Abstract
Glucocorticoids are commonly used in anti-inflammatory and immunomodulatory therapies, but glucocorticoid withdrawal can result in life-threatening risk of adrenal insufficiency. Chinese patent pharmaceutical product Jinkui Shenqi (JKSQ) pill has potent efficacy on clinical adrenal insufficiency resulted from glucocorticoid withdrawal. However, the underlying molecular mechanism remains unclear. We used an animal model to study JKSQ-induced metabolic changes under adrenal insufficiency and healthy conditions. Sprague-Dawley rats were treated with hydrocortisone for 7 days, with or without 15-day JKSQ pre-treatment. Sera were collected after 72-hour hydrocortisone withdrawal and used for global and free fatty acids (FFAs)-targeted metabolomics analyses using gas chromatography/time-of-flight mass spectrometer (GC/TOFMS) and ultra-performance liquid chromatograph/quadruple time-of-flight mass spectrometer (UPLC/Q-TOFMS). Rats without hydrocortisone treatment were used as controls. JKSQ pre-treatment normalized the significant changes of 13 serum metabolites in hydrocortisone withdrawal rats, involving carbohydrates, lipids and amino acids. The most prominent effect of JKSQ was on the changes of FFAs and some [product FFA]/[precursor FFA] ratios which represent estimated desaturase and elongase activities. The opposite metabolic responses of JKSQ in adrenal insufficiency rats and normal rats, highlighted the “Bian Zheng Lun Zhi” (treatment based on ZHENG Differentiation) guideline of TCM, and suggested that altered fatty acid metabolism was associated with adrenal insufficiency after glucocorticoid withdrawal and the protective effects of JKSQ.
Keywords: glucocorticoid-induced adrenal insufficiency, glucocorticoid withdrawal, Jinkui Shenqi pill, TCM, metabolomics, fatty acid metabolism
TOC image
INTRODUCTION
Glucocorticoids are steroid hormones that are widely used for the treatment of a variety of diseases due to their anti-inflammatory and immunomodulatory properties. However, long-term treatment with supraphysiological doses of glucocorticoids in both human1–5 and adrenal-intact animals6–9 could result in a systemic suppression of the function of the hypothalamus-pituitary-adrenal (HPA) axis. When the drug is abruptly discontinued, recovery of the HPA axis function may be delayed with increased risk of temporary adrenal insufficiency, which is one of the most serious and unpredictable adverse effects in clinical practice.10–12 Currently the most common treatment for adrenal insufficiency after glucocorticoid withdrawal is chronic glucocorticoids replacement therapy. Despite optimized glucocorticoid-tapering schedule, patients withdrawing glucocorticoids still suffer from fatigue, lack of stamina, loss of energy, reduced muscle strength, weight loss, anorexia, nausea, depression, and anxiety, among other symptoms.13–16 In particular, due to the immediate release of orally administered hydrocortisone, glucocorticoid replacement regimens does not fully mimic the cortisol diurnal rhythm regulated by the central biological clock, which leads to an increased mortality and impaired quality of life in patients with adrenal insufficiency.17,18 Therefore, new treatments are needed for the management of adrenal insufficiency patients withdrawing glucocorticoids.
In traditional Chinese medicine (TCM), the similar clinic state of adrenal insufficiency after glucocorticoid withdrawal is usually diagnosed as the Kidney-Yang deficiency syndrome (KDS-Yang).19 Jinkui Shenqi (JKSQ) pill [金匮肾气丸], an ancient traditional Chinese formula first recorded in the Synopsis of Prescriptions of the Golden Chamber [金匮要略], has been applied for treatment of patients with Kidney-Yang deficiency syndrome for thousands of years in China. JKSQ pill consists of Aconiti Lateralis Rddix Praeparata (Fu Zi), Cinnamomi Ramulus (Gui Zhi), Achyranthis Bidentatae Radix (Niu Xi), Rehmanniae Radix (Di Huang), Corni Fructus (Shan Zhu Yu), Dioscoreae Rhizoma (Shan Yao), Poria (Fu Ling), Aliamria Rhizoma (Ze Xie), Plantaginis Semen (Che Qian Zi), and Moutan Cortex (Mu Dan Pi). Recent research has shown, JKSQ possessed satisfactory therapeutic effects on adrenal insufficiency and related complications resulted from glucocorticoids withdrawal using scores of TCM syndrome and biochemistry analyses.20–23 Nevertheless, the metabolic and molecular mechanisms underlying the protective effect of JKSQ on stimulation of HPA axis function remain unclear.
In this study, a high dose of hydrocortisone was used to induce a pathophysiological condition in rats that mimiced the clinical adrenal insufficiency after glucocorticoids withdrawal based on our previous study.24 The rats were either pre-treated with JKSQ or with saline before the hydrocortisone administration to investigate the metabolic responses of normal and adrenal insufficiency rats to JKSQ treatment. Metabolites in sera were determined using both untargeted global profiling and free fatty acids (FFAs)-targeted metabolomics techniques using gas chromatography/time-of-flight mass spectrometer (GC/TOFMS) and ultra-performance liquid chromatograph/quadruple time-of-flight mass spectrometer (UPLC/Q-TOFMS).
MATERIALS AND METHODS
Reagents and pharmaceutical product
Hydrocortisone solution for injection (0.5%) was purchased from Shanghai Xinyi Pharmaceutical Company (Shanghai, China). The targeted fatty acids and the isotopically labelled internal standards (nonadecanoic-d37 acid and tridecanoic-d25 acid) were obtained from Nu-Chek Prep (Elysian, MN, USA), Sigma–Aldrich (St. Louis, MO, USA) and Cambridge Isotopes Laboratories (Andover, MA, USA). L-2-Chlorophenylalanine, BSTFA (1% TMCS), methoxyamine, leucine-enkephalin and all other chemical standards for metabolites annotation were purchased from Sigma–Aldrich (St. Louis, MO, USA). Methanol, acetonitrile, chloroform, isopropanol, n-hexane and pyridine with HPLC grade were purchased from Merck Chemicals (Darmstadt, Germany) and Fisher (Loughborough, UK). Other chemicals were of analytical grade and obtained from China National Pharmaceutical Group Corporation (Shanghai, China).
JKSQ pill (state drug approval document No: Z11020147) was manufactured and supplied by Beijing Tongrentang Science and Technique Development Co., Ltd. (Beijing, China). It is a well-established product with S-FDA approved preparation procedures and quality control protocols that are strictly carried out according to Drug Standards of People’s Republic of China (WS3-B-3892-98-2011, State Food and Drug Administration). As shown in Supporting Information Table S1, 24 components in JKSQ were identified, mainly involving phenylpropanoids, flavonoid, terpene, phenols and their glycosides.
Animals
The animal experiment was performed at the Center for Laboratory Animals, Shanghai Jiao Tong University, and the protocol was approved by the Animal Ethics Committee of the Shanghai Jiao Tong University. Eight-week-old male Sprague−Dawley rats (200±20 g) were purchased from Shanghai Laboratory Animal Co. Ltd. (SLAC, Shanghai, China). All rats were housed individually in a barrier system with regulated temperature (21±1°C), humidity (60±10%), 12/12-h light/dark cycle, and provided with certified standard rat chow and tap water ad libitum. After two weeks of acclimatization, rats were randomly assigned into four groups (7 in each group): (1) the normal control group (N), (2) the model group (M), (3) the JKSQ model group (JM), and (4) the JKSQ normal group (JN). Each rats in group JM were pretreated with JKSQ (dissolved in saline) orally at 6 g·kg−1 body weight once a day for 15 days, and then received an intraperitoneal injection of hydrocortisone in saline (5%) at 50 mg·kg−1 of body weight once a day from days 16 to 22. The dosage of JKSQ used in rat is determined according to an established formula for human−rat drug conversion.25 Rats in group JN were also pretreated with the same dose of JKSQ orally once a day for 15 days, and then injected with the same volume of saline intraperitoneally once a day from days 16 to 22. Model group rats were orally administered the same volume of saline daily from days 1 to 15, then 5% hydrocortisone 50 mg·kg−1 body weight once a day through intraperitoneal injection from days 16 to 22. Normal control rats received saline daily in the same way as mentioned above. Rats were sacrificed by cervical dislocation on day 25, and hydrocortisone or saline were not administered during the last 72 h. Sera and 24-h urine samples were collected at the end of the experiment and stored at −80°C, pending for biochemical, global and target metabolomics analysis. The 24-h food consumption and body weight of each rat were recorded on days 0, 7, 15, 22 and 25, and the general behavior of rats were also observed.
