Opinion Statement
A high proportion of suicides visit their medical provider in the month prior to death, but depression, suicidal thoughts, and substance use are seldom addressed. For the clinicians routinely treating a substantial patient population with allergic diseases, there are additional concerns, as allergy has been linked with both depression and suicidal behavior. While psychotropic medications may affect diagnosis of allergies, medications used to treat allergies impact mood and behavior. Thus, we present an overview of the overlap of allergic rhinitis with depression and suicidal behavior in adults, based on clinical and epidemiological data, and our research and clinical experience. In summary, we suggest: 1) inquiring among patients with allergies about personal and family history of depression, substance use disorders, suicidal ideation and attempts 2) increased mindfulness regarding the potential effects of allergy medications on mood and behavior; and 3) for people identified with certain types of depression or increased suicide risk, a systematic multilevel collaborative approach. While for practical reasons the majority of patients with depression will continue to be treated by general or family practitioners, the allergy-treating provider should always consider integrated care for bipolar, psychotic or suicidal depression and incomplete remission, or relapsing and highly recurrent course. While awaiting results of much needed basic and clinical research to guide clinical approach for patients with comorbid allergic rhinitis and depression, the simple steps recommended here are expected to improved clinical outcomes in depression, including, on a large scale, reduced premature deaths by suicide.
Keywords: Allergic rhinitis, Depression, Inflammation, Suicidal behavior, suicidality
Introduction
Allergic rhinitis (AR), colloquially referred to as hay fever, is among the most chief complaints among patients worldwide. According to the U.S. Department of Health and Human Services survey, in 2012, 19.1 million individuals in the US [8%] above 18 years of age, and 6.1 million under the age of 18 [10%] reported being diagnosed with hay fever [1, 2]; thereby, making it the most common chronic disease in children, and the fifth most common disorder overall. Reported incidence rates in other countries vary between 10–30% over all age groups [3, 4]. Treating those in the US with AR results in substantial healthcare costs, with direct costs including prescriptions totaling about $3.4 billion. The cost of associated loss of productivity is estimated at between $2–3 billion [5, 6]. Apart from being a ‘nuisance disease’ leading to a significant decrease quality of life [7], AR can be comorbid with other respiratory and non-respiratory disorders such as asthma [8], chronic otitis media [9], rhinosinusitis [10], turbinate hypertrophy [11], secondary obstructive sleep apnea, and sleep difficulties [12]. Evidence suggests that AR often occurs co-morbidly with mood disorders, such as depression [13], and suicidal behaviors [14]. AR is more prevalent in patients with major depression and those having family history of depressive disorder [15–17]. In adolescents, a pediatric diagnosis of AR is predictive of an adult diagnosis of major depression [18] or bipolar disorder [19]. Additionally, in Finnish twins a potentially shared genetic risk between allergic disorders and depressive symptoms has been described [20]. However, the association is likely not only genetic, as clinical studies involving symptomatic subjects with AR secondary to ragweed pollen exposure have reported worsening mood and energy levels during exposure [21]. Exposure to aeroallergens has also been associated with death by suicide [22, 23]
The prevalence of AR has increased over the last several decades, and is expected to continue to increase due to environmental influences [24, 25]. One hypothesis that has been put forward to explain the dramatic increases in incidence of allergic disorders, including AR, over the last few decades is the hygiene hypothesis, or the “old friends” hypothesis. Strachan proposed that the lower incidence of infection in early childhood could be an explanation for the rapid rise in allergic diseases, including allergic asthma and AR, documented during the 20th century [26, 27]. The “old friends” hypothesis was proposed to emphasize that it is not a lack of exposure to childhood infections or outright pathogens per se that is harmful, but instead, it is a lack of exposure to harmless environmental microorganisms and symbiotic organisms, as well as a lack of exposure to specific chronic infections that were common during the hunter gatherer period of human evolution, referred to as the “old infections”, that is harmful. It has since been proposed that this lack of exposure to “old friends”, and thus lack of suppression of inappropriate inflammation, increases vulnerability to both allergic disorders [28••] and anxiety and affective disorders [29].
Here we provide a brief overview of our current understanding of AR, and review associated literature that connects it with psychiatric disorders, namely anxiety and affective disorders and suicidality. We also propose possible mechanisms linking them.
Allergic rhinitis [AR]: manifestations and diagnosis
AR is an inflammatory, IgE-mediated disease characterized by inflammation of the mucosal lining of the nose that occurs when a person inhales airborne allergens to which he or she is sensitized to, such as animal dander, dust mite fecal particles, and/or pollen. The resultant immune reaction leads to characteristic AR-associated clinical symptoms of nasal congestion, rhinorrhea (nasal drainage), sneezing, and/or nasal itching [30]. Two or more of these symptoms, evident for at least one hour per day for more than 2 weeks, are diagnostic, especially when a detailed history reveals a specific allergen trigger [31]. Alternative causes of rhinitis include infectious, structural (secondary to mechanical airway aberrations like deviated nasal septum) or vasomotor causes, but episodes of sneezing, and an itchy nose and palate, with clear bilateral discharge and eye involvement (rhino-conjunctivitis) are particularly suggestive of an allergic origin.
A detailed clinical history and physical examination is required to make the diagnosis, and this includes differentiation between seasonal and non-seasonal AR [32]. Individuals with history of AR may also reveal common comorbidities such as conjunctivitis, chronic otitis media, significant sleep impairment and sleep apnea, hyposmia, asthma, and laryngeal involvement [33, 34]. All these factors can have a negative impact on one’s health and overall performance, as well as on their quality of life and psychological wellbeing [35••]. In vivo and laboratory-based IgE antibody testing for allergen sensitivity are used to confirm the clinical suspicion of AR [36]. These tests are useful in pinpointing the offending allergen(s) and making a definitive diagnosis. Skin prick testing (SPT) with aeroallergens common to a particular geographic area is a widely accepted, safe, convenient and cost effective [37] procedure, and one of the most widely used diagnostic screening tests [38, 39]. SPT is less useful in conditions involving generalized eczema, dermographism, in individuals with recent uses of medications that have significant antihistaminic properties like tricyclic antidepressants, or undergoing high dose corticosteroid treatment. In these cases, allergen-specific IgE serological testing is an equally informative alternative for identifying allergic sensitization [36, 40]. Additionally, in patients with needle phobias or acute psychiatric conditions limiting their cooperation with SPT, or those on medications with antihistaminic properties (e.g., many psychotropic medications), measuring allergen-specific IgE may be preferable. State-of-the-art IgE antibody serology autoanalzyers have shown comparable diagnostic sensitivity and specificity to SPT [36]. A serum IgE test is also indicated in the case of an equivocal SPT, or a negative SPT with strong clinical suspicion, or in the case of a history of severe allergic reactions when allergen is administered to the skin. A nasal provocation test (NPT), using a specific allergen that is administered to the nasal cavity, may be indicated when discrepancies remain between the clinical evaluation, skin or serological IgE antibody tests. This technique is considered more of a research procedure, as it is often used in research studies to evaluate the magnitude of allergic sensitivity to an allergen [41].
Aeroallergens: sources and seasonality
Aeroallergens most relevant to AR are inhaled following their suspension in air, mostly as the suspended particles (e.g., mold spores, pollen grains, dust mite fecal particles). Some are seasonal in nature, such as pollen (the male gametophytes from wind-pollinated plants). Plants pollinated by insects form pollen that is too large to remain suspended in air, and thus are not relevant to eliciting AR symptoms. Depending on the allergen source (e.g., trees, grass, weeds), the pollen counts in the air are generally highest during mid-spring to early summer when 75% of environmental pollen exposure occurs. The link between spring peaks in highly allergenic trees (such as oak) and allergic rhinitis in spring is confirmed by multiple lines of evidence, including over-the-counter allergy medication purchases [42]. In temperate regions, grass pollen is principally released during the summer months. Although it represents only about 10% of the annual pollen load, grass-derived pollen has significant cross reactivity between different grasses, and food allergens [43]. The most important source of pollen in late summer-early fall is the common ragweed (Ambrosia artemisiifolia), possibly the most allergenic taxa in the northern Americas [44] accounting for 15% of the yearly pollen load. Ragweed pollen production is controlled by the photoperiod and is therefore latitude dependent [45]. Recent studies indicate an increasing duration of allergen presence and increased pollen load of these plants potentially secondary to global warming and increased CO2 levels [46]. Fungal spores are also important seasonal aeroallergens, with peak concentrations that occur in spring (Ascomycete spp.) and fall (Basidiomycetes e.g., mushrooms, puffballs and bracket fungus) [47, 48]
Apart from these seasonal allergens, AR also results from chronic or episodic exposure of sensitized individuals to animal dander from pets, cockroach-related proteins, dust mites, and non-seasonal indoor fungi. These allergens are ubiquitous and may be responsible for a significant proportion of AR cases. One study found that a high percentage of North American homes have moderate to high levels of dust mites [e.g. 78.5% in San Diego, California], and detectable cockroach antigen [e.g. 21.5% in Boston, Massachusetts], which were strongly associated with a positive SPT in children [49]. A recent review concluded that dust mite is the most important indoor non-seasonal allergen [50]. Allergens causing AR may also be food items like flour, through occupational exposure, such as bakers sensitized to enzymes or flour in bread mix [51].
Comorbidity of AR and certain psychiatric disorders
Clinicians have long observed co-existence of allergic diseases like AR and psychological problems, such as “allergic toxemia”; a syndrome described in 1930s and characterized by fatigue, slow psychomotor and thought process, poor memory, irritability and depression [52]. The current body of literature suggests that this association is likely be secondary to cytokines and other macromolecules associated with inflammation in AR.
Depression: overview
In the Diagnostic and Statistical Manual 5 (DSM5) [53], all psychiatric disorders with predominant sad, empty or, in younger individuals, irritable mood accompanied by characteristic somatic and cognitive symptoms that cause significant disruption of an individual’s capacity to function have been grouped under “Depressive Disorders” [54]. Another class of disorders with a predominant mood component is “Bipolar and Related Disorders” [55]. Depressive disorders may have identifiable underlying medical-surgical, gynecological, or substance-related precipitating factors and different time course-/severity-related variables for diagnosing specific categories. Major depressive disorder (MDD), also referred to as unipolar depression or clinical depression, represents the classic condition for this group. An individual is diagnosed with MDD if for two or more consecutive weeks he/she reports or is reported by others to experience for most of the day, at least one of the two defining symptoms of 1] sadness or 2] loss of interest or pleasure in previously enjoyable activities (anhedonia), together with at least five of the following seven symptoms for most of the days in a week: 1] significant unintentional loss of weight and/or appetite, 2] insomnia or hypersomnia, 3] psychomotor agitation or retardation confirmed by objective observation, 4] fatigue or loss of energy, 5] feelings of worthlessness or excessive guilt, 6] diminished ability to concentrate or think and indecisiveness, or 7] recurrent thoughts of death (not just fear of dying), recurrent suicidal ideation or plans/attempts. Depression may be precipitated by psychosocial stressors such as loss of loved ones or financial difficulties. It can also be precipitated and perpetuated or worsened by underlying medical conditions or physiological stressors like peri-partum period or seasonal changes in vulnerable individuals. Diagnostic specifiers such as ‘seasonal’ or ‘peri-partum’ allow for accurately capturing these factors. Symptoms may be episodic or recurrent. There may be associated psychotic or anxiety symptoms with depression [54].
