Abstract
Carnitine acetyltransferase (CRAT) deficiency has previously been shown to result in muscle insulin resistance due to accumulation of long-chain acylcarnitines. However, differences in the acylcarnitine profile and/or changes in gene expression and protein abundance of CRAT in myotubes obtained from obese patients with type 2 diabetes mellitus (T2DM) and glucose-tolerant obese and lean controls remain unclear. The objective of the study was to examine whether myotubes from obese patients with T2DM express differences in gene expression and protein abundance of CRAT and in acylcarnitine species pre-cultured under glucose and insulin concentrations similar to those observed in healthy individuals in the over-night fasted, resting state. Primary myotubes obtained from obese persons with or without T2DM and lean controls (n=9 in each group) were cultivated and harvested for LC-MS-based profiling of acylcarnitines. The mRNA expression and protein abundance of CRAT were determined by qPCR and Western Blotting, respectively. Our results suggest that the mRNA levels and protein abundance of CRAT were similar between groups. Of the 14 different acylcarnitine species measured by LC-MS, the levels of palmitoylcarnitine (C16) and octadecanoylcarnitine (C18) were slightly reduced in myotubes derived from T2DM patients (p<0.05) compared to glucose-tolerant obese and lean controls. This suggests that the CRAT function is not the major contributor to primary insulin resistance in cultured myotubes obtained from obese T2DM patients.
Abbreviations: CRAT, carnitine acetyltransferase; LC-MS, Liquid Chromatography Mass Spectrometry; PDH, Pyruvate Dehydrogenase; T2DM, type 2 diabetes mellitus; ZDF, Zucker diabetic fatty
Keywords: Carnitine acetyltransferase, Type 2 diabetes mellitus, Insulin, Resistance, Acylcarnitine, LC-MS, Myotubes
Highlights
-
•
Gene expression and protein abundance of CRAT are not altered in myotubes derived from T2D patients.
-
•
Palmitoylcarnitine (16:0) and octadecanoylcarnitine (C18) are reduced in myotubes derived from T2D patients.
-
•
CRAT function is not the major contributor to primary insulin resistance.
1. Introduction
Carnitine acetyltransferase (CRAT), a mitochondrial matrix enzyme proposed as a potent regulator of metabolic inflexibility, has been found to impact whole-body glucose homeostasis and muscle-specific loss of function results in reduced metabolic control, which resembles the insulin resistant state [1]. A study conducted by Noland and colleagues suggests that CRAT overexpression in primary human skeletal myocytes increased glucose uptake and improved lipid-induced suppression of glucose oxidation [2]. In this regard, CRAT could be involved in the compensatory response to glucose intolerance. However, the potential role of CRAT within skeletal muscle insulin resistance is poorly understood.
Recently, Seiler and colleagues proposed that CRAT activity was decreased in response to genetic diabetes, high-fat diet and lipid exposure [3]. They also stated that reduced CRAT activity was accompanied by accumulation of long chain acylcarnitines in skeletal muscle and a decline in the acetylcarnitine/acetyl coenzyme A (CoA) ratio [3]. Since long chain acylcarnitines have been shown to be associated with insulin resistance, and reflect increased lipid flux in T2DM [4], it is possible that carnitine supplementation is an ideal solution for treating T2DM [1]. However, contradictory results have shown that muscular lipid oxidation is normal or even increased in T2DM patients and mice fed a high-fat diet [5].
Previously, we have shown that myotubes established from T2DM patients express primary insulin resistance at the level of glucose uptake, storage and oxidation [6], however, the molecular background is unclear. In the present study, we took advance of this model to investigate CRAT expression and acylcarnitine distribution under basal conditions, in order to clarify whether changes in mRNA levels and protein abundance of CRAT and/or the present acylcarnitine profile can explain primary (genetic) insulin resistance in these cells.
