Abstract
Liver disease can alter the disposition of xenobiotics and endogenous substances. Regulatory agencies such as the Food and Drug Administration (FDA) and the European Medicines Evaluation Agency (EMEA) recommend, if possible, studying the effect of liver disease on drugs under development to guide specific dose recommendations in these patients. While extensive research has been conducted to characterize the effect of liver disease on drug-metabolizing enzymes, emerging data have implicated that the expression and/or function of hepatobiliary transport proteins also are altered in liver disease. This review summarizes recent developments in the field, which may have implications for understanding altered disposition, safety, and of efficacy of new and existing drugs. A brief review of liver physiology and hepatic transporter localization/function is provided. Then, the expression and function of hepatic transporters in cholestasis, hepatitis C infection, hepatocellular carcinoma (HCC), human immunodeficiency virus (HIV) infection, non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), and primary biliary cirrhosis (PBC) are reviewed. In the absence of clinical data, nonclinical information in animal models is presented. This review aims to advance the understanding of altered expression and function of hepatic transporters in liver disease and the implications of such changes on drug disposition.
INTRODUCTION
The liver is an important organ in the biotransformation, disposition, and elimination of endogenous (e.g., macromolecules, bile acids) and exogenous molecules (e.g., drugs). Drug metabolizing enzymes play a central role in hepatic drug elimination; however, efforts over the past decade have revealed that transport proteins are also important determinants of hepatic clearance. Hepatic transporters are transmembrane proteins anchored in polarized hepatocytes, the primary parenchymal cell type of the liver, that facilitate the transport of molecules to and from sinusoidal blood and hepatocytes (basolateral transporters) or from hepatocytes into the biliary canaliculus (canalicular transporters). These transporters are classified into two superfamilies: the solute carrier (SLC) protein family and the adenosine triphosphate (ATP)-binding cassette (ABC) protein family.
The expression and function of transporters are subject to complex regulatory mechanisms and are contributing factors to interindividual variability. Characterizing sources that contribute to individual variability in hepatic transporter expression/function will improve the efficient development of new and safer pharmacological interventions and advance our mechanistic understanding of the genesis/progression of some diseases. The identification of drug-drug interactions (DDIs) and genetic polymorphisms that influence the expression and/or function of drug transporters have been under extensive investigation in recent years. However, DDIs and genetic polymorphisms represent only a portion of the total contribution to interindividual variability.
Liver disease may influence the expression and function of hepatic transporters. Chronic liver disease is a significant source of morbidity and mortality in both industrialized and developing nations. It is estimated that one million deaths worldwide were attributed to liver cirrhosis alone in 2010, while many more were due to liver cancer and hepatitis. 1 The etiologies of liver disease are diverse and can include chronic alcohol abuse, viral/bacterial infection, fatty liver disease, and drug-induced injury – all of which may have genetic components that can influence the development, manifestation, and severity of disease. The purpose of this article is to review the literature characterizing and quantifying the effects of liver disease on the expression and function of hepatic transporters in humans. First, a brief overview of hepatic transporters will be provided. Then, the effects of cholestasis, hepatitis C (HCV) infection, hepatocellular carcinoma (HCC), human immunodeficiency virus (HIV) infection, non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), and primary biliary cholangitis (PBC) on hepatic transporter expression and function will be summarized. In addition to highlighting current knowledge regarding the effect of liver disease on hepatic transporters, this review will identify knowledge gaps in our understanding of the contribution of liver disease to human variability in drug disposition and elimination. 2
Basolateral Uptake Transporters
Sodium-Taurocholate Co-transporting Polypeptide (NTCP)
The SLC10A1 gene encodes NTCP, a key transport protein involved in the enterohepatic recirculation of bile acids.3 NTCP is homogenously expressed throughout the liver acinus and specifically coordinates the sodium-dependent uptake of bile acids from the sinusoidal blood to hepatocytes by co-transporting two sodium ions and one bile acid molecule.4,5 NTCP transports a variety of bile acids but appears to have higher affinity for glycine- and taurine-conjugated bile acids compared to their unconjugated counterparts, and a higher affinity for dihydroxy bile acids (e.g., chenodeoxycholate and deoxycholate conjugates) than trihydroxy bile acids (e.g., cholate conjugates). Although little emphasis is placed on NTCP-mediated interactions during drug development, NTCP has been shown to transport drugs. For example, drug-conjugated bile acids have been proposed as a potential strategy for drug delivery.6 Approximately 35% of total rosuvastatin uptake into hepatocytes was accounted for by NTCP 7; however, it is not clear whether this contribution is relevant in the presence of systemic bile acids, which may selectively outcompete rosuvastatin in vivo. Interestingly, NTCP was shown to be a functional receptor for the hepatitis B virus and thus, may serve as a novel therapeutic target to inhibit the viral entry mechanism.8,9
Organic Anion Transporters (OATs)
OATs, members of the SLC22A superfamily, are a ubiquitously expressed group of organic anion transporters that have broad distribution in the kidney, liver, brain, pancreas, salivary glands, and skeletal muscle.10 These proteins generally mediate the transport of negatively charged endogenous and exogenous molecules in a bidirectional manner in exchange for dicarboxylate ions 11–13, although uncharged and even cationic molecules have been reported as substrates.14,15 OATs also transport endogenous substances including cGMP, bile acids, and hormone derivatives.16–18 Prostaglandins and glutamate are examples of endogenous substrates of OAT2, whereas methotrexate, valproic acid, and allopurinol are examples of drug substrates.17–20 OAT7 is another liver-specific OAT that appears to have similar and overlapping substrate specificity with other OATs.18,20 Despite the expression and function of OATs in the liver, there is little evidence demonstrating the clinical relevance of these transporters in the context of DDIs and/or genetic polymorphisms, although pravastatin recently was identified as a novel OAT7 substrate.21,22
Organic Anion-Transporting Polypeptides (OATPs)
OATPs are a class of proteins that belong to the SLCO superfamily with 12-transmembrane spanning domains that are localized on the basolateral membrane of many epithelial cells. OATPs are highly expressed in the liver (predominantly OATP1B1, OATP1B3, and OATP2B1).23–26 OATPs transport a wide variety of amphipathic and anionic substances in a bidirectional manner through counter transport with either bicarbonate or reduced glutathione.27–29 OATP substrates include endogenous compounds such as bile acids, bilirubin, thyroid hormones, and steroid conjugates. Several drug classes have been characterized, or even specifically designed, as substrates for OATPs including 3-hydroxy-3-methylglutaryl coenzyme A HMG-Co-A reductase inhibitors (i.e., statins), angiotensin II receptor antagonists, angiotensin converting enzyme inhibitors, and cardiac glycosides.29,30 The liver-specific OATP1B1, OATP1B3, and to a lesser extent, OATP2B1, are considered clinically relevant drug transporters by the Food and Drug Administration (FDA) and are routinely evaluated during drug development.30
Organic Cation Transporters (OCTs)
The first OCT (i.e., OCT1) was isolated and cloned from rat kidney by Gründemann et al. in 1994 and was later shown to display a 12-transmembrane structure that mediates the bidirectional transport of various small molecules (~60–350 Da) in an electrogenic manner.31,32 Similar to other transporters of the SLC family, OCTs display broad tissue distribution in such organs as the kidney, liver, and intestine. Three OCT isoforms have been identified in humans. OCT1 exhibits greatest expression in the liver. OCTs generally mediate the transport of small hydrophilic compounds with at least one positive charged amine moiety, although uncharged or anionic substrates have been reported.32 Classic substrates include tetraethylammonium (TEA) and the parkinsonian neurotoxin 1-methyl-4-phenylpyridinium (MPP+); clinically relevant drug substrates include famotidine, ranitidine, and metformin.33–35
Canalicular Efflux Transporters
Breast Cancer Resistance Protein (BCRP)
BCRP (ABCG2) was first cloned by Doyle et al. (2003) and although its name suggests otherwise, BCRP is expressed in a variety of tissues and cell types (e.g., intestine, brain, liver, cardiac muscle).36 BCRP is a half transporter hypothesized to function as a homodimer or potentially a tetramer.37,38 In the liver, BCRP is localized to the canalicular membrane with broad substrate specificity, which includes organic ions, sulfate conjugates, and both negatively and positively charged molecules. BCRP substrates have been characterized primarily in the context of oncology due to the resistance of certain cancer types against chemotherapeutic agents. Some examples include mitoxantrone, SN-38, topotecan, and doxorubicin.39–41 However, BCRP can transport sulfate and glucuronide bile acid conjugates and may play a compensatory role when the function of other transporters (e.g., MRP2) is impaired.42,43
Bile Salt Export Pump (BSEP)
ABCB11 encodes BSEP, a member of the ABC transporter family, which is the primary hepatocellular transporter responsible for secretion of bile acids into the bile canaliculus. BSEP is predominantly expressed throughout the human liver acinus. Bile acid transport requires the hydrolysis of ATP, which drives the conformational change in the transmembrane domain of BSEP, thereby translocating bile acids from hepatocytes to the canalicular space. In addition to bile acids, BSEP has been reported to transport pravastatin.44 Mono-anionic and conjugated bile acids represent the majority of BSEP substrates; unconjugated bile acids do not appear to be substrates as evidenced by in vitro and in vivo human data.45,46 Differences in substrate specificity between species have been reported; for example, human BSEP is able to transport taurolithocholate-3-sulfate although this is not true for the rodent orthologue.47 The affinity (i.e., Km) for bile acids is generally in the low micromolar range across species, but differences in rank-order and specificity have been noted. For example, human BSEP has a higher affinity for taurocholate than mouse BSEP (human Km: 4.25 μM compared to mouse Km: 15–30 μM).47
Multidrug and Toxin Extrusion Protein 1 (MATE1)
MATE1 (SLC47A1) is an H+/organic cation transporter first identified in 2005 that mediates the electrogenic transport of organic cations independent of a sodium gradient.48 It is primarily expressed on the canalicular membrane of hepatocytes and the luminal membrane of the proximal tubules and is thought to play a physiological role in the excretion of toxic endogenous substances, although several drugs have been identified as substrates (e.g., metformin, cimetidine, and acyclovir).49
Multidrug Resistance-Associated Protein 2 (MRP2)
MRP2 is a member of the ABCC family and one of nine recognized MRPs. First identified in the canalicular membrane of hepatocytes, MRP2 has been characterized in the kidney, small intestine, gallbladder, and placenta.50–53 MRP2 is a major driving force for bile-acid independent bile flow through the secretion of reduced glutathione, although it transports divalent and glucuronide- and sulfate-conjugated bile acids into the bile canaliculus. MRP2 is responsible for the biliary transport of many anionic drugs/metabolites including methotrexate, acetaminophen-glucuronide, and etoposide.54,55 MRP2 plays an important physiological role in the biliary excretion of bilirubin (specifically, the conjugated species); patients with Dubin-Johnson Syndrome, who harbor polymorphisms in the ABCC2 gene, exhibit impaired secretion of conjugated bilirubin into bile.56,57 Naturally occurring Mrp2-deficient rats (i.e., Eisai hyperbilirubinemic and CY/TR− mutant rat strains) have been a valuable in vivo model for understanding the role of Mrp2 in drug disposition.55,58
Multidrug Resistance Protein 1 (MDR1)
MDR1, commonly known as P-glycoprotein (P-gp), was one of the first ABC proteins identified due to its important role in drug resistance in chemotherapy. Like other ABC transporters, P-gp is comprised of two membrane-bound domains each consisting of six transmembrane helices.59 Expression has been quantified in many tissues such as the liver, brain, testis, gut, and colon.60 Although the physiological role of P-gp has been debated, it likely plays a general role in the protection from xenobiotics and disposition of endogenous substances, as evidenced by its ability to transport a variety of amphipathic drugs, natural products, and peptides. P-gp transports immunosuppressants, hormones, calcium channel blockers, and cardiac glycosides, but P-gp expression and function have been studied the most in the context of cancer chemotherapy. Apart from being expressed in major organs governing drug disposition (e.g., the liver, gut), P-gp is overexpressed in many cancer cell types, which limits the distribution/exposure of chemotherapeutics to the site of action.61–63
Basolateral Efflux Transporters
Multidrug Resistance-Associated Protein 1 (MRP1)
The clinical relevance of MRP1, encoded by the ABCC1 gene, was first recognized in the transport of hydrophobic and hydrophilic anticancer agents such as vincristine and etoposide.64–66 Under normal conditions, the expression of MRP1 in the liver is low.67,68 In vitro studies have established that MRP1 can transport a variety of physiological organic anions including drugs and their conjugated metabolites. For example, MRP1 can transport endogenous substances such as 17β-estradiol glucuronide, glutathione, leukotriene C4, folic acid, and vitamin B12, which may indicate a physiological role in cellular defense and oxidative stress.67,68
Multidrug Resistance-Associated Protein 3 (MRP3)
MRP3, encoded by the ABCC3 gene, actively transports organic ions using energy generated by the hydrolysis of ATP. MRP3 is localized on the basolateral membrane of hepatocytes and is expressed in other tissues such as the gut, kidney, and adrenal cortex.69 In the context of the liver, MRP3 is thought to play an adaptive role in response to hepatocellular stress as evidenced by the upregulation of MRP3 in cholestatic conditions.70,71 MRP3 transports bulky organic anions from the hepatocyte to the systemic circulation and preferentially mediates the transport of glucuronide and, to a lesser extent, sulfate conjugates (e.g., bilirubin glucuronide, bile acid conjugates).72,73 Although MRP3 has been studied in the context of bile acid transport, several drugs and/or metabolites have been identified as MRP3 substrates including acetaminophen glucuronide, methotrexate, and sorafenib.74–77
Multidrug Resistance-Associated Protein 4 (MRP4)
In contrast to MRP3, MRP4 (ABCC4) transports a dynamic range of endogenous molecules such as folate, bile acids, uric acid, eicosanoids, steroid hormones, and cyclic nucleotides. 72,78 Although MRP4 was first characterized as a transporter that confers resistance to cytotoxic agents, MRP4 has since shown broad substrate specificity to drugs from many classes including antibiotics, antivirals, and cardiovascular agents. MRP4 is generally expressed in tissues with a barrier function such as the liver, brain, and intestine. In the liver, MRP4 is localized on the basolateral membrane and translocates molecules from the hepatocyte to the systemic circulation. Similar to MRP3, it is thought that the physiological role of MRP4 is to protect the liver from toxic accumulation of bile acids as evidenced by marked increases in MRP4 expression in cholestasis.79 This hypothesis is further supported by the finding that Mrp4-knockout mice exhibited more severe liver injury due to obstructive cholestasis than wild-type mice with functional Mrp4.80
Liver Disease
The effects of liver disease on hepatic transporter expression and function in humans are summarized in the following section. Table 1 highlights reports of altered hepatic transporter expression in liver diseases where data in diseased and healthy subjects were compared. Table 2 summarizes published reports of altered transporter-mediated drug disposition in liver disease resulting in changes in pharmacokinetics and/or efficacy.
