Abstract
Because cartilage lacks nerves, blood vessels, and lymphatic vessels, it is thought to contain factors that inhibit the growth and development of those tissues. Chondroitin sulfate proteoglycans (CSPGs) are a major extracellular component in cartilage. CSPGs contribute to joint flexibility and regulate extracellular signaling via their attached glycosaminoglycan, chondroitin sulfate (CS). CS and CSPG inhibit axonal regeneration; however, their role in blood vessel formation is largely unknown. To clarify the function of CSPG in blood vessel formation, we tested salmon nasal cartilage proteoglycan (PG), a member of the aggrecan family of CSPG, for endothelial capillary-like tube formation. Treatment with salmon PG inhibited endothelial cell adhesion and in vitro tube formation. The anti-angiogenic activity was derived from CS in the salmon PG but not the core protein. Salmon PG also reduced matrix metalloproteinase expression and inhibited angiogenesis in the chick chorioallantoic membrane. All of these data support an anti-angiogenic role for CSPG in cartilage.
Abbreviations: BME, basement membrane extract; BSA, bovine serum albumin; CAM, chorioallantoic membrane; CS, chondroitin sulfate; CSPG, chondroitin sulfate proteoglycan; ECM, extracellular matrix; FAK, focal adhesion kinase; FBS, fetal bovine serum; GAG, glycosaminoglycan; GalNAc, N-acetylgalactosamine; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GdnHCl, guanidine hydrochloride; GlcUA, glucuronic acid; HSPG, heparan sulfate proteoglycan; KSPG, keratin sulfate proteoglycan; MMP, matrix metalloproteinase; OA, osteoarthritis; PBS, phosphate-buffered saline; PG, proteoglycan; UA, uronic acid
Keywords: Chondroitin sulfate proteoglycan, Glycosaminoglycan, Aggrecan, Angiogenesis, Vascular endothelial cell, Matrix metalloproteinase
Highlights
-
•
The role of CSPGs in blood vessel formation in cartilage is largely unknown.
-
•
Treatment of salmon PG inhibited in vitro and in vivo angiogenesis.
-
•
The CS portion of salmon PG was responsible for the anti-angiogenic activity.
-
•
Salmon PG also reduced MMP expression and inhibited cell adhesion.
-
•
Our results support an anti-angiogenic role for CSPG in cartilage.
1. Introduction
Chondroitin sulfate proteoglycans (CSPGs) are major components of cartilage, together with collagens and hyaluronan. Proteoglycan (PG) is a glycoprotein comprising a single core protein and attached glycosaminoglycan (GAG) polysaccharides. The GAG of CSPGs is mainly chondroitin sulfate (CS). Although CSPG is a major PG in cartilage, other PGs such as heparan sulfate PGs (HSPGs), dermatan sulfate PGs, and keratin sulfate PGs (KSPGs) are widely distributed in the cell surface and the extracellular matrix (ECM) of animal tissues [1].
CSPG and CS are major inhibitors of neuronal migration [2], [3]. Cartilage lacks neurons, blood vessels, and lymphatic vessels, which led to the hypothesis that cartilage contains substances that inhibit the growth of those tissues. Therefore, CSPG and CS are expected to have an inhibitory effect on blood vessel formation. Indeed, loss of PG in osteoarthritic (OA) cartilage is associated with loss of resistance to vascular invasion [4]. Human intervertebral disc aggrecan was shown to inhibit endothelial cell migration [5]. Inhibition of endothelial cell adhesion and migration would inhibit tubulogenesis. Although the anti-migration activity of aggrecan was attributed to its CS moiety, CS alone showed both anti-angiogenic [6] and pro-angiogenic activities [7]. Moreover, treatment with chondroitinase, which is a specific GAG lyase, inhibits endothelial cell proliferation and angiogenesis, indicating that endogenous CS in endothelial cells is essential for angiogenesis [8]. Thus, it remains unclear whether CSPGs play an inhibitory role in blood vessel formation.
To clarify the role of CSPG in blood vessel formation, we aimed to identify effects of salmon nasal cartilage PG on angiogenesis. The conventional procedure for extraction of PG uses 4 M guanidine hydrochloride (GdnHCl) [9]. Recently, a simple, low-toxicity procedure for extraction of PG from salmon nasal cartilage was developed using acetic acid [10]. Our previous report showed that the major PG in this salmon PG fraction is from the aggrecan family of CSPGs [11]. Based on the cDNA sequence, globular domains G1, G2, and G3 are conserved between salmon and mammalian aggrecan. However, the core protein of acetic acid-extracted salmon PG is partially fragmented compared to that of GdnHCl-extracted salmon PG [12]. Although the acetic acid-extracted PG is the fragmented CSPG fraction, it retains various biological activities and is the only commercially available PG for pharmacological use [13], [14], [15], [16], [17]. In this study, we show that salmon PG reduces the tube-like formation of vascular endothelial cells. The anti-angiogenic activity of salmon PG was derived from CS. In addition, salmon PG reduced matrix metalloproteinase (MMP) expression in endothelial cells. Our results support an anti-angiogenic function of CSPG and CS.
