Abstract
Background
The consumption of green tea catechins (GTCs) suppresses age-related cognitive dysfunction in mice. GTCs are composed of several catechins, of which epigallocatechin gallate (EGCG) is the most abundant, followed by epigallocatechin (EGC). Orally ingested EGCG is hydrolyzed by intestinal biota to EGC and gallic acid (GA). To understand the mechanism of action of GTCs on the brain, their permeability of the blood brain barrier (BBB) as well as their effects on cognitive function in mice and on nerve cell proliferation in vitro were examined.
Methods
The BBB permeability of EGCG, EGC and GA was examined using a BBB model kit. SAMP10, a mouse model of brain senescence, was used to test cognitive function in vivo. Human neuroblastoma SH-SY5Y cells were used to test nerve cell proliferation and differentiation.
Results
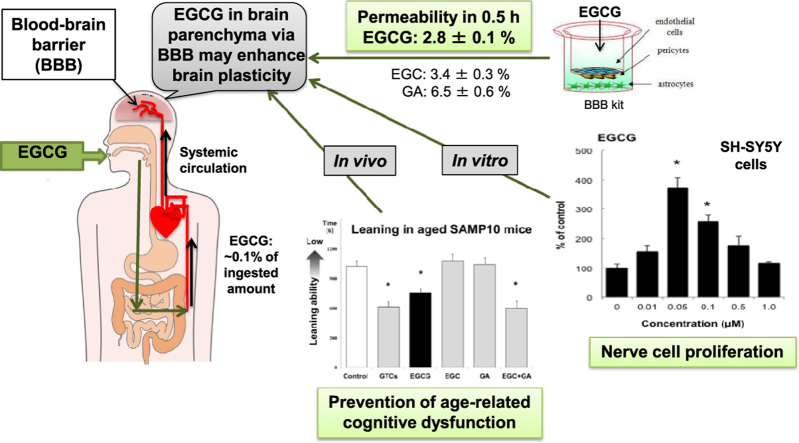
The in vitro BBB permeability (%, in 30 min) of EGCG, EGC and GA was 2.8±0.1, 3.4±0.3 and 6.5±0.6, respectively. The permeability of EGCG into the BBB indicates that EGCG reached the brain parenchyma even at a very low concentration. The learning ability of SAMP10 mice that ingested EGCG (20 mg/kg) was significantly higher than of mice that ingested EGC or GA. However, combined ingestion of EGC and GA showed a significant improvement comparable to EGCG. SH-SY5Y cell growth was significantly enhanced by 0.05 µM EGCG, but this effect was reduced at higher concentrations. The effect of EGC and GA was lower than that of EGCG at 0.05 µM. Co-administration of EGC and GA increased neurite length more than EGC or GA alone.
Conclusion
Cognitive dysfunction in mice is suppressed after ingesting GTCs when a low concentration of EGCG is incorporated into the brain parenchyma via the BBB. Nerve cell proliferation/differentiation was enhanced by a low concentration of EGCG. Furthermore, the additive effect of EGC and GA suggests that EGCG sustains a preventive effect after the hydrolysis to EGC and GA.
Abbreviations: BBB, blood-brain barrier; C, (+)-catechin; EC, (−)-epicatechin; EGC, (−)-epigallocatechin; EGCG, (−)-epigallocatechin gallate; GA, gallic acid; GTC, green tea catechin; LC-MS/MS, liquid chromatography tandem-mass spectrometry; LPO, lipid peroxidation; MRM, multiple reaction-monitoring; 8-oxodG, 8-oxodeoxyguanosine; SAMP10, senescence-accelerated mouse prone 10.
Keywords: Blood-brain barrier permeability, Brain plasticity, Cognitive dysfunction, (−)-epigallocatechin gallate, Green tea catechin, Nerve cell proliferation
Graphical abstract
Highlights
-
•
The in vitro BBB permeability of EGCG was 2.8±0.1% in 0.5 h.
-
•
EGCG is suggested to have reached into brain parenchyma.
-
•
Human neuroblastoma (SH-SY5Y) cell growth was enhanced by ~0.1 µM EGCG.