Table 1.
Altered Expression of Hepatic Transporters in Liver Disease
| Transporter | Liver Disease | Alterations (mRNA) | Alterations (protein) | Technique | References |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| NTCP/Ntcp | Cholestasis | ↓ (~2-fold, human); ↓ (~3-fold, rodent) | ↓ (~3-fold, human); ↓ (~3-fold, rodent) | RT-qPCR; NB; WB | 70,89,92,202 |
|
|
|
|
|
|
|
| HCV | ↓ (human) or ↔ (human); ↔ (rodent) | - | RT-qPCR | 108,109,203 | |
|
|
|
|
|
|
|
| HCC | ↓ (~2-fold, human) | ↓ (human) | RT-qPCR; NB | 151,204 | |
|
|
|
|
|
|
|
| HIV | - | - | - | - | |
|
|
|
|
|
|
|
| NASH | ↑ (~1.5-fold, human) or ↓ (~2–5-fold, human); ↓ (~2-fold, rodent) | ↓ (~5-fold, human) | ISH; RT-qPCR WB | 176,177,179,185 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | ↓ (~1.5–3-fold, human) | ↓ (~2-fold, human) | RT-qPCR; WB; IF | 189,191 | |
|
|
|
|
|
|
|
| OATP1B1/Oatp1b2 | Cholestasis | ↓ (~2-fold, human); ↓ (~2-fold, rodent) | ↔ (rodent) | NB; RT-qPCR; WB | 89,92,202 |
|
|
|
|
|
|
|
| HCV | ↓ (~1.5-fold human); ↔ (rodent) | - | RT-qPCR | 108,109,205 | |
|
|
|
|
|
|
|
| HCC | ↓ (~2–3-fold, human) or ↔ (human) | ↓ (human) | RT-qPCR; IF; IHC; WB | 121,129–132,204 | |
|
|
|
|
|
|
|
| HIV | - | - | - | - | |
|
|
|
|
|
|
|
| NASH | ↔ (human); ↑ (~10-fold, rodent) or ↔ (rodent) or ↓ (~2-fold, rodent) | ↑ (~5-fold, human); ↑ (~10-fold, rodent) or ↔ (rodent) | ISH; RT-qPCR; WB | 169,174,179,188 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | ↔ (human) or ↓ (~2–3-fold, human) | ↔ (human) or ↓ (~2-fold, human) | RT-qPCR; IF; WB | 189–191,205 | |
|
|
|
|
|
|
|
| OATP1B3 | Cholestasis | ↓ (~2-fold, human); ↓ (~2-fold, rodent) | ↔ (rodent) | NB; RT-qPCR; WB | 89,202 |
|
|
|
|
|
|
|
| HCV | - | - | - | - | |
|
|
|
|
|
|
|
| HCC | ↓ (~5–50-fold, human) | ↓ (human) | NB; RT-qPCR; WB; IHC; IF | 121,131,133 | |
|
|
|
|
|
|
|
| HIV | - | - | - | - | |
|
|
|
|
|
|
|
| NASH | ↔ (human) | ↓ (~10-fold, human) | ISH; WB | 175 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | ↔ (human) or ↓ (~2-fold, human) | ↔ (human) or ↓ (~2–3-fold, human) | RT-qPCR; IF; WB | 189–191 | |
|
|
|
|
|
|
|
| OATP2B1/Oatp2b1 | Cholestasis | - | - | - | - |
|
|
|
|
|
|
|
| HCV | ↓ (, human); ↑ (~2-fold rodent) | - | RT-qPCR | 109,203 | |
|
|
|
|
|
|
|
| HCC | - | - | - | - | |
|
|
|
|
|
|
|
| HIV | ↔ (rodent) | - | RT-qPCR | 164 | |
|
|
|
|
|
|
|
| NASH | ↔ (human); ↔ (rodent) | ↔ (human); ↓ (~2-fold, rodent) | ISH; WB | 174,188 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | ↑ (human) | ↑ (human) | RT-qPCR; WB | 205 | |
|
|
|
|
|
|
|
| OAT2/Oat2 | Cholestasis | - | - | - | - |
|
|
|
|
|
|
|
| HCV | ↓ (human); ↔ (rodent) | - | RT-qPCR | 109,203 | |
|
|
|
|
|
|
|
| HCC | - | - | - | - | |
|
|
|
|
|
|
|
| HIV | - | - | - | - | |
|
|
|
|
|
|
|
| NASH | ↓ (~2-fold, rodent) | RT-qPCR | 179 | ||
|
|
|
|
|
|
|
| PBC/cirrhosis | - | - | - | - | |
|
|
|
|
|
|
|
| OCT1/Oct1 | Cholestasis | ↓ (~3-fold rodent) | ↓ (~2-fold rodent) | RT-qPCR; WB | 88 |
|
|
|
|
|
|
|
| HCV | ↔ (human) or ↓ (human); ↓ (~2-fold rodent) | - | RT-qPCR | 108,109,203 | |
|
|
|
|
|
|
|
| HCC | ↓ (~2–3-fold, human) | ↓ (human) | RT-qPCR; WB; IF | 137,138 | |
|
|
|
|
|
|
|
| HIV | - | - | - | - | |
|
|
|
|
|
|
|
| NASH | - | - | - | - | |
|
|
|
|
|
|
|
| PBC/cirrhosis | - | - | - | - | |
|
|
|
|
|
|
|
| MRP1/Mrp1 | Cholestasis | - | - | - | - |
|
|
|
|
|
|
|
| HCV | ↑ (10-fold human) | ↑ (human) | RT-qPCR; IHC | 112 | |
|
|
|
|
|
|
|
| HCC | ↑ (human) | ↑ (human) | RT-qPCR; WB | 112,158,206 | |
|
|
|
|
|
|
|
| HIV | ↔ (rodent) | - | RT-qPCR | 164 | |
|
|
|
|
|
|
|
| NASH | ↑ (~2-fold, human); ↑ (~2-fold, rodent) | ↑ (~2-fold, human) | ISH; RT-qPCR; WB | 74,176,180 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | ↑ (human) | ↑ (human) | RT-qPCR; IHC | 112,205 | |
|
|
|
|
|
|
|
| MRP3/Mrp3 | Cholestasis | ↑ (~3-fold, human) | ↑ (~3-fold, human) | RT-qPCR; IHC | 70 |
|
|
|
|
|
|
|
| HCV | ↑ (~10-fold, human); ↔ (rodent) | ↑ (~2–20-fold, human) | RT-qPCR; WB | 111,112,203 | |
|
|
|
|
|
|
|
| HCC | ↑ (~2-fold, human); ↔ (human) | - | RT-qPCR | 112,139,158,206 | |
|
|
|
|
|
|
|
| HIV | ↑ (~3-fold, rodent) | - | RT-qPCR | 164 | |
|
|
|
|
|
|
|
| NASH | ↑ (~2-fold, human); ↓ (~2-fold, rodent) or ↑ (~5-fold, rodent) | ↑ (~2-fold, human); ↓ (~2-fold, rodent) or ↑ (~3-fold, rodent) | ISH; WB | 74,174,180 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | ↑ (~10-fold, human) or ↓ (human, diabetic cirrhosis); ↔ (human, alcohol cirrhosis) | ↑ (human) and ↑ (human, diabetic and alcohol cirrhosis) | RT-qPCR; IHC | 112,189,191,205 | |
|
|
|
|
|
|
|
| MRP4/Mrp4 | Cholestasis | ↑ (~1.5-fold, human) | RT-qPCR | 70,188,202 | |
|
|
|
|
|
|
|
| HCV | ↑ (~2-fold, human); ↑ (~2-fold, rodent) | ↑ (~3-fold, human) | RT-qPCR; WB | 103,111,203 | |
|
|
|
|
|
|
|
| HCC | ↑ (~ 2-fold, human) | - | RT-qPCR | 139 | |
|
|
|
|
|
|
|
| HIV | ↔ (rodent) | - | RT-qPCR | 164 | |
|
|
|
|
|
|
|
| NASH | ↑ (~3-fold, human); ↑ (~3–40-fold, rodent) | ↑ (~3-fold, human); ↑ (~3-fold, rodent) | ISH; RT-qPCR; WB | 74,174,176,180 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | ↔ (human) or ↑ (human) | ↑ (human) | RT-qPCR; IHC; WB | 190,205 | |
|
|
|
|
|
|
|
| BCRP/Bcrp | Cholestasis | - | - | - | - |
|
|
|
|
|
|
|
| HCV | ↓ (~2-fold, human); ↓ (~2-fold, rodent) | - | RT-qPCR | 103,203 | |
|
|
|
|
|
|
|
| HCC | ↑ (human); ↔ (human) | ↑ (human) | RT-qPCR; IHC | 139–141 | |
|
|
|
|
|
|
|
| HIV | ↔ (rodent) | - | RT-qPCR | 164 | |
|
|
|
|
|
|
|
| NASH | ↑ (~2-fold, human); ↑ (~5-fold, rodent) | ↑ (~2-fold, human); ↔ (rodent) | RT-qPCR; WB | 74,180 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | ↑ (~ 2-fold, human) | ↑ (human) | RT-qPCR; WB; IHC | 205,207 | |
|
|
|
|
|
|
|
| BSEP/Bsep | Cholestasis | ↔ (human) or ↓ (~2-fold, human); ↓ (~2-fold, rodent) | ↔ (human); ↓ (~2-fold, rodent) | RT-qPCR; WB; IHC | 70,85,188,202 |
|
|
|
|
|
|
|
| HCV | ↑ (~2-fold human) or ↓ (human); ↔ (rodent) | ↔ (human) | RT-qPCR; IHC | 109,111,112,203 | |
|
|
|
|
|
|
|
| HCC | ↓ (human) | ↓ (human) | RT-qPCR; WB | 143,144 | |
|
|
|
|
|
|
|
| HIV | - | - | - | - | |
|
|
|
|
|
|
|
| NASH | ↑ (~5-fold, human) or ↓ (~2-fold, human); ↔ (rodent) | - | RT-qPCR | 176,177,185 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | ↔ (human) or ↓ (human) | ↔ (human) or ↓ (human) | IHC; RT-qPCR; WB | 190,191 | |
|
|
|
|
|
|
|
| MRP2/Mrp2 | Cholestasis | ↔ (human) or ↓ (~2-fold, human) | ↓ (human) | RT-qPCR; IHC | 70,92,188,202 |
|
|
|
|
|
|
|
| HCV | ↔ (human) or ↓ (~2-fold human); ↔ (rodent) | ↔ (human) | RT-qPCR; IHC | 103,109,111,112,203 | |
|
|
|
|
|
|
|
| HCC | ↔ (human) | ↔ (human) | RT-qPCR; IHC | 109,155 | |
|
|
|
|
|
|
|
| HIV | ↑ (~2-fold, rodent) | ↑ (~1.5-fold, rodent) | qPCR; WB | 164 | |
|
|
|
|
|
|
|
| NASH | ↔ (human) or ↓ (~2-fold, human); ↔ (rodent) or ↑ (~2-fold, rodent) | ↑ (~3-fold, human); ↑ (~2-fold, rodent) or ↔ (rodent) | ISH; RT-qPCR; WB | 74,174,176,179,180,185 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | ↔ (human) | ↔ (human) | RT-qPCR; WB; IHC | 189–191 | |
|
|
|
|
|
|
|
| P-gp | Cholestasis | - | ↔ (human) | IHC | 188 |
|
|
|
|
|
|
|
| HCV | ↑ (~2–5-fold, human); ↔ (rodent) | ↑ (~20-fold, human) | RT-qPCR; IHC | 103,111,112,203 | |
|
|
|
|
|
|
|
| HCC | ↓ (human) | ↑ (human) | RT-qPCR; WB | 150–153 | |
|
|
|
|
|
|
|
| HIV | - | ↑ (~1.5-fold, rodent) | WB | 164 | |
|
|
|
|
|
|
|
| NASH | ↑ (~2-fold, rodent) | ↑ (~2-fold, rodent) | ISH; WB | 188 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | ↑ (human) | ↑ (5-fold, human) | RT-qPCR; WB; IHC | 112,189 |
Abbreviations: no data available (-); hepatocellular carcinoma (HCC); hepatitis C virus (HCV); human immunodeficiency virus (HIV); immunofluorescence microscopy (IF); immunohistochemistry (IHC); in situ hybridization (ISH); nonalcoholic steatohepatitis (NASH); northern blot (NB); primary biliary cholangitis (PBC); quantitative reverse transcription polymerase chain reaction (RT-qPCR); western blot (WB); ↑ increased expression compared to normal tissues, ↓ decreased expression compared to normal tissues, ↔ no change in expression compared to normal tissues. Although humans have two different OATP1B transporters (OATP1B1 and OATP1B3), mice have a single Oatp1b2 ortholog, which was grouped with OATP1B1 in this table for simplicity.
Table 2.