2. Materials and methods
2.1. Cell culture
The immortalized human umbilical vein endothelial cell line EA.hy926 was obtained from ATCC (CRL-2922, Manassas, VA, USA). The cells were maintained in Medium 199 (Life Technologies Japan, Tokyo, Japan) supplied with 10% fetal bovine serum (FBS) (GE Healthcare Japan, Tokyo, Japan) and penicillin/streptomycin (Life Technologies Japan). The cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
2.2. Purification of proteoglycan
Acetic acid-extracted salmon nasal cartilage PG was purchased from Ichimaru Pharcos Co., Ltd. (Gifu, Japan). The PG was repurified by ion-exchange chromatography as described previously with minor modifications [12]. Briefly, PG was dissolved in 7 M urea in 50 mM Tris-HCl buffer (pH 7.5) and applied to a column (2.5 cm×10 cm) filled with DEAE-sephacel (GE Healthcare Japan) at a flow rate of 0.5 mL/min. The column was washed with five column volumes of 7 M urea in 50 mM Tris–HCl buffer (pH 7.5) and eluted with five column volumes of 0.0–1.0 M NaCl in a linear gradient, and 5-mL fractions were collected. Uronic acid (UA)-rich fractions were pooled, dialyzed against pure water, and concentrated by Speedvac (Thermo Fisher Scientific, Waltham, MA, USA). The PG was sterilized with a 0.2-μm filter and stored at −80 °C until use. UA and protein contents in the purified PG were determined by the carbazole sulfuric acid method [18] and using Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA, USA), respectively. In this study, we used UA as a unit of PG and CS. The composition of CS in salmon PG was approximately 14.4% ΔDi-0S [glucuronic acid (GlcUA)- N-acetylgalactosamine (GalNAc)], 27.0% ΔDi-4S [GlcUA-GalNAc(4S)], 57.8% ΔDi-6S [GlcUA-GalNAc(6S)], 0.8% ΔDi-SD [GlcUA(2S)-GalNAc(6S)], and 0.0% ΔDi-SE [GlcUA-GalNAc(4S, 6S)].
2.3. Cell viability test
EA.hy926 cells were seeded in a 96-well microplate at 16,000 cells/cm2 and incubated in the culture medium at 37 °C for 24 h. After washing with phosphate-buffered saline (PBS), 0.1 mL of serum-free medium containing various concentrations of PG was added to the wells. After 24 or 48 h of incubation, 0.01 mL of WST-8 reagent (Cell count reagent SF, Nacalai Tesque Inc., Kyoto, Japan) was added to each well. The plate was incubated at 37 °C for 1 h. The absorbance at 450 nm of each well was measured using a Benchmark microplate reader (Bio-Rad).
2.4. Cell adhesion assay
A 96-well culture plate was incubated with 2 μg/well human plasma fibronectin (Life Technologies Japan) in PBS at 4 °C overnight. To block the uncoated area, the fibronectin-coated plate was incubated with 0.5% bovine serum albumin (BSA) (Sigma-Aldrich Japan, Tokyo, Japan) in PBS at 37 °C for 30 min and then washed with PBS three times. Thereafter, various amounts of PG in PBS (100 μL) were added to the well, and the plate was incubated at 37 °C for 30 min and then rinsed with PBS three times. Single-cell suspensions in serum-free media (20,000 cells/100 μL/well) were added to the well and incubated at 37 °C for 1 h. After incubation, the plate was vortexed on a plate shaker and washed with PBS three times to remove unattached cells. The numbers of attached cells were estimated by crystal violet assay. The crystal violet solution (0.2% in 25% methanol) was added to the well and incubated for 10 min. The plate was washed with water and then dried. Sodium dodecyl sulfate solution (1%) was added to the well to solubilize the stain. The number of cells was estimated by the absorbance at 570 nm of each well.
2.5. Enzymatic digestion of salmon PG
The PG (3 mg/mL) was incubated with 1 mg/mL Actinase E (Kaken Pharmaceutical Co. Ltd., Tokyo, Japan) for 16 h at 50 °C in 100 mM Tris–HCl (pH 8.0) and 10 mM CaCl2. The reaction was terminated by boiling for 3 min. The PG (3 mg/mL) was incubated with 250 mU/mL protease-free chondroitinase ABC (C3667, Sigma-Aldrich Japan) for 16 h at 37 °C in 50 mM Tris–HCl (pH 8.0), 60 mM CH3COONa, and 0.02% BSA. The reaction was terminated by boiling for 3 min. To remove digested CS oligosaccharides, the reaction was purified by ultrafiltration with Amicon Ultracel 30 kDa filters (Merck Millipore Ltd., Darmstadt, Germany). All reactions were sterilized with a Coaster Spin-X centrifuge filter unit (Corning Japan K.K., Tokyo, Japan). The efficiency of enzymatic digestion was evaluated using a combination of agarose gel electrophoresis and Stains-All staining (Sigma-Aldrich Japan).
2.6. In vitro tube formation
The in vitro angiogenesis assay was performed as described by Arnaoutova and Kleinman with minor modifications [19]. Briefly, 50 μL of Basement Membrane Extract (BME, Trevigen Inc., Gaithersburg, MD, USA) was added to a 96-well plate on ice. The plate was incubated for 30 min at 37 °C for gelling. EA.hy926 cells (5000 cells) in 100 μL of serum-free media with or without PG were added to the plate and cultured on the BME gel for 16–20 h. In some experiments, the enzyme-digested PG (mentioned above), CS4S (400655, Seikagaku Corp., Tokyo, Japan), or CS6S (400675, Seikagaku Corp.) was used instead of PG. The composition of CS4S was 1.7% ΔDi-0S, 74.1% ΔDi-4S, 23.0% ΔDi-6S, 1.0% ΔDi-SD, and 0.2% ΔDi-SE and that of CS6S was 1.9% ΔDi-0S, 12.7% ΔDi-4S, 75.0% ΔDi-6S, 7.6% ΔDi-SD, and 2.8% ΔDi-SE. Photographs were taken using a digital camera (E-330, Olympus Corp., Tokyo, Japan) attached to an Olympus CKX41 microscope. Tube formation was analyzed with ImageJ [20] using the Angiogenesis Analyzer Plug-in [21].
2.7. Real-time quantitative PCR and gelatin zymography
Confluent cells were incubated in media supplemented with 0.5% FBS and 0.5% BSA with or without PG for 24 h. Culture supernatants were used for gelatin zymography to analyze MMP expression. Gelatin zymography was performed as described previously [22]. To analyze the mRNA level, total RNA was isolated from the cells using an RNeasy mini kit (QIAGEN Japan, Tokyo, Japan) according to manufacturer's procedure. To generate cDNA, reverse transcription was performed with 1 μg of RNA using a High-Capacity cDNA Reverse Transcription Kit (Life Technologies Japan). Quantitative real-time PCR was performed using a StepOnePlus Real-time PCR system (Life Technologies Japan). The reaction mixtures contained each cDNA, FastStart Universal Probe Master [ROX] (Roche Diagnostics GmbH, Mannheim, Germany), and TaqMan Probes (Life Technologies Japan) for each gene (Table 1). The reaction was performed for 40 cycles of 95 °C for 10 s and 60 °C for 30 s followed by 95 °C for 10 min. The expression level was analyzed by the comparative Ct method and normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Table 1.