Urine 17-hydroxycorticosteroids Measurement
24-h urine 17-hydroxycorticosteroids (17-OHCS) was measured using ELISA kits (Groundwork Biotechnology Diagnosticate Ltd., San Diego, CA, USA) following manufacturer’s instruction.
Global Metabolomics Study by GC/TOFMS
Serum samples were extracted and derivatized according to our previously published procedures.26 An Agilent 6890N gas chromatography (GC) coupled with a Pegasus HT time-of-flight mass spectrometer (TOFMS) (Leco Corporation, St. Joseph, MI, USA) was used for sample analysis. Samples were splitlessly injected into a DB-5 MS capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness; (5%-phenyl)-methylpolysiloxane bonded and cross-linked; Agilent J&W Scientific, Folsom, CA, USA), with helium as the carrier gas at a constant flow rate of 1.0 mL·min−1. The injection volume was 1 μL. The GC temperature programming for the analysis serum samples was published in our previous reports.26 Temperatures of injection, transfer interface and ion source were set to 270, 260, and 200 °C, respectively. Electron impact ionization (70 eV) at full scan mode (m/z 30−600) was used. The acquisition rate was 20 spectra·second−1.
The acquired MS files were exported in NetCDF format by ChromaTOF software (v3.30, Leco Co., CA, USA). The CDF files were extracted using custom scripts (revised Matlab toolbox HDA) in the MATLAB 7.1 (The MathWorks, Inc., MA, USA) for data pretreatment procedures such as baseline correction, denoising, smoothing, alignment, time-window splitting, and peak feature extraction (based on multivariate curve resolution algorithm).27 Three-dimension data sets including sample information, peak retention time and peak intensities were obtained. In addition, metabolite identification was processed by comparing the mass fragments with NIST 11 Standard mass spectral databases in ChromaTOF software (v4.34, LECO, USA) with a similarity of more than 70%, and then further verified by comparing with the mass fragments and retention time of available reference standards in our in-house library.
Targeted Metabolomics Study of Serum FFAs by UPLC/Q-TOFMS
In the targeted analysis, FFAs were extracted from serum samples according to published methods with modifications.28 Briefly, 40 μL of serum sample was mixed with 10 μL isotope labeled internal standard solution (nonadecanoic-d37 acid in methanol, 5 μg·mL−1; tridecanoic-d25 acid in methanol, 25 μg·mL−1) in a micro-centrifuge tube. 500 μL of the modified Dole’s mixture (methanol/n-hexane/phosphoric acid (2M), 40:10:1 (v/v)) was added and vortexed for 2 min. After incubating at room temperature for 20 min, 400 μL of n-hexane and 300 μL of water were added, vortexed and centrifuged at 12,000 rpm for 10 min. Then 400 μL of the upper organic layer was transferred into another micro-centrifuge tube, and 400 μL of n-hexane was added to the lower layer for further extraction. After vortexing and centrifugation, all of the upper organic phase was combined with the supernatant from the first-round extraction and dried under vacuum. The residue was reconstituted with 80 μL of methanol and subjected to UPLC/Q-TOFMS analysis.
An ACQUITY-ultra-performance liquid chromatography (UPLC) system (Waters Corporation, Milford, USA) equipped with a binary solvent delivery system and an auto-sampler (Waters Corporation, Milford, USA) was used for the separation on a 100 cm × 2.1 mm BEH C18 column with 1.7 μm particles at 40 °C (Waters Corporation, Milford, USA). The optimal mobile phase consisted of water (solvent A) and acetonitrile/isopropyl alcohol (v/v=8:2) (solvent B) and the flow rate was set at 400 μL·min−1. The injection volume was of 5 μL. A gradient elution condition was applied as follows: 70% B over 0 ~ 2 min, 70% ~ 75% B over 2 ~ 5 min, 75% ~ 80% B over 5 ~ 10 min, 80% ~ 90% B over 10 ~ 13min, 90% ~ 100% B over 13 ~ 16 min, maintained for an additional 5 min, then returned to 70% B to re-equilibrate during over 21 ~ 22.5 min. The mass spectrometric data was collected using a tandem quadrupole-time-of-flight mass spectrometry (Q-TOFMS) (Manchester, UK). ESI was used as the ionization source and the analysis was carried out in the negative mode. The following parameters were used: capillary voltage, 2500 V; sampling cone, 55 V; extraction cone, 4 V; desolvation temperature, 450 °C; source temperature, 120 °C; desolvation gas flow, 650 L·h−1; cone gas flow, 50 L·h−1; Lm resolution, 4.7; Hm resolution, 15; scan time, 0.35 s, and inter scan time 0.02s. Leucine enkephalin was used as the lock mass (m/z 554.2615) at a concentration of 100 ng·mL−1 and flow rate of 0.2 mL·min−1, with a lockspray frequency of 20 s.
UPLC/Q-TOFMS raw data were analyzed using TargetLynx applications manager version 4.1 (Waters Corp., Milford, MA) to obtain calibration equations and the quantitative concentration of each FFA. Data were manually examined and corrected if errors were found for quality control. Compound identification was performed using the accurate mass and retention time in our in-house library containing about 100 fatty acids standards.
Statistical Analysis
Data from the biochemistry determination were expressed as mean±SE; Student’s two-tailed, unpaired t test was used to compare the means of the groups. P value less than 0.05 was considered as statistically significant.
Multivariate statistical analysis of orthogonal partial least-squares projection to latent structures-discriminant analysis (OPLS-DA) was carried out by SIMCA-P 14.0 version (Umetrics, Umeå, Sweden) after mean centering and unit variance scaling. Variable importance in the projection (VIP) values of all the variables from the 7-fold cross-validated OPLS-DA model were ranked, and those variables with VIP larger than 1 are considered relevant for group discrimination. All the differentially expressed metabolites between two groups were selected using the Mann−Whitney U test with the critical P value set to 0.05. The fold change (FC) shows the relative intensity ratio of the differential or representative metabolites in normal control or model rats whether or not pretreated with JKSQ. The box plots were generated in SPSS 16.0 (Statistical Package for the Social Sciences, SPSS Ins., IL, USA).
RESULTS
Hormone Level and General Observation
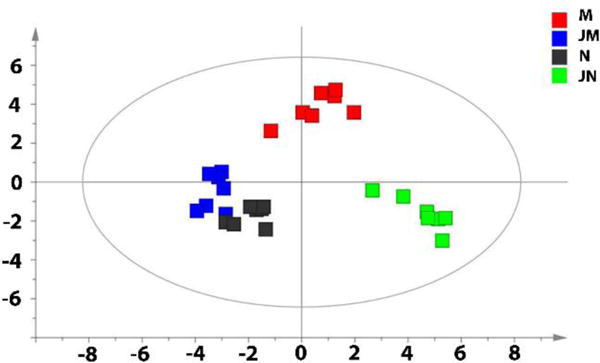
After 72 h hydrocortisone withdrawal, the 17-OHCS concentration in the 24-h urinary was lower in group M compared with group N (P<0.01) and group JM (P <0.05), while the urine 17-OHCS concentration of group JM was comparable to that of group N, suggesting the beneficial effect of JKSQ on the rapid recovery of HPA axis function following cessation of hydrocortisone treatment (Figure 1). There was no significant difference in urine 17-OHCS levels between group JN and group N, suggesting that JKSQ treatment did not increase the function of HPA axis under the normal control condition.
Figure 1.
Concentration of twenty-four-hour urinary 17-OHCS in each group (mean ± SE). * P < 0.05; ** P < 0.01 compared to M group. There was no statistically significant difference between JM and N.