Altered Transporter-Mediated Drug Disposition in Liver Disease Resulting in Changes in Pharmacokinetics and/or Efficacy
| Liver Disease | Drug | Drug Class | Altered Drug Disposition | Implicated Transport Protein | References |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| Cholestasis | Doxorubicin | Chemotherapeutic | ↑ systemic concentrations | Mrp2 (rodent) | 100 |
| Metformin | Antidiabetic | ↓ systemic clearance and hepatic uptake; ↓ suppression of glucose production | Oct1 (rodent) | 99 | |
|
|
|
|
|
|
|
| HCV | Nelfinavir | Antiretroviral | ↑ systemic concentrations | P-gp (human) | 115 |
| 99mTc-mebrofenin | Hepatobiliary imaging agent | ↓ hepatic uptake, ↑ Tmax, ↓ hepatic excretion | OATP, MRP2(human) | 116 | |
|
|
|
|
|
|
|
| HCC | Gd-EOB-DTPA | Hepatobiliary contrast agent | ↑ or ↓ signal intensity | OATP1B1 (human) OATP1B3(human) MRP2 (human) |
19,20 |
| Methotrexate | Antimetabolite | ↑ or ↓ systemic concentrations | OATP1B1 (transgenic mice) | 208 | |
|
|
|
|
|
|
|
| HIV | Indinavir, Nelfinavir, Saquinavir | Protease inhibitors | ↑ or ↓ systemic concentrations | Mdr1a (rodent) | 209 |
|
|
|
|
|
|
|
| NASH | APAP/APAP-glucuronide | Analgesic | ↑ systemic concentrations; ↓ biliary excretion | MRP3 (human); Mrp2/Mrp3 (rodent) | 74 |
| Morphine/morphine-glucuronide | Analgesic | ↑ systemic concentrations | MRP3 (human) | 183 | |
| 99mTc-mebrofenin | Hepatobiliary imaging agent | ↑ systemic concentrations; ↑ hepatic exposure | OATP, MRP2, MRP3 (human) | 2 | |
| Gd-EOB-DTPA | Hepatobiliary contrast agent | ↑ hepatic exposure | Mrp2 (rodent) | 184 | |
| Methotrexate | Chemotherapeutic | ↑ liver and ↑ GI toxicity | Mrp1–4, Mdr1 P-gp, Bcrp (rodent) | 207 | |
| Pemetrexed | Chemotherapeutic | ↑ systemic concentrations; ↓ biliary excretion | Mrp2 (rodent) | 210 | |
| Simvastatin | Cholesterol-lowering | ↑ systemic concentrations | Oatp (rodent) | 175 | |
|
|
|
|
|
|
|
| PBC/cirrhosis | Gd-EOB-DTPA | Hepatobiliary contrast agent | ↓ hepatic signal intensity | OATP (human) | 192 |
Abbreviations: data not available (-); hepatocellular carcinoma (HCC); hepatitis C virus (HCV); human immunodeficiency virus (HIV); nonalcoholic steatohepatitis (NASH); primary biliary cholangitis (PBC), ↑ increased, ↓ decrease
Cholestasis
Cholestasis is the impairment of bile formation (hepatocellular) and/or flow (obstructive) that can arise from a number of intrinsic and extrinsic factors. In response to accumulating bile constituents, adaptive mechanisms induce hepato-protective systems (e.g., bile acid transporter expression) aimed at reducing intracellular accumulation of bile acids, which can be hepatotoxic due to their detergent-like properties.
The functional role of transporters in cholestasis is highlighted in patients harboring mutations in the ABC11 (BSEP) gene known as progressive familial intrahepatic cholestasis 2 (PFIC2). First described by Clayton et al. (1969), the PFIC-2 phenotype includes pruritus, jaundice, and clinical signs of cholestasis (discolored stools, dark urine), which often leads to fulminant liver failure and death early in life.81,82 Hepatic bile acid uptake transporters (e.g., NTCP, OATP1B1, OATP1B3) were downregulated compared to control samples, whereas OATP2B1 was unaffected based on mRNA and protein analysis.83 Interestingly, MRP4, but not MRP3 was strongly upregulated in PFIC-2 livers, consistent with an adaptive response to increased hepatocellular bile acid concentrations. Although it is not clear why the authors did not observe increased MRP3, as would be expected in cholestasis, it must be noted that the sample size was small (n=4 PFIC-2 and n=3 control samples). MRP2 was localized to the canalicular membrane and appeared to be downregulated in PFIC-2; however, this result did not reach statistical significance.83
Because the etiologies of cholestasis can be diverse, altered transporter function may be causal or an adaptive response to cholestasis, or both. For example, intrahepatic cholestasis of pregnancy (ICP) is an acute form of cholestasis that typically presents in the third trimester of pregnancy and is associated with premature delivery, respiratory distress, and intrauterine death.84 In vivo studies revealed that the transcriptional dynamics of BSEP were inversely correlated with serum 17β-estradiol (E2) levels, implicating E2 levels in the repression of BSEP, which may increase susceptibility to ICP.85,86 In the same study, repression of BSEP expression also was demonstrated in human primary hepatocytes.85 The procholestatic effects of estrogens and progesterone may also alter Mrp2; E2 administration causes endocytic internalization, as well as decreased expression and function of Mrp2 in rodents.87,88 Downregulation of hepatic sinusoidal transporters (i.e., Oatp1a1, Oatp1a4, and Oatp1b2) are likely an adaptive response to increased bile acid concentrations and cholestasis.89,90 Although ethical considerations may preclude the quantification of hepatic transporter expression in ICP, the expression of OATP1B3 in placenta was decreased in pregnant women diagnosed with ICP, consistent with altered transporter expression revealed in animal studies.91 Zollner et al. (2001) reported that NTCP mRNA correlated with serum bile acids in patients with inflammatory cholestasis.92 Similar observations were reported for OATP1B1 and BSEP, but MRP2 mRNA was unchanged. 92
Given the limited availability of human samples, the application of animal models has provided information about the mechanisms underlying transporter expression/function in cholestasis. For example, ligation of the common bile duct in rodents leading to cholestasis decreased Mrp2 protein levels without a corresponding change in mRNA expression, suggestive of an impaired posttranslational modification effect.93 Immunofluorescent staining revealed intracellular localization of Mrp2, which may indicate that Mrp2 trafficking to the canalicular membrane is compromised resulting in the endocytic retention of Mrp2 and possible lysosomal degradation. Indeed, retrieval of MRP2 from the canalicular membrane into the cytoplasm of hepatocytes has been reported in patients with cholestasis.94 Although the exact mechanism underlying this observation has yet to be fully elucidated, altered binding and subsequent improper anchoring in the canalicular membrane via radixin and/or other actin filament interactions appears to play a role.94–97
Few studies have investigated the effect of cholestasis on the pharmacokinetics and hepatobiliary disposition of drugs. Bile duct ligation in rats significantly increased the systemic concentrations of morphine-3-glucuronide, the active metabolite of the analgesic morphine due, in part, to increased expression of hepatic Mrp3.98 mRNA and protein expression of MRP3 were significantly increased by ~3-fold in patients with obstructive cholestasis70; hence, it is conceivable that increased systemic exposure of morphine glucuronide(s) could impact pharmacodynamic activity and/or toxicity in this patient population. In experimental intrahepatic cholestasis induced by 17α-ethynylestradiol in rats, the antidiabetic effect of metformin was reduced due to impaired hepatic uptake of Oct1.99 Although clinical data are needed, these observations suggest that metformin efficacy (e.g., in gestational diabetes) may be impaired in diabetic patients with estrogen-associated ICP. In the same rodent model, systemic concentrations of doxorubicin were increased by ~60%, which was partially attributed to a ~67% reduction in Mrp2-mediated biliary excretion.100 Lastly, the apparent half-life and systemic exposure of digoxin were decreased in a rodent model of cholestasis, presumably through impaired P-gp-mediated enterohepatic recirculation.101
Hepatitis C Infection
Hepatitis C virus (HCV) is a prevalent form of viral hepatitis. The risk of developing cirrhosis, liver cancers, or both is increased in HCV-infected patients.102 HCV induces hepatoprotective response pathways in response to fibrosis and hepatocellular injury that can affect normal gene expression patterns.103 The World Health Organization estimates that more than 150 million individuals are chronically infected with HCV.104 Thus, understanding altered expression and/or function of proteins involved in absorption, distribution, metabolism, and excretion (ADME) is crucial to pharmacotherapeutic intervention in this population.
Due to inflammation associated with viral infection, prevailing evidence implicates the coordinate downregulation of ADME genes by nuclear transcription factors [e.g., PXR, CAR, aryl hydrocarbon receptor (AHR), RXR], although interaction(s) with the HCV virus and the transcriptional factor RXR have be reported.105–107 Therefore, the pathophysiological role of HCV in altered ADME expression cannot be dismissed. Relative mRNA expression of OATP1B1 and OATP2B1 was reduced in the livers of patients with chronic HCV infection, although the mechanistic link between inflammatory cytokines and OATP downregulation is not clear.108,109 Downregulation of NTCP, OAT2, and OCT1 correlated with fibrosis state, but another study observed no difference compared to control samples.108,109 In the presence of HCV-induced cirrhosis, expression of NTCP, OATP1B3, and OCT1 are decreased compared to control samples using proteomic analysis.110
Hepatic basolateral efflux transporters MRP3 and MRP4 are elevated compared to the livers of non-infected controls using mRNA analysis. These data are consistent with Ogasawara et al. (2010) who identified MRP4 mRNA as a potential marker for liver disease.103,111,112 Under normal physiological conditions, MRP3 and MRP4 protein are expressed at low levels but are significantly increased in cholestatic and inflammatory conditions, possibly due to the activation of AHR and/or Nrf2 observed in end-stage liver disease.71,80,112–114 In contrast, the canalicular transporters BCRP and BSEP appeared to be downregulated whereas MRP1 and P-gp were upregulated. Some observations report that MRP2 is unaffected, although one study found a negative correlation between fibrosis stage and MRP2 mRNA expression.103,109,111 The impact of altered mRNA expression on protein expression has yet to be quantified, although immunohistochemistry shows increased MRP1 and P-gp staining in HCV liver biopsies.112 In HCV-induced cirrhosis, protein expression of BSEP, MRP2, and P-gp are downregulated whereas BCRP and MATE1 are unchanged.110 To date, the clinical relevance of these findings in HCV-infected patients is unclear. The systemic clearance of nelfinavir, a P-gp substrate, was reduced in HIV/HCV-co-infected patients compared to HIV-infected patients without HCV.115 However, P-gp is expressed in other tissues (e.g., gut, blood brain barrier) and this must be considered as a contributing factor to altered pharmacokinetics due to HCV-associated changes in P-gp function. Hepatic uptake and excretion of 99mTc-mebrofenin was decreased in HCV-infected patients, suggestive of altered OATP-, MRP2-, and MRP3-mediated function.116
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is a malignancy of the liver cells that often occurs in patients with hepatic diseases such as cirrhosis or chronic hepatitis associated with HCV. There is a substantial body of evidence indicating that the expression of hepatic uptake and efflux transporters is altered in HCC. NTCP and OATP1B1 are the key transporters responsible for the uptake of drugs linked to bile acids as carrier molecules.117–119 Studies have shown that the expression of NTCP and OATP1B1 is significantly reduced in patients with HCC.117 This is consistent with previous in vitro studies that demonstrated reduced bile acid uptake in hepatoma cell lines. These studies highlight the risk of liver toxicity in nonmalignant cells with preserved expression levels of NTCP and OATP1B1 that would be overloaded with bile salt-coupled chemotherapy whereas the carcinoma cells, with reduced expression of NTCP and OATP1B1, would have impaired accumulation of bile salt-coupled drugs.117 OATP1B1 and OATP1B3 have been studied extensively in different types of cancers including HCC.120–122 Although initially considered to be liver specific, subsequent studies have shown that OATP1B1 and OATP1B3 are frequently expressed in multiple types of cancer tissues.120,123–128 After the initial study reporting the reduced expression of OATP1B1 protein in HCC cell lines, many other in vitro studies reported similar findings.117,129–132 However, one study reported no significant change in expression of OATP1B1 compared to normal liver.121 These apparent differences may be attributed to different detection methods.117,121 Similarly, OATP1B3 mRNA and protein are also reduced in HCC.121,131,133 Although studies in other cancer types have implied a potential association between OATP1B3 expression and clinical outcomes, the functional role of OATP1B3 in HCC remains to be investigated.124,134
Interestingly, mRNA and protein expression of some other OATPs (e.g., OATP2A1, OATP3A1, OATP4A1 and OATP5A1) are reported to be upregulated in primary and metastatic liver cancer suggesting the potential role of these OATPs in supplying nutrients and hormones to tumor cells.135,136 OCT1 is downregulated by epigenetic mechanisms in HCC compared to nonmalignant tissues.137 The downregulation of OCT1 expression is clinically associated with tumor progression and poor outcomes in patients with HCC.137,138
Several studies indicate that the expression of canalicular and basolateral efflux transporters can be altered in HCC. Although BCRP mRNA was unchanged in a study by Borel et al.139, other studies showed a significant elevation of BCRP mRNA and protein expression in HCC compared to normal hepatocyte tissues.140,141 Interestingly, BCRP expression increased following chemotherapy in hepatoblastoma patients highlighting its potential role in drug resistance in HCC.140,142 On the other hand, BSEP expression was reported to be reduced secondary to inflammation-induced decreases in Farnesoid X receptor (FXR) 143. Although the role of BSEP in resistance to chemotherapy is unclear, many studies have shown that high bile acid levels in the liver contribute to HCC pathogenesis.143–148 In fact, children with BSEP deficiency demonstrated a higher risk of cholestasis and HCC.144,145 Collectively, these findings highlight the potential role of BSEP and altered bile acid homeostasis in HCC progression.143
P-gp expression in HCC showed divergent results at mRNA and protein levels in some in vitro and in vivo studies, which may be related to different detection methods as well as the tumor heterogeneity.139,149–153 P-gp protein expression appeared to be reduced or expressed less extensively in HCC tissues compared to normal hepatocytes.151–153 In regard to its function, P-gp expression was inversely correlated with chemotherapeutic response in patients with inoperable HCC. 152,154 MRP2 expression in HCC was unchanged compared to normal hepatocyte tissues.117,155,156 MRP1, which is not expressed in normal hepatocytes, is overexpressed at both the mRNA and protein levels in HCC.112,157,158 One study showed that a MRP1 promoter (-1666GG) polymorphism was a predictor of poor survival in patients with HCC from Southeast China 158. MRP3 and MRP4 mRNA levels were reported to be upregulated in HCC.112,139,159 Although several studies have noted the altered expression of drug transporters in HCC, further investigations are necessary to examine their clinical significance in HCC.
