristics of these techniques.
In conclusion, mAb Das-1 is a sensitive and highly specific biomarker for the detection of high-risk and malignant IPMNs. The inclusion of the Das-1 marker into the analysis of tissue and cyst fluid may aid in the preoperative diagnosis and risk stratification of patients with IPMN to plan effective therapeutic strategies.
Supplementary Material
Significance of this study.
What is already known on this subject?
-
▶
Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas comprise distinct morphological subtypes: gastric (IPMN-G), intestinal (IPMN-I), pancreatobiliary (IPMN-PB) and oncocytic (IPMN-O). Similarly, invasive carcinomas arising in IPMN (invasive IPMN) consist of three histological subtypes: tubular, colloid and oncocytic.
-
▶
The risk of developing invasive carcinoma and prognosis of IPMNs correlates with the epithelial morphology as well as histological grade. For instance, IPMN-G carries significantly favourable outcomes in comparison with the other types.
-
▶
Clinical guidelines have been adopted to assist clinicians in determining when a lesion should be surgically resected. However, while sensitive (97%–100%), these guidelines have proven to be highly non-specific (23%–30%), especially among branch-duct IPMN.
-
▶
mAb Das-1 is a monoclonal antibody reactive against a colonic phenotype of epithelium that has been shown to be highly sensitive and specific for premalignant conditions of the upper GI tract including Barrett’s oesophagus and incomplete-type gastric intestinal metaplasia.
What are the new findings?
-
▶
mAb Das-1 reactivity is absent in tissue from normal pancreatic ducts and pancreatitis, and minimally reactive in low-grade (1/17) and intermediate-grade (1/23) IPMN-G. In comparison, mAb Das-1 reacts specifically with tissue from intermediate and high-grade IPMN-I, high-grade IPMN-G, IPMN-O and IPMN-PB, all at high risk of developing invasive carcinoma, as well as tubular, colloid and oncocytic carcinoma arising in IPMN.
-
▶
The sensitivity and specificity of mAb Das-1 in segregating high-risk/malignant IPMN from low-risk lesions were 85% and 95% among pathological specimens.
-
▶
There was a significant difference in mAb Das-1 expression in lesional fluid aspirated from high-risk and malignant IPMN when compared with low-risk IPMN-G (p<0.0001), low-grade, non-mucinous cystic neoplasms of the pancreas (p<0.0001) and pseudocysts (p<0.0001).
-
▶
Among lesional fluid samples, the sensitivity and specificity of Das-1 for segregating high-risk/malignant IPMNs from all the low-risk/benign lesions are 89% and 100%, respectively.
How might it impact on clinical practice in the forseeable future?
-
▶
Evaluation of mAb Das-1 reactivity in cyst fluid samples may be a useful tool to differentiate high-risk/malignant IPMNs from low-risk lesions and help in clinical decision-making.
Acknowledgments
We would like to thank Kelly Walton for her technical advice regarding immunohistochemical staining.
Funding Development of mAb Das-1 was supported in part by research grants NIDDK, R01 DK47673 and R01 DK63618 to KMD from the National Institutes of Health, Bethesda, MD, USA. MMK was supported by National Cancer Institute grants P50 CA127003 and R01 CA169086.
Footnotes
This work was presented in part at the annual meetings of the American Gastroenterological Association 2011 and the USA and Canadian Academy of Pathology 2011, and the American Pancreatic Association/International Academy of Pancreatology 2012 joint meeting.
Contributors Conception and design: KKD, MMK and KMD. Acquisition of data: KKD, HX, XG, CFDC, VMO, ED, DGF, BCB, WRB, MBP and MMK. Analysis and interpretation of data: KKD, MMK and XG. Drafting the manuscript: KKD and MMK. Critical review of the manuscript: HX, XG, CFDC, VMO, ED, DGF, BCB, WRB, MBP and KMD. Final approval of the manuscript: KKD, HX, XG, CFDC, VMO, ED, DGF, BCB, WRB, MBP, MMK and KMD.
Competing interests A patent for the use of mAb Das-1 in the detection of precancerous lesions of the oesophagus has been awarded (KMD) and a patent for its possible use in the detection of pancreatic cancer is currently pending (KKD, MMK, KMD). These patents have not been licensed and these authors hold no commercial interests at this time.