2. Methods
2.1. Participants and cell culture
The present study examined myotubes obtained and cultured from muscle biopsies collected in a previously reported study [7]. In brief, 10 obese T2DM patients were matched to 10 obese and 10 lean control participants according to BMI, age and gender. Their clinical characteristics have previously been published [7] (Table 1). All participants gave written, informed consent, and the local ethics committee of Funen and Vejle County approved the study. Human myotubes cultures were established as previously described [6], [7], [8] and allowed to differentiate under basal conditions of insulin (25 pmol/l) and glucose (5.5 mmol/l) for 8 days, similar to those observed in healthy individuals in the over-night fasted, resting state. However, one person in each group was lost under sample preparation. Therefore, only nine persons in each group were used.
Table 1.
Clinical characteristics.
| Control, lean | Control, obese | T2DM | |
|---|---|---|---|
| N | 9 | 9 | 9 |
| Age (years) | 50.8±1 | 48.1±1 | 50±1 |
| Weight (kg) | 71.6±3.0 | 105.5±6.4* | 102.2±4.1* |
| BMI (kg/m2) | 24.2±0.5 | 34.0±1.4* | 33.5±1.1* |
| Fasting plasma glucose (mM) | 5.7±0.1 | 5.7±0.2 | 10.0±0.7# |
| Fasting serum insulin (pM) | 23.9±5.7 | 52.6±5.0* | 96.7±10.1# |
| Glucose infusion rate (mg/min) | 383.3±20.4 | 254.1±28.3* | 117.8±18.6# |
| HbA1c(%) | 5.5±0.1 | 5.4±0.1 | 7.5±0.5# |
| Fasting total cholesterol (mM) | 5.29±0.22 | 5.33±0.41 | 5.42±0.37 |
| Fasting LDL cholesterol (mM) | 2.94±0.22 | 3.18±0.33 | 3.20±0.27 |
| Fasting HDL cholesterol (mM) | 1.85±0.15 | 1.54±0.15 | 1.36±0.03* |
| Fasting plasma triglyceride (mM) | 1.12±0.16 | 1.34±0.18 | 1.93±0.40 |
Data are means±SEM.* Significant different from the lean controls (p<0.05),# significant different from the lean and obese controls (p<0.05).
2.2. Metabolic profiling of acylcarnitines
The human myotubes were harvested in cold PBS and sonicated for 10–15 min. Protein concentrations were determined using the Pierce BCA protein assay kit. Internal standards were added (5pmol Hexadecanoyl-L-carnitine chloride (71–1732-5 Larodan), 5 pmol Octadecanoyl (18,18,18-D3)-L-carnitine chloride (71–1748 Larodan), 250 pmol Acetyl (D3)-L-carnitine chloride (71–1746-5 Larodan), 20 pmol Butyryl (4,4,4-D3)-carnitine chloride (71–1734 Larodan)). The cell lysate was extracted in 3:1 ACN: MeOH, vortexed and spun down for 10 min, 10.000 rpm, 4 °C. The sample was freeze-dried and re-dissolved in 50 µl 0.1% formic acid. Sample (10 µl) was injected into the HPLC-MS system. Samples were analysed on an Agilent 1290 HPLC coupled to an Agilent 6530 mass spectrometer. Analyte separation was achieved using a Agilent ZORBAX RRHD Eclipse Plus C18 column (2.1×150 mm2, 1.8 µm) with a Agilent ZORBAX Eclipse Plus C18 guard column (2.1 mm, 1.8 µm) both heated to 40 °C, 300 µl/min flow of mobile phase 0.1% formic acid (A) and 0.1% formic acid in acetonitrile (B) with the following gradient: 3% B for 5 min, to 15% B over 3 min, to 30% B over 2 min, to 97% B over 9 min and 97% B for 3 min. Reference ions were infused with a flow of 10 µl/min. The mass spectrometer was operated in positive ion mode with the following settings: 2 GHz extended dynamic range from 100 to 1500 m/z, gas temperature: 300 °C, drying gas flow (nitrogen): 10 l/min, nebulizer: 35 psi, sheath gas temperature: 350 °C, Sheath gas flow:11 L/min, capillary voltage: 3500 V, fragmentor: 125 V, acquisition rate: 3 spectra/s, reference correction two points at m/z 121.050873 and m/z 922.009798. MassHunter Profinder, Agilent Technologies was used to detect and quantify 14 different acylcarnitine species. These were C0, C2, C3, C4, C6, C8, C10, C12, C14, C16:1, C16, C18:2, C18:1, C18:0.