Human Immunodeficiency Virus (HIV) Infection
Similar to viral hepatitis, HIV is associated with chronic inflammation as evidenced by the presence of elevated proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α.160 The mRNA and protein expression of major sinusoidal uptake transporters (i.e., OATP1B1, OATP1B3, OATP2B1, OCT1, OCT2, and NTCP) were downregulated in human hepatocytes when incubated with TNF-α, which resulted in reduced functional activity.161 mRNA and protein levels of MRP2 were increased by IL-6 or IL-1β exposure in sandwich-cultured human hepatocytes whereas BSEP protein expression appeared to decrease.162 In another study, MRP2 and BCRP were reported to be downregulated in primary human hepatocytes after exposure to IL-6 or IL-1β; therefore, additional mechanistic studies are required to definitively conclude that canalicular transporters are upregulated or downregulated.161 Based on these data, it is conceivable that liver transporter expression and/or function may be impacted in HIV-infected patients because such signaling molecules can modulate transporters in vitro. It also must be noted that the administration of antiretroviral therapy is associated with a reduction in cytokine levels that may contribute to interindividual variability in hepatic transporter expression in HIV-infected patients.163
Despite the aforementioned data, the effect of HIV infection on the expression of transporters has not been evaluated systematically. In addition, coinfection (e.g., bacterial infection) could contribute to altered transporter regulation. For example, gene expression of hepatic Mrp2, Bcrp, and Oatps was downregulated in HIV-1 transgenic rats co-infected with endotoxin.164 P-gp and MRP2 protein levels in the rectal-sigmoid colon were lower in antiretroviral-naïve patients compared to non-infected subjects; whether similar expression patterns occur in the liver remains to be evaluated.165,166 Data quantifying hepatic transporter protein expression followed by subsequent functional studies are needed. Altered ADME processes in clearance organs (i.e., intestine, liver, and kidney) in combination with the potential for induction by co-medications (e.g., protease inhibitors) highlights the complexity of predicting transporter-mediated drug disposition in HIV-infected patients.167,168
Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH)
NAFLD consists of liver pathology ranging from simple steatosis to NASH, an advanced inflammatory state of liver disease. NAFLD has become the most common form of liver disease in Western and industrialized nations driven by the prevailing obesity epidemic; it is estimated that ~20–30% of the general population is affected, although some reports are as high as 50%.169–172 For reasons not fully known, some patients with NAFLD develop NASH, which may lead to HCC, cirrhosis, and/or fulminant liver failure. Due to the increasing prevalence of NAFLD and NASH, considerable research has been dedicated to quantifying how NASH affects ADME enzymes/transporters. These findings may have important implications in the treatment and disposition of drugs in this patient population.
Global gene expression analysis first implicated a coordinated downregulation of hepatic uptake transporters (e.g., OATPs) in patients with NASH.173 Further reports by Cherrington and colleagues revealed a significant increase in OATP1B1, but a decrease in OATP1B3, and no change in OATP2B1 in human liver biopsies using immunoblot techniques. Interestingly, no differences were observed in these transporters based on mRNA analysis.174 These findings are in contrast to data obtained from a commonly used diet-induced rodent model of NASH; mRNA and protein analyses were in agreement that rodent orthologues of human OATPs were decreased.175,176 These results support increased systemic concentrations of simvastatin acid, an OATP/Oatp substrate, in the rodent model of NASH compared to wild-type rats.175 NTCP, the primary hepatic bile acid transporter, is decreased in patients with NASH compared to patients with simple steatosis based on real-time reverse transcription polymerase chain reaction and immunoblot techniques; these data are consistent with a recent publication reporting that serum bile acids are increased in patients with NASH.177,178 Rat Ntcp and Oat2 also were shown to decrease in an experimental rodent model of NASH, presumably mediated through pro-inflammatory cytokines known to decrease hepatic transporter expression. Pharmacokinetic studies that quantify the functional impact of altered uptake transporter expression are limited in humans. However, our group recently reported increased systemic concentrations of 99mTc-mebrofenin, a hepatobiliary diagnostic agent and OATP, MRP2 and MRP3 substrate, in patients with biopsy-confirmed NASH.2
In what appears to be an adaptation to prevent further damage by toxicants, hepatic efflux transporters are increased in NASH. For example, MRP3-6 are induced in liver biopsies from NASH patients, similar to findings in a rodent model of NASH.176,180–182 Similarly, Mrp1 mRNA is increased ~5-fold compared to control animals.176 Functional drug disposition studies corroborate these reports as evidenced by increased plasma acetaminophen glucuronide concentrations in diet-induced NASH animals attributable to increased Mrp3 expression.181 Administration of morphine and acetaminophen to patients with NASH resulted in increased systemic concentrations of the glucuronide conjugates, consistent with increased function of MRP3 in this patient population.74,183 Although MRP2 is increased, a growing body of evidence supports a functional decrease in MRP2-mediated biliary excretion due to disruption of cellular trafficking and/or improper localization away from the canalicular membrane.74,180 This is supported by reports of reduced biliary excretion of acetaminophen glucuronide and pemetrexed in rats, consistent with impaired Mrp2 function.181,184 These findings corroborate enhanced hepatic retention and a prolonged half-life of the liver contrast agent gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) in NASH-induced rats.184 In contrast to Hardwick et al., who reported an increase in total MRP2 in human liver biopsies, a recent study reported decreased MRP2 protein in livers from NASH patients, which correlated inversely with progression of NAFLD.185,180 However, much of the increased MRP2 in this study was only partially glycosylated; complete glycosylation of MRP2 is required for plasma membrane trafficking.186 Decreased Mrp2 mRNA and protein expression were reported in a diet-induced rodent model of hypercholesterolemia.187 These apparent discrepancies may be due to species differences in composition of the bile acid pool, cytotoxicity of the predominant bile acid species, differences in regulation of Mrp2, or to the heterogeneous population of NASH patients and interindividual variability in MRP2 expression. Regardless, functional studies in humans are needed to evaluate the impact of altered MRP2 expression and/or localization. Although pharmacokinetic studies are limited, our group recently reported increased hepatic exposure of 99mTc-mebrofenin in patients with biopsy-confirmed NASH, which lends credence to the hypothesis that altered trafficking and localization of MRP2 result in a functional decrease in MRP2-mediated efflux.2 BCRP mRNA and protein expression were increased in NASH liver biopsies, but this finding was not observed in rodent models of NASH.180,188 Finally, rodent P-gp expression appears to be increased in NASH but protein expression has not been quantified.177,185,188
Primary Biliary Cholangitis
Primary biliary cholangitis (PBC) is a disease that causes bile duct inflammation and chronic damage over time. This leads to accumulation of bile acids and toxins that can cause cholestasis, fibrosis and cirrhosis. Hepatic transporters play an important role in the movement of solutes from the blood to the bile, and PBC may lead to alterations in these transport processes. The following section summarizes the literature studies that have examined the altered transporter expression in PBC. Several studies have reported that the mRNA and protein expression of NTCP, OATP1B1, and OATP1B3 are downregulated in PBC as compared to normal liver.189–192 These findings suggest the potential adaptation by hepatocytes to limit the accumulation of toxic bile acids.189
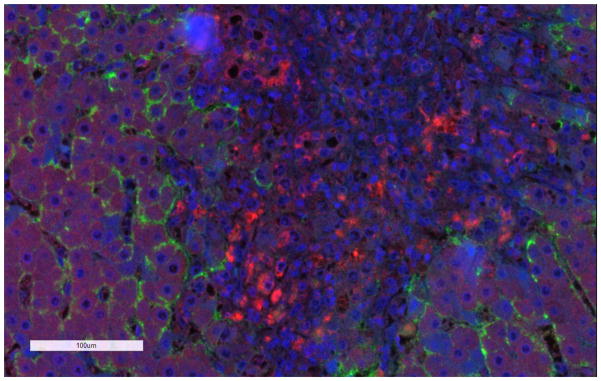
Studies performed using in vitro systems and human tissues confirmed that expression of efflux transporters was either maintained or tended to increase in PBC as compared to normal liver.189–191 One study reported that BSEP and MRP2 expression were unchanged whereas P-gp and MRP3 expression were upregulated in PBC.189 Interestingly, it was reported that these alterations may be related to the adaptation of transporters, and that expression patterns may change from early to late stage PBC.190,193 In addition to the changes in expression, the localization of MRP2 was altered in PBC stage III.191 MRP1 and OST α/β, which are not expressed or expressed at low levels in normal hepatocytes, are upregulated in patients with PBC.149,190,193,194 Consistent with the literature findings, data from our laboratory indicated that MRP1 protein was expressed in liver tissue from a patient with PBC (Figure 1). Interestingly, our findings suggest that MRP1 was expressed primarily in the inflamed tissues in macrophages around the hepatocytes in PBC. These findings are consistent with previous reports of MRP1 expression in macrophages.195 Further studies are required to better understand the functional role of MRP1 and OST α/β in PBC.
Figure 1. Immunofluorescence staining of MRP1 in a liver tissue section from a patient with primary biliary cirrhosis.
Dual staining of MRP1 (red) and Na+/K+ ATPase (green), which was used as a basolateral membrane marker. The nuclei are stained in blue. The general methods are described in Pomozi et al.201 MRP1 and Na+/K+ ATPase antibodies were purchased from Abcam and SantaCruz Biotechnology, respectively.
Summary and Perspective
Understanding the impact of liver disease on hepatic transporter function and the disposition of clinically-relevant drugs is one of the major challenges in treating liver disease. Genetic factors (e.g., single nucleotide polymorphisms, Dubin-Johnson syndrome, PFIC diseases, Rotor syndrome), bile acid accumulation, epigenetic and environmental factors (e.g., xenobiotics, toxins) also may play an important role in the etiology of liver diseases thereby affecting the expression of drug transporters. These features must be considered in order to accurately predict drug disposition and develop optimal drug dosage regimens. 196,197
Inflammation is prevalent among different forms of liver disease discussed in this review and appears to be a key regulator in liver disease-mediated alterations in drug transporter expression and function. Hence, evaluating the activation of Toll-like receptor signaling pathways in viral infections, for example, and other downstream proinflammatory cytokines (e.g., IL-1β, IL-6 and TNF-α) will be critical to understanding altered drug disposition in patients with various types of liver disease.198–200 The effects of inflammation on drug transporter function are likely to be disease specific and will require extensive knowledge regarding the mechanisms of immuno-stimulation, the varying degrees of inflammatory challenge, as well as patient-specific clinical factors.
The field of drug transport continues to evolve as more transporter-specific substrates and inhibitors are identified. However, clinically relevant probes are lacking due, in part, to the multiplicity of drug transporters (i.e., overlapping substrate specificity between transporters), the dynamic interplay between uptake, efflux, and metabolism processes, and the inability to quantify tissue-specific drug concentrations. Advances in magnetic resonance imaging (MRI) and positron emission tomography (PET), coupled with development of imaging probes that are substrates for specific transporters, will continue to facilitate the quantification of hepatic transporter function, particularly canalicular efflux transporters. These tools will improve our ability to assess transporter function in diseases afflicting the liver. Finally, the amalgamation of such functional data can serve as input for the development of pharmacokinetic modeling and systems pharmacology approaches to better understand and predict the disposition of drugs and endogenous substrates of hepatic transporters in patients with liver disease. This will improve our ability to select safe and effective drugs and design optimal dosage regimens for patients with liver disease.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under award number R01 GM041935 and R35 GM122576 (K.L.R.B).