TaqMan probes for quantitative PCR and relative gene expression levels.
| Gene | TaqMan probe | Ct (mean) | SD | ΔCt |
|---|---|---|---|---|
| GAPDH | Hs02758991_g1 | 19.0 | 0.68 | 0.00 |
| MMP-1 | Hs00899658_m1 | 20.6 | 0.80 | 1.61 |
| MMP-2 | Hs01548727_m1 | 22.2 | 0.34 | 3.13 |
| MMP-3 | Hs00968305_m1 | 36.3 | 0.95 | 17.29 |
| MMP-7 | Hs01042796_m1 | 35.8 | 0.77 | 16.78 |
| MMP-9 | Hs00957555_m1 | 35.3 | 0.89 | 16.24 |
| MMP-14 | Hs01037009_g1 | 26.3 | 0.36 | 7.32 |
The Ct values for each gene were calculated based on eight individual samples.
ΔCt = Ct (each gene) – Ct (GAPDH)
2.8. Chick chorioallantoic membrane (CAM) assay
Fertilized white leghorn chicken eggs (Hasegawa Natural Farm, Aomori, Japan) were incubated in a humidified incubator at 38 °C. On day 2 of incubation, a circular window was opened following removal of 3 ml of albumen by using a needle. The window was sealed with a cellophane tape, and the eggs were returned to the incubator. On day 7, silicon rings (6.8 mm inner diameter) were placed on the CAM. Then, 50 μl of PG in PBS was applied into the ring. After 48 h of incubation, whipping cream was injected under the CAM to facilitate visualization of blood vessels. The density of the vessel area in the ring was quantified using AngioTool software [23].
3. Results
3.1. Effects of salmon PG on endothelial cell viability and adhesion
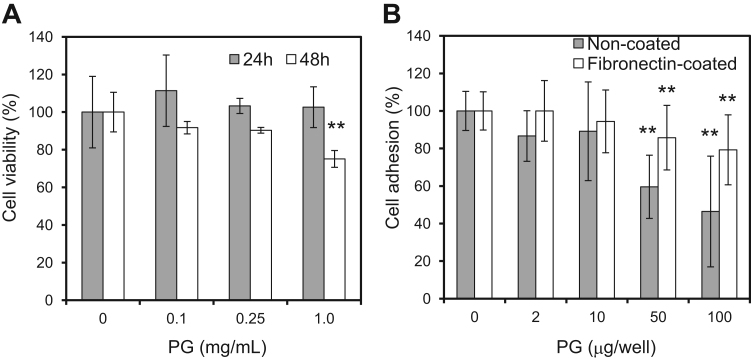
Endothelial cells were cultured with salmon PG to examine the effect of CSPG on cell growth and viability. There were no significant differences between the control and each concentration of PG (0–1 mg/mL) treatment after 24 h (Fig. 1A). However, after 48 h, the highest concentration of PG (1 mg/mL) reduced the number of cells compared to the control. These results indicate that the addition of salmon PG does not affect endothelial cell viability and growth for 24 h, but a high concentration of PG slightly reduced cell viability at longer culture times.
Fig. 1.
Effect of salmon PG on cell viability and adhesion of endothelial cells. A. EA.hy926 cells were cultured with PG in serum-free media for 24 or 48 h. The viability was measured by WST-8 assay. The columns show the mean values±SD (n =3). **p<0.01 versus control (0 mg/mL) (Student's t-test). B. EA.hy926 cells were cultured on a PG-coated dish (non-coated plastic surface or fibronectin-coated surface) for 1 h. The number of attached cells was estimated by crystal violet assay. The columns show the mean values±SD (n =9). **p<0.01 versus control (0 mg/mL) (Student's t-test).
CSPG extensively inhibits the adhesion of various cells to ECM proteins [24]. Therefore, we examined the effect of salmon PG on the adhesion of endothelial cells to fibronectin. PG was used to coat non-coated dish (plastic) and fibronectin-coated dishes. PG inhibited the adhesion of endothelial cells to the plastic surface and fibronectin in a concentration-dependent manner (Fig. 1B). These results indicate that salmon PG has anti-adhesive activity against endothelial cells as seen for other CSPGs.
3.2. Effect of salmon PG on endothelial tube formation
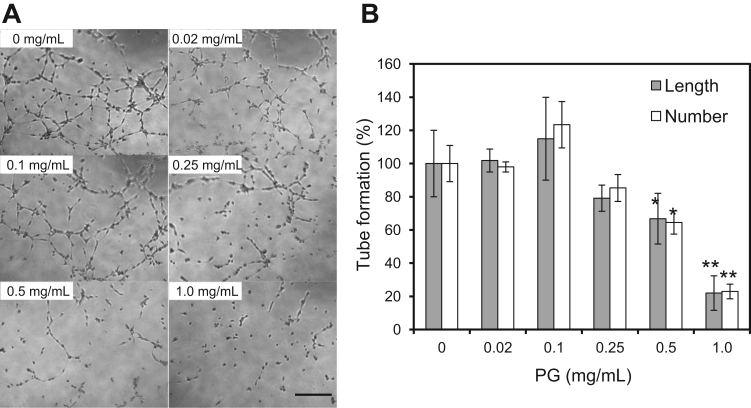
To examine whether CSPGs inhibit angiogenesis, we evaluated the effect of salmon PG on in vitro tube formation. The endothelial cells were cultured on BME from an Engelbreth-Holm-Swarm mouse and formed a capillary-like structure after 16–18 h of incubation under serum-free condition (Fig. 2A, 0 mg/mL). Media containing >0.5 mg/mL salmon PG significantly decreased both the length and number of the capillary-like structures (Fig. 2) in a concentration-dependent manner (Fig. 2B). These results suggest that salmon PG inhibits in vitro endothelial tube formation.
Fig. 2.