Ethics approval The Massachusetts General Hospital and Rutgers-Robert Wood Johnson Medical School institutional review boards.
Provenance and peer review Not commissioned; externally peer reviewed.
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/gutjnl-2013-306219).
References
- 1.Hruban RH, Pitman MB, Klimstra DS. Intraductal neoplasms. In: Hruban RH, Pitman MB, Klimstra DS, editors. Tumors of the Pancreas. Washington, DC: American Registry of Pathology; 2007. pp. 75–110. [Google Scholar]
- 2.Ohashi K, Murakami Y, Maruyama M. Four cases of mucin-producing cancer of the pancreas on specific findings of the papilla of Vater. Prog Dig Endoscopy. 1982;20:348–52. [Google Scholar]
- 3.Farrell JJ, Brugge WR. Intraductal papillary mucinous tumor of the pancreas. Gastrointest Endosc. 2002;55:701–14. doi: 10.1067/mge.2002.123641. [DOI] [PubMed] [Google Scholar]
- 4.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–7. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crippa S, Fernandez-Del Castillo C, Salvia R, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213–19. doi: 10.1016/j.cgh.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–85. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–7. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 8.Maire F, Hammel P, Terris B, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717–22. doi: 10.1136/gut.51.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raimondo M, Tachibana I, Urrutia R, et al. Invasive cancer and survival of intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 2002;97:2553–8. doi: 10.1111/j.1572-0241.2002.06022.x. [DOI] [PubMed] [Google Scholar]
- 10.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal Papillary Mucinous Neoplasms of the Pancreas. Annals of Surgery. 2004;239:788–99. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 12.Nagai K, Doi R, Ito T, et al. Single-institution validation of the international consensus guidelines for treatment of branch duct intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2009;16:353–8. doi: 10.1007/s00534-009-0068-8. [DOI] [PubMed] [Google Scholar]
- 13.Tang RS, Weinberg B, Dawson DW, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008;6:815–19. doi: 10.1016/j.cgh.2008.04.005. quiz 719. [DOI] [PubMed] [Google Scholar]
- 14.Pelaez-Luna M, Chari ST, Smyrk TC, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759–64. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Ono J, Yaeger KA, Genevay M, et al. Cytological analysis of small branch-duct intraductal papillary mucinous neoplasms provides a more accurate risk assessment of malignancy than symptoms. Cytojournal. 2011;8:21. doi: 10.4103/1742-6413.90084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bausch D, Mino-Kenudson M, Fernandez-Del Castillo C, et al. Plectin-1 is a biomarker of malignant pancreatic intraductal papillary mucinous neoplasms. J Gastrointest Surg. 2009;13:1948–54. doi: 10.1007/s11605-009-1001-9. discussion 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095–102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–9. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 20.Adsay NV, Adair CF, Heffess CS, et al. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980–94. doi: 10.1097/00000478-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26–42. doi: 10.1097/00000478-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2010;139:708–13. 713 e1–2. doi: 10.1053/j.gastro.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Das KM, Sakamaki S, Vecchi M, et al. The production and characterization of monoclonal antibodies to a human colonic antigen associated with ulcerative colitis: cellular localization of the antigen by using the monoclonal antibody. J Immunol. 1987;139:77–84. [PubMed] [Google Scholar]
- 24.Halstensen TS, Das KM, Brandtzaeg P. Epithelial deposits of immunoglobulin G1 and activated complement colocalise with the M(r) 40 kD putative autoantigen in ulcerative colitis. Gut. 1993;34:650–7. doi: 10.1136/gut.34.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das KM, Prasad I, Garla S, et al. Detection of a shared colon epithelial epitope on Barrett epithelium by a novel monoclonal antibody. Ann Intern Med. 1994;120:753–6. doi: 10.7326/0003-4819-120-9-199405010-00006. [DOI] [PubMed] [Google Scholar]
