Abstract
Objective
To determine in a 48-month longitudinal study the association of thigh muscle cross-sectional area (CSA) and strength on progression of morphologic knee cartilage degeneration using 3T magnetic resonance imaging (MRI).
Design
Seventy Osteoarthritis Initiative (OAI) subjects aged 50-60 years, with no radiographic evidence of osteoarthritis (OA) and constant muscle strength over 48 months as measured by isometric knee extension testing were included. Baseline right thigh muscle CSAs were assessed on axial T1-weighted MR images, and extensor to flexor CSA ratios were calculated. Degenerative knee abnormalities at baseline and 48-months were graded on right knee 3T MRIs using a modified whole organ MRI score (WORMS). Statistical analysis employed Student's t-tests and multivariable regression models adjusted for age, body mass index and gender.
Results
Extension strength was significantly and positively correlated with baseline thigh muscle CSA (r=0.65, p<0.001). Greater baseline total thigh muscle CSA was significant associated with increase of cartilage WORMS scores over 48 months in patellar (p=0.027) and trochlear (p=0.038) compartments, but not in other knee compartments. Among specific muscle groups, CSA of extensors (p=0.021) and vastus medialis (VM) (p=0.047) were associated with patellar cartilage increase in WORMS. Baseline E/F ratio had a significant positive association with patellar WORMS cartilage score increase over 48 months, p=0.0015. There were no other significant associations between muscle CSA/ratios and increase in WORMS scores.
Conclusion
Maintenance of proper extensor to flexor muscle balance about the knee through decreased E/F ratios may slow patellofemoral cartilage deterioration, while higher extensor and VM CSA may increase patellofemoral cartilage loss.
Introduction
Conservative treatment modalities for knee osteoarthritis (OA) are limited, despite the fact that radiographic knee OA affects nearly 19% of the general population 1. Invasive treatments such as total knee arthroplasty (TKA), while having successful short- to intermediate-term outcomes, can often be prone to late failure, with reported revision rates of up to 18.9% at 15 years 2. For this reason, a focus has been placed on OA prevention and identification of modifiable risk factors in the treatment of knee OA.
Part of this focus has turned to the role played by knee stability, and specifically static and dynamic stabilizers of the knee. The integrity of static stabilizers (i.e. bones, ligaments) of the knee has been implicated as protective against degenerative disease of the cartilage 3. Recent literature has turned to the importance of dynamic stabilizers of the knee as well, with studies showing associations of increased muscle strength about the knee joint with both increased risk and a protective effect with respect to knee OA progression 4-10. However there is currently no firm agreement regarding the basis of these associations, or the definitive recommendations that should be derived from them with regards to the role of muscle strength in prevention of knee OA progression.
Previous studies exploring the relationship between thigh muscle and knee OA progression have mostly used strength testing measures, which can be exceedingly limited in terms of reliability and validity as they are prone to patient effort and comfort levels, to characterize muscle strength in study subjects. Muscle cross-sectional area (CSA) can be accurately and objectively quantified using magnetic resonance (MR) imaging, and this has been shown in several studies to have a close correlation with muscle strength measurements 10, 11. However, few previous studies have used this technology for characterizing and quantifying muscle strength when evaluating its relationship to osteoarthritis progression 12-14.
The purpose of our study was to determine the relationship between baseline muscle CSA and strength with longitudinal progression of degenerative, morphologic abnormalities of the knee joint in individuals with stable muscle strength over four years and no signs of radiographic osteoarthritis in the tibiofemoral joint. An additional focus of our study was to examine the role that imbalance of agonist and antagonist thigh muscle groups potentially plays in the progression of degenerative changes of the knee joint.
Materials and Methods
Subjects
Seventy individuals from the Osteoarthritis Initiative (OAI) incidence and progression cohorts were included in this study as outlined in Figure 1. All subjects included had risk factors for OA without symptomatic knee OA in at least one knee. Risk factors included previous knee injury or surgery, family history of TKA, Heberden's nodes, or knee symptoms within the past 12 months. All subjects provided informed consent. The study was HIPAA-compliant and approved by the local committees on human research. Inclusion criteria were no radiographic OA defined by a Kellgren-Lawrence (KL) grade of 0 or 1 15. To minimize the effect of age as a confounding variable, only subjects age 50-60 years were included. Exclusion criteria were those implemented by the OAI and included contraindications to MR imaging, bilateral end-stage OA, inflammatory arthritis, pregnancy, and co-morbidities that could interfere with study participation 16, 17.
Figure 1.
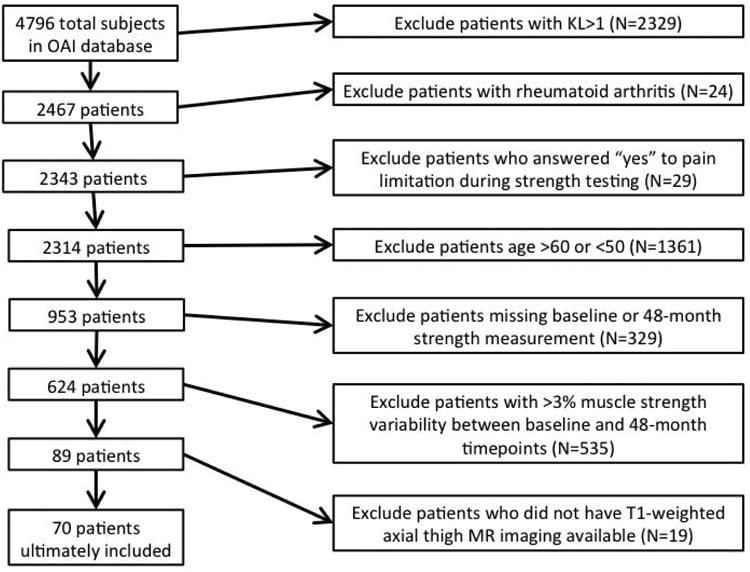
Flowsheet demonstrating patient exclusion criteria.
As part of the clinical data collection for the OAI, maximum extension strength was measured using an isometric force testing chair at each visit. Patients who endorsed being limited in strength measurement due to pain were excluded, as were patients who did not have isometric strength testing performed at both baseline and 48-months. Only patients who demonstrated less than 3% variability in extension strength testing were included, in an effort to eliminate changes in muscle strength over time (and factors that may contribute to these changes) as confounding variables in our relationship assessments. A threshold of 3% variability was used because of previous studies which report that 5% variability in muscle strength reflects significant changes in Western Ontario and McMaster Universities (WOMAC) functional knee scores 18.