Effect of salmon PG on in vitro endothelial tube formation. A. EA.hy926 cells were incubated on the BME gel for 16–18 h. Incubation with PG significantly inhibited tube formation in a concentration-dependent manner (0–1.0 mg/mL PG). The scale bar indicates 0.1 mm. B. Quantitative analysis of branch length and number. The columns show values relative to the control (0 mg/mL)±SD (n =4). *p<0.05, **p<0.01 (Student's t-test).
3.3. Effect of salmon PG in the chick CAM assay
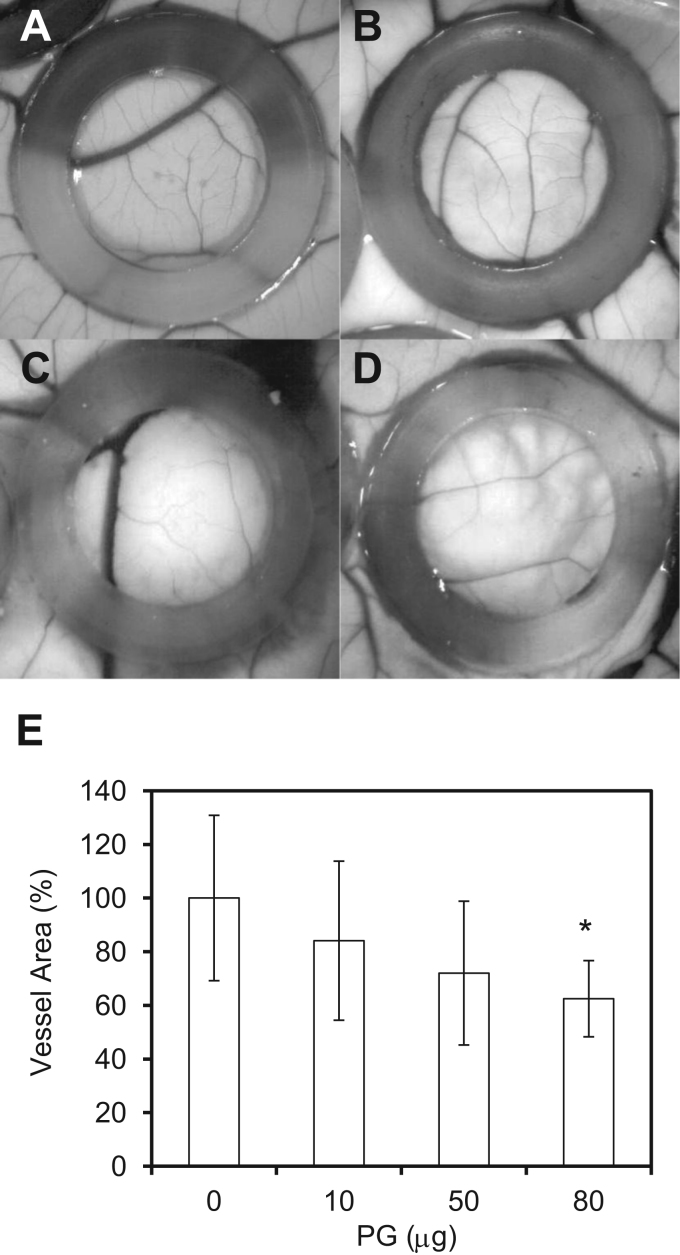
We next investigated the effect of salmon PG in the chick CAM assay as an in vivo angiogenesis model. Application of salmon PG inhibited the formation of blood vessels in the CAM in a dose-dependent manner (Fig. 3). At 80 μg/ring, PG reduced the vessel area by approximately 40% compared to the control (Fig. 3E). These results suggest that salmon PG also inhibits in vivo CAM angiogenesis.
Fig. 3.
PG inhibits angiogenesis on chick CAM. A-D. PG diluted in PBS was applied in the CAMs of 7 days old chick embryos. After 48 h of incubation, developing vessels on the CAM were observed under a microscope. E. Quantitative analysis of vessel area. The columns show values relative to the control (A)±SD. (A) 0 μg of PG / ring (n=10), (B) 10 μg (n=4), (C) 50 μg (n=8), (D) 80 μg (n=4). *p<0.05 (Student's t-test).
3.4. GAG is the active component for the anti-angiogenic activity of salmon PG
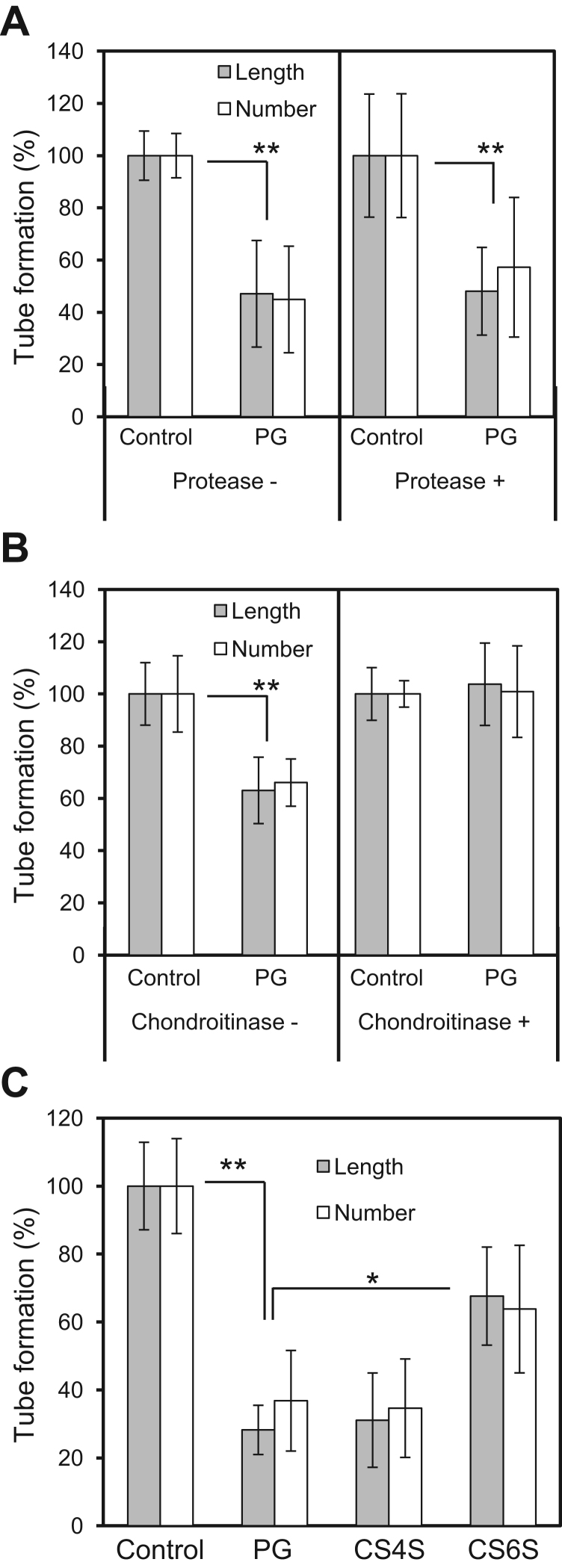
Most functions of PGs are derived from their attached GAGs, but the core protein is functional in some cases [1]. Therefore, we clarified which component in PG is essential for its anti-angiogenic activity. The protein components in salmon PG were digested by protease. To inactivate the enzyme, the reaction mixture was boiled; boiled PG did not lose its anti-angiogenic activity (Supplemental Fig. 1). The protease-treated PG retained anti-angiogenic activity (Fig. 4A) similar to that of untreated PG. Next, the CS chains of salmon PG were removed using chondroitinase ABC. The chondroitinase ABC-treated PG showed no inhibitory activity for tube formation (Fig. 4B). Thus, the CS chains in salmon PG are the active component underlying its anti-angiogenic activity.