In addition, significantly decreased body weight was found on day 22 in the model group, and continued to the end of study compared to controls (P <0.01), similar to the weight loss experienced by patients withdrawing glucocorticoids.29 Although the body weight increased in group JM compared to group M, no significant variation was found between the two groups. Food consumption of rats had no significant differences among the groups. (Supporting Information Table S2).
Quality Control of Global and Targeted Metabolomics Analyses
The data quality in global and targeted metabolomics analyses were evaluated by the intensities of internal standards added during the samples preparation. The relative standard derivations (RSDs) for the internal standard of L-2-chlorophenylalanine were 12.0 % in global metabolomics study by GC/TOFMS. For quantitative analysis of targeted metabolomics, the RSDs for the internal standards were 14.9% for tridecanoic-d25 acid and 16.3% for nonadecanoic-d37 acid in UPLC/Q-TOFMS over all samples. The results demonstrated that the metabolomics data were of sufficient quality.
Identification of Significantly Differential Metabolites through Global Metabolomics
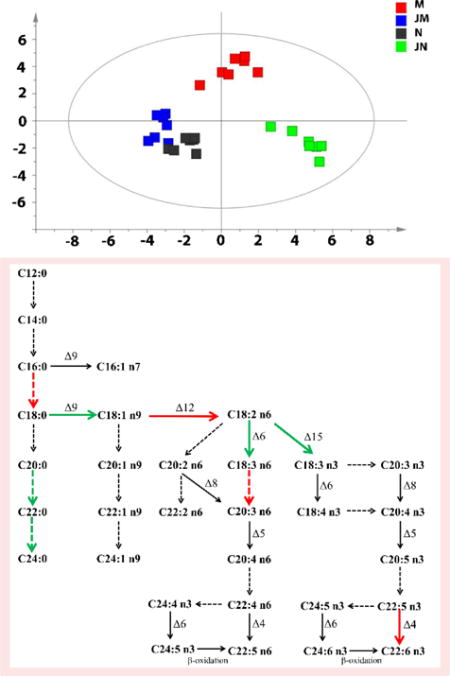
Figure 2 shows the metabolic phenotypes of the 4 groups of rats using the multiple pattern recognition method. Metabolic phenotype separations were observed between group N (grey boxes) and group M (red boxes), group M and group JM (blue boxes), and group JN (green boxes) and group N. The global metabolic pattern of group JM was similar to that of group N. The fact that group N and group JM had similar metabolic phenotype suggested that JKSQ pre-treatment had prevented the changes of serum metabolites in adrenal insufficiency rats with abrupt glucocorticoids withdrawal.
Figure 2.
Differential metabolic responses to JKSQ treatment in normal (green boxes) and model rats (blue boxes) illustrated by multiple pattern recognition OPLS-DA model (R2X = 0.754, R2Y=0.649, Q2 = 0.173).
As shown in Table 1 and Supporting Information Table S3, the serum levels of 13 metabolites were significantly changed in group M compared to group N, but all of these metabolites were found similar between group JM and group N. When group JM was compared with group M, the levels of 10 out of the 13 metabolites were found significantly changed in directions that were opposite to those in the M-verse-N comparison. The altered carbohydrate, lipids and amino acid metabolism were thought to be responsible for the protective effects of JKSQ on acute hydrocortisone withdrawal. Further, JKSQ induced significant metabolic alterations that were independent of hydrocortisone withdrawal. Some metabolites showed no changes between group M and group N, but changed significantly between group JM and M; these compounds included norepinephrine, oxoglutaric acid, succinic acid, malic acid, aspartic acid, pipecolinic acid, uracil, sorbitol, etc., which mainly participate in catecholamine biosynthetic, tricarboxylic acid (TCA) cycle, amino acid and polyol metabolism. Besides, different concentrations of 4 metabolites were found between group JN and group N after 10 days of washout period (from day 16 to day 25). The differential metabolites between two groups were selected based on the VIP values (VIP>1.0) by multiple pattern recognition OPLS-DA models (Supporting Information Figure S1), P values (P <0.05) by the Mann−Whitney U test, and FC of the arithmetic mean values (FC>1.2 or <0.8), as shown in Supporting Information Table S3.
Table 1.
Identification of Significantly Differential Metabolites in the Sera of Normal and Acute Glucocorticoid Withdraw Induced Adrenal Insufficiency Rats with or without Pretreatment of JKSQ.
| RT (min) | Metabolites | Formula | ID | M vs. N | JM vs. M | JN vs. N | Pathway |
|---|---|---|---|---|---|---|---|
| 5.54 | L-lactic acid | C3H6O3 | HMDB00190 | *(↓) | *(↑) | gluconeogenesis | |
| 6.19 | L-alanine | C3H7NO2 | HMDB00161 | *(↑) | ** (↓) | alanine metabolism; glutamate metabolism; glycine and serine metabolism | |
| 6.39 | acetylcarnitine | C9H17NO4 | HMDB00201 | *(↓) | *(↑) | beta oxidation of very long chain fatty acids | |
| 6.77 | glyceraldehyde | C3H6O3 | HMDB01051 | *(↓) | *(↑) | *(↓) | glycerolipid metabolism; fructose and mannose degradation |
| 8.57 | ethanolamine | C2H7NO | HMDB00149 | *(↑) | **(↓) | phospholipid biosynthesis | |
| 17.29 | L-histidine | C6H9N3O2 | HMDB00177 | **(↓) | beta-alanine metabolism; histidine metabolism | ||
| 21.12 | 3-phosphoglyceric acid | C3H7O7P | HMDB00807 | *(↓) | *(↑) | gluconeogenesis; glycerolipid metabolism; | |
| 21.18 | linoleic acid | C18H32O2 | HMDB00673 | *(↓) | *(↓) | fatty acid metabolism | |
| 21.23 | oleic acid | C18H34O2 | HMDB00207 | *(↓) | *(↓) | fatty acid metabolism | |
| 21.42 | stearic acid | C18H36O2 | HMDB00827 | **(↓) | **(↑) | fatty acid metabolism | |
| 22.15 | arachidonic acid | C20H32O2 | HMDB01043 | *(↓) | **(↑) | fatty acid metabolism | |
| 22.36 | MG(18:2(9Z,12Z)/0:0/0:0) | C21H38O4 | HMDB11568 | **(↓) | *(↑) | glycerolipid metabolism; | |
| 23.63 | lactose | C12H22O11 | HMDB00186 | *(↓) | **(↑) | galactose metabolism | |
| 5.41 | pyruvic acid | C3H4O3 | HMDB00243 | *(↓) | alanine metabolism; citric acid cycle | ||
| 9.25 | succinic acid | C4H6O4 | HMDB00254 | *(↓) | citric acid cycle; | ||
| 9.56 | uracil | C4H4N2O2 | HMDB00300 | *(↓) | beta-alanine metabolism; pyrimidine metabolism | ||
| 9.95 | pipecolinic acid | C6H11NO2 | HMDB00070 | *(↓) | lysine metabolism | ||
| 11.51 | malic acid | C4H6O5 | HMDB00744 | *(↓) | gluconeogenesis | ||
| 11.92 | L-aspartic acid | C4H7NO4 | HMDB00191 | *(↓) | arginine and proline metabolism; | ||
| 12.37 | creatinine | C4H7N3O | HMDB00562 | *(↓) | creatine metabolism | ||
| 12.63 | oxoglutaric acid | C5H6O5 | HMDB00208 | *(↓) | alanine metabolism; arginine and proline metabolism | ||
| 17.46 | sorbitol | C6H14O6 | HMDB00247 | *(↓) | fructose and mannose degradation | ||
| 19.16 | palmitic acid | C16H32O2 | HMDB00220 | *(↓) | fatty acid metabolism | ||
| 22.61 | norepinephrine | C8H11NO3 | HMDB00216 | *(↑) | catecholamine biosynthesis | ||
| 27.85 | cholesterol | C27H46O | HMDB00067 | *(↑) | bile acid biosynthesis |
“↑” means increase of metabolite level, “↓”means decrease of metabolite level.