This work was submitted to the Graduate School of the University of North Carolina at Chapel Hill in partial fulfillment of requirements for the Doctor of Philosophy degree in Pharmaceutical Sciences (J.R.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12(1):145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slizgi JR. Thesis. 2016. Hepatic Transporter Function in Liver Disease: Impact on Hepatobiliary Disposition and Pharmacokinetics. [Google Scholar]
- 3.Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest. 1994;93(3):1326–1331. doi: 10.1172/JCI117091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinman SA. Electrogenicity of Na(+)-coupled bile acid transporters. [Accessed February 8, 2017];Yale J Biol Med. 70(4):331–340. http://www.ncbi.nlm.nih.gov/pubmed/9626753. [PMC free article] [PubMed] [Google Scholar]
- 5.Hagenbuch B, Meier PJ. Sinusoidal (basolateral) bile salt uptake systems of hepatocytes. Semin Liver Dis. 1996;16(2):129–136. doi: 10.1055/s-2007-1007226. [DOI] [PubMed] [Google Scholar]
- 6.Vallejo M, Castro MA, Medarde M, et al. Novel bile acid derivatives (BANBs) with cytostatic activity obtained by conjugation of their side chain with nitrogenated bases. Biochem Pharmacol. 2007;73(9):1394–1404. doi: 10.1016/j.bcp.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Ho RH, Tirona RG, Leake BF, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: Function, expression, and pharmacogenetics. Gastroenterology. 2006;130(6):1793–1806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012:1. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watashi K, Urban S, Li W, Wakita T. NTCP and Beyond: Opening the door to unveil hepatitis B virus entry. Int J Mol Sci. 2014;15(2):2892–2905. doi: 10.3390/ijms15022892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleasby K, Castle JC, Roberts CJ, et al. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica. 2006;36(10–11):963–988. doi: 10.1080/00498250600861751. [DOI] [PubMed] [Google Scholar]
- 11.Simonson GD, Vincent AC, Roberg KJ, Huang Y, Iwanij V. Molecular cloning and characterization of a novel liver-specific transport protein. [Accessed February 9, 2017];J Cell Sci. 1994 Apr;:1065–1072. doi: 10.1242/jcs.107.4.1065. http://www.ncbi.nlm.nih.gov/pubmed/8056831. [DOI] [PubMed]
- 12.Burckhardt G, Burckhardt BC. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb Exp Pharmacol. 2011;(201):29–104. doi: 10.1007/978-3-642-14541-4_2. [DOI] [PubMed] [Google Scholar]
- 13.Van Aubel RA, Masereeuw R, Russel FG. Molecular pharmacology of renal organic anion transporters. [Accessed February 9, 2017];Am J Physiol Renal Physiol. 2000 279(2):F216–32. doi: 10.1152/ajprenal.2000.279.2.F216. http://www.ncbi.nlm.nih.gov/pubmed/10919840. [DOI] [PubMed] [Google Scholar]
- 14.Sweet DH, Pritchard JB. The molecular biology of renal organic anion and organic cation transporters. Cell Biochem Biophys. 1999;31(1):89–118. doi: 10.1007/BF02738157. [DOI] [PubMed] [Google Scholar]
- 15.Cropp CD, Komori T, Shima JE, et al. Organic Anion Transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol Pharmacol. 2008;73(4):1151–1158. doi: 10.1124/mol.107.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi Y, Ohshiro N, Shibusawa A, et al. Isolation, characterization and differential gene expression of multispecific organic anion transporter 2 in mice. [Accessed February 9, 2017];Mol Pharmacol. 2002 62(1):7–14. doi: 10.1124/mol.62.1.7. http://www.ncbi.nlm.nih.gov/pubmed/12065749. [DOI] [PubMed] [Google Scholar]
- 17.Sun W, Wu RR, van Poelje PD, Erion MD. Isolation of a Family of Organic Anion Transporters from Human Liver and Kidney. Biochem Biophys Res Commun. 2001;283(2):417–422. doi: 10.1006/bbrc.2001.4774. [DOI] [PubMed] [Google Scholar]
- 18.Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. [Accessed February 9, 2017];J Biol Chem. 1997 272(30):18526–18529. doi: 10.1074/jbc.272.30.18526. http://www.ncbi.nlm.nih.gov/pubmed/9228014. [DOI] [PubMed] [Google Scholar]
- 19.Enomoto A, Takeda M, Shimoda M, et al. Interaction of human organic anion transporters 2 and 4 with organic anion transport inhibitors. [Accessed February 9, 2017];J Pharmacol Exp Ther. 2002 301(3):797–802. doi: 10.1124/jpet.301.3.797. http://www.ncbi.nlm.nih.gov/pubmed/12023506. [DOI] [PubMed] [Google Scholar]
- 20.Shin HJ, Anzai N, Enomoto A, et al. Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology. 2007;45(4):1046–1055. doi: 10.1002/hep.21596. [DOI] [PubMed] [Google Scholar]
- 21.Emami Riedmaier A, Burk O, van Eijck BAC, et al. Variability in hepatic expression of organic anion transporter 7/SLC22A9, a novel pravastatin uptake transporter: impact of genetic and regulatory factors. Pharmacogenomics J. 2016;16(4):341–351. doi: 10.1038/tpj.2015.55. [DOI] [PubMed] [Google Scholar]
- 22.International Transporter Consortium KM. Giacomini KM, Huang S-M, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9(3):215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badée J, Achour B, Rostami-Hodjegan A, Galetin A. Meta-analysis of expression of hepatic organic anion-transporting polypeptide (OATP) transporters in cellular systems relative to human liver tissue. Drug Metab Dispos. 2015;43(4):424–432. doi: 10.1124/dmd.114.062034. [DOI] [PubMed] [Google Scholar]
- 24.Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica. 2008;38(7–8):778–801. doi: 10.1080/00498250801986951. [DOI] [PubMed] [Google Scholar]
- 25.Mikkaichi T, Suzuki T, Onogawa T, et al. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc Natl Acad Sci U S A. 2004;101(10):3569–3574. doi: 10.1073/pnas.0304987101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satlin LM, Amin V, Wolkoff AW. Organic anion transporting polypeptide mediates organic anion/HCO3- exchange. [Accessed February 9, 2017];J Biol Chem. 1997 272(42):26340–26345. doi: 10.1074/jbc.272.42.26340. http://www.ncbi.nlm.nih.gov/pubmed/9334206. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Lee TK, Meier PJ, Ballatori N. Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. [Accessed February 9, 2017];J Biol Chem. 1998 273(26):16184–16191. doi: 10.1074/jbc.273.26.16184. http://www.ncbi.nlm.nih.gov/pubmed/9632674. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Meier PJ, Ballatori N. Oatp2 mediates bidirectional organic solute transport: a role for intracellular glutathione. [Accessed February 9, 2017];Mol Pharmacol. 2000 58(2):335–340. doi: 10.1124/mol.58.2.335. http://www.ncbi.nlm.nih.gov/pubmed/10908301. [DOI] [PubMed] [Google Scholar]
- 29.Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158(3):693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.FDA. [Accessed May 19, 2017];Guidance for Industry Drug Interaction Studies — Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. 2012 Available at: https://www.fda.gov/downloads/drugs/guidances/ucm292362.pdf.
- 31.Gründemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994;372(6506):549–552. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- 32.Koepsell H, Schmitt BM, Gorboulev V. Organic cation transporters. Rev Physiol Biochem Pharmacol. 2003;150:36–90. doi: 10.1007/s10254-003-0017-x. [DOI] [PubMed] [Google Scholar]
- 33.Bourdet DL, Pritchard JB, Thakker DR. Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3) J Pharmacol Exp Ther. 2005;315(3):1288–1297. doi: 10.1124/jpet.105.091223. [DOI] [PubMed] [Google Scholar]
- 34.Han TK, Proctor WR, Costales CL, Cai H, Everett RS, Thakker DR. Four cation-selective transporters contribute to apical uptake and accumulation of metformin in Caco-2 cell monolayers. J Pharmacol Exp Ther. 2015;352(3):519–528. doi: 10.1124/jpet.114.220350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai H, Zhang Y, Han TK, Everett RS, Thakker DR. Cation-selective transporters are critical to the AMPK-mediated antiproliferative effects of metformin in human breast cancer cells. Int J cancer. 2016;138(9):2281–2292. doi: 10.1002/ijc.29965. [DOI] [PubMed] [Google Scholar]
- 36.Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22(47):7340–7358. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 37.Litman T, Druley TE, Stein WD, Bates SE. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci. 2001;58(7):931–959. doi: 10.1007/PL00000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Liu Y, Yang Y, Bates S, Zhang J-T. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem. 2004;279(19):19781–19789. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 39.Kawabata S, Oka M, Shiozawa K, et al. Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells. Biochem Biophys Res Commun. 2001;280(5):1216–1223. doi: 10.1006/bbrc.2001.4267. [DOI] [PubMed] [Google Scholar]
- 40.Maliepaard M, van Gastelen MA, de Jong LA, et al. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. [Accessed February 9, 2017];Cancer Res. 1999 59(18):4559–4563. http://www.ncbi.nlm.nih.gov/pubmed/10493507. [PubMed] [Google Scholar]
- 41.Nakagawa M, Schneider E, Dixon KH, et al. Reduced intracellular drug accumulation in the absence of P-glycoprotein (mdr1) overexpression in mitoxantrone-resistant human MCF-7 breast cancer cells. [Accessed February 9, 2017];Cancer Res. 1992 52(22):6175–6181. http://www.ncbi.nlm.nih.gov/pubmed/1358431. [PubMed] [Google Scholar]
- 42.Pauli-Magnus C, Meier PJ. Hepatobiliary transporters and drug-induced cholestasis. Hepatology. 2006;44(4):778–787. doi: 10.1002/hep.21359. [DOI] [PubMed] [Google Scholar]
- 43.Zamek-Gliszczynski MJ, Hoffmaster KA, Nezasa K, Tallman MN, Brouwer KLR. Integration of hepatic drug transporters and phase II metabolizing enzymes: mechanisms of hepatic excretion of sulfate, glucuronide, and glutathione metabolites. Eur J Pharm Sci. 2006;27(5):447–486. doi: 10.1016/j.ejps.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Hirano M, Maeda K, Hayashi H, Kusuhara H, Sugiyama Y. Bile salt export pump (BSEP/ABCB11) can transport a nonbile acid substrate, pravastatin. J Pharmacol Exp Ther. 2005;314(2):876–882. doi: 10.1124/jpet.105.084830. [DOI] [PubMed] [Google Scholar]
- 45.Gerloff T, Stieger B, Hagenbuch B, et al. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. [Accessed February 9, 2017];J Biol Chem. 1998 273(16):10046–10050. doi: 10.1074/jbc.273.16.10046. http://www.ncbi.nlm.nih.gov/pubmed/9545351. [DOI] [PubMed] [Google Scholar]
- 46.Carlton VEH, Harris BZ, Puffenberger EG, et al. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet. 2003;34(1):91–96. doi: 10.1038/ng1147. [DOI] [PubMed] [Google Scholar]
- 47.Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. [Accessed February 9, 2017];Gastroenterology. 2002 123(5):1649–1658. doi: 10.1053/gast.2002.36591. http://www.ncbi.nlm.nih.gov/pubmed/12404239. [DOI] [PubMed] [Google Scholar]
- 48.Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci. 2005;102(50):17923–17928. doi: 10.1073/pnas.0506483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011;(201):105–167. doi: 10.1007/978-3-642-14541-4_3. [DOI] [PubMed] [Google Scholar]
- 50.Keppler D, Kartenbeck J. The canalicular conjugate export pump encoded by the cmrp/cmoat gene. [Accessed February 9, 2017];Prog Liver Dis. 1996 14:55–67. http://www.ncbi.nlm.nih.gov/pubmed/9055574. [PubMed] [Google Scholar]
- 51.Schaub TP, Kartenbeck J, König J, et al. Expression of the MRP2 gene-encoded conjugate export pump in human kidney proximal tubules and in renal cell carcinoma. [Accessed February 9, 2017];J Am Soc Nephrol. 1999 10(6):1159–1169. doi: 10.1681/ASN.V1061159. http://www.ncbi.nlm.nih.gov/pubmed/10361853. [DOI] [PubMed] [Google Scholar]
- 52.Sandusky GE, Mintze KS, Pratt SE, Dantzig AH. Expression of multidrug resistance-associated protein 2 (MRP2) in normal human tissues and carcinomas using tissue microarrays. [Accessed February 9, 2017];Histopathology. 2002 41(1):65–74. doi: 10.1046/j.1365-2559.2002.01403.x. http://www.ncbi.nlm.nih.gov/pubmed/12121239. [DOI] [PubMed] [Google Scholar]
- 53.Meyer zu Schwabedissen HE, Jedlitschky G, Gratz M, et al. Variable expression of MRP2 (ABCC2) in human placenta: influence of gestational age and cellular differentiation. Drug Metab Dispos. 2005;33(7):896–904. doi: 10.1124/dmd.104.003335. [DOI] [PubMed] [Google Scholar]
- 54.Fahrmayr C, König J, Auge D, Mieth M, Fromm MF. Identification of drugs and drug metabolites as substrates of multidrug resistance protein 2 (MRP2) using triple-transfected MDCK-OATP1B1-UGT1A1-MRP2 cells. Br J Pharmacol. 2012;165(6):1836–1847. doi: 10.1111/j.1476-5381.2011.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Büchler M, König J, Brom M, et al. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMrp, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. [Accessed February 9, 2017];J Biol Chem. 1996 271(25):15091–15098. doi: 10.1074/jbc.271.25.15091. http://www.ncbi.nlm.nih.gov/pubmed/8662992. [DOI] [PubMed] [Google Scholar]
- 56.Jemnitz K, Heredi-Szabo K, Janossy J, Ioja E, Vereczkey L, Krajcsi P. ABCC2/Abcc2: a multispecific transporter with dominant excretory functions. Drug Metab Rev. 2010;42(3):402–436. doi: 10.3109/03602530903491741. [DOI] [PubMed] [Google Scholar]
- 57.Keppler D. The roles of MRP2, MRP3, OATP1B1, and OATP1B3 in conjugated hyperbilirubinemia. Drug Metab Dispos. 2014;42(4):561–565. doi: 10.1124/dmd.113.055772. [DOI] [PubMed] [Google Scholar]