Fig. 4.
Effect of enzymatic digestion of salmon PG on tube formation. A. Effect of protease-treated PG (1 mg/mL) on tube formation (n =6). B. Effect of chondroitinase ABC-treated PG (1 mg/mL) on tube formation (n =6). C. Effect of salmon PG, whale CS4S, or shark CS6S (1 mg/mL) on tube formation (n =4). The columns show values relative to the control (buffer)±SD. *p<0.05, **p<0.01 (Student's t-test).
3.5. Comparison with CS from other sources
Our data indicate that the CS chain in salmon PG is responsible for its anti-angiogenic activity. Since CS is structurally heterogeneous, we compared the anti-angiogenic activity of salmon PG to that of CS4S from whale and CS6S from shark to determine what structure of CS exhibits this activity. CS4S and PG showed higher anti-angiogenic activity than CS6S, with the inhibitory activity of CS4S similar to that of salmon PG (Fig. 4C). These results suggest that CS compositions are essential for anti-angiogenic activity.
3.6. Effects of salmon PG on MMP expression
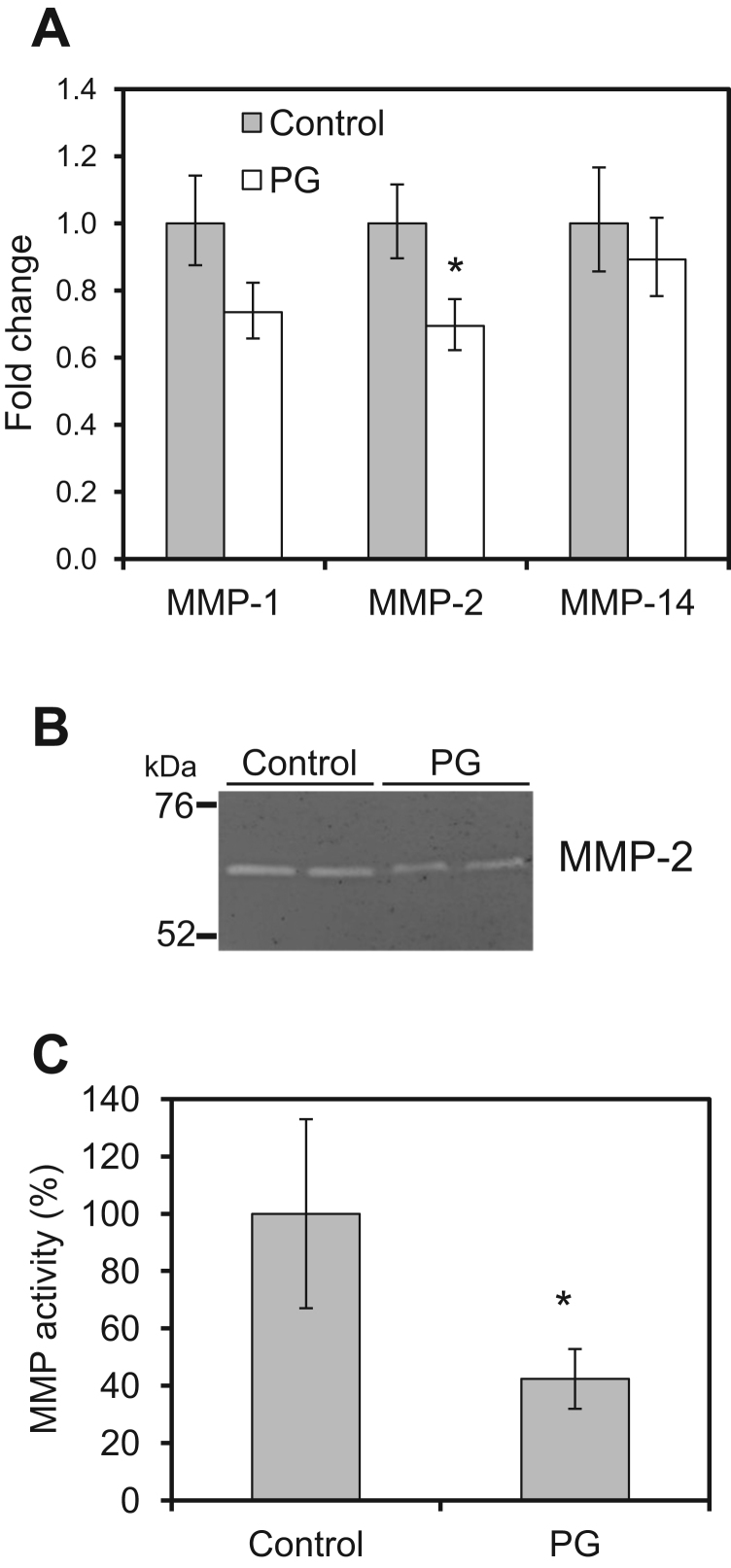
MMPs play important roles in angiogenesis. PGs are known to suppress the expression of MMP-2 and MMP-9 in vitro [25], [26]. Therefore, we examined the expression level of MMPs in endothelial cells treated with salmon PG. Under normal conditions, EA.hy926 cells expressed relatively high levels of MMP-1, -2, and -14 mRNA and far lower levels of MMP-3, -7, and -9 (Table 1). After 24 h of incubation with PG, the expression of MMP-1 and -2 mRNA was reduced by 15–20%, and that of MMP-14 was reduced slightly (Fig. 5A). In addition, gelatin zymography showed that the level of MMP-2 was significantly reduced by PG treatment; the 64–68 kDa intermediate form of MMP-2 was abundant, with less of the pro-from (72 kDa) and fully-activated form (62 kDa) observed, as reported previously (Fig. 5B) [27], [28]. PG treatment significantly reduced the amount of the intermediate form and did not increase the amount of the pro- or fully-activated form, suggesting that PG reduced the total amount and not the activation of MMP-2 (Fig. 5B, C). These results suggest that salmon PG inhibits the expression of MMPs, which contributes to its anti-angiogenic activity.