Major depressive disorder affects 6.7% of US adults or estimated 15.7 million US citizens in 2014 [56]. Depression, particularly when exhibiting atypical symptoms of increased sleep and appetite, affects women more than men, [57]. Women also experience more somatic symptoms like sleep and appetite difficulties, fatigue, and anxiety as compared to men, possibly due to underlying biological predispositions such as hormonal differences [58]. Depression can result in significant personal and societal costs due to significant negative impacts on performance, productivity and resultant, absenteeism, and disability. The annual cost of depression in the US exceeds $26 billion for medical care, $5.4 billion due to suicide-related mortality, and $50 billion due to loss of productivity [59].
Bipolar disorder
Depressive episodes can occur as a manifestation of bipolar disorder, which requires at least one episode of mania and/or hypomania (Bipolar II includes only hypomania) for diagnosis. Mania is diagnosed when there is at least one week (or less, if the patient is admitted) of a distinct period of abnormally and persistently elevated, expansive or irritable mood and abnormally increased goal directed activity or energy, grandiosity, pressured speech, decreased need for sleep, psychomotor excitation and distractibility, or excessive involvement in hedonistic activity, which may have painful consequences. All of which must lead to significant functional impairment. Hypomania is diagnosed with similar symptoms but of lower intensity with no or minimal impairment and lasts for at least four consecutive days. There may be extended periods of normal mood between bipolar mood episodes [55]. There is evidence that stress, both psychological and biological in origin, circadian disruption and sleep impairment, particularly decreased sleep, which could be the consequence of AR [60], and antidepressant medication are all important precipitating factors for manic or hypomanic episodes [61]. The 12-month prevalence of bipolar disorder in the US is 2.6% in the adult population [62]. Obtaining collateral information from family members and health care providers may be very important in bipolar disorder, considering that treatment differs from that of major depression, and that a sizable proportion of patients do not have an adequate insight into their history of hypomania or mania.
Suicide: overview
Suicide (or fatal suicidal self-directed violence) is death caused by self-directed injurious behavior with intent to die as a result of the behavior. It may be preceded by suicide attempt/s, defined as non-fatal, suicidal self-directed violence. Suicidal behavior includes suicide (i.e. death by suicide) or suicide attempt generally takes place after the engaging individual had been thinking about, considering or planning suicide, also termed as suicidal ideation. [63]. Suicidal ideas often stem from feelings of worthlessness, hopelessness and helplessness and cognitive biases such as fortune telling [predicting outcomes of a situation, mostly the worst one] [64, 65], which often can be estimated with good clinical interview. According to the Center for Disease Control (CDC), suicide was the tenth most common cause of death in all ages in the USA in 2013, with almost 13 suicides every minute. In the USA more than 41,000 individuals die by suicide, and 494,000 receive medical care for self-inflicted injuries annually, with an age-adjusted suicide rate of 13 per 100,00 individuals. There has been a gradual increase in suicide-related deaths since 1999 [66]. Men died by suicide 4 times more frequently than women, though women were much more likely to attempt suicide. Nearly 24% of those who died by suicide were taking antidepressants at the time of their death [67]. The World Health Organization (WHO) in 2004 describes suicide as a “Huge but preventable public health problem [68]. Suicide is most frequently associated with mood disorders such as MDD, or the depressive phase of bipolar disorder, but also occurs among those with other psychiatric disorders like schizophrenia, personality disorders and substance use disorders [69]. A history of anxiety disorders, either by themselves or comorbid with other psychiatric disorders, may also increase an individual’s risk for death by suicide [70]. Nevertheless, in individual patients it is possible that treating anxiety disorders, in particular with anxiolytics, may elevate suicide risk through disinhibition, or lowering of fear of death or dying, a protective factor.
Past suicide attempts and current suicidal ideation are considered the most important clinical predictors of future attempts/death by suicide, particularly in individuals previously diagnosed with psychiatric illness [71]. For every suicide, there are about 25 suicide attempts [72]. Risk of suicide post-suicide attempt is highest within the first 6 months, though it remains significantly elevated for at least a few years [73]. A large national cohort-based study involving 2.46 million people from Denmark, with follow-up data from age 15 years onwards, showed that bipolar disorder presented maximum risk while comorbid substance use and unipolar depression were associated with increased absolute risk, and past history of self-harm nearly doubled the risk. The study also highlighted that risk of suicide was greatest within the first few years of contact with mental health care [74] [75]
Clinical approach to suicidal behavior
A thorough clinical interview covering current or past wishes to be dead, “active” suicidal thoughts with frequency, intent, plan with specificity and severity, past history of suicidal behavior and resultant injury or damage is essential in suicide risk evaluation. Inquiry must be made as to the availability of means to engage in suicidal behavior such as guns, other weapons or stored medications. Validated scales such as the Columbia-Suicide Severity Rating Scale (CSSRS) help in clinical documentation of suicidality and potential for suicidal behavior [76] [Available at http://www.cssrs.columbia.edu/]
The stress-diathesis model of suicidal behavior
The stress-diathesis model conceptualizes suicidal behavior as interplay between vulnerability and triggering [77], and is currently one of the most accepted models of suicidal behavior. The model posits that, in a predisposed individual, a psychological or bio-physiological stressor leads to exacerbation of suicide risk, through mechanisms like precipitating or worsening of psychiatric illness thus triggering suicide attempts. Further, the interplay between supportive factors (family, friends, religion) and deterrents (e.g. minor children), availability of means (e.g. firearms), and the presence or absence of adequate social and healthcare services, moderates suicidal risk and behaviors increasing or limiting the probability of death by suicide. Another model gaining traction is interpersonal theory of suicide, which attempts to explain desire towards suicidality as an outcome of perceived loss of belongingness, burdensomeness with associated hopelessness, and repeated painful or fear inducing experiences leading to acting on these desires [78]. It has been proposed that substantial increases in exposure to aeroallergens, especially in allergen-sensitized individuals such as those suffering from AR and asthma, may represent environmental stressors that could lead to precipitation of suicidal behavior through biological and psychological factors [79, 22, 80] Prevention of suicidal behavior by immediate safety measures like inpatient admission, removing access to lethal means like guns, adequate and prompt treatment of underlying psychiatric condition and psychosocial stabilization remains the cornerstone of therapeutic approaches to managing risk for suicide.
Immune mechanisms underlying AR
Individuals with a predisposition for allergic disorders like AR have predominant activation of CD4+ T helper type 2 (Th2) cells in response to otherwise innocuous specific antigen exposure, when presented by dendritic cells in the nasal epithelium. Th2 cells are important in differentiation and recruitment of other immune cells such as eosinophils that participate in anti-parasitic and allergic immunity and they secrete various cytokines including interleukin (IL)-4, IL-5, IL-6, IL-10 and IL-13 that are involved in stimulating the humoral and cellular immune responses. IL-4 from Th2 cells promotes B cell transformation to immunoglobulin-secreting plasma cells and class switching of immunoglobulin G (IgG) to immunoglobulin E [81], peripherally or locally [82]. IL-5 is a major maturation and differentiation factor for eosinophils [83]. The IgE molecules circulate in the bloodstream and bind to high affinity Fc receptors (FcεRI) on mast cells in the nasal mucosa, which are now “armed”. The other important receptors now identified to be involved in IgE activity and dysregulation as observed in AR and other allergic disorders, are CD23 as well as galactin-3, and several other CD23 co-receptors like CD21 and other integrins [84, 85]. AR is triggered when re-exposure to the sensitizing allergen occurs and it cross-links preformed IgE bound to nasal mucosal mast cells. The reaction is classified as either “early” or “delayed” according to time sequence in relation to the allergen exposure. The early reaction develops within 30 minutes and is mediated by mast cells activated by allergen exposure, which secrete chemical inflammatory mediators like histamine, tryptase, kininogenase, and prostaglandins that are stored in preformed granules. The late reaction is mainly characterized by eosinophil chemotaxis induced by synthesized leukotrienes and cytokines. These inflammatory mediators cause increased vascular permeability, contraction of local smooth muscles and increased mucus secretion and nerve irritation, which all lead to the observed characteristic clinical features of AR. There is influx of inflammatory cells including eosinophils and more Th2 cells. Activated mast cells and Th2 cells secrete cytokines that augment eosinophil activation and degranulation, leading to further increases in inflammatory cells and more differentiation of B cells to plasma cells and IgE production. The mast cells also release cytokines normally associated with Th1 cells, such as tumor necrosis factor (TNF) and IL-6, which promote more vascular permeability, tissue edema and watery rhinorrhea. As the exposure to allergen continues, this cascade of inflammatory responses persists, with a further increase in pro-inflammatory cytokines like IL-1α, IL-1β, IL-6 and TNF [86, 87]. The aeroallergen-nasal epithelial cell interactions may have a role to play in allergic sensitization and airway inflammation, through increased permeability, and the epithelial cells themselves release inflammatory mediators that can lead to preferential Th2 responses. Chronic exposure to inflammatory mediators like TNF secondary to the AR may cause tight junction barrier dysfunction in the nasal epithelium, and contribute to further worsening of AR by making allergen readily available to underlying immune cells, and ultimately leading to airway remodeling [88, 89]. Despite several years of research and an increasing understanding of the allergic processes, the exact role and mechanism of IgE antibodies and mast cells remain to be fully described [87]. To summarize, current knowledge suggests that AR is associated with both Th2- (IL-4, IL-10, IL-13) and Th1- (IL-1, IL-6, TNF) pro- and anti-inflammatory cytokine release [90] with potential effects on psychological health.