P < 0.05;
P < 0.01 from the Mann−Whitney U test. M, model group; N, normal control group; JM, JKSQ-treated model group; JN, JKSQ-treated normal group. The levels of VIP, FC and P value are listed in Supporting Information Table S3.
Targeted Metabolomics Analyses of Serum FFAs
The global metabolomics study showed that the most notable metabolic effect of JKSQ in adrenal insufficiency and normal rats was fatty acid metabolism as evidenced by altered concentrations of stearic acid, linoleic acid, oleic acid and arachidonic acid (P<0.05), and possibly palmitic acid (P =0.085). To monitor the metabolic changes of circulating FFAs in the 4 groups of rats more comprehensively and accurately, the absolute concentrations of 42 FFAs in the sera were quantified through a targeted metabolomics analysis using a special pretreatment protocol. As shown in Table 2 and Supporting Information Table S4, the concentrations of 30 FFAs decreased significantly in group M compared to group N. Among them, the concentrations of 2 FFAs significantly increased in group JM compared to group M, and the concentrations of 18 FFAs were higher in group JM compared to group M, but the differences were not statistically significant; and out of these 20 metabolites, 16 had similar concentrations between group JM and group N. Furthermore, the concentrations of 27 FFAs significantly decreased in group JN compared to group N (P<0.05), even after 10 days of washout period. The results suggested that JKSQ had different regulatory effects on fatty acid metabolism under healthy and HPA axis suppression conditions.
Table 2.
Quantitative Analysis of Serum Free Fatty Acids using Targeted Metabolomics (μg·mL−1).
| No. | FFA | N group | JN group | M group | JM group |
|---|---|---|---|---|---|
| SFAs | |||||
| 1 | C12:0 | 0.06±0.03 | 0.04±0.02 | 0.07±0.02 | 0.05±0.02 |
| 2 | C14:0 | 3.51±0.27 | 3.37±0.22 | 3.16±0.19* | 3.28±0.29 |
| 3 | C14:0 iso | 0.09±0.01 | 0.09±0.01 | 0.08±0.003 | 0.08±0.01 |
| 4 | C15:0 | 1.04±0.13 | 0.82±0.07† | 0.77±0.10* | 0.87±0.18 |
| 5 | C15:0 iso | 0.29±0.04 | 0.29±0.02 | 0.27±0.02 | 0.26±0.02 |
| 6 | C16:0 | 45.33±5.13 | 35.10±2.24† | 35.90±4.74* | 39.11±7.94 |
| 7 | C16:0 iso | 0.28±0.03 | 0.22±0.03† | 0.21±0.02** | 0.21±0.03 § |
| 8 | C17:0 | 1.37±0.11 | 1.13±0.07‡ | 1.13±0.13* | 1.14±0.14§ |
| 9 | C17:0 iso | 0.33±0.03 | 0.26±0.02‡ | 0.26±0.02* | 0.26±0.05 |
| 10 | C17:0 anteiso | 0.61±0.04 | 0.59±0.01 | 0.57±0.04 | 0.53±0.04 § |
| 11 | C18:0 | 35.86±2.78 | 31.46±0.68‡ | 33.53±3.55 | 30.67±2.33 § |
| 12 | C18:0 iso | 1.92±0.21 | 1.38±0.17‡ | 1.37±0.18** | 1.25±0.23 § |
| 13 | C19:0 | 0.30±0.04 | 0.24±0.02† | 0.23±0.01** | 0.23±0.06 |
| 14 | C20:0 | 1.24±0.34 | 0.84±0.03‡ | 0.91±0.14* | 0.83±0.12 § |
| 15 | C22:0 | 0.14±0.03 | 0.08±0.01‡ | 0.08±0.008** | 0.08±0.02 § |
| 16 | C23:0 | 0.02±0.004 | 0.01±0.002‡ | 0.01±0.001** | 0.02±0.01 |
| 17 | C24:0 | 0.10±0.02 | 0.05±0.01‡ | 0.04±0.003** | 0.06±0.03 § |
| MUFAs | |||||
| 18 | C16:1 n9 | 0.19±0.07 | 0.186±0.058 | 0.14±0.02* | 0.14±0.05 |
| 19 | C16:1 n7 | 2.06±0.63 | 1.69±0.43 | 1.23±0.41* | 1.83±0.76 |
| 20 | C17:1 n7 | 0.19±0.15 | 0.15±0.11 | 0.11±0.07 | 0.18±0.10 |
| 21 | C18:1 n9 | 30.89±5.79 | 18.10±1.20‡ | 16.52±2.84** | 21.83±7.71 |
| 22 | C19:1 n9 | 0.05±0.008 | 0.02±0.01‡ | 0.02±0.01** | 0.03±0.02 |
| 23 | C20:1 n9 | 1.17±0.27 | 0.60±0.07‡ | 0.57±0.07** | 0.73±0.26 § |
| 24 | C22:1 n9 | 0.24±0.06 | 0.15±0.05† | 0.13±0.02** | 0.15±0.04 § |
| 25 | C23:1 | 0.02±0.003 | 0.01±0.002‡ | 0.01±0.002** | 0.01±0.01 § |
| 26 | C24:1 n9 | 0.21±0.03 | 0.09±0.02‡ | 0.10±0.01** | 0.10±0.04 § |
| PUFAs | |||||
| n6 PUFAs | |||||
| 27 | C16:2 | 0.07±0.03 | 0.05±0.01 | 0.03±0.01** | 0.06±0.02 ¶ |
| 28 | C18:2 n6 | 62.59±8.51 | 45.74±4.94‡ | 43.27±4.78** | 46.63±10.72 § |
| 29 | C18:3 n6 | 0.74±0.15 | 0.45±0.07‡ | 0.38±0.08** | 0.52±0.15§ |
| 30 | C20:2 n6 | 1.01±0.27 | 0.81±0.07† | 0.75±0.10* | 0.88±0.19 |
| 31 | C20:3 n6 | 1.35±0.36 | 1.21±0.07 | 1.11±0.28 | 1.10±0.17 |
| 32 | C20:4 n6 | 32.15±4.78 | 28.39±2.85 | 27.82±3.73 | 26.78±5.83 |
| 33 | C22:2 n6 | 0.04±0.01 | 0.03±0.003 | 0.03±0.006 | 0.03±0.01 |
| 34 | C22:4 n6 | 2.07±0.28 | 1.61±0.23† | 1.70±0.19* | 1.85±0.57 |
| 35 | C22:5 n6 | 0.25±0.03 | 0.23±0.03 | 0.22±0.05 | 0.21±0.05 |
| 36 | C24:5 n3 | 0.05±0.01 | 0.03±0.003 | 0.04±0.007 | 0.04±0.02 |
| 37 | C24:6 n3 | 0.17±0.03 | 0.13±0.01‡ | 0.14±0.03 | 0.15±0.04 |
| n3 PUFAs | |||||
| 38 | C18:3 n3 | 2.40±0.52 | 1.48±0.17‡ | 1.33±0.18** | 1.71±0.46 |
| 39 | C18:4 n3 | 0.31±0.10 | 0.20±0.03† | 0.16±0.05** | 0.21±0.05 § |
| 40 | C20:5 n3 | 4.98±1.27 | 3.62±0.51 | 2.91±0.79* | 3.83±0.50 ¶ |
| 41 | C22:5 n3 | 4.82±1.58 | 2.70±0.30‡ | 2.64±0.56* | 3.37±0.62 |
| 42 | C22:6 n3 | 29.10±4.33 | 21.25±2.12† | 22.16±3.81* | 21.31±3.44 § |
Data are expressed as mean ± SD.
P < 0.05;
P < 0.01 for the difference between M and N;
P<0.05,
P<0.01 between JN and N.
P<0.05 between JM and N; and
P<0.05 between JM and M. The P values are listed in Supporting Information Table S4. SFAs, saturated fatty acids; MUFAs, monounsaturated fatty acid; PUFAs, polyunsaturated fatty acids.