2.3. qPCR
Total RNA was purified and cDNA was synthesized as previously described [9]. qPCR was performed on The LightCycler® 480 Real-Time PCR system (Roche) using commercially available SYBR Green Jumpstart Taq Ready Mix (Sigma) according to the manufacturer's instructions. The following conditions were used: 2 min at 95 °C (hot start), 40 cycles of 15 s at 95 °C, 45 s at 60 °C, and 45 s at 72 °C. The CRAT expression was normalized against the expression of the housekeeping gene TBP. The following primers were used: CRAT fwd: 5′-GACACAGTCAGCAACTTCAGC, CRAT rev: 5′ GCTGCACAAAGATCTGATCCG; TBP fwd: 5′-GCCCGAAACGCCGAATAT, TBP rev: 5′CCTCATGATTACCGCAGCAAA.
2.4. Western Blot
Proteins were extracted and analysed as previously described [9]. Protein concentration was determined using the Pierce BCA protein assay kit and equal amounts of cell protein (10 μg) were loaded in each well. Rabbit Anti-CRAT (1:250) (Sigma HPA022815) and Rabbit Anti-VDAC1 (1:1000) were used as primary antibodies. VDAC1 was used as loading control in order to normalize the protein abundance of CRAT to the mitochondrial content. Horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2000) (Promega W401B) was used as secondary antibody.
2.5. Statistics
Statistical analysis was calculated as One-way ANOVA with Dunnett's multiple comparisons test using GraphPad Prism version 5.01. p<0.05 was considered statistically significant; n used for analysis is the total number of individuals. Mean±SEM is shown.
3. Results
The clinical characteristics show that fasting glucose, serum insulin and HbA1c levels were significantly higher, whereas the fasting HDL cholesterol level was significantly lower in T2DM patients compared to lean controls, (Table 1). Serum insulin level was higher in obese compared to lean controls, and the glucose infusion rate (GIR) measured during the hyperinsulinemic euglycemic clamp was significantly lower in obese with and without T2DM compared to lean controls (Table 1).
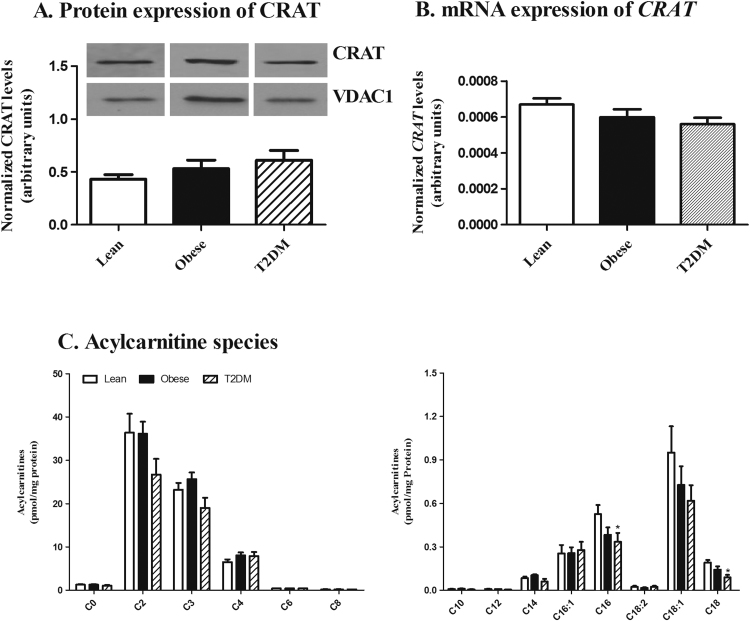
The protein and mRNA levels of CRAT were determined in cultured myotubes from obese with and without T2DM and lean persons (Fig. 1 A and B). Our results show no significant difference between the investigated groups. By using LC-MS, no significant difference was found in the total acylcarnitine level (166.32±8.37, lean; 170±7.1, obese; 130±15.5, T2D, Mean±SEM, pmol/mg protein, p<0.05) (data not shown) or among individual short- and medium-chain acylcarnitines between groups (Mean±SEM, pmol/mg protein, and p<0.05) (Fig. 1C). Muscular levels of C16 and C18 carnitine (Mean±SEM, pmol/mg protein, p<0.05) were significantly reduced 36% and 53% in T2DM patients compared to lean controls (Fig. 1C). No significant differences were observed among other long-chain acylcarnitines (Mean±SEM, pmol/mg protein, p>0.05) (Fig. 1C).