Other than cytokines, prostaglandins are also important in mediating an individual’s response involving AR, with prostaglandin D2 (PGD2) produced by mast cells as possibly the most important inflammatory mediator. One study based on a mouse model of AR suggested that PGD2 contributes to AR symptomatology by stimulating vascular and other inflammatory responses by interaction with D prostanoid receptors and a chemoattractant receptor-like molecule expressed on Th2 cell receptors through possible CD3/CD28 co-stimulation. They preferentially elicit production of the cytokines IL-4, IL-5 and IL-13, without affecting anti-inflammatory IL-10 [91]. This may also contribute to psychological difficulties.
Nose-brain connection
Available research suggests that pro-inflammatory cytokines from the periphery are able to reach the CNS and they have the capacity to interact with pathophysiological domains such as neurotransmitter metabolism, neuroendocrine function, sleep disturbances and behaviors that characterize psychiatric disorders like depression and anxiety [92]. Research has shown that environmental macromolecules with inflammatory potential as well as peripherally formed endogenous inflammatory molecules are able to reach the brain through various routes, with the critical step being passing through the blood-brain barrier (BBB). Animal models have demonstrated that peptides [93] and interleukins [94] can travel from the nose to the brain along the trigeminal or olfactory pathways [95, 96], bypassing the BBB. Review of present evidence suggests potential neuro-immune mechanisms underlie the association between peripheral inflammation and psychiatric symptoms [97]. In AR, neuroinflammation may be mediated through passage of inflammatory molecular and cellular mediators from the nasal cavities to the brain, though exact mechanism remains to be explained [98].
AR and depression
Causal pathways involving induction of inflammation by depression, or precipitation of depression by inflammation, or a bidirectional relationship, can be expected, as both AR and depression have been associated with activation of inflammatory pathways involving similar mediators. AR presents with significant social and interpersonal difficulties and loss of productivity, which itself may induce stress and lead to sadness [references in Part 1]. Apart from these psychological stressors, the causal association may be biologically driven, which can be inferred from the observation that there are similar cytokine-mediated neuroinflammatory processes in depression and AR, or AR markers like seasonality and absolute peaks of aeroallergens, as well as the ability of these cytokine markers to induce a depressive mood.
Medically healthy patients with major depression often have elevated levels of neuroinflammatory markers that are also observed in AR. For example, they have been reported to have increased levels of IL-6 [99] [100, 101], IL-1β, and TNF [102]. A meta-analysis of 24 studies showed significantly higher concentrations of the proinflammatory cytokines TNF and IL-6 in depressed subjects compared with control subjects [103]. Another systematic review of the literature showed positive associations between inflammatory biomarkers IL-1, IL-6 and C-reactive protein (CRP) and depression [104]. Some of these immune abnormalities may be responsive to effective treatment of depression [105, 106].
Evidence for peripheral and neuroimmune inflammatory dysregulation being able to produce depression comes from the association of immune inflammatory conditions with depressive symptoms, as observed in studies of treatment of animals with lipopolysaccharide (LPS) from bacterial cell walls. LPS induces expression of cytokines such as IL-1β in the brain meninges, CVOs and brain parenchyma [107–109], responses that have been linked to “sickness behavior”, displaying depressive features like loss of appetite, inhibition of sexual activity, increased sleepiness, concentration and memory difficulties along with fever and hypothalamic-pituitary-adrenal (HPA) axis activation. Cytokines in circulation, even in concentrations not sufficient to produce the sickness syndrome, have been associated with depressed mood, anxiety and memory difficulty [97]. One recent study examined effect of intraperitoneal injection of LPS in rats on sickness and depression like behavior. The study showed early increase (2 hours) in cytokine levels in serum and CSF coinciding with sickness behavior with specific alterations in mRNA transcription of IL-1β, IL-6 and TNF-α in frontal cortex, hippocampus and striatum; and late onset of depression like behavior paralleled by increasing iL-1β transcription and CSF levels. The study also reported Cyclooxygenase (COX) enzyme downregulation in hippocampus. The study indicated persistence of central inflammation even after peripheral inflammation and acute sickens behavior had ceased. [110] Circulating cytokines can also lead to increased glucocorticoid output and receptor dysfunction under stressful situations[111], which has been shown to be associated with MDD [112]. Some cytokines (i.e., IL-1β, IL-2, frequently elevated in AR patients [113] can induce expression of the CNS enzyme indoleamine 2,3-dioxygenase [114], which induces production of kynurenine from tryptophan. The kynurenine: tryptophan ratio in turn is linked to depression and suicidal behavior through neurotoxic, glutamatergic and possibly serotonergic dysregulation in the CNS [115••–117]. Peripheral immune activation is capable of central up-regulation of proinflammatory cytokine expression, microglia activation, and the production of neurotoxic substances like kynurenine metabolites [118]. It is possible, therefore, that cytokines released during the allergic reactions like AR may have similar neurobehavioral effects leading to precipitating or perpetuating psychiatric and behavioral difficulties, particularly depression, [119].
Evidence of AR being associated with exacerbation of depression comes from studies showing temporal associations between aeroallergen exposure, a precipitating and perpetuating factor for AR, and depressive mood. In a self-report study, self-reported lower moods were associated with high pollen counts, with seasonality different than seasonal affective disorder (i.e., fall-winter mood worsening) [120]. Subjective worsening of mood with high pollen counts, which is similar to season of pollen exposure, has been reported previously in college students [121]. More specific evidence comes from studies demonstrating that pollen-specific IgE positivity is associated with worsening of depressive scores in bipolar disorder patients during high pollen season [122]. AR in early life is associated with depression later in life [18]. Moreover, bipolar disorder in later life [19] has also been associated with AR, with a possible immune link involving a likely immune causation.
AR and suicidal behavior
Associations have been reported and replicated between AR/atopy and surrogate markers of allergen exposure, such as season of highest aeroallergen (pollen) load or measured pollen count, which are linked to triggering peaks in AR, biomarkers like inflammatory cytokines, and suicidal ideation and behavior. Multiple studies on seasonality of suicide have confirmed a highly replicated spring peak of suicide [123] across continents and hemispheres [124••]. This peak overlaps with peaks in AR and exposure to airborne aeroallergens, in particular atmospheric pollen. We hypothesized that, via immune mediators in the upper airways reaching the brain and affecting modulation of suicidal behavior, allergen exposure and seasonal rhinitis lead to a seasonal exacerbation of suicide risk. To further support our hypothesis of a potential predictive association between exposure to atmospheric tree pollen in vulnerable individuals and suicidal behavior, analysis of a 13-year database of all suicides in Finland showed that those with hospital-treated atopy died by suicide predominantly (78%) during spring and summer when pollen counts are highest, as compared to non-atopic individuals who had a very similar distribution of completed suicides across half-yearly intervals with different pollen exposure [125]. In a first test of our hypothesis, we reported, ecologically, an increase in non-violent suicides in women associated with peak tree pollen exposure in the US, after adjustment for surrogates of light exposure, a potential confounder [22]. Later, we were unable to replicate our findings in a subsequent interval of time with a slightly modified methodology [126]. Yet, a year later, in the first population study that connected death by suicide individually linked to allergen exposure, we confirmed a relationship between measured pollen counts and suicides that remained significant after correcting for other environmental factors like regional distribution, temperature, humidity and cloud cover. We also identified sex difference, with women requiring an increase in pollen count beyond a certain threshold [> 30 grains/M3] before increasing the suicide risk significantly in contrast to men who experienced more uniform increases in relative risk [23]. A more recent study analyzing data of nan-fatal self-directed violence (SDV) from emergency department medical records over three years from Dallas, Texas found significant positive temporal association between tree pollen peaks and SDV in women (p=0.04) and grass pollen peaks and SDV in both men (p =0.03) and women (p < 0.0001), but no association with ragweed pollen [127••].
Later, suicidal ideation has also been linked with history of allergy after controlling for age, sex, race, smoking, comorbid asthma and previous diagnosis of depression [128]. We have also identified an association between allergy, including AR and suicide, independent of seasonal exposure to allergens, even when accounting for the psychiatric history or socio-economic status of individuals. The risk was slightly higher in women, and moderated by history of mood disorders, contrary to our expectations, higher in individual without prior diagnosis of mood disorders [14].
The question that persists is as follows: Is the association between suicidal behavior and allergen exposure or allergy driven by psychological symptoms of illness, discomfort, or medications for rhinitis, or is it a biological connection linked with mediators of inflammation reaching the brain? Our evidence argues for the latter. First, we reported an increased gene expression for allergy-related cytokines in the prefrontal cortex of individuals who died by suicide as compared with those who died by other causes [129]. Second, we identified in rodents an increased expression of similar cytokine profiles in the prefrontal cortex, and an amplification of anxiety-like behavior (though not depression-like behavior) and impairment in social interactions as a result of sensitization and exposure to allergens [130]. Third, in a pharmacoecological study we reported that intranasal corticosteroids, known to reduce molecular mediators of inflammation in AR; were associated with significantly lower rates of suicide, the effect not observed with second generation antihistamines which are equally effective for symptoms, but do not affect molecular mediators of inflammation in AR. This suggests that the connection between upper airway inflammation with suicidality is more than just psychological [131].
AR and sleep impairment as a potential mediator
Sleep disturbances are very common among those with AR, with 48% of seasonal AR and 68% of perennial AR patients reporting sleep disturbances [81]. Allergic rhinitis can impair sleep through obstructive and molecular processes (e.g., production of cytokines), and sleep impairment may be an important pathophysiological link between AR and mood, anxiety disorders, and suicidal behavior [132]. Cytokines involved in allergic reactions such as IL-4 and IL-10 have been shown to inhibit spontaneous sleep in rodent models [133, 134]. Considering that sleep loss appears to be a consistent predictor of suicidal behavior [135, 136] and one that is more rapidly modifiable, interrupting sleep disruption in individuals with vulnerability for both AR and suicidal behavior is worth investigating. Supporting this line of thinking, it had been reported that intranasal corticosteroids, previously associated in our pharmacoecological study with lower rates of suicide, have a stronger effect on improving sleep disruption than systemic antihistamines [137, 138]
Psychopharmacological management of depression
Clinical principles
In the following paragraphs, we are presenting a summary of our experience in treating mood disorders, which is by no means exclusive. While the first line treatment of depression is well documented by adequate placebo controlled trials, we do not have the same evidence base when it comes to treating patients who fail or partially fail to respond to one intervention and especially several interventions. Also, the clinical practice of psychopharmacologists who often treat patients who have failed previous antidepressants, is often very divergent from evidence-based practice, and involves multiple combinations of medications for augmentation strategies as well as reciprocally minimizing side effects. These medication cocktails are often puzzling for non-psychopharmacologists, and yet, often, in experienced hands, they represent rational polypharmacy rather than a cavalier approach to practice.