MR imaging
MR imaging was obtained using identical 3T MRI systems (Trio, Siemens, Erlangen, Germany). Identical knee coils were used for all studies. Four sequences were used for image analysis: coronal intermediate-weighted (IW) 2D fast spin-echo (FSE) sequence (echo time (TE) 29 ms, repetition time (TR) 3700 ms, slice thickness 3.0 mm, bandwidth 352 Hz/pixel), sagittal 2D IW FSE sequence with fat suppression (TE 30 ms, TR 3200 ms, slice thickness 3.0 mm, bandwidth 248 Hz/pixel), sagittal 3D dual-echo in steady state (DESS) sequence (TE 4.7 ms, TR 16.3 ms, flip angle 25 degrees, slice thickness 0.7 mm, bandwidth 185 Hz/pixel) with axial multiplanar reconstruction. Further details about image acquisition are provided in the OAI MR protocol 19.
Semi-quantitative morphological imaging analysis
All images were viewed on PACS workstations (Agfa, Ridgefield Park, NJ, USA). Baseline and 48-month MR images were obtained of the right knee for all seventy patients included in this study as part of the OAI database. Images were reviewed by a radiologist with three years of experience who was blinded to the initial strength measurement of the subjects. Unclear readings were reviewed by a second board-certified musculoskeletal radiologist with 22 years of experience. Images were semi-quantitatively graded based on the modified Whole Organ Magnetic Resonance Imaging Score (WORMS) assessing morphologic lesions of cartilage, menisci, or ligaments, in addition to presence of bone marrow edema pattern, loose bodies, subchondral cysts, effusion, or popliteal cysts. Cartilage lesions were graded on a scale of 1 to 6, with 1 representing an intracartilaginous signal abnormality, 2 representing a partial thickness defect of the cartilage surface measuring <1 cm, 2.5 representing a full-thickness cartilage defect measuring <1 cm, 3 representing a partial thickness lesion measuring >1 cm but <75% of the region, 4 representing diffuse cartilage thinning involving >75% of the region, 5 representing a full thickness lesion >1 cm but <75% of the region, and 6 involving diffuse full thickness cartilage loss involving >75% of the region. The maximum cartilage score was defined as the highest cartilage score from any measured compartment (patella, trochlea, medial or lateral femur, or medial or lateral tibia). Meniscal lesions were graded on a 5-point scale as normal (0), intrasubstance signal abnormality (1), meniscal tear without deformity (2), meniscal tear with deformity (3), or meniscal maceration (4). Ligament/tendon lesions were graded as normal (0), increased surrounding signal abnormality (1), increased signal abnormality within the tendon/ligament (2), partial tear (3), or complete tear (4). Subchondral bone marrow edema pattern was graded as none (0), mild (<5 mm, grade 1), moderate (>5 mm but <20 mm, grade 2), or severe (>20 mm, grade 3). Loose bodies were graded as present or absent. Subchondral cysts were graded as none (0), mild (<3 mm, grade 1), moderate (3-5 mm, grade 2), or severe (>5 mm, grade 3). Joint effusion was graded as none (0), mild (<33% maximal distention, grade 1), moderate (33-66% maximal distention, grade 2), or severe (>66% maximal distention, grade 3). Popliteal cysts were also graded on a 4-point scale as none, mild, moderate, or severe 20.
Baseline thigh muscle CSA measurements
Each included subject had standing full-length anterior-posterior radiographs of the right femur obtained. The femoral length was measured from the intercondylar notch to the superior femoral head. A T1W axial MR slice corresponding to the distal-middle third junction of the femur based on the measurement obtained in full-length radiographs was localized on the coronal MR scout view. This was considered the index slice for muscle segmentation; two slices proximal were also segmented for a total of three slices. Manual spline-based segmentation was performed using Matlab software (Figure 2). Eight regions of interest were included for segmentation: rectus femoris, vastus medialis, vastus lateralis, vastus intermedialis, biceps femoris (short head), biceps femoris (long head), semitendinosus, and semimembranosus, and their CSAs were calculated as the average of the CSAs in all segmented MR slices. Extensor CSA was calculated as the sum of the individual CSAs of the rectus femoris, vastus medialis, vastus lateralis, and vastus intermedialis. Flexor CSA was similarly calculated as the sum of the biceps femoris (long and short heads), semitendinosus, and semimembranosus CSAs. Extensor flexor ratio (E/F ratio) was calculated as the ratio of extensor to flexor CSA (Figure 3). MR images were graded based on a Goutallier scale by one radiologist with three years of experience as Grade 0 (no fatty streaks), Grade 1 (some fatty streaks), Grade 2 (more muscle than fat), grade 3 (as much fat as muscle) and grade 4 (less muscle than fat) 21, 22. A Goutallier-derived correction factor was applied to the CSA values to obtain an adjusted value accounting for intramuscular fat (Grade 0=1, Grade 1=0.90, Grade 2=0.85, Grade 3=0.82, Grade 4=0.70, which is analogous to previously-published studies investigating the relationship between Goutallier grade and fat composition 23, 24).
Figure 2.
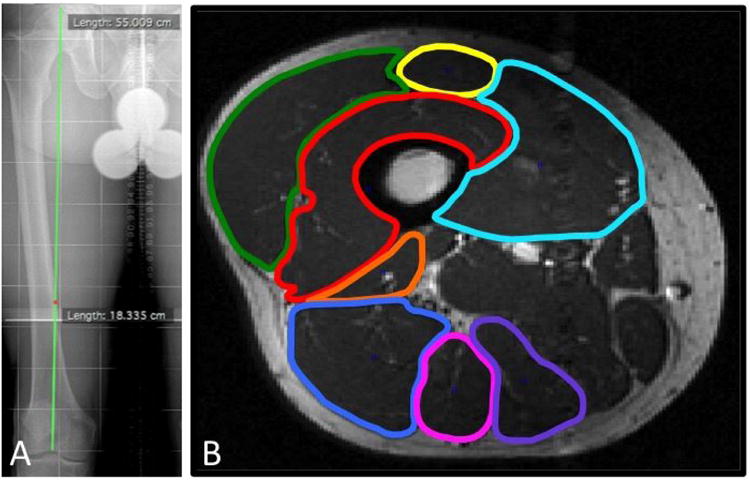
Full-length femur radiograph (A) demonstrates method of obtaining slice selection in uniform manner that accounted for individual height/body size differences. The length of the femur from the superior aspect of the femoral head to the intercondylar notch was first measured, and the obtained measurement was divided by three to obtain the location of the distal-middle third junction of the femur. This length (18.335 cm) was then correlated with the appropriate axial MR slice (B).