-
•
Age-related cognitive dysfunction was prevented in mice that ingested EGCG.
-
•
A low concentration of EGCG in the brain may enhance brain plasticity.
1. Introduction
A number of studies on green tea catechins (GTCs) have demonstrated their beneficial effects on health. We have shown that long-term intake of GTCs prevents aging-related cognitive dysfunction, using a mouse model of brain senescence (SAMP10) [1], [2], [3]. SAMP10 mice, which are a useful model for studying the aging brain, are characterized by an atrophied forebrain and cognitive impairment such as lowered learning and memory abilities [4], [5]. Since the regression of the mouse brain is similar to that of an aged human [6], SAMP10 was used in our study.
GTCs are composed of several catechins, among which epigallocatechin gallate (EGCG) is the main molecule, followed by epigallocatechin (EGC). EGCG was widely distributed in various organs of mouse using labeled EGCG [7]. Kohri and coworkers defined the metabolic profile of EGCG [8], [9] in which orally administered EGCG is first hydrolyzed to EGC and gallic acid (GA) by intestinal bacteria. Although most EGCG and its degradation products are excreted in the bile, a part of intact EGCG enters the blood circulation, peaking at 1–2 h after administration [8], [9], [10], [11], [12], [13].
The regional distribution of EGCG in the brain was reported to be ~4.95% using brain homogenate but it is not clear whether EGCG was transferred from blood vessels into the parenchyma [14]. The blood-to-brain distribution ratios of (+)-catechin (C) and (-)-epicatechin (EC) were measured by microdialysis sampling coupled to HPLC and detected by chemiluminescence [15]. In addition, the transporting efficiency of C and EC was reported using BBB models [16]. These results indicate that C and EC pass through the BBB. However, there is no reliable data of the permeability of EGCG and EGC into the BBB. Furthermore, the interaction between these catechins in BBB permeability has not been examined.
Oxidative damage contributes to aging [17]. The brain is very sensitive to the accumulation of oxidative damage of lipids, proteins and DNA. We have found that production of the superoxide anion increased with aging in the brain in mice, rats and birds [18]. The level was higher in the brains of SAMP10 mice than in the brains of same-aged control mice (SAMR1) with normal longevity. The consumption of GTCs, a potent antioxidant, suppressed oxidative damage in proteins and DNA of aged SAMP10 mice brains [1], [2], [19], suggesting that GTCs improve the balance between oxidation and reduction. However, it is unclear whether the suppression of oxidative damage can fully explain the suppression of cognitive function.
In this study, we first examined the permeability of BBB by EGCG, EGC and GA using a BBB model. Next, we compared the effects of EGCG, EGC and GA on cognitive function in aged SAMP10 mice, and neuronal cell proliferation and differentiation. In addition, the relationship between the suppression of oxidative damage and the suppression of cognitive dysfunction was examined. Based on these data, we considered the concentration and role of GTCs incorporated into the brain parenchyma.
2. Materials and methods
2.1. Measurement of BBB permeability
A BBB kit (RBT-24, PharmaCo-Cell, Nagasaki, Japan), consisting of co-cultures of endothelial cells, pericytes, and astrocytes, was used to determine BBB permeability rates and coefficients of EGCG, EGC and GA. According to this method's protocol, the BBB kit was first activated. After transepithelial electrical resistance reached >150 Ω×cm2, the kit was used to measure permeability.
EGCG, EGC and GA were dissolved in DMSO and an aliquot of 1 µL was added to the blood-side of the BBB kit at a final concentration of 30 µM. Caffeine was used as the positive control. The plate was incubated for 30 min with gentle stirring, then 190 µL of blood-side medium and 380 µL of brain-side medium were added to 10 µL and 20 µL of McIlvain buffer (pH 3.0), respectively in a tube, and frozen immediately. To reduce variability among plates, the same experiment was carried out three times. The samples were stored at −80 °C before measurement of the three catechins. The concentrations of EGCG, EGC and GA were measured using liquid chromatography tandem-mass spectrometry (LC-MS/MS).