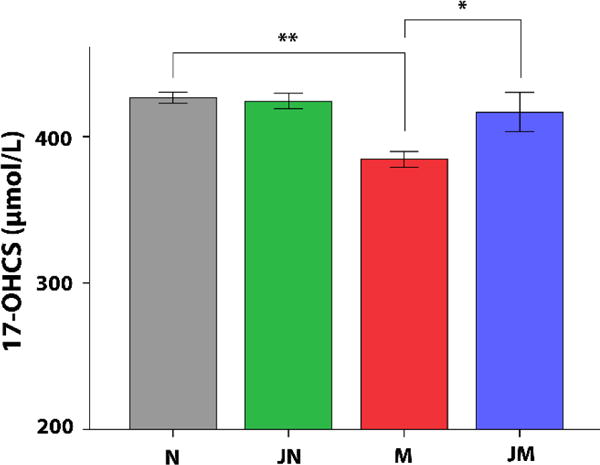
The FFAs composition in circulating to some extent depends on the endogenous metabolism of FAs through elongation (lengthened by 2 carbon atoms) and desaturation (insertion of a double bond).30 The ratios of product/precursor FFAs in serum were traditionally used to estimate elongase and desaturase activitie.31 In this study, 23 serum product/precursor FFA ratios were analyzed and compared between the 4 groups (Supporting Information Table S5). Among them, 9 ratios altered significantly in group M compared to group N, include 5 ratios reflecting the estimated desaturases activities and 4 ratios which reflect the estimated elongase activities (Figure 3 and Figure 4). Out of the 9 ratios, 4 ratios including [stearic acid]/[palmitic acid] (C18:0/C16:0), [linoleic acid]/[oleic acid] (C18:2n6/C18:1n9), [dihomo-γ-linolenic acid]/[γ-linolenic acid] (C20:3n6/C18:3n6), and [docosahexaenoic acid]/[docosapentaenoic acid] (C22:6n3/C22:5n3), increased in group M relative to group N, while 5 ratios of [oleic acid]/[stearic acid] (C18:1n9/C18:0), [α-linolenic acid]/[linoleic acid] (C18:3n3/C18:2n6), [γ-linolenic acid]/[linoleic acid] (C18:3n6/C18:2n6), [behenic acid]/[arachidic acid] (C22:0/C20:0) and [lignoceric acid]/[behenic acid] (C24:0/C22:0) decreased in group M compared to group N. Opposite changes of these ratios were found in group JM and group JN when compared with group N. Exactly, all of the 9 ratios were similar between group JM and group N. However, 6 out of the 9 ratios significantly changed in group JN (P<0.05) compared to group N, and the other 3 ratios also showed the change but no statistical significance (P=0.091, 0.089, 0.272).
Figure 3.
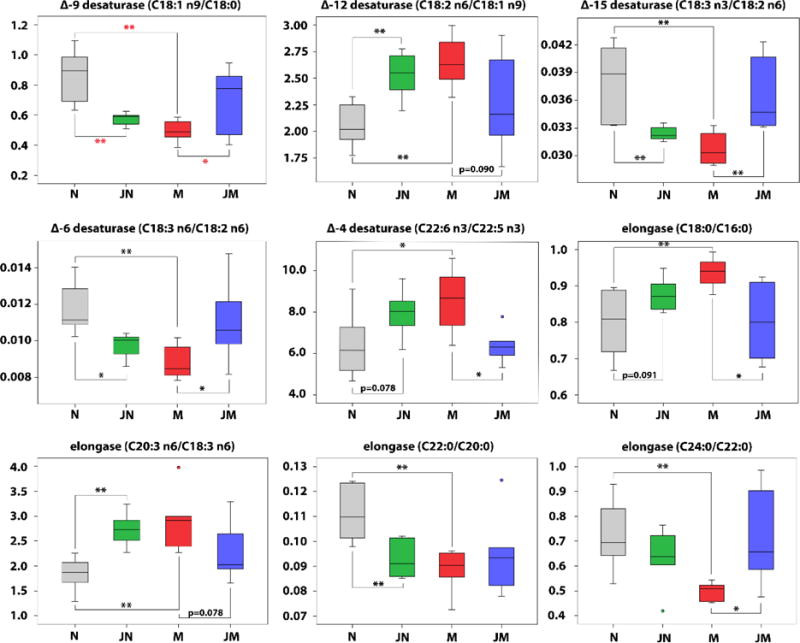
Box plots of 9 serum [product FFA]/[precursor FFA] ratios that were significantly changed in model group compared to controls. * P < 0.05; ** P < 0.01.
Figure 4.
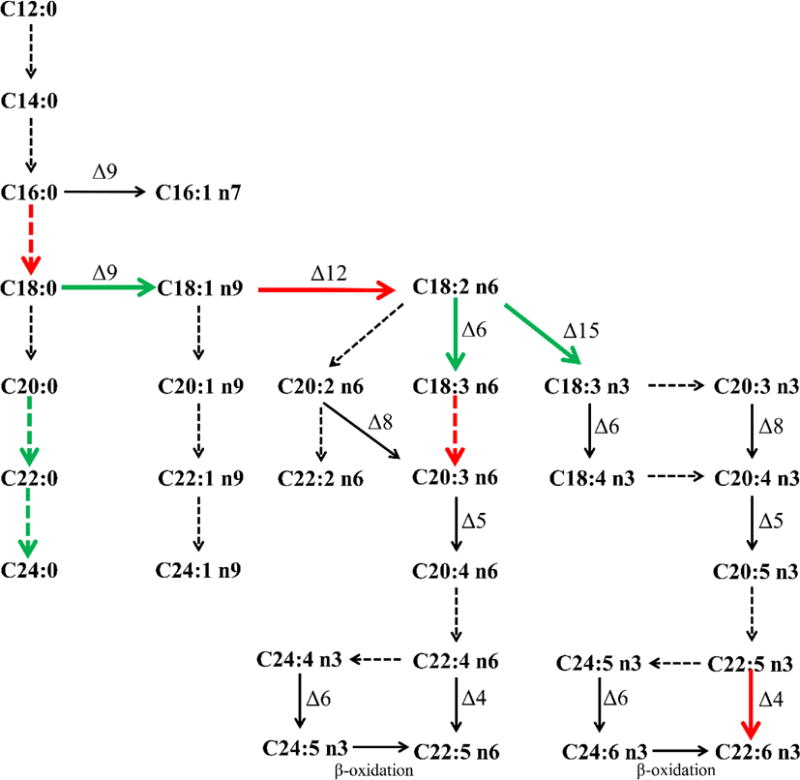
FFAs biosynthesis pathway and changes of estimated desaturase (solid arrow) and elongase (dotted arrow) activities in adrenal insufficiency rats after hydrocortisone withdrawal. Green (or red) arrows: the ratios by product to precursor significantly decreased (or increased) in model rats relative to controls (P<0.05).
DISCUSSION
Endogenous secretion of glucocorticoids by the adrenal cortex is under the control of the neuroendocrine feedback system of HPA axis. Due to the suppression of endogenous secretion of corticotropin releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), treatment with supraphysiological doses of glucocorticoids, sometimes even the physiological doses of exogenous glucocorticoids, may suppress the HPA axis with consecutive atrophy of the adrenal cortex. During the course of glucocorticoid therapy, the body could adjust to a special status of “pathologic homeostasis”. When the drug is suddenly discontinued, this equilibrium is disturbed. The rate of recovery of the individual components of the HPA axis varies, which can be as short as several days,6–8, 32, 33 or as long as a year or even longer.3, 34 Many factors influence restoration of appropriate responsiveness, such as the specific glucocorticoids used, the route, the duration and the dose of treatment.3, 6–8, 32–34 In our pilot study,24 we induced the KDS-Yang animal model using 72-hour withdrawal after hydrocortisone treatment for 7 consecutive days in rats, and found three hormonal levels, including cortisone and ACTH in fasting sera, and 17-OHCS in 24-h urine, which are regarded as standard clinical measurements in diagnosing the suppression of HPA axis (HPAS) resulted from glucocorticoid therapy and in monitoring the duration of HPAS,35, 36 all significantly decreased in model rats relative to control. These results demonstrated that this animal model is suitable for our present study.