Figure 3.
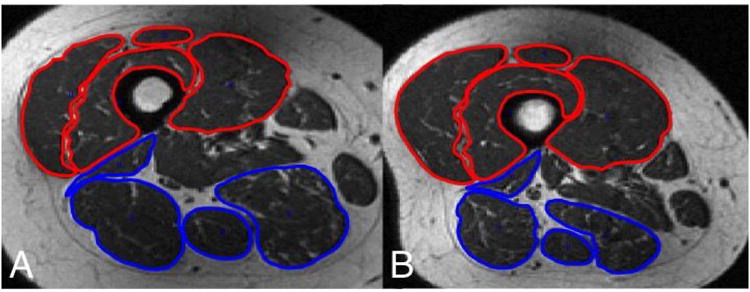
Axial T1-weighted thigh MRIs at the distal one-third junction in patient (A) demonstrates extensor compartment (highlighted in red) and flexor compartment (highlighted in blue) with an E/F ratio of 1.01, in contrast to an axial image in a different patient (B) showing a much larger E/F ratio of 2.17.
Baseline patellofemoral alignment
In order to determine the variation among our study population in terms of patellofemoral alignment factors, each patient was assigned a value for patellofemoral alignment factors including presence/absence of patella alta as defined by Insall-Salvati ratio of greater than 1.3 25. Presence of patellar tilt was defined as lateral facet angle less than 8 degrees. Patellar subluxation was also assessed (determined through measurement of bisect offset), and trochlear dysplasia as defined by sulcus angle greater than 145 degrees or sulcus depth less than 3 mm was recorded 26.
Statistical analyses
Statistical analyses were performed using JMP software version 11 (SAS Institute, Cary, NC, USA). Gender-specific Z-scores were calculated for each muscle CSA compartment/ratio (total, extensors, flexors, VM, and E/F ratio). Multivariable linear regression models adjusted for age, gender, and BMI were used to assess the relationship between gender-specific Z-scores for compartmental muscle CSA/ratios (predictor variables) and subcompartmental cartilage, BMEP, and meniscus WORMS scores (outcome variables), after testing that the appropriate relevant assumptions had been met (specifically regarding normality of variables, which was assessed using Q-Q plots). Analysis of WORMS maximum cartilage score progression and non-progression subgroups (where progression defined as WORMS maximum cartilage score increase ≥ 1) was performed using Student's t-tests. Statistical significance was defined as p<0.05.
Our group has previously analyzed inter- and intra-reader reproducibility for WORMS measurements in a subset of 15 participants with demonstration of high inter-observer reliabilities within various WORMS subcategories 19, 27, 28. Coefficient of variance (CV) for thigh muscle CSA has also been described previously in our group, and was calculated as <2% for all regions of interest measured (vastus medialis, vastus lateralis, vastus intermedialis, rectus femoris, biceps femoris-short head, biceps femoris-long head, semimembranosus, and semitendinosus) 13. Specifically, CV for total thigh muscle CSA, quadriceps, hamstring, vastus medialis, and vastus lateralis were 0.72%, 0.92%, 0.96%, 1.34%, and 1.67%, respectively). Additionally, our group has recently demonstrated high intra- and inter-observer agreement for semi-quantitative grading of fat infiltration using Goutallier's classification (0.83 and 0.81, respectively)23.
Results
Subject characteristics
Baseline demographic variables of the study population are depicted in Table 1. Seventy patients were included, 47.1% of whom were male. Average body mass index was 27.5±4.3 kg/m2. Average maximum right knee extension force among the study cohort was 428.8±148.6 N, with an average speed of extension force of 580±465 N/s.
Table 1. Demographics in relation to total CSA group.
| All | Low Total CSA* | High Total CSA* | P-value^ | |
|---|---|---|---|---|
| Number | 70 | 35 | 35 | |
| Males (%, number) | 47.1 (33) | 22.9 (8) | 71.4 (25) | <0.0001 |
| Age (years) | 55.3±3.1 | 55.1 ±3.3 | 55.4±2.9 | 0.648 |
| Height (mm) | 1708.6±100.2 | 1664.8±69.4 | 1752.4±107.7 | <0.001 |
| BMI (kg/m2) | 27.5±4.3 | 26.5±4.0 | 28.5±4.4 | 0.046 |
| Right knee maximum extension (N) | 428.8±148.6 | 376.6±95.1 | 481.1±173.6 | 0.003 |
| Maximum extension speed (N/s) | 579.8±465.1 | 589.5±465.1 | 570.8±472.1 | 0.874 |
Calculated using student's t-test, with statistical significance defined as p < 0.05.
Groups determined using cut-off value of 10,000 mm2, which separated upper and lower 50 percentile for corrected total muscle CSA
The majority of individuals had Goutallier grade 0 (40.0%, 28/70) or 1 (48.6%, 34/70). The remaining eight subjects had Goutallier grade 2. Baseline values for patellar and patellofemoral joint WORMS were compared to previously published data from the OAI normal control cohort, and the incidence of cartilage lesions of various WORMS grades was not found to vary significantly29.
Baseline data
Baseline maximum knee extension strength had a significant positive correlation with both total CSA (r=0.64, 95% CI 0.48, 0.76, p<0.0001), and Goutallier-corrected total CSA (r=0.65, 95% CI 0.49, 0.77, p<0.0001). No significant correlations were found between baseline muscle strength and Goutallier grade, or between baseline PASE (Physical Activity Scale for the Elderly) scores and extensor/flexor ratio.
There were no statistically significant relationships between baseline WORMS score for any given cartilage compartment, and baseline muscle CSA for total thigh muscle, extensor compartments, or vastus medialis (VM). However, baseline (and 48-month) trochlear cartilage WORMS scores did have a significant positive association with E/F ratios (p=0.048 and p=0.031, respectively). Interestingly, CSA had a significant association with meniscal WORMS score at baseline: individuals with lower CSA had higher maximum meniscus WORMS scores for total CSA, flexor CSA, and extensor CSA (p<0.05).