Fig. 5.
Effect of salmon PG on MMP expression. EA.hy926 cells were treated with 1 mg/mL PG for 24 h. A. Quantitative analysis of MMP-1, -2, and -14 mRNA by real-time quantitative PCR. The columns show values relative to each control±SD (n =4). B. Expression of MMP-2 was analyzed by gelatin zymography. An active intermediate form (66 kDa) of MMP-2 appeared on the gel. C. Quantification of MMP-2 in gelatin zymography. The columns show values relative to each control±SD (n =4). *p<0.05 (Student's t-test).
4. Discussion
The mechanisms underlying avascularity in cartilage remain unclear. Normal human cartilage is avascular, whereas OA cartilage loses its avascularity. The avascularity of OA cartilage has been associated with PGs and their GAGs [4], [29]. CSPG, the major PG in cartilage, is thought to inhibit vascularization. Angiogenesis involves basement membrane degradation, endothelial cell proliferation, migration, and tube formation, which are important targets for anti-angiogenic therapy. A previous study showed that human invertebrate disc aggrecan inhibits endothelial cell adhesion and migration [5]. However, it is still unclear whether CSPG inhibits tube formation.
The mechanisms underlying inhibition of angiogenesis by CSPG are unclear, although CS in salmon PG was essential for the anti-angiogenic activity (Fig. 4B). CSPG is also a well-known inhibitor of axon regeneration [2], [3]. Inhibition of focal adhesion kinase (FAK) phosphorylation in integrin signaling is one of the inhibitory mechanisms by which CSPG inhibits axonal regeneration [30]. This inhibition depends on CS in CSPG because chondroitinase pretreatment abolished the inhibition. FAK signaling is also important for angiogenesis [31]. Thus, inhibition of angiogenesis by CSPG might also occur through inhibition of FAK phosphorylation by the CS chain. Furthermore, the core protein could be involved in angiogenesis. It has been reported that Lumican, a KSPG, inhibits angiogenesis by reducing MMP-14 expression [25]. This anti-angiogenic activity of Lumican depends on direct binding between the core protein and the α2β1 integrin [32]. Herein, chondroitinase ABC-treated PG did not inhibit tube formation (Fig. 4B), suggesting that the core protein of salmon aggrecan has no anti-angiogenic activity. However, acetic acid-extracted salmon nasal PG lacks the C-terminal G3 domain of the core protein, as we reported previously [12]. Therefore, the function of the intact aggrecan core protein requires further evaluation.
In previous reports, administrations of CS had both pro- and anti-angiogenic effects [6], [7], although endogenous CS in endothelial cells is essential for angiogenesis [8]. The concentrations of CS/CSPG used in this study and others [6] are quite higher than the amount of endogenous CS in the endothelial cells. Therefore, such a large amount of CS, as present in the cartilage environment, would have an inhibitory effect against angiogenesis. However, high concentrations of CS also show pro-angiogenic activity [7], indicating that the composition of CS is also important. CS is a linear, non-branching sugar chain that comprises a disaccharide repeat of glucuronic acid (GlcUA) and N-acetylgalactosamine (GalNAc). GlcUA and GalNAc are modified with O-sulfation at C-2 and C-4 and/or C-6, respectively [2], [3]. Our previous and present data indicate that salmon PG contains approximately 21–27% ΔDi-4S and 58–63% ΔDi-6S [12]. For comparison, the composition of whale CS4S is 70–75% ΔDi-4S and 20–23% ΔDi-6S, and that of shark CS6S is 13–18% ΔDi-4S and 75–80% ΔDi-6S [33]. Accordingly, the CS composition in salmon PG is closer to that of shark CS6S than to that of whale CS4S. However, salmon PG and CS4S showed similar anti-angiogenic activity, whereas CS6S showed much lower activity (Fig. 4C). There are two possible reasons for this conflict between CS structure and activity. First, the PG form (core protein with GAG) might be more effective than CS alone. Our results indicate that salmon PG decreases adhesion of endothelial cells (Fig. 1B). Although CS is the main contributor to the anti-adhesive activity of CSPG, CS alone has no inhibitory activity [24]. Indeed, shark cartilage CS was shown to have no inhibitory effect on either cell attachment or spreading of endothelial cells, whereas shark cartilage extract inhibited the adhesion of endothelial cells [34]. Because the CS structures for salmon and shark are similar, salmon PG might have a greater effect on inhibition of adhesion. Therefore, the PG would have more anti-angiogenic activity than CS6S. The other possibility is that minor disaccharide units in shark CS6S could have a pro-angiogenic role. Shark CS6S contains 7–8% ΔDi-SD and 0.5–2.8% ΔDi-SE, whereas whale CS4S and salmon PG contain much lower contents of ΔDi-SD (1.0–1.8% and 0.6–0.8%, respectively) and undetectable levels of ΔDi-SE[12], [35]. These ΔDi-SD and ΔDi-SE units in shark CS6S might promote pro-angiogenic factors like heparin/HSPG. For example, ΔDi-SD and ΔDi-SE units have a high or moderate affinity for fibroblast growth factor family proteins [36]. Further investigations are needed to determine the relationship between CS structures and their role in angiogenesis.