The clinical value of JKSQ on activating HPA axis function and alleviating adrenal insufficiency during glucocorticoid taper and withdrawal has been accepted using traditional diagnostics of TCM. A randomized controlled trial involving 52 primary nephrotic syndrome children demonstrated that, compared with routine prednisone-tapering remedy, adjuvant treatment with JKSQ while prednisone-tapering for 1 year significantly reduced TCM symptoms score, increased the total effective rate from 65 to 94%, and decreased the common adverse effects induced by glucocorticoid taper and withdrawal.23 Also, JKSQ adjuvant treatment has been shown to prevent the recurrence and aggravation of nephrotic syndrome in patients with “Yang Deficiency of Spleen and Kidney” during prednisone withdrawal when evaluated using TCM syndrome scores and biochemistry analyses.22 In animal studies, the possible mechanism of JKSQ in treating KDS-Yang animal model included increased endogenous secretion of CRH and ACTH,37 prevented the degeneration of adrenal gland cells38 and up-regulated the gene expressions of ACTH and 17-hydroxylase.39, 40 The contribution of our present study is that we provide more insights into the prophylactic efficacy and molecular mechanisms of JKSQ in the experimental adrenal insufficiency rats, using global and targeted metabolomics approach. The results were valuable not only increasing to an understanding of adrenal insufficiency at molecular level, but also dissect the underlying mechanisms of the prophylactic treatment of JKSQ in reducing the risk of developing adrenal insufficiency after glucocorticoid withdrawal.
Glucocorticoids are metabolic hormones, which increase availability of all fuel substrates by mobilization of glucose,41 free fatty acids,42 and amino acids43 from endogenous stores. Therefore adrenal insufficiency is associated with decreased metabolic rates of carbohydrates, lipids and amino acids. In our study, down-regulated gluconeogenesis pathway, altered amino acids metabolism, decreased glycerolipid metabolism, decreased fatty acids metabolism and beta oxidation of long chain fatty acids were found in rats after glucocorticoids withdrawal, compatible with the previous finding that glucocorticoids deficiency led to decreased energy expenditure.44 JKSQ could effectively normalize all of the differentially expressed metabolites in model rats compared to controls, suggesting its beneficial effect as a glucocorticoid mimetics on adrenal insufficiency after glucocorticoid withdrawal.
Activation of HPA axis increased the release of circulating FFAs and glycerol from adipose tissue.45, 46 Both in vivo and in vitro studies demonstrated that metabolic disorders with elevated concentrations of circulating FFAs were associated with hyperactivation of HPA axis.47–50 Also, fatty acids supplementation influenced in vivo HPA activation and changed serum cortisol concentrations, although the results were inconsistent.51–53 Our results showed differential metabolic responses to JKSQ in adrenal insufficiency and normal rats. As shown in Table 2 and Figure 3, JKSQ effectively attenuated or normalized most of the decreased concentrations of serum FFAs and altered product/precursor FFAs ratios in model rats. However, when JKSQ was administered to healthy rats, significant changes of 27 FFAs and 6 product/precursor FFAs ratios were observed between the treatment group and controls, even after 10 days of washout period. These results suggest that JKSQ could exert harmful and beneficial metabolic regulatory actions on fatty acids metabolism under healthy and pathological conditions, respectively.
According to TCM theory of “Yi Pian Jiu Pian (以偏纠偏)” [use biased treatment to correct the imbalanced system], traditional Chinese herbs with “Pian Xing (偏性)” [biased properties and function]54 can be used to moderate excess or deficiency of Yin and Yang in diseased conditions. However, when these herbs are prescribed to healthy individuals, they will induce imbalance between Yin and Yang and result in adverse reactions, which is described as “Yi Pian Zhi Pian (以偏致偏)” [disturb a balanced system with a biased treatment] in TCM. Traditionally, JKSQ possessed the powerful effect in reinforcing Kidney-Yang. Therefore, it is not surprising that JKSQ was observed to exert opposite regulatory effects on fatty acid metabolism under healthy and adrenal insufficiency conditions. However, further studies are needed to unveil the bioactive components of JKSQ and molecular mechanisms underlying the regulatory effects on fatty acid metabolism.
An important strength of the present study is that, the metabolic alterations as a result of JKSQ exposure in healthy rats were compared with the findings from the JKSQ treatment in the rat model. The key metabolites and pathways which were disturbed in healthy status but moderated in pathologic condition by JKSQ treatment were considered the most valuable findings to associate with the functional mechanisms of JKSQ.
The present study has some limitations. This was a pilot study which investigated whether metabolomics data were meaningful for the understanding of the pathophysiologic mechanism of adrenal insufficiency after glucocorticoids withdrawal and the functional mechanism of TCM prophylactic treatment. The sample size of the experiment was relatively small. The duration of hydrocortisone treatment (7 days) was much shorter than the clinical duration of glucocorticoids administration; although this short-term treatment might have been partially compensated by the higher dosage of hydrocortisone used in the rats, a regimen that more closely simulates the clinical condition should be considered in future studies. Even so, results from this animal model suggested that metabolomics is a useful tool in the study of adrenal insufficiency, and can be used in future clinical studies on the metabolic responses of JKSQ in preventing adrenal insufficiency resulting from glucocorticoid therapy and withdrawal.
CONCLUSIONS
In conclusion, both the standard endocrine testing and the global and targeted metabolomics results revealed the protective effects of JKSQ in an experimental adrenal insufficiency rat model. The significantly altered serum metabolites were associated with carbohydrate, lipids and amino acids metabolism, especially FFA metabolism. The differential metabolic responses to JKSQ in adrenal insufficiency and normal rats confirmed the TMC principle of “treatment based on ZHENG Differentiation”. The results also suggest that metabolomics analyses of the differential metabolic responses in both pathologic and healthy conditions are valuable in understanding the functional mechanisms of TCM formulas in modern pharmacological studies.
Supplementary Material
Table S1. The main constituents in Chinese patent medicine JKSQ pill. Table S2. Body Weight and Food Consumption of Rats on Days 0, 7, 15, 22 and 25. Table S3. Identification of Significantly Differential Metabolites in the Sera of Normal and Acute Glucocorticoid Withdraw Induced Adrenal Insufficiency Rats with or without Pretreatment of JKSQ. Table S4. List of P Values Representing the Different Changes of Serum Free Fatty Acids in Normal and Model Rats with or without Pretreatment of JKSQ. Table S5. Serum Product FFA/Precursor FFA Concentration Ratios in Normal and Model Rats with or without Pretreatment of JKSQ. Figure S1. The scores plots of the OPLS-DA models using data from normal control group (N, gray boxes), model group (M, red boxes), JKSQ-treated normal group (JM, blue boxes) and JKSQ-treated model group (JN, green boxes), respectively. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This study was financially supported by International Science & Technology Cooperation Program of China (2014DFA31870), China Scholarship Council (201408310049) and Infinitus (China) Company Ltd., a member of LKK Health Products Group (B9109E).