This being said, the majority of patients with depression are not adequately treated. It is estimated that approximately half of patients who would require treatment are not diagnosed, and half of those diagnosed are not adequately treated. Most common issues with treatment are the lack of sufficient education about side effects (and their often-transient nature), the lack of addressing side effects when they occur, the absence of tailoring the treatment to patients’ response and side effect profile, the insufficient push for remission, and the lack of reevaluation for adjustment of treatment. Treatment of depression is often too short. It has been reported that majority of patients receive less than two month of treatment [139] rather than the recommended minimum of 4 to 6 month [140] (going up to at least 2 years in the recurrent major depression). Patient’s preferences are sometimes disregarded, and psychotherapy options as monotherapy for milder forms of depression, or in combination with pharmacology in moderate or more severe depression are not considered.
The principle is to start with a first line agent (such as an SSRI), increase slowly to therapeutic response to use for an adequate amount of time before moving to step 2, augmentation of treatment with another agent (e.g., bupropion, buspirone, a second-generation neuroleptic, or replacing the SSRI with an SNRI in monotherapy). Duration of treatment and any changes in treatment, as well as the rapidity of changes, is governed by side effects and treatment response. Agents with potentially more severe side effects, and with higher lethality in overdose, such as tricyclic antidepressants and monoamine oxidase inhibitors, represent further steps. The goal of treatment is to induce remission, defined as improvement in symptoms such that the individuals no longer meets the criteria for clinical diagnosis and has minimal symptoms at the most, and then to maintain it by preventing recurrence. Another useful term is treatment response, which is defined as 50 % improvement in depression scores. After response but before remission, a worsening of depression symptoms back to a full-blown episode is called relapse, while after remission is called recurrence. In all research and clinical trials settings, and also in clinical settings that desire thriving for best outcomes, the use of standardized self-report or clinically administered scales in clinical determination of remission and response [141] is paramount. In particular, a number of self-report tools have shown adequate psychometric properties and convenience of use [142]. Among them, perhaps the most used is Patient Health Questionnaire-9 (PHQ-9), a self-report survey containing nine questions, is useful in identifying and monitoring depression treatment in primary care. [143]. It is important to emphasize that, in reading literature of depression, that majority of studies have been powered for improvement in depression scores vs. placebo, not for remission, and yet, remission remains the most important research outcome.
Mild to moderate depressive symptoms respond well to psychotherapeutic interventions like interpersonal therapy, CBT, mindfulness-based therapy and behavioral therapy, and these interventions also prove effective in preventing relapse. Common clinical considerations for pharmacological therapy, are based on the relative efficacy and safety. In the choice of a first or “next” line agent, the clinician should factor in the nature of prior response to medication, tolerability, anticipated side effects, co-occurring psychiatric or general medical conditions with potential drug interactions, half-life of medication deciding frequency of administration and possibly compliance, patient preference, and cost of treatment. Management of depression in special populations like children, adolescents, pregnant women, the medically ill, those with a history of substance abuse and the elderly, involves additional considerations. In severe and resistant cases, or in depressed patients with psychotic symptoms, neurostimulation like electroconvulsive therapy [ECT] may play a substantial role in successful treatment. Recently, newer forms of treatment such as transcranial magnetic stimulation have gained indication in certain forms of treatment resistant depression. All in all, considering the importance of remission as a goal, even in fragile patients, the principle is to “START LOW, GO SLOW, BUT GET THERE”. Among those with bipolar disorders, those with depression with psychotic features, those with imminent danger of suicidal behavior or a history of suicidal behavior, are also best managed through referral to mental health specialists. We have included a list with resources for treatment of depression in particular in primary care. Evidence based approaches are summarized in those tables. (see Table 1
Table 1.
Management of Depression: Useful Links
What to expect when treating depression in real life: Effectiveness data
National Institute of Mental Health conducted the Sequenced Treatment Alternative to Relieve Depression [STAR*D], a federally funded, large-scale, long-term effectiveness study, directly comparing treatment strategies, which has improved understanding of sequenced psychopharmacological treatment of depression. It emphasizes on measuring treatment at regular intervals using validated scales for dose adjustment, titration and optimization of medication under trial before switching or augmenting. The trial involved four different treatment levels, and patients were encouraged to enter the next level of treatment if they failed to achieve remission or response (50% reduction in symptoms) after a specified number of weeks. In level one, patients received the selective serotonin reuptake inhibitor (SSRI) citalopram for up to 14 weeks, with adjustment of the dose being managed by their own physicians. If patients achieved remission or response during that time period, they could enter a 12-month naturalistic follow-up, during which time the researchers did not have any influence over the treatment plan. Non-remitters were encouraged to enter level two. In level two, there were seven different treatment options, and cognitive behavioral therapy (CBT) was included as the psychotherapy option. There were three combination options (either an antidepressant or CBT added to citalopram), and four switch options (to either a different antidepressant or CBT). Those who remitted or responded were offered 12-month naturalistic follow-up; non-remitters after two medication trials were encouraged to enter level 3; other non-remitters entered level 2A, which involved a second antidepressant trial. In level three, patients were offered the addition of lithium or triiodothyronine (a thyroid hormone) to their antidepressant, or a switch to another antidepressant (mirtazapine or nortriptyline) for 12 weeks. Level four consisted of the monoamine oxidase inhibitor tranylcypromine or a combination of venlafaxine and mirtazapine Per STAR*D outcome: [ available for review at: http://www.nimh.nih.gov/funding/clinical-research/practical/stard/allmedicationlevels.shtml]
Level 1] SSRI medication [Citalopram], were effective in about 1/3rd participants.
Level 2] if First treatment with one SSRI fails, about one in four people who choose to switch to another medication will get better, regardless of whether the second medication is another SSRI or a medication of a different class [SNRI/Bupropion]. Psychological interventions [CBT, CBT+ Citalopram] were equally effective. And if patients choose to add a new medication to the existing SSRI, about one in three people will get better. It makes modest difference if second medication is an antidepressant from a different class (e.g. bupropion) or if it is a medication that is meant to enhance the SSRI (e.g. buspirone). Whether patients are more likely to get better by switching medications or by adding another medication could not be investigated.
Level 3] those who do not get better after two medication treatment steps. By switching to a different antidepressant medication [TCA, Mirtazapine], about one in seven people will get better. By adding a new medication to the existing one, about one in five people will get better. Adding T3 may have some advantages over adding lithium for patients who have tried two other treatments without success.
Level 4] patients with the most treatment-resistant depression, level 4 results suggest that tranylcypromine [MAO inhibitor] is limited in its tolerability and that up to 10 percent may benefit from the combination of venlafaxine/mirtazapine [SNRI +Atypical].
There is no proven pharmacology that acutely reduces suicide risk. Recently much hope has been generated from the rapid antidepressant response to ketamine and the reduction of suicidal ideation beyond its antidepressant effects [144]. At the time this review is finalized, ketamine is not yet ready to be used clinically, as more studies regarding efficacy and safety are needed.
Bipolar Depression Treatment
Treatment principles based on clinical practice
The cornerstone of pharmacological management of bipolar disorder is the use of mood stabilizers such as valproate, lithium, or more commonly recently, lamotrigine, oxcarbazepine, and second-generation neuroleptics (olanzapine, aripiprazole, quetiapine, etc.). Analogous to unipolar depression treatment, a stepped approach is adopted. It is important to obtain a detailed history whenever depression is suspected in patients, especially to attempt to differentiate unipolar depression from depressive phase of bipolar disorder, as the management differs significantly.
This is not always easy even in experienced hands, mainly because of reduced insight into mania, concerns for stigma, and side effects of mood stabilizer treatments. In a depressed phase, mood stabilizers with known antidepressant properties (lithium, lamotrigine) are preferred, frequently with addition of antidepressants and second-generation neuroleptics, sometimes at lower dosages than when used for manic episodes (quetiapine, aripiprazole, olanzapine, etc.) A non-judicious use of antidepressants may induce mood cycling and precipitate further manic episodes followed by episodes of depression [145]. While, in general, long-term use of antidepressants for patients with bipolar depression type 1 is contraindicated, the long-term use of antidepressants for patients with type II or bipolar disorder (exclusively hypomanic rather than manic episodes) is a matter of debate. Psychotherapeutic approaches that have been found to be useful for bipolar disorder (in contrast to major depression, – always in combination with psychopharmacology), are CBT and family focused therapies may help in improving compliance, reducing precipitating stressors and decreasing relapse [146].
At this point- mood stabilizing treatment in bipolar disorder should be life long, and requires a very intense effort to address non-compliance, in particularly when patient is euthymic. We are not detailing steps for the treatment for bipolar depression, as most often physicians treating allergy are advised to request a mental health consultation for specific patients. Occasionally, a patient who was considered unipolar treated in a primary or medical settings will manifest hypomanic or manic symptoms as a result of treatment with antidepressants. Similarly, a patient with allergy taking systemic decongestants (e.g. pseudoephedrine) or systemic corticosteroids may have emerging hypomanic or manic symptoms. In contrast to previous versions- DSM5 is now recognizing the medication induced condition as true bipolar disorder, and thus a referral to a mental health professional for possible mood stabilizing treatment is recommended.
For useful guidelines by various agencies, please refer to Table 1
Management of AR: special considerations in patients with mood disorders and increased risk for suicide
An important element in the treatment of AR limiting exposure to responsible allergens. This can be achieved by physically removing the allergen from the indoor environment [e.g., by frequent use of HEPA filter-assisted cleaning of households and workplaces] or avoiding environments where exposure is likely [i.e., avoiding outings during pollen season]. These interventions can add to academic and occupational limitations and result in reducing social interactions. This may contribute to lowering quality of life and worsening of mood difficulties. Moreover, as isolation is a prosuicidal factor, and access to social networks a protective factor, it might further increase suicide risk. It remains unclear at this point to what degree virtual communication (e.g. Skype) would correct social isolation in the context of active avoidance of seasonal allergen exposure., Furthermore allergen avoidance interventions have limited efficacy [Bernstein, 2016].
There may be a need to modify the diagnostic approach in patients with mood disorders, such as preferring IgE antibody serology over SPT. This is based on the considerations of modifying effects of psychotropic medications as well as compliance and comfort of patients.