The reduction of MMP expression by PG contributed to inhibition of angiogenesis. Interestingly, PG fractions from shark cartilage have inhibitory activity for MMP-2 and MMP-9, but CS6S from shark cartilage shows no significant inhibitory activity against MMP-2 and MMP-9 [26]. These data suggest that the protein component in PG or the PG form is important for inhibition of MMP expression. In this study, protease-treated PG still showed anti-angiogenic activity, whereas the chondroitinase ABC-treated PG did not (Fig. 4B). Because the protease-treated PG comprises GAG and small remaining peptides, this semi-PG form might be essential for the reduction of MMP expression. In fact, protein is necessary for the anti-adhesive effect of CSPG, but the structure of the protein is not important [24]. Thus, our data suggest that the CS in CSPG is the source of inhibitory activity, but the PG form could enhance the anti-angiogenic activity.
In conclusion, we show that salmon PG (aggrecan) inhibits not only endothelial cell adhesion but also tube formation and expression of MMPs. Our findings support the anti-angiogenic activity of CSPG and CS from cartilage.
Acknowledgments
Funding: This work was supported by the Regional Innovation Strategy Support Program from the Ministry of Education, Culture, Sports, Science and Technology in Japan, and Hirosaki University Grant for Exploratory Research by Young Scientists.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.11.009.
Transparency document associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2016.11.009.
Appendix A. Transparency document
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Appendix B. Supplementary material
Supplemental Figure 1. Effect of boiling on anti-angiogenic activity. Salmon PG was boiled for 5 min before in vitro tube formation. The boiled PG showed inhibitory activity similar to that of non-boiled PG. A. Number of branching tubes. B. Length of branching tubes. The columns show values relative to the control (0 mg/mL)±SD (n =3)
References
- 1.Iozzo R.V., Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maeda N. Proteoglycans and neuronal migration in the cerebral cortex during development and disease. Front. Neurosci. 2015;9:98. doi: 10.3389/fnins.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyata S., Kitagawa H. Mechanisms for modulation of neural plasticity and axon regeneration by chondroitin sulphate. J. Biochem. 2015;157:13–22. doi: 10.1093/jb/mvu067. [DOI] [PubMed] [Google Scholar]
- 4.Smith J.O., Oreffo R.O., Clarke N.M., Roach H.I. Changes in the antiangiogenic properties of articular cartilage in osteoarthritis. J. Orthop. Sci. 2003;8:849–857. doi: 10.1007/s00776-003-0717-8. [DOI] [PubMed] [Google Scholar]
- 5.Johnson W.E., Caterson B., Eisenstein S.M., Roberts S. Human intervertebral disc aggrecan inhibits endothelial cell adhesion and cell migration in vitro. Spine. 2005;30:1139–1147. doi: 10.1097/01.brs.0000162624.95262.73. [DOI] [PubMed] [Google Scholar]
- 6.Cornejo M.C., Cho S.K., Giannarelli C., Iatridis J.C., Purmessur D. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthr. Cartil. 2015;23:487–496. doi: 10.1016/j.joca.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Souza Lins Borba F.K., Felix G.L., Costa E.V., Silva L., Dias P.F., de Albuquerque Nogueira R. Fractal analysis of extra-embryonic vessels of chick embryos under the effect of glucosamine and chondroitin sulfates. Microvasc. Res. 2016;105:114–118. doi: 10.1016/j.mvr.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Denholm E.M., Lin Y.Q., Silver P.J. Anti-tumor activities of chondroitinase AC and chondroitinase B: inhibition of angiogenesis, proliferation and invasion. Eur. J. Pharmacol. 2001;416:213–221. doi: 10.1016/s0014-2999(01)00884-6. [DOI] [PubMed] [Google Scholar]
- 9.Sajdera S.W., Hascall V.C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J. Biol. Chem. 1969;244:77–87. [PubMed] [Google Scholar]
- 10.Majima M., Takagaki K., Sudo S., Yoshihara S., Kudo Y., Yamagishi S. Effect of proteoglycan on experimental colitis. Int. Congr. Ser. 2001;1223:221–224. [Google Scholar]
- 11.Kakizaki I., Tatara Y., Majima M., Kato Y., Endo M. Identification of proteoglycan from salmon nasal cartilage. Arch. Biochem. Biophys. 2011;506:58–65. doi: 10.1016/j.abb.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Kakizaki I., Mineta T., Sasaki M., Tatara Y., Makino E., Kato Y. Biochemical and atomic force microscopic characterization of salmon nasal cartilage proteoglycan. Carbohydr. Polym. 2014;103:538–549. doi: 10.1016/j.carbpol.2013.12.083. [DOI] [PubMed] [Google Scholar]
- 13.Sashinami H., Takagaki K., Nakane A. Salmon cartilage proteoglycan modulates cytokine responses to Escherichia coli in mouse macrophages. Biochem. Biophys. Res. Commun. 2006;351:1005–1010. doi: 10.1016/j.bbrc.2006.10.146. [DOI] [PubMed] [Google Scholar]
- 14.Kashiwakura I., Takahashi K., Takagaki K. Application of proteoglycan extracted from the nasal cartilage of salmon heads for ex vivo expansion of hematopoietic progenitor cells derived from human umbilical cord blood. Glycoconj. J. 2007;24:251–258. doi: 10.1007/s10719-007-9033-4. [DOI] [PubMed] [Google Scholar]
- 15.Asano K., Yoshimura S., Nakane A. Alteration of intestinal microbiota in mice orally administered with salmon cartilage proteoglycan, a prophylactic agent. PLoS One. 2013;8:e75008. doi: 10.1371/journal.pone.0075008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuyama A., Tanaka K., Kakizaki I., Kasai K., Chiba M., Nakamura T., Mizunuma H. Anti-inflammatory effect of proteoglycan and progesterone on human uterine cervical fibroblasts. Life Sci. 2012;90:484–488. doi: 10.1016/j.lfs.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Ohshika S., Ishibashi Y., Kon A., Kusumi T., Kijima H., Toh S. Potential of exogenous cartilage proteoglycan as a new material for cartilage regeneration. Int. Orthop. 2012;36:869–877. doi: 10.1007/s00264-011-1335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bitter T., Muir H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 19.Arnaoutova I., Kleinman H.K. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 2010;5:628–635. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 20.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.G.Carpentier, M.Martinelli, J.Courty, I.Cascone, Angiogenesis Analyzer for ImageJ, in: Proceedings of the 4th ImageJ User and Developer Conference proceedings, Mondorf-les-Bains, Luxembourg, pp. 198–201, 2012.