2.2. Measurement of catechins using LC-MS/MS
Each brain and blood side sample (90 µL) was mixed with 10 µL of McIlvain buffer (pH 3.0). After centrifugation of the solutions at 12,000× g for 5 min at room temperature, the resulting supernatants were filtered with a 0.45 µm hydrophilic PTFE filter (DISMIC-13HP, ADVANTEC Toyo, Tokyo, Japan), and then subjected to LC-MS/MS analysis. LC-MS/MS analysis was performed using a model Agilent 1100 series LC system (Agilent Technologies, CA, USA) coupled with a 3200 QTRAP LC-MS/MS system (AB SCIEX, MA, USA). HPLC conditions were based on a previous protocol [20]. The mass detector was equipped with a turbo-ion spray (electrospray ionization, ESI) source, and multiple reaction-monitoring (MRM) mode was used for quantification. EGCG, EGC and GA were analyzed in the negative ion polarity mode. Voltage was maintained at −4000 V and curtain gas, collision gas and capillary temperature were set at 10, 5 and 600 °C, respectively. Caffeine was analyzed in the positive ion polarity mode with voltage maintained at 5000 V, and curtain gas, collision gas and capillary temperature were set at 10, 5 and 450 °C, respectively. The optimized instrument settings for quantification are listed in Table 1.
Table 1.
Molecular weight (MW) and optimized instrument settings for LC/MS/MS measurement.
| Compound | MW | Transition (m/z) |
Polarity | DP | CE | CXP | |
|---|---|---|---|---|---|---|---|
| Precursor | Product | ||||||
| Caffeine | 194 | 195.1 | 138.0 | positive | 45.0 | 23.0 | 4.0 |
| EGCG | 458 | 457 | 168.9 | negative | −255.0 | −24.0 | −4.0 |
| EGC | 306 | 305.1 | 161.1 | negative | −45.0 | −18.0 | −4.0 |
| Gallic acid | 170 | 168.9 | 125.1 | negative | −25.0 | −24.0 | −2.0 |
DP; declustering potential, CE; collision energy, CXP; collision cell exit potential.
2.3. Animals, diets and green tea catechins
Male SAMP10/TaSlc (SAMP10) mice were purchased from Japan SLC Co., Ltd. (Shizuoka, Japan) and bred under conventional conditions in a temperature- and humidity-controlled room with a 12-h light/dark cycle. Experimental mice between the ages of 2–12 months had free access to a normal diet (CE-2; Clea Co Ltd., Tokyo, Japan) and water containing catechin. Six mice were housed per cage. Water containing GTCs was prepared fresh every third day at a concentration of 0.2 mg/mL. EGCG, EGC and GA were prepared at a concentration of 0.1 mM. Control mice were given free access to a normal diet and water. The volume of ingested water was recorded. The effects of GTCs, EGCG, EGC and GA were examined in six groups of mice (control and EGCG; n=24, GTCs; n=30, EGC, GA, and EGC+GA; n=12 for each). All experimental protocols were approved by the University of Shizuoka Laboratory Animal Care Advisory Committee (approval No. 136068) and were in accordance with the guidelines of the US National Institutes of Health for the care and use of laboratory animals.
The source of GTCs was Polyphenon 70S (Mitsui Norin Co. Ltd., Tokyo, Japan), which contains ~70% catechin without caffeine. The composition is as follows: 31.7% EGCG, 15.7% EGC, 10.0% ECG, 8.5% (−)-EC, 4.5% (−)-gallocatechin gallate, and 1.0% (−)-catechin gallate. EGCG (Sunphenon EGCg, Taiyo Kagaku Co. Ltd., Yokkaichi, Japan; purity 95.11%), EGC (Sunphenon EGC, purity 81.42%) and gallic acid (MP Biomedicals, LLC, Illkirch, France) were used in this study.