ABBREVIATIONS
- 17-OHCS
17-hydroxycorticosteroids
- ACTH
adrenocorticotropic hormone
- CRH
corticotropin releasing hormone
- FC
fold change
- FFAs
free fatty acids
- GC/TOFMS
gas chromatography/time-of-fight mass spectrometry
- HPA
hypothalamus-pituitary-adrenal
- HPAS
hypothalamus-pituitary-adrenal axis suppression
- JKSQ
Jinkui Shenqi Pill
- OPLS-DA
orthogonal partial least-squares projection to latent structures-discriminant analysis
- TCA cycle
tricarboxylic acid cycle
- TCM
traditional Chinese medicine
- UPLC/Q-TOFMS
ultra-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry
- VIP
variable importance in the projection
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Ford LR, Willi SM, Hollis BW, Wright NM. Supression and recovery of the neonatal hypothalamic-pituitary-adrenal axis after prolonged dexamethasone therapy. The Journal of Pediatrics. 1997;131(5):722–726. doi: 10.1016/s0022-3476(97)70100-8. [DOI] [PubMed] [Google Scholar]
- 2.Ng PC, Lam CW, Lee CH, Chan IH, Wong SP, Fok TF. Suppression and recovery of the hypothalamic function after high-dose corticosteroid treatment in preterm infants. Neonatology. 2008;94(3):170–175. doi: 10.1159/000143396. [DOI] [PubMed] [Google Scholar]
- 3.Huber BM, Bolt IB, Sauvain MJ, Flück CE. Adrenal insufficiency after glucocorticoid withdrawal in children with rheumatic diseases. Acta Paediatrica. 2010;99(12):1889–1893. doi: 10.1111/j.1651-2227.2010.01936.x. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy MJ, Carpenter JM, Lozano RA, Castile RG. Impaired recovery of hypothalamic-pituitary-adrenal axis function and hypoglycemic seizures after high-dose inhaled corticosteroid therapy in a toddler. Annals of Allergy, Asthma, & Immunology. 2002;88(5):523–526. doi: 10.1016/S1081-1206(10)62393-9. [DOI] [PubMed] [Google Scholar]
- 5.Graber AL, Ney RL, Nicholson WE, Island DP, Liddle GW. Natural history of pituirary-adrenal recovery following long-term supperssion with corticosteroids. The Journal of Clinical Endocrinology and Metabolism. 1965;25:11–16. doi: 10.1210/jcem-25-1-11. [DOI] [PubMed] [Google Scholar]
- 6.Calogero AE. Recovery of the rat hypothalamic-pituitary-adrenal axis after discontinuation of prolonged treatment with the synthetic glucocorticoid agonist dexamethasone. Endocrinology. 1990;127(4):1574–1579. doi: 10.1210/endo-127-4-1574. [DOI] [PubMed] [Google Scholar]
- 7.Huang TS. Corticotropin secretagogues facilitate recovery of the hypothalamus-pituitary-adrenal axis suppressed by prolonged treatment with dexamethasone. Metabolism. 1994;43(5):544–548. doi: 10.1016/0026-0495(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson S, Campbell E, Torrellas A, Beckford U, Altaher R, Sandford R, Scraggs R, Gillham B, Jones M. Recovery of the hypothalamo-pituitary-adrenocortical axis in the rat after long-term dexamethasone treatment. Neuroendocrinology. 1984;39(4):343–349. doi: 10.1159/000124002. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson SA, Campbell EA, Gillham B, Jones MT. Recovery of the components of the hypothalamo-pituitary-adrenocortical axis in the rat after chronic treatment with prednisolone. Journal of Endocrinology. 1987;113(2):239–247. doi: 10.1677/joe.0.1130239. [DOI] [PubMed] [Google Scholar]
- 10.Dinsen S, Baslund B, Klose M, Rasmussen AK, Friis-Hansen L, Hilsted L, Feldt-Rasmussen U. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. European Journal of Internal Medicine. 2013;24(8):714–720. doi: 10.1016/j.ejim.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Neidert S, Schuetz P, Mueller B, Christ-Crain M. Dexamethasone suppression test predicts later development of an impaired adrenal function after a 14-day course of prednisone in healthy volunteers. European Journal of Endocrinology. 2010;162(5):943–949. doi: 10.1530/EJE-09-0930. [DOI] [PubMed] [Google Scholar]
- 12.Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacology and Therapeutics. 2002;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 13.Hunt PJ, Gurnell EM, Huppert FA, Richards C, Prevost AT, Wass JA, Herbert J, Chatterjee VKK. Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison’s disease in a randomized, double blind trial. Journal of Clinical Endocrinology & Metabolism. 2000;85(12):4650–4656. doi: 10.1210/jcem.85.12.7022. [DOI] [PubMed] [Google Scholar]
- 14.Arlt W, Allolio B. Adrenal insufficiency. The Lancet. 2003;361(9372):1881–1893. doi: 10.1016/S0140-6736(03)13492-7. [DOI] [PubMed] [Google Scholar]
- 15.Løvås K, Loge JH, Husebye ES. Subjective health status in Norwegian patients with Addison’s disease. Clinical Endocrinology. 2002;56(5):581–588. doi: 10.1046/j.1365-2265.2002.01466.x. [DOI] [PubMed] [Google Scholar]
- 16.Arlt W, Rosenthal C, Hahner S, Allolio B. Quality of glucocorticoid replacement in adrenal insufficiency: clinical assessment vs. timed serum cortisol measurements. Clinical Endocrinology. 2006;64(4):384–389. doi: 10.1111/j.1365-2265.2006.02473.x. [DOI] [PubMed] [Google Scholar]
- 17.Debono M, Ross RJ. What is the best approach to tailoring hydrocortisone dose to meet patient needs in 2012? Clinical Endocrinology. 2013;78(5):659–664. doi: 10.1111/cen.12117. [DOI] [PubMed] [Google Scholar]
- 18.Debono M. Optimal glucocorticoid therapy. Pediatric Adrenal Diseases: Workshop, Turin, May 2010. 2010;20:173–180. [Google Scholar]
- 19.Shen Z. The location of deficiency syndrome of kidney Yang. Chinese Medical Journal. 1999;112(11):973–975. [PubMed] [Google Scholar]
- 20.Zhang D, Yu G, Peng C. Effect of Jinkuishenqi wan on adrenal insufficiency induced by prednisone. Henan Zhongyi. 1992;12(6):262. [Google Scholar]
- 21.Shen J. Effect of Jinkui shenqi wan on complications by prednisone therapy. Zhongyi Zazhi. 1987;(1):45–46. [Google Scholar]
- 22.Lv Y, Wang Y. Effect of Jinkui Shenqi Bolus during Hormone Withdrawal in Nephrotic Syndrome Patients with Yang Deficiency of Spleen and Kidney. Anhui Zhongyi Xueyuan Xuebao. 2004;23(3):15–17. [Google Scholar]
- 23.Mao X, Han L. Clinical observation of effect of combing traditional and western medicine treatment in 32 children with nephrotic syndrome during hormone withdrawl. Zhongguo Zhongxiyi Jiehe Erkexue. 2013;5(6):533–534. [Google Scholar]
- 24.Zhao L, Wu H, Qiu M, Sun W, Wei R, Zheng X, Yang Y, Xin X, Zou H, Chen T, Liu J, Lu L, Su J, Ma C, Zhao A, Jia W. Metabolic Signatures of Kidney Yang Deficiency Syndrome and Protective Effects of Two Herbal Extracts in Rats Using GC/TOF MS. Evidence-Based Complementary and Alternative Medicine. 2013;2013:540957. doi: 10.1155/2013/540957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q. Experimental Methodology of Pharmacological Research in Traditional Chinese Medicine. People’s Health Publishing House; Beijing: 1993. [Google Scholar]
- 26.Qiu Y, Cai G, Su M, Chen T, Zheng X, Xu Y, et al. Serum metabolite profiling of human colorectal cancer using GC-TOFMS and UPLC-QTOFMS. Journal of Proteome Research. 2009;8(10):4844–4850. doi: 10.1021/pr9004162. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson P, Gullberg J, Nordström A, Kusano M, Kowalczyk M, Sjöström M, et al. A Strategy for Identifying Differences in Large Series of Metabolomic Samples Analyzed by GC/MS. Analytical Chemistry. 2004;76(6):1738–1745. doi: 10.1021/ac0352427. [DOI] [PubMed] [Google Scholar]
- 28.Puttmann M, Krug H, von Ochsenstein E, Kattermann R. Fast HPLC determination of serum free fatty acids in the picomole range. Clinical Chemistry. 1993;39(5):825–832. [PubMed] [Google Scholar]
- 29.Hochberg Z. Glucocorticoid withdrawal syndrome. The Endocrinologist. 2004;14(3):152–160. [Google Scholar]