Fig. 1.
CRAT expression and the acylcarnitine profile in human myotubes. (A) The protein abundance of CRAT (70 kDa) was determined by western blotting using VDAC1 (31 kDa) as loading control. Band intensities were quantified by densitometry and expressed as the ratio between CRAT and VDAC band intensities. (B) The mRNA level of CRAT was determined by qPCR and normalized to TBP levels. (C) Acylcarnitine species in human myotubes obtained from obese persons with and without T2DM and lean controls were analysed by LC-MS. Mean±SEM is shown, n =9. Statistical analysis was assessed by One-way ANOVA with Dunnett's multiple comparisons test, *(p<0.05).
4. Discussion
T2DM myotubes cultured under basal conditions express primary insulin resistance and show several signs of impaired mitochondrial function [6]. In the present study, we aimed to investigate to which extent CRAT and acylcarnitine levels express alterations in myotubes established from obese T2DM patients. The mRNA levels and protein abundance of CRAT were similar between groups and C16- and C18-carnitines were significantly reduced in myotubes derived from T2DM patients compared to glucose-tolerant obese and lean controls. These data provide evidence that mRNA levels and proteins abundance of CRAT are unaltered in T2DM muscle and does not result in accumulation of acylcarnitine species. However, a reduction in C16 and C18 carnitine was seen, which suggests that CRAT is not important for primary insulin resistance in T2DM muscle.
Our findings are in accordance with an early study with Zucker diabetic fatty (ZDF) rats showing that the protein abundance of CRAT was unchanged in ZDF rats on a normal diet, but lipid surplus inhibited CRAT in order to counter-regulate glucose oxidation [3]. One could speculate that CRAT activity is reduced despite that similar mRNA expression and protein expression of CRAT are similar between groups. Nevertheless, we did not see any significant increase in the acetylcarnitine levels between groups, indicating that CRAT activity is unaltered.
Muoio and colleagues have shown that muscle-specific loss of functional CRAT in mice could not regulate the flux of acetyl CoA, which resulted in accumulation of acetyl CoA within the mitochondria and allosteric inhibition of pyruvate dehydrogenase (PDH), which subsequently impaired glucose utilization [1]. Glucose intolerance, insulin resistance, and metabolic inflexibility were seen in these mice [1]. Interestingly, medium- and long-chain acylcarnitines accumulated in muscle, which could suggest dysregulation in carnitine metabolism. L-Carnitine supplementation for six month in transgenic mice with muscle-specific loss of functional CRAT improved the insulin sensitivity suggesting that metabolic inflexibility was partially ameliorated by carnitine supplementation [1]. In addition, previous studies have shown that myotubes exposed to surplus of palmitate express secondary insulin resistance with concomitant increased acyl CoA levels [10], and would expect that acylcarnitine will be increased based on mass action. The exact mechanism for lipid-induced insulin resistance is unknown, but both long-chain acyl-CoAs and acylcarnitines may be part hereof giving the distinct functions of acylcarnitines in mitochondrial lipid metabolism such as preventing accumulation of noxious long-chain acyl-CoAs and reducing CoA trapping. The increase in long-chain acyl-CoA, diacylglycerol, and lipid oxidation products will promote induction of insulin resistance though atypical protein kinase C activation and decreases insulin stimulated activity of insulin receptor substrate-1 associated phosphatidylinositol-3-kinase and subsequently reduce glucose uptake and storage. Thus, CRAT may be an important pathophysiological factor for the development of lipid-induced insulin resistance, but not primary insulin resistance. Further, studies have to clarify if CRAT activity is partly impaired by lipids per se and thereby enhance acylcarnitine levels.