Longitudinal data
No significant difference was found regarding demographic and strength measurement variables in groups that experienced increase in WORMS maximum cartilage scores (defined as total 48-month increase ≥ 1, n=31) compared to those who had no increase (change in maximum cartilage score equal to <1, n=38). With progression defined as an increase in WORMS cartilage score of at least 1 over the study period, only baseline Goutallier grade and E/F ratio were found to have a significant association with progressive degeneration, with increased values for both being associated with increased progression rate (p < 0.05). No other individual muscle group CSA values were found to have a significant correlation with rate of progression.
Tables 2 and 3 summarize the results of a multiple linear regression model adjusted for age, gender, and BMI, to investigate the associations between gender-normalized muscle CSA and compartmental change in WORMS cartilage scores, as well as global maximum cartilage, meniscus, and BMEP WORMS scores. No significant associations were seen with regards to baseline muscle strength measurements and longitudinal change in any WORMS score parameter. However, increase in patellar and trochlear WORMS scores were found to be significantly positively associated with total muscle CSA (p=0.027 and p=0.038, respectively) (Figure 4). Total extensor CSA and specifically VM CSA were similarly found to be significantly associated with increase in patellar cartilage WORMS (p=0.021 and p=0.038, respectively). No other longitudinal WORMS parameters reached significance. Similarly, vastus intermedialis and vastus lateralis CSA were not found to have significant relationships with 48-month increase in WORMS score. Baseline WOMAC scores were analyzed for correlation with both changes in WORMS scores as well as baseline WORMS values for patellar cartilage and maximum cartilage score, and no significant associations were found.
Table 2. Relationship of muscle CSA parameters to WORMS cartilage scores.
| Time-point | Baseline MS* | Total CSA* | Flexor CSA* | Extenstor CSA* | VM CSA* | E/F ratio* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low^ | High^ | Low | High | Low | High | Low | High | Low | High | Low | High | ||
| Patella | Baseline | 1.66±1.64 | 1.91±1.54 | 1.91±1.77 | 1.66±1.39 | 1.77±1.55 | 1.8±1.64 | 1.97±1.77 | 1.6±1.38 | 1.63±1.82 | 1.94±1.33 | 2.11±1.84 | 1.46±1.52 |
| 48-mo | 2.19±1.87 | 2.73±1.73 | 2.46±1.94 | 2.46±1.70 | 2.36±1.81 | 2.56±1.83 | 2.51±1.92 | 2.4±1.72 | 2.18±1.90 | 2.73±1.70 | 2.50±1.91 | 2.41±1.73 | |
| Change | 0.53±0.67 | 0.76±0.92 | 0.49±068 | 0.80±0.90 | 0.59±0.75 | 0.71±0.87 | 0.49±0.68 | 0.80±0.90 | 0.50±0.79 | 0.79±0.82 | 0.32±0.59 | 0.96±0.88 | |
| Trochlea | Baselne | 1.43±1.63 | 0.91±1.04 | l.00±1.33 | 1.34±1 43 | 1.29±1.43 | 1.06±1.35 | 1.14±1.48 | 1.20±1.30 | 0.94±1.21 | 1.40±1.52 | 0.86±1.19 | 1.49±1.50 |
| 48 mo | 1.77±1.88 | 1.39±1.23 | 1.32±1 63 | 1.93±1 54 | 1.67±1.65 | 1.59±1.56 | 1.47±1.73 | 1.69±1.47 | 1.29±1.40 | l.96±1.73 | 1.21±1.47 | 1.94±1.64 | |
| Change | 0.34±0.68 | 0.44±0.79 | 0.29±1.72 | 0.49±0.74 | 0.29±0.71 | 0.50±0.75 | 0.29±0.7? | 0.49±0.74 | 0.32±0.68 | 0.46±0.78 | 0.32±0.68 | 0.46±0.78 | |
Groups were defined according to assigned gender-specific Z-scores for the study population. Goutallier-adjusted values were used where appropriate.
All analyses were performed using a multiple regression model adjusted for age. BMI, and gender. Significance was defined as p < 0.05. and statistically significant values are depicted in red.
Table 3. Relationship of muscle CSA parameters to WORMS maximum cartilage, meniscus, and BMEP scores.
| Time-point | Baseline MS | Total CSA* | Flexor CSA* | Extensor CSA* | VM CSA* | E/F ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low^ | High^ | Low | High | Low | High | Low | High | Low | High | Low | High | ||
| Global max cartilage score | Baseline | 2.81±1.47 | 2.29±1.38 | 2.74±1.48 | 2.36±1.39 | 2.54±1.36 | 2.56±154 | 2.86±1.50 | 2.24±1.33 | 2.57±1.50 | 2.53±1 40 | 2.69±1.64 | 2.41±1.22 |
| 48-mo | 3.34±1.41 | 3.06±1.48 | 3.32±1.43 | 3.09±1.46 | 3.20±1.35 | 3.21±1.55 | 3.41±1.43 | 3.00±1.43 | 3.03±1.38 | 3.37±1.50 | 3.09±1.56 | 3.31±1.32 | |
| Change | 0.53±0.70 | 0.74±0.83 | 0.53±0.71 | 0.73±0.82 | 0.66±0.80 | 0.60±0.74 | 0.60±0.71 | 0.76±0.81 | 0.41±0.66 | 0.84±0.81 | 0.35±0.60 | 0.90±0.82 | |
| Meniscus | Baseline | 1.20±1.21 | 0.71±1.10 | 1.26±1.29 | 0.66±0.97 | 1.17±1.34 | 0.74±0.95 | 1.26±1.29 | 0.66±0.97 | 1.06±1.26 | 0.86±1.09 | 0.86±1.14 | 1.06±1.21 |
| 48-mo | 1.46±1.31 | 1.38±1.28 | 1.68±1.39 | 1.17±1.15 | 1.69±1.32 | 1.15±1.21 | 1.62±1.41 | 1.23±1.14 | 1.56±1.31 | 1.29±1.27 | 1.24±1.26 | 1.60±1.31 | |
| Change | 0.26±0.92 | 0.68±0.88 | 0.41±0.86 | 0.51±0.98 | 0.51±0.85 | 0.41±0.99 | 0.35±0.95 | 0.57±0.89 | 0.50±0.86 | 0.43±0.98 | 0.38±0.99 | 0.54±0.86 | |
| Bone marrow edema pattern | Baseline | 1.06±0.87 | l.20±0.96 | 1.11±0.87 | 1.14±0.97 | 1.20±0.83 | 1.06±1.00 | 1.14±0.89 | 1.11±0.96 | 1.11±0.93 | 1.14±0.91 | 1.00±0.84 | 1.26±0.98 |
| 48-mo | 1.23±0.88 | 1.35±1.01 | 1.18±0.97 | 1.40±0.91 | 1.26±0.92 | 1.32±0.98 | 1.21±0.99 | 1.37±0.91 | 1.09±0.97 | 1.49±0.89 | 0.97±0.97 | 1.60±0.81 | |
| Change | 0.17±0.82 | 0.15±0.82 | 0.06±0.81 | 0.286±0.82 | 0.06±0.80 | 0.26±0.83 | 0.06±0.81 | 0.26±0.82 | -0.03±0.80 | 0.34±0.80 | -0.03±0.72 | 0.34±0.87 | |
Groups were defined according to assigned gender-specific Z-scores for the study population, Goulallier-adjusted values were used where appropriate.