Pharmacoecological data suggest that intranasal corticosteroids might be associated with lower risk for suicide, as opposed by a slightly increased risk with second generation antihistamine [131] However, ecological studies contain a too high potential for bias to draw clinical conclusions from these data. There is need to advance large scale, preferentially longitudinal pharmacoepidemiological studies to confirm these results, and ultimately, clinical trials to compare the use of intranasal corticosteroids vs. second generation antihistamine in clinical samples with depression, and perhaps, suicidal ideation. Systemic corticosteroids are associated with depression, mania, and psychosis. [147], and an inquiry about these conditions, and monitoring treatment is important when initiating systemic corticosteroids. Dosages of antidepressants, mood stabilizers and neuroleptics may have to be increased or augmenting agents added when systemic corticosteroids are administered to a psychiatric patient. Antihistamines may act additively or synergistically with certain psychotropic medications in beneficial (treatment of insomnia, potentiation of anxiolytic effects) or detrimental (worsening of sedation- elevating risk of accidents). Insomnia, present in many patients during exacerbation of allergy and in patients with mood disorder, exacerbating both depression and potentially triggering cycling and manic episodes is a treatment target, more-rapidly modifiable than reduction of depressive symptoms. It should always be an early element of attention and treatment in comorbid patients. Systemic decongestants with α-Adrenergic agonist mechanism of action can precipitate hypomania or mania, and exacerbate insomnia and anxiety in vulnerable individuals. Also, SNRIs, TCAs and stimulants (medications used for ADD) may have additive effects with decongestants in elevating their arrythmogenic potential. There have been reports that montelukast can precipitate depression, anxiety and behavioral changes, and thus titration of the medication, in patients with history of those conditions, needs to be more closely monitored. Suicidality has also been reported, but reports have not been further substantiated. [148]. Yet the practice of inquiring about depressive symptoms and suicidal ideation when initiating or restarting treatment will in the very long run benefit an occasional patient at increased risk.
It is recommended to include a psychiatrist in the team management cases with history of bipolar disorder, psychotic depression, history of suicide attempts or suicidal ideation, and depression that appears resilient to treatment, of often reoccurs.
Managing suicide risk in primary care
It is impossible to fully predict or prevent suicide. However, many suicides can be prevented, and although the yield of identifying patients at risk is low, the expected impact on mortality is high. Suicide of a patient has a lifelong effect on his friends, family especially offspring, and his treating medical professionals. It is important to remember that patients who died by suicide visit a primary physician twice as often than a mental health professional prior to suicide [149]. Approximately 45% of patients who die by suicide visit their primary physician during the month preceding death. Although the great majority of antidepressants are prescribed in primary care, half of simulated patients with depression are not being asked about suicide ideation and history[150]. In an actual treatment of depression, it appears that majority of patients are treated for too short duration of treatment, that only a quarter are asked about suicidal ideation [139], and when asked, a positive response most commonly leads to no further workup or treatment.
We recommend that in allergy treating settings patients be asked about suicide ideation at the initial evaluation and follow-up. A stepwise approach, starting with hopelessness and desire to be dead, moving towards active thoughts of suicide intent, plan, and finally history of prior attempts and family history is particularly recommended. Sometimes, patients may be more likely to endorse ideation on a questionnaire. However, responses on these questionnaires must be checked during the visit, rather than at a later time. Resources such as CSSRS and training in its administration and decision point are freely available to clinicians CSSRS: Information and Training available at: http://www.cssrs.columbia.edu/training_cssrs.html. However, physicians are advised to not rule out risk of suicide based on negative responses on any questionnaire. A clinical interview is also necessary. Sometimes, getting collateral information is also important, as patients are not always forthcoming about history or ideation. Primary physicians should obtain consent to contact mental health professionals (psychiatrist, psychotherapist or councilor) upon intake and during routine visits, and should contact them in case of emergency even if patient does not offer consent in acute situations. Involving family members/significant others is beneficial when acute suicide risk is suspected, and considerations for patient’s life outweigh considerations for confidentiality.
Thus, ask primary care, such as the settings where allergy is treated the most, to promise a potential role in reduction of the resilient worldwide suicide epidemics. Physicians should be mindful of risk factors, as warning signs. Risk factors for suicide, as being a Caucasian male, divorced or single, having previous suicide attempts and family history of suicide, are generally considered to be relatively stable and less modifiable. However, warning signs, or precipitating factors- such as increased isolation, increased substance use, psychomotor agitation are state-dependent, and could be investigated and addressed in their own right. One of these factors, substance use is rarely investigated in primary care. For example, less than ¼ of individuals in primary care are asked about alcohol use [139] and only 11 % of people with alcohol use disorders receive recommendations for treatment [151]. Considering the importance of identifying and addressing alcohol use disorders, as individuals often more easily reveal substance use on questionnaires rather than face to face, we argue in favor of using the Alcohol Use Disorders Identification Test (AUDIT) in its longer 10 question form or, under more challenging time pressure circumstances, the brief 3-question version (AUDIT-C). The AUDIT is free, used worldwide, and has relatively good psychometric properties in identifying use, abuse and dependence with at least 50% sensitivity for ruling in and 80% specificity for ruling out. [152]
It is recommended that a mental health professional is consulted in patients with suicidal ideation, or history of attempt, and that with patients with acute intent, plan, or suspicion of unrevealed intent or plan (e.g. report of writing “good bye” letters, distributing belongings) in patients with increasing isolation, increased substance use, worsening depression should lead to continuous observation until examined by a mental health professional (e.g. escorting to an emergency room). In those cases, whenever the location of the treating facility is not in the proximity of an emergency room, calling 911 may be the correct intervention. For all patients, the National Suicide Prevention Lifeline is available 24 hours a day regardless of location by call or text. It is important to also educate family or significant others that they can call suicide hotline at any time. As an important piece of information, suicide hotline can be accessed anonymously, and can provide further resources for the patient or family. Suicide Hotline information for acutely suicidal patients:
|
|
| National Suicide Prevention Lifeline: 1-800-273-TALK (8255) |
| Text: “GO” to 741741 |
|
|
Lithium is the only psychotropic used to treat mood disorders (bipolar disorder, and augmentation of major depression, in particular highly recurrent depression) that has also demonstrated a reduction in risk of suicidal behavior, and as such may be considered in high-risk patients. Nevertheless, its potential suicide protective benefit should be weighed against its narrow therapeutic margin and relatively high potential for lethal overdose. As such, we do not suggest that primary clinicians initiate treatment with Lithium for this purpose. Clozapine is FDA approved for lowering suicide risk, but only for patients with schizophrenia, Ketamine, a medication that holds promise for acute lowering of suicide ideation (as well as rapid antidepressant response), should wait further research until clinically indicated.
No pharmacological treatments for suicide risk
No pharmacological treatments for suicide risk: although multiple counseling/psychotherapeutic interventions have been proposed for reduction of suicide risk, and for having positive effects for suicide ideation, in a medical setting, ER, as well as a general hospital setting, we believe that a brief intervention focus on safety planning makes most immediate sense and is easily implemented by non-mental health providers in an individualized mode [153]. It is long term focused and includes considerations for warning signs, individual coping strategies, social situations, people and professionals who can be helpful, and increasing safety of the environment (such as not keeping guns or alcohol at home, only having minimal number of pills available).
A role for an integrated collaborative care
Literature on collaborative approaches in primary care supports an increased depression remission rate and suicidal ideation (at least in older adults) [154]. Metanalyses further documents benefits in terms of effectiveness [155], and, in the longer run, cost-effectiveness [156]. Although the setting was not focused on patients for allergy, it supports the idea that a collaborative care implementation in an allergy setting is likely to improve depression outcomes and potentially lower suicide risk, with no additional costs in the long run. Screening for depression, alcohol use disorders and suicide risk alone, even with education efforts are not likely to make a major impact in the absence of adequately referring and treating those conditions, ideally through integration of care.
Conclusions
Allergic rhinitis is associated with higher rates of depression and suicidal behavior than those found in the general population. Reciprocal directions of causality between allergy and mood anxiety disorders are possible, and likely, with potential for cascading in a vicious circle. Associations between aeroallergen outdoor exposure and suicide peaks have been reported and replicated, with animal, postmortem and pharmacoecological studies suggesting at least a component of biological mediation of these links. Allergists and general practitioners should therefore actively inquire about depression, anxiety symptoms, suicidal ideation and history of suicidal behavior in patients with AR and other manifestations of allergic disease. Certain conditions (bipolar disorder, psychotic depression, treatment resistant depression or anxiety disorder) are criteria for referring the patient to a mental health professional. Alcohol use disorders must be identified and addressed. There is a major need of experimental and longitudinal investigations, as well as clinical trials to further our understanding of best practices in patients with comorbid allergy and depression and to find new therapeutic and preventative interventions. Ultimately, increases in collaborations between allergists and mental health professionals are expected to lead to synergistic results, with potential reduction in mortality and morbidity, and improvement in functioning and quality of life.
Figure 1. Allergy, depression and suicidal behavior.
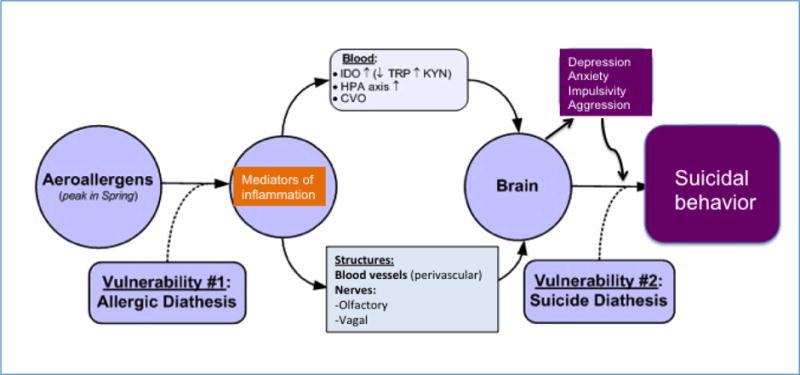
CVO, circumventricular organs; HPA axis, hypothalamic-pituitary-adrenal axis; IDO, indoleamine 2,3-dioxygenase; KYN, kynurenine; TRP, tryptophan.
Learning Objectives.
Present overlaps between allergy, allergen exposure, depression, and suicidal behavior in adults
Familiarize allergists with the principles of diagnosis and treatment of depression in adults and importance to monitor suicide risk
Acknowledgments
Supported by the Military and Veteran Microbiome Consortium for Research and Education (MVM-CoRE), Denver, CO, USA; Rocky Mountain Mental Illness Research Education and Clinical Center (MIRECC), Denver, CO, USA; Tuning Inc., Silver Spring, MD, USA; and the DC Department of Mental Health, St. Elizabeths Hospital Residency Training Program, Washington DC, USA
The authors thank to Ms. Aline Dagdag and Dr. Naila Karim for their comments on an earlier draft of the manuscript, and to Ms. Winny Mwaura for her excellent administrative support.