- 30.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Progress in Lipid Research. 2008;47(5):348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Yary T, Voutilainen S, Tuomainen TP, Ruusunen A, Nurmi T, Virtanen JK. Serum n-6 polyunsaturated fatty acids, Delta5- and Delta6-desaturase activities, and risk of incident type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. The American Journal of Clinical Nutrition. 2016;103(5):1337–1343. doi: 10.3945/ajcn.115.128629. [DOI] [PubMed] [Google Scholar]
- 32.Carella MJ, Srivastava LS, Gossain VV, Rovner DR. Hypothalamic-pituitary-adrenal function one week after a short burst of steroid therapy. The Journal of Clinical Endocrinology and Metabolism. 1993;76(5):1188–1191. doi: 10.1210/jcem.76.5.8388401. [DOI] [PubMed] [Google Scholar]
- 33.Streck WF, Lockwood DH. Pituitary adrenal recovery following short-term suppression with corticosteroids. The American Journal of Medicine. 1979;66(6):910–914. doi: 10.1016/0002-9343(79)90444-3. [DOI] [PubMed] [Google Scholar]
- 34.Harrison BD, Rees LH, Cayton RM, Nabarro JD. Recovery of hypothalamo-pituitary-adrenal function in asthmatics whose oral steroids have been stopped or reduced. Clinical Endocrinology. 1982;17(2):109–118. doi: 10.1111/j.1365-2265.1982.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 35.Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics. 2007;120(3):e575–e586. doi: 10.1542/peds.2006-2402. [DOI] [PubMed] [Google Scholar]
- 36.Weston WL, Fennessey PV, Morelli J, Schwab H, Mooney J, Samson C, Huff L, Harrison LM, Gotlin R. Comparison of hypothalamus-pituitary-adrenal axis suppression from superpotent topical steroids by standard endocrine function testing and gas chromatographic mass spectrometry. Journal of Investigative Dermatology. 1988;90(4):532–535. doi: 10.1111/1523-1747.ep12461062. [DOI] [PubMed] [Google Scholar]
- 37.Xu C, Sun J, Zhu Q, Song J, Zhang D, Li Z. Effect of Jinkuishenqi wan on the hypothalamic-pituitary-adrenal axis in Kidney-Yang Dificiency mice. Shandong Zhongyiyao Daxue Xuebao. 2009;33(3):248–249. [Google Scholar]
- 38.Xu C, Li Z, Song J, Zhang D, Zhu Q. Effect of traditional Chinese medicine for kidney-reinforcing of morphological alerations of the adrenal gland in mice with Kidney-Yang deficiency. Shandong Daxue Xuebao (Yixueban) 2011;49(2):67–70. [Google Scholar]
- 39.Zheng XW, Bao SZ, Liu MZ, Song H, Li RQ. Effect of Jinkui Shenqi pill on pituitary adrenocorticotropic hormone gene expression in Shen-Yang deficieney rats. Zhongguo Zhongxiyi Jiehe Zazhi. 2004;24(3):238–240. [PubMed] [Google Scholar]
- 40.Zheng DS. Effect of Jinkui Shenqi pill on 17-hydroxylase gene expression in kidney yang deficiency rats. Zhongguo Zhongyiyao Xinxi Zazhi. 2006;13(10):42–43. [Google Scholar]
- 41.Rizza RA, Mandarino LJ, Gerich JE. Cortisol-Induced Insulin Resistance in Man: Impaired Suppression of Glucose Production and Stimulation of Glucose Utilization due to a Postreceptor Defect of Insulin Action. The Journal of Clinical Endocrinology & Metabolism. 1982;54(1):131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- 42.Djurhuus C, Gravholt CH, Nielsen S, Mengel A, Christiansen J, Schmitz O, Møller N. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. American Journal of Physiology-Endocrinology And Metabolism. 2002;283(1):E172–E177. doi: 10.1152/ajpendo.00544.2001. [DOI] [PubMed] [Google Scholar]
- 43.Horber FF, Haymond MW. Human growth hormone prevents the protein catabolic side effects of prednisone in humans. Journal of Clinical Investigation. 1990;86(1):265–272. doi: 10.1172/JCI114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christiansen JJ, Djurhuus CB, Gravholt CH, Iversen P, Christiansen JS, Schmitz O, Weeke J, Jørgensen JOL, Møller N. Effects of cortisol on carbohydrate, lipid, and protein metabolism: studies of acute cortisol withdrawal in adrenocortical failure. The Journal of Clinical Endocrinology & Metabolism. 2007;92(9):3553–3559. doi: 10.1210/jc.2007-0445. [DOI] [PubMed] [Google Scholar]
- 45.Björntorp P. Neuroendocrine perturbations as a cause of insulin resistance. Diabetes/Metabolism Research and Reviews. 1999;15(6):427–441. doi: 10.1002/(sici)1520-7560(199911/12)15:6<427::aid-dmrr68>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 46.Ottosson M, Vikman-Adolfsson K, Enerbäck S, Olivecrona G, Björntorp P. The effects of cortisol on the regulation of lipoprotein lipase activity in human adipose tissue. Journal of Clinical Endocrinology and Metabolism. 1994;79(3):820–825. doi: 10.1210/jcem.79.3.8077367. [DOI] [PubMed] [Google Scholar]
- 47.Widmaier EP, Rosen K, Abbott B. Free fatty acids activate the hypothalamic-pituitary-adrenocortical axis in rats. Endocrinology. 1992;131(5):2313–2318. doi: 10.1210/endo.131.5.1330498. [DOI] [PubMed] [Google Scholar]
- 48.Widmaier E, Margenthaler J, Sarel I. Regulation of pituitary-adrenocortical activity by free fatty acids in vivo and in vitro. Prostaglandins, Leukotrienes and Essential Fatty Acids. 1995;52(2):179–183. doi: 10.1016/0952-3278(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 49.Benthem L, Keizer K, Wiegman CH, de Boer SF, Strubbe JH, Steffens AB, Kuipers F, Scheurink AJ. Excess portal venous long-chain fatty acids induce syndrome X via HPA axis and sympathetic activation. American Journal of Physiology-Endocrinology and Metabolism. 2000;279(6):E1286–E1293. doi: 10.1152/ajpendo.2000.279.6.E1286. [DOI] [PubMed] [Google Scholar]
- 50.Mocking RJ, Ruhé HG, Assies J, Lok A, Koeter MW, Visser I, Bockting CL, Schene AH. Relationship between the hypothalamic–pituitary–adrenal-axis and fatty acid metabolism in recurrent depression. Psychoneuroendocrinology. 2013;38(9):1607–1617. doi: 10.1016/j.psyneuen.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Caroprese M, Ciliberti MG, Annicchiarico G, Albenzio M, Muscio A, Sevi A. Hypothalamic-pituitary-adrenal axis activation and immune regulation in heat-stressed sheep after supplementation with polyunsaturated fatty acids. Journal of Dairy Science. 2014;97(7):4247–4258. doi: 10.3168/jds.2013-7696. [DOI] [PubMed] [Google Scholar]
- 52.Oh YT, Kim J, Kang I, Youn JH. Regulation of hypothalamic-pituitary-adrenal axis by circulating free fatty acids in male Wistar rats: Role of individual free fatty acids. Endocrinology. 2014;155(3):923–931. doi: 10.1210/en.2013-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jazayeri S, Keshavarz SA, Tehrani-Doost M, Djalali M, Hosseini M, Amini H, Chamari M, Djazayery A. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Research. 2010;178(1):112–115. doi: 10.1016/j.psychres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 54.Cui Y, Changi Z, Liu J. A Look at “pianxing” of traditonal Chinese medicine. Zhongguo Zhongyao Zazhi. 2009;34(6):774–775. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The main constituents in Chinese patent medicine JKSQ pill. Table S2. Body Weight and Food Consumption of Rats on Days 0, 7, 15, 22 and 25. Table S3. Identification of Significantly Differential Metabolites in the Sera of Normal and Acute Glucocorticoid Withdraw Induced Adrenal Insufficiency Rats with or without Pretreatment of JKSQ. Table S4. List of P Values Representing the Different Changes of Serum Free Fatty Acids in Normal and Model Rats with or without Pretreatment of JKSQ. Table S5. Serum Product FFA/Precursor FFA Concentration Ratios in Normal and Model Rats with or without Pretreatment of JKSQ. Figure S1. The scores plots of the OPLS-DA models using data from normal control group (N, gray boxes), model group (M, red boxes), JKSQ-treated normal group (JM, blue boxes) and JKSQ-treated model group (JN, green boxes), respectively. This material is available free of charge via the Internet at http://pubs.acs.org.