- 58.Oswald S, Westrup S, Grube M, Kroemer HK, Weitschies W, Siegmund W. Disposition and sterol-lowering effect of ezetimibe in multidrug resistance-associated protein 2-deficient rats. J Pharmacol Exp Ther. 2006;318(3):1293–1299. doi: 10.1124/jpet.106.104018. [DOI] [PubMed] [Google Scholar]
- 59.Loo TW, Clarke DM. Membrane topology of a cysteine-less mutant of human P-glycoprotein. [Accessed February 9, 2017];J Biol Chem. 1995 270(2):843–848. doi: 10.1074/jbc.270.2.843. http://www.ncbi.nlm.nih.gov/pubmed/7822320. [DOI] [PubMed] [Google Scholar]
- 60.Cordon-Cardo C, O’Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. [Accessed February 9, 2017];J Histochem Cytochem. 1990 38(9):1277–1287. doi: 10.1177/38.9.1974900. http://www.ncbi.nlm.nih.gov/pubmed/1974900. [DOI] [PubMed] [Google Scholar]
- 61.Mechetner E, Kyshtoobayeva A, Zonis S, et al. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. [Accessed February 9, 2017];Clin Cancer Res. 1998 4(2):389–398. http://www.ncbi.nlm.nih.gov/pubmed/9516927. [PubMed] [Google Scholar]
- 62.Vredenburg MR, Ojima I, Veith J, et al. Effects of orally active taxanes on P-glycoprotein modulation and colon and breast carcinoma drug resistance. [Accessed February 9, 2017];J Natl Cancer Inst. 2001 93(16):1234–1245. doi: 10.1093/jnci/93.16.1234. http://www.ncbi.nlm.nih.gov/pubmed/11504769. [DOI] [PubMed] [Google Scholar]
- 63.Amin ML. P-glycoprotein Inhibition for Optimal Drug Delivery. Drug Target Insights. 2013;7:27–34. doi: 10.4137/DTI.S12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou S-F, Wang L-L, Di YM, et al. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. [Accessed February 9, 2017];Curr Med Chem. 2008 15(20):1981–2039. doi: 10.2174/092986708785132870. http://www.ncbi.nlm.nih.gov/pubmed/18691054. [DOI] [PubMed] [Google Scholar]
- 65.Keppler D. Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. Handb Exp Pharmacol. 2011;(201):299–323. doi: 10.1007/978-3-642-14541-4_8. [DOI] [PubMed] [Google Scholar]
- 66.Slot AJ, Molinski SV, Cole SPC. Mammalian multidrug-resistance proteins (MRPs) Essays Biochem. 2011;50(1):179–207. doi: 10.1042/bse0500179. [DOI] [PubMed] [Google Scholar]
- 67.Deeley RG, Cole SPC. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1) FEBS Lett. 2006;580(4):1103–1111. doi: 10.1016/j.febslet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 68.Stride BD, Grant CE, Loe DW, Hipfner DR, Cole SP, Deeley RG. Pharmacological characterization of the murine and human orthologs of multidrug-resistance protein in transfected human embryonic kidney cells. [Accessed February 9, 2017];Mol Pharmacol. 1997 52(3):344–353. doi: 10.1124/mol.52.3.344. http://www.ncbi.nlm.nih.gov/pubmed/9281595. [DOI] [PubMed] [Google Scholar]
- 69.Scheffer GL, Kool M, de Haas M, et al. Tissue distribution and induction of human multidrug resistant protein 3. [Accessed February 9, 2017];Lab Invest. 2002 82(2):193–201. doi: 10.1038/labinvest.3780411. http://www.ncbi.nlm.nih.gov/pubmed/11850532. [DOI] [PubMed] [Google Scholar]
- 70.Chai J, He Y, Cai S-Y, et al. Elevated hepatic MRP3/ABCC3 expression in human obstructive cholestasis is mediated through TNFα and JNK/SAPK signaling pathway. Hepatology. 2012;55(5):1485–1494. doi: 10.1002/hep.24801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donner MG, Keppler D. Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology. 2001;34(2):351–359. doi: 10.1053/jhep.2001.26213. [DOI] [PubMed] [Google Scholar]
- 72.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71(1):537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 73.Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3) [Accessed February 9, 2017];J Biol Chem. 2000 275(4):2905–2910. doi: 10.1074/jbc.275.4.2905. http://www.ncbi.nlm.nih.gov/pubmed/10644759. [DOI] [PubMed] [Google Scholar]
- 74.Canet MJ, Merrell MD, Hardwick RN, et al. Altered regulation of hepatic efflux transporters disrupts acetaminophen disposition in pediatric nonalcoholic steatohepatitis. Drug Metab Dispos. 2015;43(6):829–835. doi: 10.1124/dmd.114.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeng H, Chen ZS, Belinsky MG, Rea PA, Kruh GD. Transport of methotrexate (MTX) and folates by multidrug resistance protein (MRP) 3 and MRP1: effect of polyglutamylation on MTX transport. [Accessed February 9, 2017];Cancer Res. 2001 61(19):7225–7232. http://www.ncbi.nlm.nih.gov/pubmed/11585759. [PubMed] [Google Scholar]
- 76.Tomonari T, Takeishi S, Taniguchi T, et al. MRP3 as a novel resistance factor for sorafenib in hepatocellular carcinoma. Oncotarget. 2016;7(6):7207–7215. doi: 10.18632/oncotarget.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiong H, Suzuki H, Sugiyama Y, Meier PJ, Pollack GM, Brouwer KLR. Mechanisms of impaired biliary excretion of acetaminophen glucuronide after acute phenobarbital treatment or phenobarbital pretreatment. [Accessed February 9, 2017];Drug Metab Dispos. 2002 30(9):962–969. doi: 10.1124/dmd.30.9.962. http://www.ncbi.nlm.nih.gov/pubmed/12167560. [DOI] [PubMed] [Google Scholar]
- 78.Zelcer N, Reid G, Wielinga P, et al. Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4) Biochem J. 2003;371(Pt 2):361–367. doi: 10.1042/BJ20021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gradhand U, Lang T, Schaeffeler E, et al. Variability in human hepatic MRP4 expression: influence of cholestasis and genotype. Pharmacogenomics J. 2008;8(1):42–52. doi: 10.1038/sj.tpj.6500451. [DOI] [PubMed] [Google Scholar]
- 80.Mennone A, Soroka CJ, Cai S-Y, et al. Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43(5):1013–1021. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- 81.Clayton RJ, Iber FL, Ruebner BH, McKusick VA. Byler disease. Fatal familial intrahepatic cholestasis in an Amish kindred. [Accessed February 9, 2017];Am J Dis Child. 1969 117(1):112–124. http://www.ncbi.nlm.nih.gov/pubmed/5762004. [PubMed] [Google Scholar]
- 82.Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2012;36(Suppl 1):S26–35. doi: 10.1016/S2210-7401(12)70018-9. [DOI] [PubMed] [Google Scholar]
- 83.Keitel V, Burdelski M, Warskulat U, et al. Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology. 2005;41(5):1160–1172. doi: 10.1002/hep.20682. [DOI] [PubMed] [Google Scholar]
- 84.Rook M, Vargas J, Caughey A, Bacchetti P, Rosenthal P, Bull L. Fetal outcomes in pregnancies complicated by intrahepatic cholestasis of pregnancy in a Northern California cohort. In: Middleton P, editor. PLoS One. 3. Vol. 7. 2012. p. e28343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song X, Vasilenko A, Chen Y, et al. Transcriptional dynamics of bile salt export pump during pregnancy: mechanisms and implications in intrahepatic cholestasis of pregnancy. Hepatology. 2014;60(6):1993–2007. doi: 10.1002/hep.27171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y, Vasilenko A, Song X, et al. Estrogen and estrogen receptor-α-mediated transrepression of bile salt export pump. Mol Endocrinol. 2015;29(4):613–626. doi: 10.1210/me.2015-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mottino AD, Cao J, Veggi LM, Crocenzi F, Roma MG, Vore M. Altered localization and activity of canalicular Mrp2 in estradiol-17beta-D-glucuronide-induced cholestasis. Hepatology. 2002;35(6):1409–1419. doi: 10.1053/jhep.2002.33327. [DOI] [PubMed] [Google Scholar]
- 88.Trauner M, Arrese M, Soroka CJ, et al. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. [Accessed February 9, 2017];Gastroenterology. 1997 113(1):255–264. doi: 10.1016/s0016-5085(97)70103-3. http://www.ncbi.nlm.nih.gov/pubmed/9207286. [DOI] [PubMed] [Google Scholar]
- 89.Geier A, Dietrich CG, Gerloff T, et al. Regulation of basolateral organic anion transporters in ethinylestradiol-induced cholestasis in the rat. [Accessed February 9, 2017];Biochim Biophys Acta. 2003 1609(1):87–94. doi: 10.1016/s0005-2736(02)00657-0. http://www.ncbi.nlm.nih.gov/pubmed/12507762. [DOI] [PubMed] [Google Scholar]
- 90.Simon FR, Fortune J, Iwahashi M, Gartung C, Wolkoff A, Sutherland E. Ethinyl estradiol cholestasis involves alterations in expression of liver sinusoidal transporters. [Accessed February 9, 2017];Am J Physiol. 1996 271(6 Pt 1):G1043–52. doi: 10.1152/ajpgi.1996.271.6.G1043. http://www.ncbi.nlm.nih.gov/pubmed/8997249. [DOI] [PubMed] [Google Scholar]
- 91.Wang H, Yan Z, Dong M, Zhu X, Wang H, Wang Z. Alteration in placental expression of bile acids transporters OATP1A2, OATP1B1, OATP1B3 in intrahepatic cholestasis of pregnancy. Arch Gynecol Obstet. 2012;285(6):1535–1540. doi: 10.1007/s00404-011-2183-4. [DOI] [PubMed] [Google Scholar]
- 92.Zollner G, Fickert P, Zenz R, et al. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology. 2001;33(3):633–646. doi: 10.1053/jhep.2001.22646. [DOI] [PubMed] [Google Scholar]
- 93.Paulusma CC, Kothe MJ, Bakker CT, et al. Zonal down-regulation and redistribution of the multidrug resistance protein 2 during bile duct ligation in rat liver. Hepatology. 2000;31(3):684–693. doi: 10.1002/hep.510310319. [DOI] [PubMed] [Google Scholar]
- 94.Kojima H, Sakurai S, Uemura M, et al. Disturbed colocalization of multidrug resistance protein 2 and radixin in human cholestatic liver diseases. J Gastroenterol Hepatol. 2008;23(7 Pt 2):e120–8. doi: 10.1111/j.1440-1746.2007.05109.x. [DOI] [PubMed] [Google Scholar]
- 95.He X-J, Wang W-R, Zhang Y, Yang Q. The effect of radixin knockdown on the expression and efflux function of MRP2 in SGC-7901 cells. Eur J Pharm Sci. 2012;46(5):426–434. doi: 10.1016/j.ejps.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Elferink RPJO, Paulusma CC. MRP2 in cholestasis: Putting down the anchor. J Hepatol. 2015;63(6):1309–1310. doi: 10.1016/j.jhep.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 97.Guo C, He L, Yao D, et al. Alpha-naphthylisothiocyanate modulates hepatobiliary transporters in sandwich-cultured rat hepatocytes. Toxicol Lett. 2014;224(1):93–100. doi: 10.1016/j.toxlet.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 98.Hasegawa Y, Kishimoto S, Takahashi H, Inotsume N, Takeuchi Y, Fukushima S. Altered expression of MRP2, MRP3 and UGT2B1 in the liver affects the disposition of morphine and its glucuronide conjugate in a rat model of cholestasis. J Pharm Pharmacol. 2009;61(9):1205–1210. doi: 10.1211/jpp/61.09.0010. [DOI] [PubMed] [Google Scholar]
- 99.Jin H-E, Hong S-S, Choi M-K, et al. Reduced antidiabetic effect of metformin and down-regulation of hepatic Oct1 in rats with ethynylestradiol-induced cholestasis. Pharm Res. 2009;26(3):549–559. doi: 10.1007/s11095-008-9770-5. [DOI] [PubMed] [Google Scholar]
- 100.Choi YH, Lee YK, Lee MG. Effects of 17α-ethynylestradiol-induced cholestasis on the pharmacokinetics of doxorubicin in rats: reduced biliary excretion and hepatic metabolism of doxorubicin. Xenobiotica. 2013;43(10):901–907. doi: 10.3109/00498254.2013.783250. [DOI] [PubMed] [Google Scholar]
- 101.Harrison LI, Gibaldi M. Influence of cholestasis on drug elimination: pharmacokinetics. [Accessed February 9, 2017];J Pharm Sci. 1976 65(9):1346–1348. doi: 10.1002/jps.2600650921. http://www.ncbi.nlm.nih.gov/pubmed/966151. [DOI] [PubMed] [Google Scholar]
- 102.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45–57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 103.Kurzawski M, Dziedziejko V, Post M, et al. Expression of genes involved in xenobiotic metabolism and transport in end-stage liver disease: up-regulation of ABCC4 and CYP1B1. [Accessed February 9, 2017];Pharmacol Rep. 2012 64(4):927–939. doi: 10.1016/s1734-1140(12)70888-5. http://www.ncbi.nlm.nih.gov/pubmed/23087145. [DOI] [PubMed] [Google Scholar]
- 104.Chu C-J, Lee S-D. Hepatitis B virus/hepatitis C virus coinfection: epidemiology, clinical features, viral interactions and treatment. J Gastroenterol Hepatol. 2008;23(4):512–520. doi: 10.1111/j.1440-1746.2008.05384.x. [DOI] [PubMed] [Google Scholar]
- 105.Congiu M, Mashford ML, Slavin JL, Desmond PV. Coordinate regulation of metabolic enzymes and transporters by nuclear transcription factors in human liver disease. J Gastroenterol Hepatol. 2009;24(6):1038–1044. doi: 10.1111/j.1440-1746.2009.05800.x. [DOI] [PubMed] [Google Scholar]
- 106.Tsutsumi T, Suzuki T, Shimoike T, et al. Interaction of hepatitis C virus core protein with retinoid X receptor alpha modulates its transcriptional activity. Hepatology. 2002;35(4):937–946. doi: 10.1053/jhep.2002.32470. [DOI] [PubMed] [Google Scholar]
- 107.Clément S, Pascarella S, Negro F. Hepatitis C virus infection: Molecular pathways to steatosis, insulin resistance and oxidative stress. Viruses. 2009;1(2):126–143. doi: 10.3390/v1020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakai K, Tanaka H, Hanada K, et al. Decreased expression of cytochromes P450 1A2, 2E1, and 3A4 and drug transporters Na+-taurocholate-cotransporting polypeptide, organic cation transporter 1, and organic anion-transporting peptide-C correlates with the progression of liver fibrosis in chro…. Drug Metab Dispos. 2008;36(9) doi: 10.1124/dmd.107.020073. [DOI] [PubMed] [Google Scholar]
- 109.Hanada K, Nakai K, Tanaka H, et al. Effect of nuclear receptor downregulation on hepatic expression of cytochrome P450 and transporters in chronic hepatitis C in association with fibrosis development. [Accessed February 9, 2017];Drug Metab Pharmacokinet. 2012 27(3):301–306. doi: 10.2133/dmpk.dmpk-11-rg-077. http://www.ncbi.nlm.nih.gov/pubmed/22166890. [DOI] [PubMed] [Google Scholar]