All analyses were performed using a multiple regression model adjusted for age. BMI, and gender. Significance was defined as p < 0.05. and statistically significant values are depicted in red.
Figure 4.
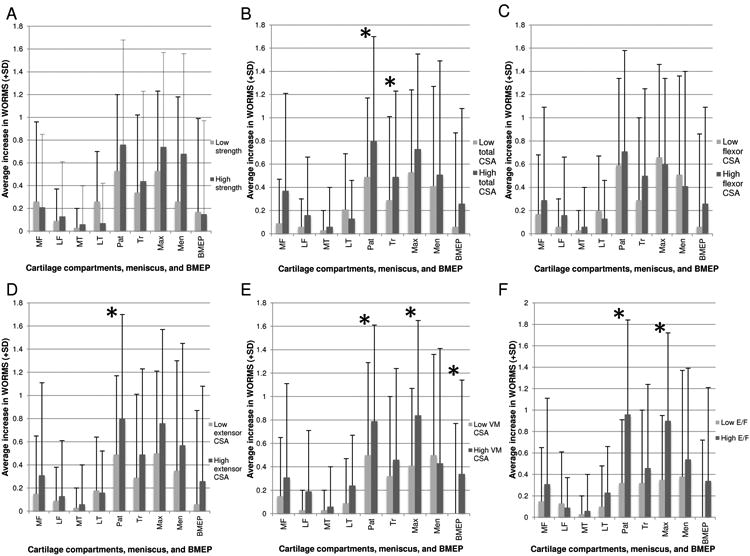
Graphical depiction of relationships between various muscle strength (A) and CSA parameters– including total CSA (B), flexor CSA (C), extensor CSA (D), VM CSA (E), and E/F ratio (F)-- and the corresponding mean increase in subcompartmental cartilage WORMS scores associated with each group. Statistical significance was defined as p<0.05 (asterisk). MF=medial femur; LF=lateral femur; MT=medial tibia; LT=lateral tibia; Pat=patella; Tr=trochlea; Max=maximum cartilage score (any compartment); Men=meniscus; BMEP=bone marrow edema pattern.
The strongest association demonstrated in our data pertained to the E/F ratio, calculated as the ratio between the sum of the extensor CSA to the sum of the flexor CSA. There was a strong positive association between E/F ratio and increase in patellar WORMS over 48-months follow-up, p=0.0015 (Figures 4D and 5). Specifically, differences between high and low E/F ratio groups demonstrate that the high E/F patients experienced a nearly 1-grade increase in patellar cartilage WORMS scores on average.
Figure 5.
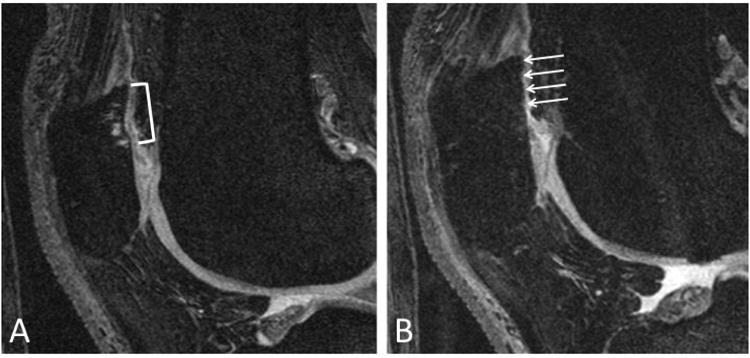
Baseline sagittal DESS image (A) demonstrating patellar cartilage with small partial-thickness defect (bracket) in patient with high extensor-flexor ratio. 48-month follow-up MRI (B) demonstrates clear progression of the patellar cartilage lesion which is now a large full-thickness defect (arrows).
To determine if our results were potentially influenced by varying Goutallier grades, all analyses were repeated with the exclusion of the eight cases which were classified as Goutallier grade 2 (i.e. only including Grades 0 or 1). The results obtained did not vary significantly regarding associations between gender-normalized muscle CSA and WORMS progression, thus confirming the validity of our analyses. Similarly, to determine if results were influenced by patellar alignment factors (patella alta, patellar tilt, patellar subluxation, or trochlear dysplasia), analyses were repeated with the exclusion of outliers in these categories (one case with patella alta, five cases with patellar tilt, three cases with patellar subluxation, and three cases with trochlear dysplasia), and the results obtained again did not demonstrate significant variation.
Of note, several longitudinal WORMS variables (48-month change in WORMS scores) were found to be significantly correlated with baseline WORMS values, specifically for maximum cartilage score (r=-0.263, p=0.029), meniscus (r=-0.266, p=0.027), and BMEP (r=-0.419, p<0.001). For these variables, separate analyses were performed in order to adjust for baseline WORMS values in addition to age, gender and BMI. This additional adjustment did not result in a significant change with regards to data provided in Tables 2 and 3.