Footnotes
Conflict of Interest
Dr. Christopher A. Lowry is a member of the Scientific Advisory Board of Immodulon Theraputics, outside of the submitted work. Dr. Ameya U. Amritwar, Dr. Lisa A Brenner, Dr. Andrew J. Hoisington, Dr. Robert Hamilton, Dr. John W. Stiller, and Dr. Teodor T. Postolache
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Resources USDoHaH, editor. Summary Health Statistics for US adults: National Health Interview Survey, 2012. Hyattsville, MD: DHHS; 2014. [Google Scholar]
- 2.Resources USDoHaH, editor. Summary Health Statistics for US Children: National Health Interview Survey, 2012. Hyattsville, MD: DHHS; 2014. [Google Scholar]
- 3.Wu WF, Wan KS, Wang SJ, Yang W, Liu WL. Prevalence, severity, and time trends of allergic conditions in 6-to-7-year-old schoolchildren in Taipei. J Investig Allergol Clin Immunol. 2011;21(7):556–62. [PubMed] [Google Scholar]
- 4.Abdulrahman H, Hadi U, Tarraf H, Gharagozlou M, Kamel M, Soliman A, et al. Nasal allergies in the Middle Eastern population: results from the “Allergies in Middle East Survey”. Am J Rhinol Allergy. 2012;26(Suppl 1):S3–23. doi: 10.2500/ajra.2012.26.3836. [DOI] [PubMed] [Google Scholar]
- 5.Blaiss MS. Allergic rhinitis: Direct and indirect costs. Allergy Asthma Proc. 2010;31(5):375–80. doi: 10.2500/aap.2010.31.3329. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(2 Suppl):S12–6. doi: 10.1016/j.anai.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Ozdoganoglu T, Songu M, Inancli HM. Quality of life in allergic rhinitis. Ther Adv Respir Dis. 2012;6(1):25–39. doi: 10.1177/1753465811424425. [DOI] [PubMed] [Google Scholar]
- 8.Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466–76. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 9.Passali D, Passali GC, Lauriello M, Romano A, Bellussi L, Passali FM. Nasal Allergy and Otitis Media: A real correlation? Sultan Qaboos Univ Med J. 2014;14(1):e59–64. doi: 10.12816/0003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirtsreesakul V, Naclerio RM. Role of allergy in rhinosinusitis. Curr Opin Allergy Clin Immunol. 2004;4(1):17–23. doi: 10.1097/00130832-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Ameli F, Brocchetti F, Tosca MA, Signori A, Ciprandi G. Adenoidal hypertrophy and allergic rhinitis: is there an inverse relationship? Am J Rhinol Allergy. 2013;27(1):e5–10. doi: 10.2500/ajra.2013.27.3854. [DOI] [PubMed] [Google Scholar]
- 12.Craig TJ, Sherkat A, Safaee S. Congestion and sleep impairment in allergic rhinitis. Curr Allergy Asthma Rep. 2010;10(2):113–21. doi: 10.1007/s11882-010-0091-5. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin RD, Demmer RT, Galea S, Lemeshow AR, Ortega AN, Beautrais A. Asthma and suicide behaviors: results from the Third National Health and Nutrition Examination Survey (NHANES III) J Psychiatr Res. 2012;46(8):1002–7. doi: 10.1016/j.jpsychires.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Qin P, Mortensen PB, Waltoft BL, Postolache TT. Allergy is associated with suicide completion with a possible mediating role of mood disorder – a population-based study. Allergy. 2011;66(5):658–64. doi: 10.1111/j.1398-9995.2010.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timonen M, Jokelainen J, Silvennoinen-Kassinen S, Herva A, Zitting P, Xu B, et al. Association between skin test diagnosed atopy and professionally diagnosed depression: a Northern Finland 1966 Birth Cohort study. Biol Psychiatry. 2002;52(4):349–55. doi: 10.1016/s0006-3223(01)01364-6. [DOI] [PubMed] [Google Scholar]
- 16.Timonen M, Jokelainen J, Hakko H, Silvennoinen-Kassinen S, Meyer-Rochow VB, Herva A, et al. Atopy and depression: results from the Northern Finland 1966 Birth Cohort Study. Mol Psychiatry. 2003;8(8):738–44. doi: 10.1038/sj.mp.4001274. [DOI] [PubMed] [Google Scholar]
- 17.Timonen M, Jokelainen J, Herva A, Zitting P, Meyer-Rochow VB, Rasanen P. Presence of atopy in first-degree relatives as a predictor of a female proband’s depression: results from the Northern Finland 1966 Birth Cohort. J Allergy Clin Immunol. 2003;111(6):1249–54. doi: 10.1067/mai.2003.1546. [DOI] [PubMed] [Google Scholar]
- 18.Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, et al. Allergic rhinitis in adolescence increases the risk of depression in later life: a nationwide population-based prospective cohort study. J Affect Disord. 2013;145(1):49–53. doi: 10.1016/j.jad.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Chen MH, Lan WH, Hsu JW, Huang KL, Chen YS, Li CT, et al. Risk of bipolar disorder among adolescents with allergic rhinitis: A nationwide longitudinal study. J Psychosom Res. 2015;79(6):533–6. doi: 10.1016/j.jpsychores.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Wamboldt MZ, Hewitt JK, Schmitz S, Wamboldt FS, Rasanen M, Koskenvuo M, et al. Familial association between allergic disorders and depression in adult Finnish twins. Am J Med Genet. 2000;96(2):146–53. doi: 10.1002/(sici)1096-8628(20000403)96:2<146::aid-ajmg4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Marshall PS, O’Hara C, Steinberg P. Effects of seasonal allergic rhinitis on fatigue levels and mood. Psychosom Med. 2002;64(4):684–91. doi: 10.1097/01.psy.0000021944.35402.44. [DOI] [PubMed] [Google Scholar]
- 22.Postolache TT, Stiller JW, Herrell R, Goldstein MA, Shreeram SS, Zebrak R, et al. Tree pollen peaks are associated with increased nonviolent suicide in women. Mol Psychiatry. 2005;10(3):232–5. doi: 10.1038/sj.mp.4001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin P, Waltoft BL, Mortensen PB, Postolache TT. Suicide risk in relation to air pollen counts: a study based on data from Danish registers. BMJ open. 2013;3(5) doi: 10.1136/bmjopen-2012-002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stipic-Markovic A, Pevec B, Pevec MR, Custovic A. [Prevalence of symptoms of asthma, allergic rhinitis, conjunctivitis and atopic eczema: ISAAC (International Study of Asthma and Allergies in Childhood) in a population of schoolchildren in Zagreb] Acta Med Croatica. 2003;57(4):281–5. [PubMed] [Google Scholar]
- 25.Bjorksten B, Clayton T, Ellwood P, Stewart A, Strachan D. Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008;19(2):110–24. doi: 10.1111/j.1399-3038.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 26.Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55(Suppl 1):S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin Immunopathol. 2004;25(3–4):237–55. doi: 10.1007/s00281-003-0148-9. [DOI] [PubMed] [Google Scholar]
- 28••.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 2016;375(5):411–21. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rook GA, Raison CL, Lowry CA. Microbiota, immunoregulatory old friends and psychiatric disorders. Adv Exp Med Biol. 2014;817:319–56. doi: 10.1007/978-1-4939-0897-4_15. [DOI] [PubMed] [Google Scholar]
- 30.Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, et al. Clinical practice guideline: Allergic rhinitis. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015;152(1 Suppl):S1–43. doi: 10.1177/0194599814561600. [DOI] [PubMed] [Google Scholar]
- 31.Bousquet J, Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108 doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton RG. Allergic sensitization is a key risk factor for but not synonymous with allergic disease. J Allergy Clin Immunol. 2014;134(2):360–1. doi: 10.1016/j.jaci.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Han DH, Rhee CS. Comorbidities of Allergic Rhinitis. In: Pereira PC, editor. Allergic Diseases – Highlights in the Clinic, Mechanisms and Treatment. InTech; 2012. [Google Scholar]
- 34.Hadley JA, Derebery MJ, Marple BF. Comorbidities and allergic rhinitis: not just a runny nose. J Fam Pract. 2012;61(2 Suppl):S11–5. [PubMed] [Google Scholar]
- 35••.Scadding GK, Scadding GW. Diagnosing Allergic Rhinitis. Immunol Allergy Clin North Am. 2016;36(2):249–60. doi: 10.1016/j.iac.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Adkinson NF, Jr, Hamilton RG. Clinical History-Driven Diagnosis of Allergic Diseases: Utilizing in vitro IgE Testing. J Allergy Clin Immunol Pract. 2015;3(6):871–6. doi: 10.1016/j.jaip.2015.08.002. quiz 7–8. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(3 Suppl 3):S1–148. doi: 10.1016/s1081-1206(10)60305-5. [DOI] [PubMed] [Google Scholar]
- 38.Antico A, Lima G, Arisi M, Ostan A, Morrica B. Assay of prick test inoculum volume. II. Average values and individual variability. Ann Allergy Asthma Immunol. 2000;85(2):145–9. doi: 10.1016/s1081-1206(10)62455-6. [DOI] [PubMed] [Google Scholar]
- 39.Chinoy B, Yee E, Bahna SL. Skin testing versus radioallergosorbent testing for indoor allergens. Clin Mol Allergy. 2005;3(1):4. doi: 10.1186/1476-7961-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton RG, Oppenheimer J. Serological IgE Analyses in the Diagnostic Algorithm for Allergic Disease. J Allergy Clin Immunol Pract. 2015;3(6):833–40. doi: 10.1016/j.jaip.2015.08.016. quiz 41–2. [DOI] [PubMed] [Google Scholar]
- 41.Dordal MT, Lluch-Bernal M, Sanchez MC, Rondon C, Navarro A, Montoro J, et al. Allergen-specific nasal provocation testing: review by the rhinoconjunctivitis committee of the Spanish Society of Allergy and Clinical Immunology. J Investig Allergol Clin Immunol. 2011;21(1):1–12. quiz follow. [PubMed] [Google Scholar]
- 42.Ito K, Weinberger KR, Robinson GS, Sheffield PE, Lall R, Mathes R, et al. The associations between daily spring pollen counts, over-the-counter allergy medication sales, and asthma syndrome emergency department visits in New York City, 2002–2012. Environ Health. 2015;14:71. doi: 10.1186/s12940-015-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin BG, Mansfield LE, Nelson HS. Cross-allergenicity among the grasses. Ann Allergy. 1985;54(2):99–104. [PubMed] [Google Scholar]
- 44.Wodehouse RP. Hayfever Plants. New York: Hafner Publishing; 1971. [Google Scholar]
- 45.Frenz DA. Volumetric ragweed pollen data for eight cities in the continental United States. Ann Allergy Asthma Immunol. 1999;82(1):41–6. doi: 10.1016/s1081-1206(10)62658-0. [DOI] [PubMed] [Google Scholar]
- 46.Stinson KA, Albertine JM, Hancock LM, Seidler TG, Rogers CA. Northern ragweed ecotypes flower earlier and longer in response to elevated CO2: what are you sneezing at? Oecologia. 2016 doi: 10.1007/s00442-016-3670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Athanasios DDV, Dimitrios G, John H. Long-term trends in airborne fungal-spore concentrations: a comparison with pollen. FUNGAL ECOL. 2015;13:150–6. http://dx.doi.org/10.1016/j.funeco.2014.09.010. [Google Scholar]