- 110.Wang L, Collins C, Kelly EJ, et al. Transporter expression in liver tissue from subjects with alcoholic or hepatitis C cirrhosis quantified by targeted quantitative proteomics. Drug Metab Dispos. 2016;44(11):1752–1758. doi: 10.1124/dmd.116.071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ogasawara K, Terada T, Katsura T, et al. Hepatitis C virus-related cirrhosis is a major determinant of the expression levels of hepatic drug transporters. [Accessed February 9, 2017];Drug Metab Pharmacokinet. 2010 25(2):190–199. doi: 10.2133/dmpk.25.190. http://www.ncbi.nlm.nih.gov/pubmed/20460825. [DOI] [PubMed] [Google Scholar]
- 112.Ros JE, Libbrecht L, Geuken M, Jansen PLM, Roskams TAD. High expression of MDR1, MRP1, and MRP3 in the hepatic progenitor cell compartment and hepatocytes in severe human liver disease. J Pathol. 2003;200(5):553–560. doi: 10.1002/path.1379. [DOI] [PubMed] [Google Scholar]
- 113.Zollner G, Wagner M, Fickert P, et al. Expression of bile acid synthesis and detoxification enzymes and the alternative bile acid efflux pump MRP4 in patients with primary biliary cirrhosis. Liver Int. 2007;27(7):920–929. doi: 10.1111/j.1478-3231.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 114.Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol. 2010;244(1):57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Regazzi M, Maserati R, Villani P, et al. Clinical pharmacokinetics of nelfinavir and its metabolite M8 in human immunodeficiency virus (HIV)-positive and HIV-hepatitis C virus-coinfected subjects. Antimicrob Agents Chemother. 2005;49(2):643–649. doi: 10.1128/AAC.49.2.643-649.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kula M, Karacavus S, Baskol M, Deniz K, Abdulrezzak U, Tutus A. Hepatobiliary function assessed by 99mTc-mebrofenin cholescintigraphy in the evaluation of fibrosis in chronic hepatitis: histopathological correlation. Nucl Med Commun. 2010;31(4):280–285. doi: 10.1097/MNM.0b013e328334bff7. [DOI] [PubMed] [Google Scholar]
- 117.Zollner G, Wagner M, Fickert P, et al. Hepatobiliary transporter expression in human hepatocellular carcinoma. Liver Int. 2005;25(2):367–379. doi: 10.1111/j.1478-3231.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 118.Briz O, Serrano MA, Rebollo N, et al. Carriers involved in targeting the cytostatic bile acid-cisplatin derivatives cis-diammine-chloro-cholylglycinate-platinum(II) and cis-diammine-bisursodeoxycholate-platinum(II) toward liver cells. [Accessed August 16, 2016];Mol Pharmacol. 2002 61(4):853–860. doi: 10.1124/mol.61.4.853. http://www.ncbi.nlm.nih.gov/pubmed/11901224. [DOI] [PubMed] [Google Scholar]
- 119.Kramer W, Wess G, Schubert G, et al. Liver-specific drug targeting by coupling to bile acids. [Accessed August 16, 2016];J Biol Chem. 1992 267(26):18598–18604. http://www.ncbi.nlm.nih.gov/pubmed/1526993. [PubMed] [Google Scholar]
- 120.Thakkar N, Lockhart AC, Lee W. Role of Organic Anion-Transporting Polypeptides (OATPs) in cancer therapy. AAPS J. 2015;17(3):535–545. doi: 10.1208/s12248-015-9740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vavricka SR, Jung D, Fried M, Grützner U, Meier PJ, Kullak-Ublick GA. The human organic anion transporting polypeptide 8 (SLCO1B3) gene is transcriptionally repressed by hepatocyte nuclear factor 3beta in hepatocellular carcinoma. [Accessed August 16, 2016];J Hepatol. 2004 40(2):212–218. doi: 10.1016/j.jhep.2003.10.008. http://www.ncbi.nlm.nih.gov/pubmed/14739090. [DOI] [PubMed] [Google Scholar]
- 122.Han S, Kim K, Thakkar N, Kim D, Lee W. Role of hypoxia inducible factor-1?? in the regulation of the cancer-specific variant of organic anion transporting polypeptide 1B3 (OATP1B3), in colon and pancreatic cancer. Biochem Pharmacol. 2013;86(6):816–823. doi: 10.1016/j.bcp.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 123.Abe T, Unno M, Onogawa T, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. [Accessed August 16, 2016];Gastroenterology. 2001 120(7):1689–1699. doi: 10.1053/gast.2001.24804. http://www.ncbi.nlm.nih.gov/pubmed/11375950. [DOI] [PubMed] [Google Scholar]
- 124.Muto M, Onogawa T, Suzuki T, et al. Human liver-specific organic anion transporter-2 is a potent prognostic factor for human breast carcinoma. Cancer Sci. 2007;98(10):1570–1576. doi: 10.1111/j.1349-7006.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thakkar N, Kim K, Jang ER, et al. A cancer-specific variant of the slco1b3 gene encodes a novel human organic anion transporting polypeptide 1B3 (OATP1B3) localized mainly in the cytoplasm of colon and pancreatic cancer cells. Mol Pharm. 2013;10(1):406–416. doi: 10.1021/mp3005353. [DOI] [PubMed] [Google Scholar]
- 126.Kounnis V, Ioachim E, Svoboda M, et al. Expression of organic anion-transporting polypeptides 1B3, 1B1, and 1A2 in human pancreatic cancer reveals a new class of potential therapeutic targets. Onco Targets Ther. 2011;4:27–32. doi: 10.2147/OTT.S16706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hamada A, Sissung T, Price DK, et al. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008;14(11):3312–3318. doi: 10.1158/1078-0432.CCR-07-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee W, Belkhiri A, Lockhart AC, et al. Overexpression of OATP1B3 confers apoptotic resistance in colon cancer. Cancer Res. 2008;68(24):10315–10323. doi: 10.1158/0008-5472.CAN-08-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cui Y, König J, Nies AT, et al. Detection of the human organic anion transporters SLC21A6 (OATP2) and SLC21A8 (OATP8) in liver and hepatocellular carcinoma. [Accessed August 16, 2016];Lab Invest. 2003 83(4):527–538. doi: 10.1097/01.lab.0000065015.02412.48. http://www.ncbi.nlm.nih.gov/pubmed/12695556. [DOI] [PubMed] [Google Scholar]
- 130.Monks NR, Liu S, Xu Y, Yu H, Bendelow AS, Moscow JA. Potent cytotoxicity of the phosphatase inhibitor microcystin LR and microcystin analogues in OATP1B1- and OATP1B3-expressing HeLa cells. Mol Cancer Ther. 2007;6(2):587–598. doi: 10.1158/1535-7163.MCT-06-0500. [DOI] [PubMed] [Google Scholar]
- 131.Libra A, Fernetti C, Lorusso V, et al. Molecular determinants in the transport of a bile acid-derived diagnostic agent in tumoral and nontumoral cell lines of human liver. J Pharmacol Exp Ther. 2006;319(2):809–817. doi: 10.1124/jpet.106.106591. [DOI] [PubMed] [Google Scholar]
- 132.Vander Borght S, Libbrecht L, Blokzijl H, et al. Diagnostic and pathogenetic implications of the expression of hepatic transporters in focal lesions occurring in normal liver. J Pathol. 2005;207(4):471–482. doi: 10.1002/path.1852. [DOI] [PubMed] [Google Scholar]
- 133.Tsuboyama T, Onishi H, Kim T, et al. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging--correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology. 2010;255(3):824–833. doi: 10.1148/radiol.10091557. [DOI] [PubMed] [Google Scholar]
- 134.Lockhart AC, Harris E, Lafleur BJ, et al. Organic anion transporting polypeptide 1B3 (OATP1B3) is overexpressed in colorectal tumors and is a predictor of clinical outcome. [Accessed August 16, 2016];Clin Exp Gastroenterol. 2008 1:1–7. doi: 10.2147/ceg.s3743. http://www.ncbi.nlm.nih.gov/pubmed/21677819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Buxhofer-Ausch V, Secky L, Wlcek K, et al. Tumor-specific expression of organic anion-transporting polypeptides: transporters as novel targets for cancer therapy. J Drug Deliv. 2013;2013:863539. doi: 10.1155/2013/863539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wlcek K, Svoboda M, Riha J, et al. The analysis of organic anion transporting polypeptide (OATP) mRNA and protein patterns in primary and metastatic liver cancer. [Accessed August 16, 2016];Cancer Biol Ther. 2011 11(9):801–811. doi: 10.4161/cbt.11.9.15176. http://www.ncbi.nlm.nih.gov/pubmed/21383546. [DOI] [PubMed] [Google Scholar]
- 137.Schaeffeler E, Hellerbrand C, Nies AT, et al. DNA methylation is associated with downregulation of the organic cation transporter OCT1 (SLC22A1) in human hepatocellular carcinoma. Genome Med. 2011;3(12):82. doi: 10.1186/gm298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heise M, Lautem A, Knapstein J, et al. Downregulation of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) in human hepatocellular carcinoma and their prognostic significance. BMC Cancer. 2012;12:109. doi: 10.1186/1471-2407-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borel F, Han R, Visser A, et al. Adenosine triphosphate-binding cassette transporter genes up-regulation in untreated hepatocellular carcinoma is mediated by cellular microRNAs. Hepatology. 2012;55(3):821–832. doi: 10.1002/hep.24682. [DOI] [PubMed] [Google Scholar]
- 140.Sukowati CH, Rosso N, Pascut D, et al. Gene and functional up-regulation of the BCRP/ABCG2 transporter in hepatocellular carcinoma. BMC Gastroenterol. 2012;12(1):160. doi: 10.1186/1471-230X-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun Z, Zhao Z, Li G, et al. Relevance of two genes in the multidrug resistance of hepatocellular carcinoma: in vivo and clinical studies. [Accessed August 14, 2016];Tumori. 96(1):90–96. doi: 10.1177/030089161009600115. http://www.ncbi.nlm.nih.gov/pubmed/20437864. [DOI] [PubMed] [Google Scholar]
- 142.Vander Borght S, van Pelt J, van Malenstein H, et al. Up-regulation of breast cancer resistance protein expression in hepatoblastoma following chemotherapy: A study in patients and in vitro. Hepatol Res. 2008;38(11):1112–1121. doi: 10.1111/j.1872-034X.2008.00381.x. [DOI] [PubMed] [Google Scholar]
- 143.Chen Y, Song X, Valanejad L, et al. Bile salt export pump is dysregulated with altered farnesoid X receptor isoform expression in patients with hepatocellular carcinoma. Hepatology. 2013;57(4):1530–1541. doi: 10.1002/hep.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Knisely AS, Strautnieks SS, Meier Y, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44(2):478–486. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- 145.Strautnieks SS, Byrne JA, Pawlikowska L, et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 2008;134(4):1203–1214. doi: 10.1053/j.gastro.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 146.Jansen PLM. Endogenous bile acids as carcinogens. J Hepatol. 2007;47(3):434–435. doi: 10.1016/j.jhep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 147.Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. [Accessed August 15, 2016];World J Gastroenterol. 2009 15(27):3329–3340. doi: 10.3748/wjg.15.3329. http://www.ncbi.nlm.nih.gov/pubmed/19610133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gadaleta RM, van Mil SWC, Oldenburg B, Siersema PD, Klomp LWJ, van Erpecum KJ. Bile acids and their nuclear receptor FXR: Relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta. 2010;1801(7):683–692. doi: 10.1016/j.bbalip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 149.Ros JE, Libbrecht L, Geuken M, Jansen PLM, Roskams TAD. High expression of MDR1, MRP1, and MRP3 in the hepatic progenitor cell compartment and hepatocytes in severe human liver disease. J Pathol. 2003;200(5):553–560. doi: 10.1002/path.1379. [DOI] [PubMed] [Google Scholar]
- 150.Teeter L, Hsu H, Curley S, Tong M, Kuo M. Expression of multidrug resistance (p-glycoprotein) mdr1 and mdr2 genes in human hepatocellular carcinomas and liver metastases of colonic tumors. [Accessed August 14, 2016];Int J Oncol. 1993 2(1):73–80. http://www.ncbi.nlm.nih.gov/pubmed/21573517. [PubMed] [Google Scholar]
- 151.Zollner G, Wagner M, Fickert P, et al. Hepatobiliary transporter expression in human hepatocellular carcinoma. Liver Int. 2005;25(2):367–379. doi: 10.1111/j.1478-3231.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 152.Ng IO, Liu CL, Fan ST, Ng M. Expression of P-glycoprotein in hepatocellular carcinoma. A determinant of chemotherapy response. Am J Clin Pathol. 2000;113(3):355–363. doi: 10.1309/AC1M-4TY4-U0TN-EN7T. [DOI] [PubMed] [Google Scholar]
- 153.Takanishi K, Miyazaki M, Ohtsuka M, Nakajima N. Inverse relationship between P-glycoprotein expression and its proliferative activity in hepatocellular carcinoma. Oncology. 2009;54(3):231–237. doi: 10.1159/000227694. [DOI] [PubMed] [Google Scholar]
- 154.Chou YY, Cheng AL, Hsu HC. Expression of P-glycoprotein and p53 in advanced hepatocellular carcinoma treated by single agent chemotherapy: clinical correlation. [Accessed August 14, 2016];J Gastroenterol Hepatol. 1997 12(8):569–575. doi: 10.1111/j.1440-1746.1997.tb00487.x. http://www.ncbi.nlm.nih.gov/pubmed/9304508. [DOI] [PubMed] [Google Scholar]
- 155.Namisaki T, Schaeffeler E, Fukui H, et al. Differential expression of drug uptake and efflux transporters in Japanese patients with hepatocellular carcinoma. Drug Metab Dispos. 2014;42(12):2033–2040. doi: 10.1124/dmd.114.059832. [DOI] [PubMed] [Google Scholar]
- 156.Pfeifer ND, Hardwick RN, Brouwer KLR. Role of hepatic efflux transporters in regulating systemic and hepatocyte exposure to xenobiotics. Annu Rev Pharmacol Toxicol. 2014;54:509–535. doi: 10.1146/annurev-pharmtox-011613-140021. [DOI] [PubMed] [Google Scholar]