2.4. Memory acquisition test
A step-through passive avoidance task was carried out using 11-month-old mice as described previously [1]. In brief, when a mouse entered the dark chamber from the light chamber, the door was closed and an electric foot-shock was delivered at 50 µA for 1 s (MST-01S; Muromachi Kikai Co., Ltd, Tokyo, Japan). Acquisition of the avoidance response was judged as successful if the mouse remained in the light chamber for 300 s. The trial was repeated until the mouse satisfied the acquisition criterion within five trials. For each trial, the time spent in the light chamber was subtracted from 300 s. This result from successive trials was summed for each mouse to give a measure of the time required for learning (“learning time”).
2.5. Cell growth assay in vitro
Human SH-SY5Y neuroblastoma cells (ACTT, CRL-2266) were plated in a 100-mm flask and cultured in D-MEM/Ham's F-12 with L-glutamine, phenol red, HEPES and sodium pyruvate (Wako Pure Chemical Industries, Ltd., Osaka, Japan), containing 10% fetal bovine serum (Mediatech, Inc., Tokyo, Japan) and a mixture of 1% penicillin-streptomycin (Nacalai Tesque, Inc. Kyoto, Japan). The cell culture was incubated at 37 °C under 5% CO2 for 48 h in an incubator (Hirasawa, Tokyo, Japan). After reaching 70–80% confluence, cells were trypsinized (Trypsin, Gibco Life Technologies, NY, USA), and then cells were plated as 1×105 cells/mL in a 24-well plate (500 µL of cell suspension/well). EGCG, EGC and GA dissolved in 0.01% DMSO were added to the culture medium to make a final concentration of 0.01–1.0 µM in triplicate for each concentration. Plates were incubated for 48 h. To determine cell viability, a cell suspension was prepared by trypsinization as indicated above and a 1:1 mixture of the cell suspension with 0.4% trypan blue dye (Cosmo Bio Co. Ltd., Tokyo, Japan) was prepared. Cells were counted with a TC10TM Automated Cell Counter (Bio-Rad, CA, USA). The experiment of each catechins was carried out twice.
2.6. Quantitation of neurite outgrowth
SH-SY5Y cells were plated as 2.5×104 cells/mL in a 24-well plate (500 µL of cell suspension/well). EGCG, EGC and GA dissolved in 0.01% DMSO were added to the culture medium to make a final concentration of 0.05 µM, and cultured for 72 h. Cells were visualized by using a phase-contrast inverted microscope (IX71; Olympus, Tokyo, Japan) with a LUCPIanFLN 20×/0.45 objective lens (Olympus) and a DP70 digital microscope camera (Olympus). Neurite length was measured by ImageJ software (Ver. 1.50i). The number of differentiated cells was determined by counting cells that had at least one neurite with a length equal to or greater than the diameter of the cell body. Assays were performed in triplicate and at least three images from each culture were taken. At least 75 neurites per treatment were assessed to measure length, and at least 100 cells per treatment were used to count neurite number.
2.7. Oxidative damage in the brain
Lipid peroxidation (LPO) in the brain was measured using a lipid hydroperoxide assay kit (Cayman Chemical Co., Ann Arbor, MI, USA) according to the manufacturer's protocol. In brief, cerebral cortex (ca. 50 mg) was homogenized in 500 µL of water (n=4/group). The hydroperoxide extracted from the homogenate was measured utilizing the redox reaction with ferrous ions. The resulting ferric ions were detected using thiocyanate ion as the chromogen. The absorbance of each sample at 500 nm was obtained in triplicate.
DNA in the cerebral cortex (ca. 100 mg) was extracted using an extraction kit (DNA Extractor® TIS kit, Wako Pure Chemical Industries, Ltd.) (n=4/group). The obtained DNA (ca. 20 µg) was treated with nuclease P1 and alkaline phosphatase according to the manufacturer's protocol. The level of 8-oxo-deoxyguanosine (8-oxodG) in DNA was measured in triplicate using an ELISA kit (8-OHdG assay preparation reagent set, Wako Pure Chemical Industries, Ltd).
2.8. Statistical analyses
Data are expressed as the mean±SEM. Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by the Bonferroni's post hoc test for multiple comparisons. ANOVA was assessed using a statistical analysis program (StatPlus, AnalystSoft Inc., online version). Differences were considered to be significant at p<0.05.