- 48.Baxi SN, Portnoy JM, Larenas-Linnemann D, Phipatanakul W. Exposure and Health Effects of Fungi on Humans. J Allergy Clin Immunol Pract. 2016;4(3):396–404. doi: 10.1016/j.jaip.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huss K, Adkinson NF, Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001;107(1):48–54. doi: 10.1067/mai.2001.111146. [DOI] [PubMed] [Google Scholar]
- 50.Pomes A, Chapman MD, Wunschmann S. Indoor Allergens and Allergic Respiratory Disease. Curr Allergy Asthma Rep. 2016;16(6):43. doi: 10.1007/s11882-016-0622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hox V, Steelant B, Fokkens W, Nemery B, Hellings PW. Occupational upper airway disease: how work affects the nose. Allergy. 2014;69(3):282–91. doi: 10.1111/all.12347. [DOI] [PubMed] [Google Scholar]
- 52.H RA. ‘ALLERGIC TOXEMIA AND MIGRAINE DUE TO FOOD ALLERGY: REPORT OF CASES’. Cal West Med. 1930;33:785–93. [PMC free article] [PubMed] [Google Scholar]
- 53.Association AP. Diagnostic and statistical manual of mental disorders. 5. Washington DC: 2013. [Google Scholar]
- 54.Association AP. Depressive Disorders Diagnostic and Statistical Manual of Mental Disorders. DSM Library: American Psychiatric Association; 2013. [Google Scholar]
- 55.Association AP. Bipolar and Related Disorders Diagnostic and Statistical Manual of Mental Disorders. DSM Library: American Psychiatric Association; 2013. [Google Scholar]
- 56.Sarra L, Hedden JK, Lipari Rachel, Medley Grace, Tice Peter. In: Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Administration SAaMHS, editor. Rockville, MD: HHS Publication; 2015. [Google Scholar]
- 57.Silverstein B. Gender differences in the prevalence of somatic versus pure depression: a replication. Am J Psychiatry. 2002;159(6):1051–2. doi: 10.1176/appi.ajp.159.6.1051. [DOI] [PubMed] [Google Scholar]
- 58.Halbreich U, Kahn LS. Atypical depression, somatic depression and anxious depression in women: are they gender-preferred phenotypes? J Affect Disord. 2007;102(1–3):245–58. doi: 10.1016/j.jad.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 59.Bender A, Farvolden P. Depression and the workplace: a progress report. Curr Psychiatry Rep. 2008;10(1):73–9. doi: 10.1007/s11920-008-0013-6. [DOI] [PubMed] [Google Scholar]
- 60.Koinis-Mitchell D, Craig T, Esteban CA, Klein RB. Sleep and allergic disease: a summary of the literature and future directions for research. J Allergy Clin Immunol. 2012;130(6):1275–81. doi: 10.1016/j.jaci.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Proudfoot J, Doran J, Manicavasagar V, Parker G. The precipitants of manic/hypomanic episodes in the context of bipolar disorder: a review. J Affect Disord. 2011;133(3):381–7. doi: 10.1016/j.jad.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 62.Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352(24):2515–23. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prevention CfDCa, editor. Control NCfIPa. Self-directed Violence Surveillance: Uniform Definitions and Recommended Data Elements. Atlanta, Georgia: 2011. [Google Scholar]
- 64.Jager-Hyman S, Cunningham A, Wenzel A, Mattei S, Brown GK, Beck AT. Cognitive Distortions and Suicide Attempts. Cognit Ther Res. 2014;38(4):369–74. doi: 10.1007/s10608-014-9613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beck AT, Steer RA, Beck JS, Newman CF. Hopelessness, depression, suicidal ideation, and clinical diagnosis of depression. Suicide Life Threat Behav. 1993;23(2):139–45. [PubMed] [Google Scholar]
- 66.Sally C, Curtin MA, Warner, PhD Margaret, Hedegaard, MD, MSPH Holly. In: Increase in Suicide in the United States; 1999–2014. Services UdoHaH, editor. 1600 Clifton Road Atlanta, GA 30329-4027 USA: 2016. [Google Scholar]
- 67.Control NCfIPa, editor. Suicide Facts at a Glance. 2015. [Google Scholar]
- 68.Suicide huge but preventable public health problem. Geneva: World Health Organization (WHO); 2004. [Google Scholar]
- 69.Fawcett J, Frontiers in Neuroscience . Diagnosis, Traits, States, and Comorbidity in Suicide. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): CRC Press/Taylor & Francis Llc; 2012. [PubMed] [Google Scholar]
- 70.Bentley KH, Franklin JC, Ribeiro JD, Kleiman EM, Fox KR, Nock MK. Anxiety and its disorders as risk factors for suicidal thoughts and behaviors: A meta-analytic review. Clin Psychol Rev. 2016;43:30–46. doi: 10.1016/j.cpr.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weiser M, Goldberg S, Werbeloff N, Fenchel D, Reichenberg A, Shelef L, et al. Risk of completed suicide in 89,049 young males assessed by a mental health professional. Eur Neuropsychopharmacol. 2016;26(2):341–9. doi: 10.1016/j.euroneuro.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 72.U.S.A. suicide 2013 Official final data. Washington, DC: American Association of Suicidology [database on the Internet] American Association of Suicidology; 2015. Available from: http://www.suicidology.org Accessed: [Google Scholar]
- 73.Cooper J, Kapur N, Webb R, Lawlor M, Guthrie E, Mackway-Jones K, et al. Suicide after deliberate self-harm: a 4-year cohort study. Am J Psychiatry. 2005;162(2):297–303. doi: 10.1176/appi.ajp.162.2.297. [DOI] [PubMed] [Google Scholar]
- 74.Hawton K, van Heeringen K. Suicide. Lancet. 2009;373(9672):1372–81. doi: 10.1016/s0140-6736(09)60372-x. [DOI] [PubMed] [Google Scholar]
- 75.Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry. 2011;68(10):1058–64. doi: 10.1001/archgenpsychiatry.2011.113. [DOI] [PubMed] [Google Scholar]
- 76.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156(2):181–9. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- 78.Van Orden KA, Witte TK, Cukrowicz KC, Braithwaite SR, Selby EA, Joiner TE., Jr The interpersonal theory of suicide. Psychol Rev. 2010;117(2):575–600. doi: 10.1037/a0018697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Postolache TT, Komarow H, Tonelli LH. Allergy: a risk factor for suicide? Curr Treat Options Neurol. 2008;10(5):363–76. doi: 10.1007/s11940-008-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Postolache TTM, Komarow HDM, Stiller JWM, Tonelli LHP. Allergy, Depression, and Suicide. Directions in Psychiatry. 2005;25 [Google Scholar]
- 81.Blaiss M, Reigel T, Philpot E. A study to determine the impact of rhinitis on sufferers’ sleep and daily routine. J Allergy Clin Immunol. 115(2):S197. doi: 10.1016/j.jaci.2004.12.800. [DOI] [Google Scholar]
- 82.KleinJan A, Vinke JG, Severijnen LW, Fokkens WJ. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur Respir J. 2000;15(3):491–7. doi: 10.1034/j.1399-3003.2000.15.11.x. [DOI] [PubMed] [Google Scholar]
- 83.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21(12):1303–9. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 84.Gushchin IS. Molecular basis of allergy control. Patol Fiziol Eksp Ter. 2005;(2):2–9. [PubMed] [Google Scholar]
- 85.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8(3):205–17. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 86.Min YG. The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2010;2(2):65–76. doi: 10.4168/aair.2010.2.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hardyman MA, Wilkinson E, Martin E, Jayasekera NP, Blume C, Swindle EJ, et al. TNF-alpha-mediated bronchial barrier disruption and regulation by src-family kinase activation. J Allergy Clin Immunol. 2013;132(3):665–75.e8. doi: 10.1016/j.jaci.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Gandhi VD, Vliagoftis H. Airway epithelium interactions with aeroallergens: role of secreted cytokines and chemokines in innate immunity. Front Immunol. 2015;6:147. doi: 10.3389/fimmu.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Commins SP, Borish L, Steinke JW. Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S53–72. doi: 10.1016/j.jaci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Hunter MG, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175(10):6531–6. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 92.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ross TM, Zuckermann RN, Reinhard C, Frey WH., 2nd Intranasal administration delivers peptoids to the rat central nervous system. Neurosci Lett. 2008;439(1):30–3. doi: 10.1016/j.neulet.2008.04.097. [DOI] [PubMed] [Google Scholar]
- 94.Kalueff AV, Lehtimaki KA, Ylinen A, Honkaniemi J, Peltola J. Intranasal administration of human IL-6 increases the severity of chemically induced seizures in rats. Neurosci Lett. 2004;365(2):106–10. doi: 10.1016/j.neulet.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 95.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–96. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 96.Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, Frey WH., 2nd Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol. 2004;151(1–2):66–77. doi: 10.1016/j.jneuroim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 97.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58(5):445–52. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 98.Tonelli LH, Postolache TT. Airborne inflammatory factors: “from the nose to the brain”. Front Biosci (Schol Ed) 2010;2:135–52. doi: 10.2741/s52. [DOI] [PubMed] [Google Scholar]
- 99.Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158(8):1252–7. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139(3):230–9. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 101.Bahrini L, Ouanes S, Ghachem R. Inflammatory profile in depression and associated clinical and sociodemographic features in a Middle-Eastern North-African population. J Affect Disord. 2016;198:122–6. doi: 10.1016/j.jad.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 102.Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40(4):171–6. doi: 10.1159/000026615. doi: 26615. [DOI] [PubMed] [Google Scholar]
- 103.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 104.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 105.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370–9. doi: 10.1016/s0893-133x(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 106.Eyre HA, Lavretsky H, Kartika J, Qassim A, Baune BT. Modulatory Effects of Antidepressant Classes on the Innate and Adaptive Immune System in Depression. Pharmacopsychiatry. 2016;49(3):85–96. doi: 10.1055/s-0042-103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buttini M, Boddeke H. Peripheral lipopolysaccharide stimulation induces interleukin-1 beta messenger RNA in rat brain microglial cells. Neuroscience. 1995;65(2):523–30. doi: 10.1016/0306-4522(94)00525-a. [DOI] [PubMed] [Google Scholar]