- 22.Nakamura T., Ishikawa T., Nanashima N., Miura T., Nozaka H., Nakaoka R., Sato T. 4-Methylumbelliferone induces the expression of membrane type 1-matrix metalloproteinase in cultured human skin fibroblasts. Biochem. Biophys. Res. Commun. 2002;298:646–650. doi: 10.1016/s0006-291x(02)02516-0. [DOI] [PubMed] [Google Scholar]
- 23.Zudaire E., Gambardella L., Kurcz C., Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One. 2011;6:e27385. doi: 10.1371/journal.pone.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamagata M., Suzuki S., Akiyama S.K., Yamada K.M., Kimata K. Regulation of cell-substrate adhesion by proteoglycans immobilized on extracellular substrates. J. Biol. Chem. 1989;264:8012–8018. [PubMed] [Google Scholar]
- 25.Niewiarowska J., Brézillon S., Sacewicz-Hofman I., Bednarek R., Maquart F.X., Malinowski M., Wiktorska M., Wegrowski Y., Cierniewski C.S. Lumican inhibits angiogenesis by interfering with α2β1 receptor activity and downregulating MMP-14 expression. Thromb. Res. 2011;128:452–457. doi: 10.1016/j.thromres.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Sato K., Murata N., Tsutsumi M., Shimizu-Suganuma M., Shichinohe K., Kitahashi T., Nishimura K., Nakamura Y., Ohtsuki K. Moderation of chemo–induced cancer by water extract of dried shark fin: anti–cancer effect of shark cartilage. Dev. Food Sci. 2004;42:159–168. [Google Scholar]
- 27.Ribatti D., Presta M., Vacca A., Ria R., Giuliani R., Dell'Era P., Nico B., Roncali L., Dammacco F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- 28.Palmieri D., Camardella L., Ulivi V., Guasco G., Manduca P. Trimer carboxyl propeptide of collagen I produced by mature osteoblasts is chemotactic for endothelial cells. J. Biol. Chem. 2000;275:32658–32663. doi: 10.1074/jbc.M002698200. [DOI] [PubMed] [Google Scholar]
- 29.Fenwick S.A., Gregg P.J., Rooney P. Osteoarthritic cartilage loses its ability to remain avascular. Osteoarthr. Cartil. 1999;7:441–452. doi: 10.1053/joca.1998.0238. [DOI] [PubMed] [Google Scholar]
- 30.Tan C.L., Kwok J.C., Patani R., Ffrench-Constant C., Chandran S., Fawcett J.W. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J. Neurosci. 2011;31:6289–6295. doi: 10.1523/JNEUROSCI.0008-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone R.L., Baggerly K.A., Armaiz-Pena G.N., Kang Y., Sanguino A.M., Thanapprapasr D., Dalton H.J., Bottsford-Miller J., Zand B., Akbani R., Diao L., Nick A.M., DeGeest K., Lopez-Berestein G., Coleman R.L., Lutgendorf S., Sood A.K. Focal adhesion kinase: an alternative focus for anti-angiogenesis therapy in ovarian cancer. Cancer Biol. Ther. 2014;15:919–929. doi: 10.4161/cbt.28882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeltz C., Brézillon S., Käpylä J., Eble J.A., Bobichon H., Terryn C., Perreau C., Franz C.M., Heino J., Maquart F.X., Wegrowski Y. Lumican inhibits cell migration through α2β1 integrin. Exp. Cell Res. 2010;316:2922–2931. doi: 10.1016/j.yexcr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Kakizaki I., Koizumi H., Chen F., Endo M. Inhibitory effect of chondroitin sulfate oligosaccharides on bovine testicular hyaluronidase. Carbohydr. Polym. 2015;121:362–371. doi: 10.1016/j.carbpol.2014.11.071. [DOI] [PubMed] [Google Scholar]
- 34.Chen J.S., Chang C.M., Wu J.C., Wang S.M. Shark cartilage extract interferes with cell adhesion and induces reorganization of focal adhesions in cultured endothelial cells. J. Cell. Biochem. 2000;78:417–428. [PubMed] [Google Scholar]
- 35.Mikami T., Yasunaga D., Kitagawa H. Contactin-1 is a functional receptor for neuroregulatory chondroitin sulfate-E. J. Biol. Chem. 2009;284:4494–4499. doi: 10.1074/jbc.M809227200. [DOI] [PubMed] [Google Scholar]
- 36.Asada M., Shinomiya M., Suzuki M., Honda E., Sugimoto R., Ikekita M., Imamura T. Glycosaminoglycan affinity of the complete fibroblast growth factor family. Biochim. Biophys. Acta. 1790;2009:40–48. doi: 10.1016/j.bbagen.2008.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplemental Figure 1. Effect of boiling on anti-angiogenic activity. Salmon PG was boiled for 5 min before in vitro tube formation. The boiled PG showed inhibitory activity similar to that of non-boiled PG. A. Number of branching tubes. B. Length of branching tubes. The columns show values relative to the control (0 mg/mL)±SD (n =3)