3. Results
3.1. BBB permeability of EGCG, EGC and GA
The BBB permeability coefficient was measured using a BBB kit. Caffeine was used as the positive control. GA exhibited a significantly higher permeability coefficient (21.97±1.92) than EGCG and EGC (9.31±0.32 and 11.56±1.05, respectively). The permeability coefficient of EGC tended to be higher than that of EGCG (Table 2). The permeability (%, in 30 min) of EGCG, EGC and GA was calculated as 2.8±0.1, 3.4±0.3 and 6.5±0.6, respectively.
Table 2.
BBB permeability of EGCG, EGC and GA.
| Sample | Coexistence sample | Permeability coefficient | BBB permeability |
|---|---|---|---|
| (10−6 cm/s) | (%) (30 min) | ||
| EGCG | – | 9.31±0.32 | 2.77±0.10 |
| EGCG | EGC | 7.29±0.35* | 2.16±0.11* |
| EGC | – | 11.56±1.05 | 3.43±0.31 |
| EGC | EGCG | 8.58±0.88 | 2.25±0.31 |
| EGC | GA | 4.16±0.89* | 1.53±0.50* |
| GA | – | 21.97±1.92 | 6.52±0.57 |
| GA | EGC | 18.68±1.56 | 5.55±0.46 |
| Caffeine | – | 31.30±2.49 | 9.30±0.74 |
*p<0.05, Bonferroni's t-test.
The interference effect of EGCG, EGC and GA permeability was examined. The permeability of EGCG was significantly lowered by the coexistence of the same concentration of EGC and the permeability of EGC was significantly lowered by the coexistence of same concentration of GA (Table 2).
3.2. Learning ability of aged SAMP10 mice that ingested EGCG, EGC and GA
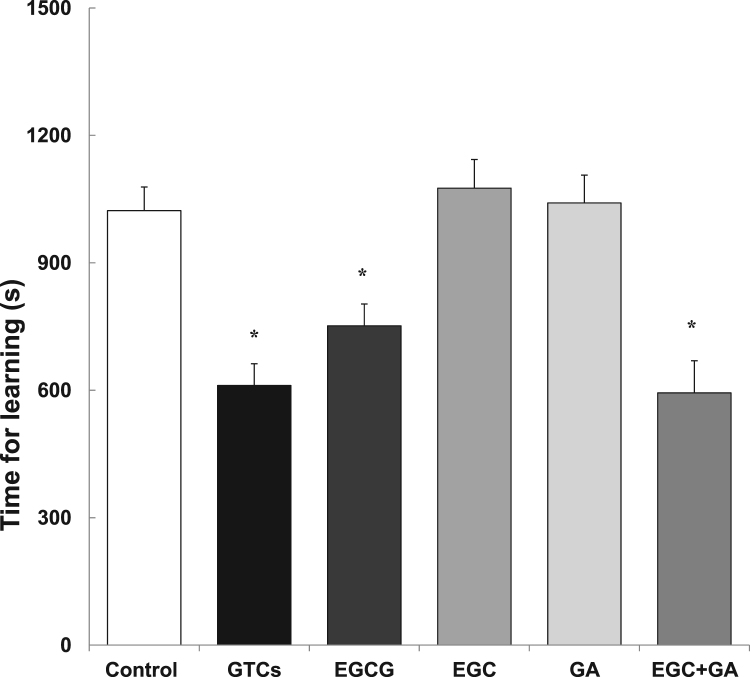
The time for learning was measured at 11 months using a step-through passive avoidance task. A longer learning time indicates lower learning ability. The learning time was significantly shorter in mice that drank GTCs (67 mg/kg) than in control mice that drank water (Fig. 1). Since the concentration of EGCG in GTCs was about 0.1 mM when EGCs were dissolved in water at 0.2 mg/mL, the effect of EGCG, EGC and GA were compared at a concentration of 0.1 mM. Mice ingested EGCG (20 mg/kg), EGC (10 mg/kg), and GA (5.7 mg/kg), respectively. The learning time of mice that ingested EGCG was as short as that of mice that ingested GTCs. EGC or GA showed no observable effect, indicating that EGCG is the most potent catechin to suppress age-related cognitive dysfunction (Fig. 1). Surprisingly, the mice that ingested both EGC and GA showed as short a learning time as those mice that ingested EGCG.