Discussion
This study explores the longitudinal effects of muscle strength on changes in knee cartilage degeneration in individuals with OA risk factors, but without preexisting radiographic evidence of OA. Our study used objectively-assessed muscle CSA by 3T MRI and objective maximal muscle force testing to find that individuals with no radiographic knee OA but high extensor CSA have an increased rate of progression of patellofemoral joint cartilage degeneration. Also notable is the role of increased extensor CSA in relation to flexor CSA (E/F ratio), as this had the most significant association with increased patellar cartilage deterioration over 48 months and could potentially provide a target for management of individuals with increased risk for OA.
Our study found an association between increased total CSA of thigh musculature with increase in WORMS scores over 48 months in trochlear and patellar regions. This is especially interesting in light of the previously published literature, which is often conflicting regarding the role of total thigh muscle strength in prevention or mitigation of OA. Global muscle weakness has previously been shown to have a close association with the development of OA in other load-bearing joints 30. However, a recent study by Ruhdorfer et al. failed to demonstrate a significant relationship between thigh muscle CSA between knees with and without radiographic OA 12. Some studies have suggested that both knee flexion and extension strength weakness can themselves be associated with knee OA 31, 32. A recent study by Mikesky et al. in which patients without radiographic knee OA underwent strength training of both hamstrings and quadriceps, demonstrated a greater incident joint space narrowing in the strength training group, suggesting an opposite effect of global muscle strength about the knee joint 33. Another study by Visseret al. supported this finding in demonstrating a positive correlation between skeletal muscle mass and severity of knee OA 34. Our study sheds light on the role that muscle strength plays in OA because it examines individuals prior to the development of OA symptoms, and shows that in this population, increased total thigh muscle CSA may actually be associated with longitudinal worsening of trochlear and patellar cartilage abnormalities.
A primary focus of this study concerned the specific role of extensor strength in the development of knee OA. A detailed regression analysis yielded information regarding the significant association between extensors as a group, and specifically the vastus medialis, in worsening cartilage lesions of the patella. Previous literature related to this topic is conflicting. Palmieri-Smith et al. have suggested that women with early OA had decreased quadriceps strength compared to a non-OA cohort, and similarly, Amin et al. have previously demonstrated a protective role of increased quadriceps strength as it relates to cartilage loss of the lateral patella 6, 35. Another recent study specific to patients with malalignment of the knee demonstrated a detrimental effect of increased quadriceps strength with respect to tibiofemoral OA progression risk 36. Studies that used quantitative MR imaging modalities such as muscle CSA and cartilage T2 quantification have also demonstrated that the effect of improved extension strength is not necessarily beneficial. In 2012, Pan et al. described increased vastus lateralis/vastus medialis CSA ratios as being inversely related to T2 values of knee cartilage, suggesting that decreased vastus medialis CSA (relative to vastus lateralis CSA) may actually be chondroprotective; this is consistent with the results of our study 13.
The strongest association demonstrated in our study involved increased E/F ratio and patellar cartilage degeneration. This finding raises the concept of hamstring strength possibly playing a protective role for knee cartilage, particularly in patients with increased quadriceps strength. The hamstrings-quadriceps ratio has been the focus of previous studies, especially as it relates to patients at risk for ACL injuries (which in turn can give rise to progressive knee OA) 37. From a biomechanical standpoint, increased E/F ratio has been implicated in anterolateral tibial subluxation, and it has been posited that a higher E/F ratio is itself a risk factor for ACL injury 38, 39. Though much literature focuses on the role of knee extension strength in knee OA, there has been little focus on the potential role of hamstring strengthening, even though studies have shown a beneficial effect of improvement of hamstring strength for pain reduction in patients with knee OA 31. Our study in many ways confirms what has been hinted at in previous literature, showing that the most important aspect of thigh musculature governing knee OA, relates to the intrinsic balance between the extensors and flexors (that is, an unbalanced decrease in hamstring strength relative to quadriceps strength, can potentially have detrimental long-term effects on the patellofemoral cartilage). Though we have only demonstrated a possible protective effect of flexor CSA with regards to meniscus health, we see that the role of flexor strength becomes much more important as it relates to balancing against high extensor strength.
It is important to note that our study set out to clarify the role of muscle CSA with overall knee cartilage health, including tibiofemoral cartilage. None of our muscle CSA parameters reached significance with regards to the longitudinal effect on the tibiofemoral joint. One possible interpretation which has been described previously, is that patellofemoral cartilage anomalies are actually a precursor for further cartilage deterioration of the tibiofemoral joint 40. Previous studies have suggested that structural changes in the patellofemoral joint provide better explanation for knee pain than those in the tibiofemoral joint, and this is also an important consideration as potential relationships between pain and muscle strength/CSA may contribute to our findings 41, 42.
Our study shows a potential direct association between E/F ratio and patellar cartilage degeneration. Confounding factors such as level of physical activity and sports participation may have played a role in this relationship, however we did not find a significant correlation between baseline PASE scores and extensor/flexor ratio to suggest that the level to which physical activity confounded these results was significant. Certain variables such as involvement in high-impact sport activities which may preferentially increase extensor muscle bulk, may also be direct contributors to patellar cartilage deterioration, and future studies on this topic may be able to shed light on whether such factors could play a role in this relationship.
Our study has several limitations which should also be discussed. We used the KL scale for subject selection and only included those with KL Grade 0 or 1. The KL grade solely pertains to radiographic OA of the tibiofemoral joint 43. However, the main findings in our study related to the effect of thigh muscle strength and CSA on the patellar and trochlear cartilage. It is therefore possible that our KL grade selection may have allowed for selection of patients who already had evidence of patellofemoral OA. However, a comparison of our MR data with a previous study by Pan et al. showed no significant difference in frequency of baseline patellar or patellofemoral joint cartilage lesions in our study cohort compared to a normal reference population from the OAI database 29. Secondly, a complicating limitation of our study pertains to our finding that baseline meniscal WORMS scores differed significantly between patients with low and high thigh muscle CSA, though longitudinal values did not differ significantly between these groups. Though the significance of this finding is unclear, it is possible that this may reflect an underlying protective effect of increased thigh muscle CSA as pertains to meniscal, and, by deduction, tibiofemoral cartilage health. The precise implications of this are beyond the scope of this study, and future investigation is warranted to further clarify this result.
Our study demonstrates potentially clinically significant findings regarding patellar cartilage deterioration in relation to muscle strength; however, the findings regarding tibiofemoral cartilage were less robust. One possible explanation may be related to the relatively lesser longitudinal changes seen in these cartilage compartments compared to those of the tibiofemoral joint amongst all included patients, unrelated to muscle CSA. Thus, a potential limiting factor of our study is that the deterioration seen in the tibiofemoral compartment over the 48 month time frame was not sufficient to allow adequate assessment of the relationship with muscle CSA. In that case, additional studies investigating longer periods of time would be needed to assess muscle strength effects on the tibiofemoral joint in particular.