Taken together, the present study shows that CRAT and short-, medium- or long-chain acylcarnitines are not important for primary insulin resistance in myotubes derived from obese T2DM patients. The precise events leading to primary insulin resistance in myotubes derived from obese T2DM patients remain unanswered.
Funding
The study was supported by grants from the Danish Medical Research Council, the Novo Nordisk Foundation and the Danish Diabetes Association.
Disclosure statement
The authors have nothing to disclose.
Acknowledgements
Irene Lynfort and Jesper Havelund provided excellent technical assistance. We thank Kurt Højlund and Klaus Levin for muscle biopsies. All authors fulfill the contribution requirements for authorship credit, S.M.Berg and M.Gaster were responsible for the conception of the study and wrote the first draft of the manuscript. S.M.Berg and N.J. Færgeman analysed the data. All authors contributed to the interpretation of the data, revised the article critically, and approved the final version of the paper to be published. N.J. Færgeman and M.Gaster are responsible for the integrity of the work as a whole and are the guarantors of this work.
Footnotes
Transparency data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2016.11.010.
Appendix A. Transparency document
Supplementary material
.
References
- 1.Muoio D.M., Noland R.C., Kovalik J.P., Seiler S.E., Davies M.N., DeBalsi K.L., Ilkayeva O.R., Stevens R.D., Kheterpal I., Zhang J., Covington J.D., Bajpeyi S., Ravussin E., Kraus W., Koves T.R., Mynatt R.L. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012;15:764–777. doi: 10.1016/j.cmet.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noland R.C., Koves T.R., Seiler S.E., Lum H., Lust R.M., Ilkayeva O., Stevens R.D., Hegardt F.G., Muoio D.M. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 2009;284:22840–22852. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seiler S.E., Martin O.J., Noland R.C., Slentz D.H., DeBalsi K.L., Ilkayeva O.R., An J., Newgard C.B., Koves T.R., Muoio D.M. Obesity and lipid stress inhibit carnitine acetyltransferase activity. J. Lipid Res. 2014;55:635–644. doi: 10.1194/jlr.M043448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mihalik S.J., Goodpaster B.H., Kelley D.E., Chace D.H., Vockley J., Toledo F.G., DeLany J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity. 2010;18:1695–1700. doi: 10.1038/oby.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mynatt R.L. Carnitine and type 2 diabetes. Diabetes/Metab. Res. Rev. 2009;25(Suppl 1):S45–S49. doi: 10.1002/dmrr.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaster M. Insulin resistance and the mitochondrial link. Lessons from cultured human myotubes, Biochimica et biophysica acta. 2007;1772:755–765. doi: 10.1016/j.bbadis.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Ortenblad N., Mogensen M., Petersen I., Hojlund K., Levin K., Sahlin K., Beck-Nielsen H., Gaster M. Reduced insulin-mediated citrate synthase activity in cultured skeletal muscle cells from patients with type 2 diabetes: evidence for an intrinsic oxidative enzyme defect. Biochim. Et. Biophys. Acta. 2005;1741:206–214. doi: 10.1016/j.bbadis.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Gaster M., Kristensen S.R., Beck-Nielsen H., Schroder H.D. A cellular model system of differentiated human myotubes. APMIS. 2001;109:735–744. doi: 10.1034/j.1600-0463.2001.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 9.Neess D., Bloksgaard M., Bek S., Marcher A.B., Elle I.C., Helledie T., Due M., Pagmantidis V., Finsen B., Wilbertz J., Kruhoffer M., Faergeman N., Mandrup S. Disruption of the acyl-CoA-binding protein gene delays hepatic adaptation to metabolic changes at weaning. J. Biol. Chem. 2011;286:3460–3472. doi: 10.1074/jbc.M110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Just M., Faergeman N.J., Knudsen J., Beck-Nielsen H., Gaster M. Long-chain Acyl-CoA is not primarily increased in myotubes established from type 2 diabetic subjects. Biochim. Et. Biophys. Acta. 2006;1762:666–672. doi: 10.1016/j.bbadis.2006.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material