- 26.Mirza ZK, Das KK, Slate J, et al. Gastric intestinal metaplasia as detected by a monoclonal antibody is highly associated with gastric adenocarcinoma. Gut. 2003;52:807–12. doi: 10.1136/gut.52.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piazuelo MB, Haque S, Delgado A, et al. Phenotypic differences between esophageal and gastric intestinal metaplasia. Mod Pathol. 2004;17:62–74. doi: 10.1038/sj.modpathol.3800016. [DOI] [PubMed] [Google Scholar]
- 28.Griffel LH, Amenta PS, Das KM. Use of a novel monoclonal antibody in diagnosis Barrett’s esophagus. Dig Dis Sci. 2000;45:40–8. doi: 10.1023/a:1005449024524. [DOI] [PubMed] [Google Scholar]
- 29.Watari J, Moriichi K, Tanabe H, et al. Biomarkers predicting development of metachronous gastric cancer after endoscopic resection: an analysis of molecular pathology of Helicobacter pylori eradication. Int J Cancer. 2012;130:2349–58. doi: 10.1002/ijc.26275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kesari KV, Yoshizaki N, Geng X, et al. Externalization of tropomyosin isoform 5 in colon epithelial cells. Clin Exp Immunol. 1999;118:219–27. doi: 10.1046/j.1365-2249.1999.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajpai M, Liu J, Geng X, et al. Repeated exposure to acid and bile selectively induces colonic phenotype expression in a heterogeneous Barrett’s epithelial cell line. Lab Invest. 2008;88:643–51. doi: 10.1038/labinvest.2008.34. [DOI] [PubMed] [Google Scholar]
- 32.Taniguchi M, Geng X, Liu J, et al. Structural diversity in the epitope shared by colon epithelium and extracolonic organs commonly involved in ulcerative colitis: Molecular analysis using monoclonal antibodies. Gastroenterology. 2001;120:A353. [Google Scholar]
- 33.Al-Rashdan A, Schmidt CM, Al-Haddad M, et al. Fluid analysis prior to surgical resection of suspected mucinous pancreatic cysts. A single centre experience. J Gastrointest Oncol. 2011;2:208–14. doi: 10.3978/j.issn.2078-6891.2011.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badve S, Logdberg L, Sokhi R, et al. An antigen reacting with das-1 monoclonal antibody is ontogenically regulated in diverse organs including liver and indicates sharing of developmental mechanisms among cell lineages. Pathobiology. 2000;68:76–86. doi: 10.1159/000028117. [DOI] [PubMed] [Google Scholar]
- 35.Onuma EK, Amenta PS, Jukkola AF, et al. A phenotypic change of small intestinal epithelium to colonocytes in small intestinal adenomas and adenocarcinomas. Am J Gastroenterol. 2001;96:2480–5. doi: 10.1111/j.1572-0241.2001.04056.x. [DOI] [PubMed] [Google Scholar]
- 36.Pantuck AJ, Murphy DP, Amenta PS, et al. The monoclonal antibody 7E12H12 can differentiate primary adenocarcinoma of the bladder and prostate. Br J Urol. 1998;82:426–30. doi: 10.1046/j.1464-410x.1998.00755.x. [DOI] [PubMed] [Google Scholar]
- 37.Mino-Kenudson M, Fernandez-Del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011;60:1712–20. doi: 10.1136/gut.2010.232272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishida M, Egawa S, Aoki T, et al. Characteristic clinicopathological features of the types of intraductal papillary-mucinous neoplasms of the pancreas. Pancreas. 2007;35:348–52. doi: 10.1097/mpa.0b013e31806da090. [DOI] [PubMed] [Google Scholar]
- 39.Sawai Y, Yamao K, Bhatia V, et al. Development of pancreatic cancers during long-term follow-up of side-branch intraductal papillary mucinous neoplasms. Endoscopy. 2010;42:1077–84. doi: 10.1055/s-0030-1255971. [DOI] [PubMed] [Google Scholar]
- 40.Matthaei H, Wylie D, Lloyd MB, et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res. 2012;18:4713–24. doi: 10.1158/1078-0432.CCR-12-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2013;62:1024–33. doi: 10.1136/gutjnl-2012-302823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol. 2013;11:719–30, e5. doi: 10.1016/j.cgh.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genevay M, Mino-Kenudson M, Yaeger K, et al. Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann Surg. 2011;254:977–83. doi: 10.1097/SLA.0b013e3182383118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maker AV, Lee LS, Raut CP, et al. Cytology from Pancreatic Cysts Has Marginal Utility in Surgical Decision-Making. Ann Surg Oncol. 2008;15:3187–92. doi: 10.1245/s10434-008-0110-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