Fig. 1.
Effect of GTCs, EGCG, EGC and GA on learning in aged SAMP10 mice. The learning time of SAMP10 mice was examined using a step-through test system. The time needed to acquire the avoidance response was measured in mice that ingested GTCs, EGCG, EGC and GA (black or gray columns) and in control mice (open column). Each value represents the mean±SEM (n=8–23). Asterisks represent a significant difference (* p<0.05, Bonferroni's t-test).
3.3. Effect of EGCG, EGC and GA on nerve cell growth and neurite outgrowth
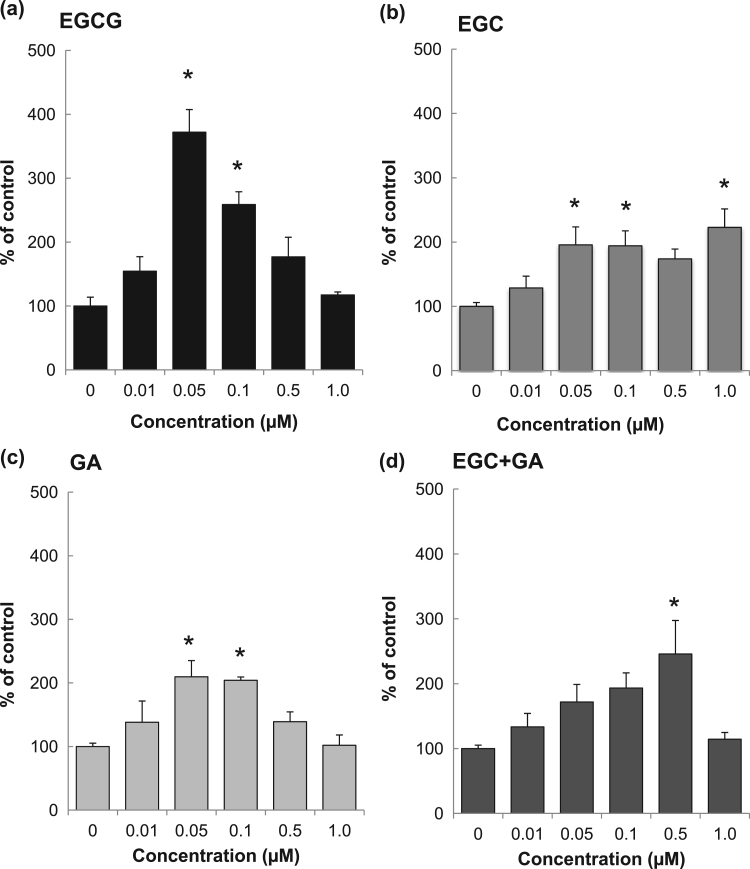
The effect of EGCG, EGC and GA on SH-SY5Y cells was examined. Although SH-SY5Y cells have been used to observe neurotrophic activity-inducing neurite outgrowth, an increase in cell number has also been reported [21], [22]. Therefore, the effects of EGCG, EGC and GA were calculated based on the number of cells after 48 h cultivation. EGCG remarkably enhanced cell number at 0.05 µM (Fig. 2a). The effect was lowered in the presence of ≥0.5 µM EGCG, indicating that there is an optimal concentration of EGCG in cell proliferation but that it is not dose-dependent. Although EGC or GA also increased cell number at 0.05–0.10 µM, the efficacy was lower than that of EGCG (Fig. 2b and c). In the presence of both EGC and GA, cell growth was enhanced at a high concentration, i.e., 0.5 µM (Fig. 2d).
Fig. 2.