- 108.Konsman JP, Kelley K, Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of Fos, interleukin-1beta and inducible nitric oxide synthase in rat brain. Neuroscience. 1999;89(2):535–48. doi: 10.1016/s0306-4522(98)00368-6. [DOI] [PubMed] [Google Scholar]
- 109.Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83(1):281–93. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- 110.Bay-Richter C, Janelidze S, Hallberg L, Brundin L. Changes in behaviour and cytokine expression upon a peripheral immune challenge. Behav Brain Res. 2011;222(1):193–9. doi: 10.1016/j.bbr.2011.03.060. [DOI] [PubMed] [Google Scholar]
- 111.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21(1):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7(3):254–75. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 113.Scadding G. Cytokine profiles in allergic rhinitis. Curr Allergy Asthma Rep. 2014;14(5):435. doi: 10.1007/s11882-014-0435-7. [DOI] [PubMed] [Google Scholar]
- 114.Bedolla-Barajas M, Morales-Romero J, Pulido-Guillen NA, Robles-Figueroa M, Plascencia-Dominguez BR. Rhinitis as an associated factor for anxiety and depression amongst adults. Braz J Otorhinolaryngol. 2016 doi: 10.1016/j.bjorl.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115••.Brundin L, Erhardt S, Bryleva EY, Achtyes ED, Postolache TT. The role of inflammation in suicidal behaviour. Acta Psychiatr Scand. 2015;132(3):192–203. doi: 10.1111/acps.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110–7. doi: 10.1016/j.bbi.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 117.Oxenkrug G. Serotonin-kynurenine hypothesis of depression: historical overview and recent developments. Curr Drug Targets. 2013;14(5):514–21. doi: 10.2174/1389450111314050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Parrott JM, Redus L, O’Connor JC. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J Neuroinflammation. 2016;13(1):124. doi: 10.1186/s12974-016-0590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maier SF. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17(2):69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 120.Guzman A, Tonelli LH, Roberts D, Stiller JW, Jackson MA, Soriano JJ, et al. Mood-worsening with high-pollen-counts and seasonality: a preliminary report. J Affect Disord. 2007;101(1–3):269–74. doi: 10.1016/j.jad.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Postolache TT, Lapidus M, Sander ER, Langenberg P, Hamilton RG, Soriano JJ, et al. Changes in allergy symptoms and depression scores are positively correlated in patients with recurrent mood disorders exposed to seasonal peaks in aeroallergens. Scientific World J. 2007;7:1968–77. doi: 10.1100/tsw.2007.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Manalai P, Hamilton RG, Langenberg P, Kosisky SE, Lapidus M, Sleemi A, et al. Pollen-specific immunoglobulin E positivity is associated with worsening of depression scores in bipolar disorder patients during high pollen season. Bipolar disord. 2012;14(1):90–8. doi: 10.1111/j.1399-5618.2012.00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Postolache TT, Mortensen PB, Tonelli LH, Jiao X, Frangakis C, Soriano JJ, et al. Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord. 2010;121(1–2):88–93. doi: 10.1016/j.jad.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124••.Coimbra DG, Pereira ESAC, de Sousa-Rodrigues CF, Barbosa FT, de Siqueira Figueredo D, Araujo Santos JL, et al. Do suicide attempts occur more frequently in the spring too? A systematic review and rhythmic analysis. J Affect Disord. 2016;196:125–37. doi: 10.1016/j.jad.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 125.Timonen M, Viilo K, Hakko H, Sarkioja T, Meyer-Rochow VB, Vaisanen E, et al. Is seasonality of suicides stronger in victims with hospital-treated atopic disorders? Psychiatry Res. 2004;126(2):167–75. doi: 10.1016/j.psychres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 126.Woo JM, Gibbons RD, Rogers CA, Qin P, Kim JB, Roberts DW, et al. Pollen counts and suicide rates. Association not replicated. Acta Psychiatr Scand. 2012;125(2):168–75. doi: 10.1111/j.1600-0447.2011.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127••.Jeon-Slaughter H, Claassen CA, Khan DA, Mihalakos P, Lee KB, Brown ES. Temporal Association Between Nonfatal Self-Directed Violence and Tree and Grass Pollen Counts. J Clin Psychiatry. 2016;77(9):1160–7. doi: 10.4088/JCP.15m09864. [DOI] [PubMed] [Google Scholar]
- 128.Messias E, Clarke DE, Goodwin RD. Seasonal allergies and suicidality: results from the National Comorbidity Survey Replication. Acta Psychiatr Scand. 2010;122(2):139–42. doi: 10.1111/j.1600-0447.2009.01518.x. [DOI] [PubMed] [Google Scholar]
- 129.Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117(3):198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tonelli LH, Katz M, Kovacsics CE, Gould TD, Joppy B, Hoshino A, et al. Allergic rhinitis induces anxiety-like behavior and altered social interaction in rodents. Brain Behav Immun. 2009;23(6):784–93. doi: 10.1016/j.bbi.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Woo JM, Gibbons RD, Qin P, Komarow H, Kim JB, Rogers CA, et al. Suicide and prescription rates of intranasal corticosteroids and nonsedating antihistamines for allergic rhinitis: an ecological study. J Clin Psychiatry. 2011;72(10):1423–8. doi: 10.4088/JCP.10m06765. [DOI] [PubMed] [Google Scholar]
- 132.Fang BJ, Tonelli LH, Soriano JJ, Postolache TT. Disturbed sleep: linking allergic rhinitis, mood and suicidal behavior. Front Biosci (Schol Ed) 2010;2:30–46. doi: 10.2741/s44. [DOI] [PubMed] [Google Scholar]
- 133.Kushikata T, Fang J, Wang Y, Krueger JM. Interleukin-4 inhibits spontaneous sleep in rabbits. Am J Physiol. 1998;275(4 Pt 2):R1185–91. doi: 10.1152/ajpregu.1998.275.4.R1185. [DOI] [PubMed] [Google Scholar]
- 134.Kushikata T, Fang J, Krueger JM. Interleukin-10 inhibits spontaneous sleep in rabbits. J Interferon Cytokine Res. 1999;19(9):1025–30. doi: 10.1089/107999099313244. [DOI] [PubMed] [Google Scholar]
- 135.Goldstein TR, Bridge JA, Brent DA. Sleep Disturbance Preceding Completed Suicide in Adolescents. J Consult Clin Psychol. 2008;76(1):84. doi: 10.1037/0022-006X.76.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bernert RA, Kim JS, Iwata NG, Perlis ML. Sleep disturbances as an evidence-based suicide risk factor. Curr Psychiatry Rep. 2015;17(3):554. doi: 10.1007/s11920-015-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Davies MJ, Fisher LH, Chegini S, Craig TJ. A practical approach to allergic rhinitis and sleep disturbance management. Allergy Asthma Proc. 2006;27(3):224–30. doi: 10.2500/aap.2006.27.2858. [DOI] [PubMed] [Google Scholar]
- 138.Craig TJ, Ferguson BJ, Krouse JH. Sleep impairment in allergic rhinitis, rhinosinusitis, and nasal polyposis. Am J Otolaryngol. 2008;29(3):209–17. doi: 10.1016/j.amjoto.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 139.Hepner KA, Rowe M, Rost K, Hickey SC, Sherbourne CD, Ford DE, et al. The effect of adherence to practice guidelines on depression outcomes. Ann Intern Med. 2007;147(5):320–9. doi: 10.7326/0003-4819-147-5-200709040-00007. [DOI] [PubMed] [Google Scholar]
- 140.Association AP. Practise Guidelines for the Treatment of Patients With Major Depressive Disorder. 2010 http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf.
- 141.Israel JA. Remission in depression: definition and initial treatment approaches. J Psychopharmacol. 2006;20(3 Suppl):5–10. doi: 10.1177/1359786806064306. [DOI] [PubMed] [Google Scholar]
- 142.Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry. 2009;70(Suppl 6):26–31. doi: 10.4088/JCP.8133su1c.04. [DOI] [PubMed] [Google Scholar]
- 143.Wittkampf KA, Naeije L, Schene AH, Huyser J, van Weert HC. Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen Hosp Psychiatry. 2007;29(5):388–95. doi: 10.1016/j.genhosppsych.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 144.Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161–6. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Goodwin GM, Haddad PM, Ferrier IN, Aronson JK, Barnes TRH, Cipriani A, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition Recommendations from the British Association for Psychopharmacology. J Psychopharmacol (Oxford, England) 2016;30(6):495–553. doi: 10.1177/0269881116636545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hollon SD, Ponniah K. A review of empirically supported psychological therapies for mood disorders in adults. Depress Anxiety. 2010;27(10):891–932. doi: 10.1002/da.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brown ES, Chandler PA. Mood and Cognitive Changes During Systemic Corticosteroid Therapy. Prim Care Companion J Clin Psychiatry. 2001;3(1):17–21. doi: 10.4088/pcc.v03n0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marchand MS, Jonville-Bera AP, Autret-Leca E. [Psychiatric disorders associated with montelukast: data from the National Pharmacovigilance Database] Arch Pediatr. 2013;20(3):269–73. doi: 10.1016/j.arcped.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 149.Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry. 2002;159(6):909–16. doi: 10.1176/appi.ajp.159.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feldman MD, Franks P, Duberstein PR, Vannoy S, Epstein R, Kravitz RL. Let’s not talk about it: suicide inquiry in primary care. Ann Fam Med. 2007;5(5):412–8. doi: 10.1370/afm.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 152.Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res. 2007;31(2):185–99. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 153.Stanley B, Brown GK. Safety Planning Intervention: A Brief Intervention to Mitigate Suicide Risk. Cogn Behav Pract. 2012;19:256–64. [Google Scholar]
- 154.Alexopoulos GS, Reynolds CF, 3rd, Bruce ML, Katz IR, Raue PJ, Mulsant BH, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Cal West Med. 2009;166(8):882–90. doi: 10.1176/appi.ajp.2009.08121779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166(21):2314–21. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 156.Glied S, Herzog K, Frank R. Review: the net benefits of depression management in primary care. Med Care Res Rev. 2010;67(3):251–74. doi: 10.1177/1077558709356357. [DOI] [PubMed] [Google Scholar]