Similarly, our study did not find significant associations between total muscle CSA values or WORMS scores and baseline or longitudinal change in WOMAC scores, which is not unexpected as previous studies have reported similar limited correlations between WORMS scores/muscle CSA values and WOMAC scores 13, 44. This may be explained by the fact that WOMAC scores report only knee functionality over the past seven days, and they may also have limitations in assessing pain/symptoms in this relatively young cohort (aged 50-60) with KL grades of 0 and 1.
In summary, our study shows increased total CSA of thigh muscle, and especially increased extensor to flexor CSA ratio, are significantly associated with worsening patellofemoral cartilage WORMS scores over 48 months. In light of our findings, more effort should be made to ensure that patients with purely patellofemoral OA maintain relatively low E/F ratios through specific exercises designed to increase hamstrings and quadriceps in the appropriate proportions. Additionally, more studies with increased numbers of patients would likely be necessary to investigate more thoroughly the exact role of flexion strength in knee OA prevention. Such knowledge would shed light on how targeted strengthening exercises for various lower extremity muscle groups might ultimately guide conservative management of knee OA.
Acknowledgments
This research was supported in part by funding from the National Institute of Arthritis, Musculoskeletal and Skin Diseases, a division of the National Institutes of Health, under award number P50AR060752. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
The authors would like to extend sincere thanks to the members of the Musculoskeletal and Quantitative Imaging Research group, a division of the Department of Radiology and Biomedical Imaging at UCSF, for their support during the duration of this project.
Role of funding source: This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Funding: This research was supported in part by funding from the National Institute of Arthritis, Musculoskeletal and Skin Diseases, a division of the National Institutes of Health, under award number P50AR060752.
Footnotes
Author contributions: Drs. Lauren H. Goldman and Thomas M. Link both had full access to all of the study data and assume responsibility for its integrity and the accuracy of the data analysis.
Conception and design: Link TM, Heilmeier U, Joseph GB.
Analysis and interpretation of the data: Goldman LH, Tang K, Nevitt MC, McCulloch CE, Joseph GB.
Drafting of the article: Goldman LH.
Critical revision of the article for important intellectual content: Souza RB, Heilmeier U, Nevitt MC, McCulloch CE, Link TM.
Final approval of the article: all authors.
Statistical expertise: Joseph GB, Nevitt MC, McCulloch CE.
Collection and assembly of data: Goldman LH, Facchetti L and Tang K.
Competing interests: Nothing to declare. There is no conflict of interest and the paper has not been submitted elsewhere.
References
- 1.Carmona-Terés V, Lumillo-Guttiérez I, Jodar-Fernández L, Rodriguez-Blanco T, Moix-Queraltó J, Pujol-Ribera E, et al. Effectiveness and cost-effectiveness of a health coaching intervention to improve the lifestyle of patients with knee osteoarthritis: cluster randomized clinical trial. BMC Musculoskelet Disord. 2015;16:1–12. doi: 10.1186/s12891-015-0501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts VI, Esler CNA, Harper WM. A 15-year follow-up study of 4606 primary total knee replacements. J Bone Joint Surg Br. 2007;89B:1452–1456. doi: 10.1302/0301-620X.89B11.19783. [DOI] [PubMed] [Google Scholar]
- 3.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 4.Øiestad BE, Juhl CB, Eitzen I, Thorlund JB. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis Osteoarthritis Cartilage. 2015;23:171–177. doi: 10.1016/j.joca.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Glass NA, Torner JC, Frey Law LA, Wang K, Yang T, Nevitt MC, et al. The relationship between quadriceps muscle weakness and worsening of knee pain in the MOST cohort: a 5-year longitudinal study. Osteoarthritis Cartilage. 2013;21:1154–1159. doi: 10.1016/j.joca.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin S, Baker K, Niu J, Clancy M, Goggins J, Guermazi A, et al. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60:189–198. doi: 10.1002/art.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segal NA, Glass NA, Torner J, Yang M, Felson DT, Sharma L, et al. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage. 2010;18:769–775. doi: 10.1016/j.joca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Esch M, Knoop J, van der Leeden M, Roorda LD, Lems WF, Knol DL, et al. Clinical phenotypes in patients with knee osteoarthritis: a study in the Amsterdam osteoarthritis cohort. Osteoarthritis Cartilage. 2015;23:544–549. doi: 10.1016/j.joca.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Segal NA, Findlay C, Wang K, Torner JC, Nevitt MC. The longitudinal relationship between thigh muscle mass and the development of knee osteoarthritis. Osteoarthritis Cartilage. 2012;29:1534–1540. doi: 10.1016/j.joca.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaric S. Muscle strength testing: use of normalisation for body size. Sports Med. 2002;32:615–31. doi: 10.2165/00007256-200232100-00002. [DOI] [PubMed] [Google Scholar]
- 11.Jaric S. Role of body size in the relation between muscle strength and movement performance. Exerc Sport Sci Rev. 2003;31:8–12. doi: 10.1097/00003677-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Ruhdorfer AS, Dannhauer T, Wirth W, Cotofana S, Roemer F, Nevitt M, et al. Thigh muscle cross-sectional areas and strength in knees with early vs. knees without radiographic knee osteoarthritis: a between-knee, within-person comparison. Osteoarthritis Cartilage. 2014;22:1634–1638. doi: 10.1016/j.joca.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan J, Stehling C, Muller-Hocker C, Schwaiger BJ, Lynch J, McCulloch CE, et al. Vastus lateralis/vastus medialis cross-sectional area ratio impacts presence and degree of knee joint abnormalities and cartilage T2 determined with 3T MRI - an analysis from the incidence cohort of the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19:65–73. doi: 10.1016/j.joca.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Wluka AE, Berry PA, Siew T, Teichtahl AJ, Urquhart DM, et al. Increase in vastus medialis cross-sectional area is associated with reduced pain, cartilage loss, and joint replacement risk in knee osteoarthritis. Arthritis Rheum. 2012;64:3917–3925. doi: 10.1002/art.34681. [DOI] [PubMed] [Google Scholar]