- 157.Bonin S, Pascolo L, Crocé LS, Stanta G, Tiribelli C. Gene expression of ABC proteins in hepatocellular carcinoma, perineoplastic tissue, and liver diseases. Mol Med. 2002;8(6):318–325. [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao J, Yu B-Y, Yang J-E, et al. Promoter polymorphism of MRP1 associated with reduced survival in hepatocellular carcinoma. 2010 doi: 10.3748/wjg.v16.i48.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nies AT, König J, Pfannschmidt M, Klar E, Hofmann WJ, Keppler D. Expression of the multidrug resistance proteins MRP2 and MRP3 in human hepatocellular carcinoma. [Accessed August 14, 2016];Int J cancer. 2001 94(4):492–499. doi: 10.1002/ijc.1498. http://www.ncbi.nlm.nih.gov/pubmed/11745434. [DOI] [PubMed] [Google Scholar]
- 160.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214(2):231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 161.Vee ML, Lecureur V, Stieger B, Fardel O. Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor- or interleukin-6. Drug Metab Dispos. 2009;37(3):685–693. doi: 10.1124/dmd.108.023630. [DOI] [PubMed] [Google Scholar]
- 162.Diao L, Li N, Brayman TG, Hotz KJ, Lai Y. Regulation of MRP2/ABCC2 and BSEP/ABCB11 expression in sandwich cultured human and rat hepatocytes exposed to inflammatory cytokines TNF-α, IL-6, and IL-1. J Biol Chem. 2010;285(41):31185–31192. doi: 10.1074/jbc.M110.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brazille P, Dereuddre-Bosquet N, Leport C, et al. Decreases in plasma TNF-alpha level and IFN-gamma mRNA level in peripheral blood mononuclear cells (PBMC) and an increase in IL-2 mRNA level in PBMC are associated with effective highly active antiretroviral therapy in HIV-infected patients. [Accessed February 9, 2017];Clin Exp Immunol. 2003 131(2):304–311. doi: 10.1046/j.1365-2249.2003.02064.x. http://www.ncbi.nlm.nih.gov/pubmed/12562393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ghoneim RH, Piquette-Miller M. Endotoxin-mediated downregulation of hepatic drug transporters in HIV-1 transgenic rats. Drug Metab Dispos. 2016;44(5):709–719. doi: 10.1124/dmd.115.067827. [DOI] [PubMed] [Google Scholar]
- 165.De Rosa MF, Robillard KR, Kim CJ, et al. Expression of membrane drug efflux transporters in the sigmoid colon of HIV-infected and uninfected men. J Clin Pharmacol. 2013;53(9):934–945. doi: 10.1002/jcph.132. [DOI] [PubMed] [Google Scholar]
- 166.Kis O, Sankaran-Walters S, Hoque MT, Walmsley SL, Dandekar S, Bendayan R. HIV-1 alters intestinal expression of drug transporters and metabolic enzymes: Implications for antiretroviral drug disposition. Antimicrob Agents Chemother. 2016;60(5):2771–2781. doi: 10.1128/AAC.02278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dixit V, Hariparsad N, Li F, Desai P, Thummel KE, Unadkat JD. Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: implications for predicting clinical drug interactions. Drug Metab Dispos. 2007;35(10):1853–1859. doi: 10.1124/dmd.107.016089. [DOI] [PubMed] [Google Scholar]
- 168.Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42(7):1048–1059. doi: 10.1345/aph.1K615. [DOI] [PubMed] [Google Scholar]
- 169.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42(1):44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 170.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 171.Lomonaco R, Sunny NE, Bril F, Cusi K. Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs. 2013;73(1):1–14. doi: 10.1007/s40265-012-0004-0. [DOI] [PubMed] [Google Scholar]
- 172.Ali R, Cusi K. New diagnostic and treatment approaches in non-alcoholic fatty liver disease (NAFLD) Ann Med. 2009;41(4):265–278. doi: 10.1080/07853890802552437. [DOI] [PubMed] [Google Scholar]
- 173.Lake AD, Novak P, Fisher CD, et al. Analysis of global and absorption, distribution, metabolism, and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2011;39(10):1954–1960. doi: 10.1124/dmd.111.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Clarke JD, Hardwick RN, Lake AD, et al. Synergistic interaction between genetics and disease on pravastatin disposition. J Hepatol. 2014;61(1):139–147. doi: 10.1016/j.jhep.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clarke JD, Hardwick RN, Lake AD, Canet MJ, Cherrington NJ. Experimental nonalcoholic steatohepatitis increases exposure to simvastatin hydroxy acid by decreasing hepatic organic anion transporting polypeptide expression. J Pharmacol Exp Ther. 2014;348(3):452–458. doi: 10.1124/jpet.113.211284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology. 2012;56(1):118–129. doi: 10.1002/hep.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Aguilar-Olivos NE, Carrillo-Córdova D, Oria-Hernández J, et al. The nuclear receptor FXR, but not LXR, up-regulates bile acid transporter expression in non-alcoholic fatty liver disease. [Accessed February 9, 2017];Ann Hepatol. 14(4):487–493. http://www.ncbi.nlm.nih.gov/pubmed/26019035. [PubMed] [Google Scholar]
- 178.Ferslew BC, Xie G, Johnston CK, et al. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60(11):3318–3328. doi: 10.1007/s10620-015-3776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fisher CD, Lickteig AJ, Augustine LM, et al. Experimental non-alcoholic fatty liver disease results in decreased hepatic uptake transporter expression and function in rats. Eur J Pharmacol. 2009;613(1–3):119–127. doi: 10.1016/j.ejphar.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2011;39(12):2395–2402. doi: 10.1124/dmd.111.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lickteig AJ, Fisher CD, Augustine LM, et al. Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metab Dispos. 2007;35(10):1970–1978. doi: 10.1124/dmd.107.015107. [DOI] [PubMed] [Google Scholar]
- 182.Yang K, Guo C, Woodhead JL, et al. Sandwich-cultured hepatocytes as a tool to study drug disposition and drug-induced liver injury. J Pharm Sci. 2016;105(2):443–459. doi: 10.1016/j.xphs.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ferslew BC, Johnston CK, Tsakalozou E, et al. Altered morphine glucuronide and bile acid disposition in patients with nonalcoholic steatohepatitis. Clin Pharmacol Ther. 2015;97(4):419–427. doi: 10.1002/cpt.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tsuda N, Matsui O. Signal profile on Gd-EOB-DTPA-enhanced MR imaging in non-alcoholic steatohepatitis and liver cirrhosis induced in rats: correlation with transporter expression. Eur Radiol. 2011;21(12):2542–2550. doi: 10.1007/s00330-011-2228-x. [DOI] [PubMed] [Google Scholar]
- 185.Okushin K, Tsutsumi T, Enooku K, et al. The intrahepatic expression levels of bile acid transporters are inversely correlated with the histological progression of nonalcoholic fatty liver disease. J Gastroenterol. 2016;51(8):808–818. doi: 10.1007/s00535-015-1148-y. [DOI] [PubMed] [Google Scholar]
- 186.Zhang P, Tian X, Chandra P, Brouwer KLR. Role of Glycosylation in Trafficking of Mrp2 in Sandwich-Cultured Rat Hepatocytes. Mol Pharmacol. 2005;67(4):1334–1341. doi: 10.1124/mol.104.004481. [DOI] [PubMed] [Google Scholar]
- 187.Liu Y, Pu Q-H, Wu M-J, Yu C. Proteomic analysis for the impact of hypercholesterolemia on expressions of hepatic drug transporters and metabolizing enzymes. Xenobiotica. 2016;46(10):940–947. doi: 10.3109/00498254.2016.1144228. [DOI] [PubMed] [Google Scholar]
- 188.Canet MJ, Hardwick RN, Lake AD, Dzierlenga AL, Clarke JD, Cherrington NJ. Modeling human nonalcoholic steatohepatitis-associated changes in drug transporter expression using experimental rodent models. Drug Metab Dispos. 2014;42(4):586–595. doi: 10.1124/dmd.113.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zollner G, Fickert P, Silbert D, et al. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. [Accessed August 16, 2016];J Hepatol. 2003 38(6):717–727. doi: 10.1016/s0168-8278(03)00096-5. http://www.ncbi.nlm.nih.gov/pubmed/12763363. [DOI] [PubMed] [Google Scholar]
- 190.Takeyama Y, Sakisaka S. Hepatobiliary membrane transporters in primary biliary cirrhosis. Hepatol Res. 2012;42(2):120–130. doi: 10.1111/j.1872-034X.2011.00912.x. [DOI] [PubMed] [Google Scholar]
- 191.Kojima H, Nies AT, König J, et al. Changes in the expression and localization of hepatocellular transporters and radixin in primary biliary cirrhosis. [Accessed August 16, 2016];J Hepatol. 2003 39(5):693–702. doi: 10.1016/s0168-8278(03)00410-0. http://www.ncbi.nlm.nih.gov/pubmed/14568249. [DOI] [PubMed] [Google Scholar]
- 192.Takeyama Y, Tsuchiya N, Kunimoto H, et al. Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging as a useful detection method for advanced primary biliary cirrhosis. Hepatol Res. 2015;45(10):E108–E114. doi: 10.1111/hepr.12470. [DOI] [PubMed] [Google Scholar]
- 193.Takeyama Y, Uehara Y, Inomata S, et al. Alternative transporter pathways in patients with untreated early-stage and late-stage primary biliary cirrhosis. Liver Int. 2009;29(3):406–414. doi: 10.1111/j.1478-3231.2008.01846.x. [DOI] [PubMed] [Google Scholar]
- 194.Boyer JL, Trauner M, Mennone A, et al. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1124–30. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- 195.Jorajuria S, Dereuddre-Bosquet N, Naissant-Storck K, Dormont D, Clayette P. Differential expression levels of MRP1, MRP4, and MRP5 in response to human immunodeficiency virus infection in human macrophages. Antimicrob Agents Chemother. 2004;48(5):1889–1891. doi: 10.1128/AAC.48.5.1889-1891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ieiri I, Takane H, Hirota T, Otsubo K, Higuchi S. Genetic polymorphisms of drug transporters: pharmacokinetic and pharmacodynamic consequences in pharmacotherapy. Expert Opin Drug Metab Toxicol. 2006;2(5):651–674. doi: 10.1517/17425255.2.5.651. [DOI] [PubMed] [Google Scholar]
- 197.Lee J, Kim Y, Friso S, Choi S-W. Epigenetics in non-alcoholic fatty liver disease. Mol Aspects Med. 2017;54:78–88. doi: 10.1016/j.mam.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 198.Cressman AM, Petrovic V, Piquette-Miller M. Inflammation-mediated changes in drug transporter expression/activity: implications for therapeutic drug response. Expert Rev Clin Pharmacol. 2012;5(1):69–89. doi: 10.1586/ecp.11.66. [DOI] [PubMed] [Google Scholar]
- 199.Hanafy S, El-Kadi AOS, Jamali F. Effect of inflammation on molecular targets and drug transporters. [Accessed April 8, 2017];J Pharm Pharm Sci. 2012 15(3):361–375. doi: 10.18433/j30300. http://www.ncbi.nlm.nih.gov/pubmed/22974786. [DOI] [PubMed] [Google Scholar]
- 200.Christensen H, Hermann M. Immunological response as a source to variability in drug metabolism and transport. Front Pharmacol. 2012;3:8. doi: 10.3389/fphar.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pomozi V, Le Saux O, Brampton C, et al. ABCC6 is a basolateral plasma membrane protein. Circ Res. 2013;112(11):e148–51. doi: 10.1161/CIRCRESAHA.111.300194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chen H-L, Liu Y-J, Chen H-L, et al. Expression of hepatocyte transporters and nuclear receptors in children with early and late-stage biliary atresia. Pediatr Res. 2008;63(6):667–673. doi: 10.1203/PDR.0b013e318170a6b5. [DOI] [PubMed] [Google Scholar]
- 203.Kikuchi R, McCown M, Olson P, et al. Effect of hepatitis C virus infection on the mRNA expression of drug transporters and cytochrome p450 enzymes in chimeric mice with humanized liver. Drug Metab Dispos. 2010;38(11):1954–1961. doi: 10.1124/dmd.109.031732. [DOI] [PubMed] [Google Scholar]
- 204.Kullak-Ublick GA, Beuers U, Paumgartner G. Molecular and functional characterization of bile acid transport in human hepatoblastoma HepG2 cells. Hepatology. 1996;23(5):1053–1060. doi: 10.1002/hep.510230518. [DOI] [PubMed] [Google Scholar]
- 205.More VR, Cheng Q, Donepudi AC, et al. Alcohol cirrhosis alters nuclear receptor and drug transporter expression in human liver. Drug Metab Dispos. 2013;41(5):1148–1155. doi: 10.1124/dmd.112.049676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bonin S, Pascolo L, Crocé LS, Stanta G, Tiribelli C. Gene expression of ABC proteins in hepatocellular carcinoma, perineoplastic tissue, and liver diseases. [Accessed August 14, 2016];Mol Med. 2002 8(6):318–325. http://www.ncbi.nlm.nih.gov/pubmed/12428063. [PMC free article] [PubMed] [Google Scholar]
- 207.Hardwick RN, Clarke JD, Lake AD, et al. Increased susceptibility to methotrexate-induced toxicity in nonalcoholic steatohepatitis. Toxicol Sci. 2014;142(1):45–55. doi: 10.1093/toxsci/kfu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.van de Steeg E, van der Kruijssen CMM, Wagenaar E, et al. Methotrexate Pharmacokinetics in Transgenic Mice with Liver-Specific Expression of Human Organic Anion-Transporting Polypeptide 1B1 (SLCO1B1) [Accessed April 8, 2017];Drug Metab Dispos. 2009 37(2) doi: 10.1124/dmd.108.024315. http://dmd.aspetjournals.org/content/37/2/277. [DOI] [PubMed] [Google Scholar]
- 209.Kim RB, Fromm MF, Wandel C, et al. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101(2):289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dzierlenga AL, Clarke JD, Klein DM, et al. Biliary elimination of pemetrexed is dependent on Mrp2 in rats: Potential mechanism of variableresponse in nonalcoholic steatohepatitis. J Pharmacol Exp Ther. 2016;358(2):246–253. doi: 10.1124/jpet.116.234310. [DOI] [PMC free article] [PubMed] [Google Scholar]