- 15.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maly MR, Calder KM, MacIntyre NJ, Beattie KA. Intermuscular fat volume in the thigh relates to knee extensor strength and physical performance in women at risk for or with knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2013;65:44–52. doi: 10.1002/acr.21868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nevitt M, Felson D, Lester G. The Osteoarthritis Initiative. Protocol for the cohort study. 2006;1.1 [Google Scholar]
- 18.Bruce-Brand RA, Walls RJ, Ong JC, Emerson BS, O'Byrne JM, Moyna NM. Effects of home-based resistance training and neuromuscular electrical stimulation in knee osteoarthritis: a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:118. doi: 10.1186/1471-2474-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterfy CG, Schneidder E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterfy CG, Guermazi A, Zaim S, Tirman J, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Goutallier D, Postel JM, Bernaeau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures: pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78. [PubMed] [Google Scholar]
- 22.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 23.Alizai H, Nardo L, Karampinos DC, Joseph GB, Yap SP, Baum T, et al. Comparison of clinical semi-quantitative assessment of muscle fat infiltration with quantitative assessment using chemical shift-based water/fat separation in MR studies of the calf of post-menopausal women. Eur Radiol. 2012;22:1592–1600. doi: 10.1007/s00330-012-2404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nardo L, Karampinos DC, Lansdown DA, Carballido-Gamio J, Lee S, Maroldi R, et al. Quantitative assessment of fat infiltration in the rotator cuff muscles using water-fat MRI. J Magn Reson Imaging. 2014;39:1178–1185. doi: 10.1002/jmri.24278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. RadioGraphics. 2010;30:961–981. doi: 10.1148/rg.304095755. [DOI] [PubMed] [Google Scholar]
- 26.Hunter DJ, Zhang YQ, Niu JB, Felson DT, Kowh K, Newman A, et al. Patella malalignment, pain and patellofemoral progression: the Health ABC Study. Osteoarthritis Cartilage. 2007;15:1120–1127. doi: 10.1016/j.joca.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the Osteoarthritis Initiative. Radiology. 2010;254:509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahl R, Jain SK, Lutz J, Wyman BT, Le Graverand-Gastineau MPH, Vignon E, et al. Osteoarthritis of the knee at 3.0 T: comparision of a quantitative and semi-quantitative score for the assessment of the extent of cartilage lesion and bone marrow edema pattern in a 24-month longitudinal study. Skeletal Radiol. 2011;40:1315–1327. doi: 10.1007/s00256-011-1156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan J, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology. 2011;261:507–515. doi: 10.1148/radiol.11102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arokoski MH, Arokoski JPA, Haara M, Kankaanpää M, Vesterinen M, Niemitukia LH, et al. Hip muscle strength and muscle cross sectional area in men with and without hip osteoarthritis. J Rheumatol. 2002;29:2185–2195. [PubMed] [Google Scholar]
- 31.Hafez AR, Al-Johani AH, Zakaria AR, Al-Ahaideb A, Buragadda S, Melam GR, et al. Treatment of knee osteoarthritis in relation to hamstring and quadricep strength. J Phys Ther Sci. 2013;25:1401–1405. doi: 10.1589/jpts.25.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heiden TL, Lloyd DG, Ackland TR. Knee extension and flexion weakness in people with knee osteoarthritis: is antagonist cocontraction a factor? J Ortho Sports Phys Ther. 2009;39:807–815. doi: 10.2519/jospt.2009.3079. [DOI] [PubMed] [Google Scholar]
- 33.Mikesky AE, Mazzuca SA, Brandt KD, Perkins SM, Damush T, Lane KA. Effects of strength training on the incidence and progression of knee osteoarthritis. Arthritis Care Res. 2006;55:690–699. doi: 10.1002/art.22245. [DOI] [PubMed] [Google Scholar]
- 34.Visser AW, de Mutsert R, Loef M, le Cessie S, den Heijer M, Bloem JL, et al. The role of fat mass and skeletal muscle mass in knee osteoarthritis is different for men and women: the NEO study. Osteoarthritis Cartilage. 2014;22:197–202. doi: 10.1016/j.joca.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers MF. Isometric quadriceps strength in women with mild, moderate, and severe knee osteoarthritis. Am J Phys Med Rehabil. 2010;89:541–548. doi: 10.1097/PHM.0b013e3181ddd5c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138:613–619. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 37.van Meer BL, Meuffels DE, van Eijsden WA, Verhaar JAN, Bierma-Zeinstra SMA, Reijman M. Which determinants predict tibiofemoral and patellofemoral osteoarthritis after anterior cruciate ligament injury? A systematic review. Br J Sports Med. 2015;0:1–11. doi: 10.1136/bjsports-2013-093258. [DOI] [PubMed] [Google Scholar]
- 38.Kannus P. Ratio of hamstring to quadriceps femoris muscle, strength in the anterior cruciate ligament insufficient knee. Relationship to long term recovery Phys Ther. 1988;6:961–965. doi: 10.1093/ptj/68.6.961. [DOI] [PubMed] [Google Scholar]
- 39.Myer GD, Ford KR, Barber KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19:3–8. doi: 10.1097/JSM.0b013e318190bddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzuca SA, Brandt KD, Katz BP, Ding Y, Lane KA, Buckwalter KA. Risk factors for progression of tibiofemoral osteoarthritis: an analysis based on fluoroscopically standardised knee radiography. Ann Rheum Dis. 2006;65:515–519. doi: 10.1136/ard.2005.039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cicuttini FM, Baker J, Hart DJ, Spector TD. Association of pain with radiological changes in different compartments and views of the knee joint. Osteoarthritis Cartilage. 1996;4:143–147. doi: 10.1016/s1063-4584(05)80323-1. [DOI] [PubMed] [Google Scholar]
- 42.Lanyon P, O'Reilly S, Jones A, Doherty M. Radiographic assessment of symptomatic knee osteoarthritis in the community: definitions and normal joint space. Ann Rheum Dis. 1998;57:595–601. doi: 10.1136/ard.57.10.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandt KD, Fife RS, Braunstein EM, Katz B. Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum. 1991;34:1381–1386. doi: 10.1002/art.1780341106. [DOI] [PubMed] [Google Scholar]
- 44.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of MRI-based knee cartilage T2 measurements and focal lesions with knee pain - data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2013;64:248–255. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]