Effect of EGCG, EGC and GA on cell growth of human SH-SY5Y neuroblastoma cells. A cell suspension (5×104 cells/well) was plated in a 24-well plate. EGCG, EGC and GA dissolved in 0.01% DMSO were added to the culture medium to make the final concentration of 0.01–1.0 µM, and cultured for 48 h at 37 °C. The number of cells treated with EGCG (a), EGC (b), GA (c), and EGC and GA combined (d) are shown. Each value represents the mean±SEM. Asterisks represent a significant difference (* p<0.05, Bonferroni's t-test).
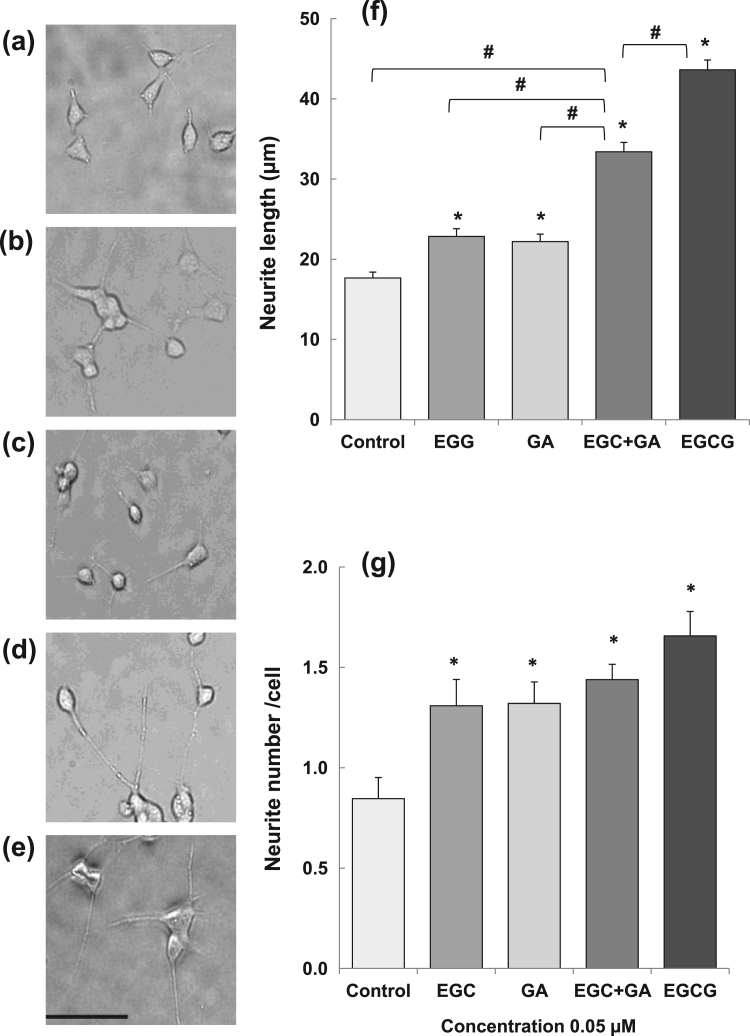
Next, the neuritogenic ability of EGCG, EGC and GA on SH-SY5Y cells was compared at 0.05 µM. The length and number of neurites were significantly higher in cells treated with catechins than in control cells (Fig. 3). Especially, neurite length was significantly longer in cells treated with EGCG than in other cells. In the presence of both EGC and GA, neurites were significantly longer than in cells treated with EGC or GA alone, suggesting that the cell differentiation was induced by the co-administration of EGC and GA.
Fig. 3.
Effect of EGCG, EGC and GA on neurite outgrowth of human SH-SY5Y neuroblastoma cells. A cell suspension (2.5×104 cells/well) was plated in a 24-well plate. EGCG, EGC and GA dissolved in 0.01% DMSO were added to the culture medium to make a final concentration of 0.05 µM and cultured for 72 h at 37 °C. Photos of control cells (a), and cells treated with EGC (b), GA (c), EGC+GA (d), or EGCG (e). Neurite length (f) and neurite number (g) of cells treated with catechins. Scale bar is 50 µm. Each value represents the mean±SEM. Asterisks and # represent significant differences with the control (*) and with EGC+GA (#) (p<0.05, Bonferroni's t-test).
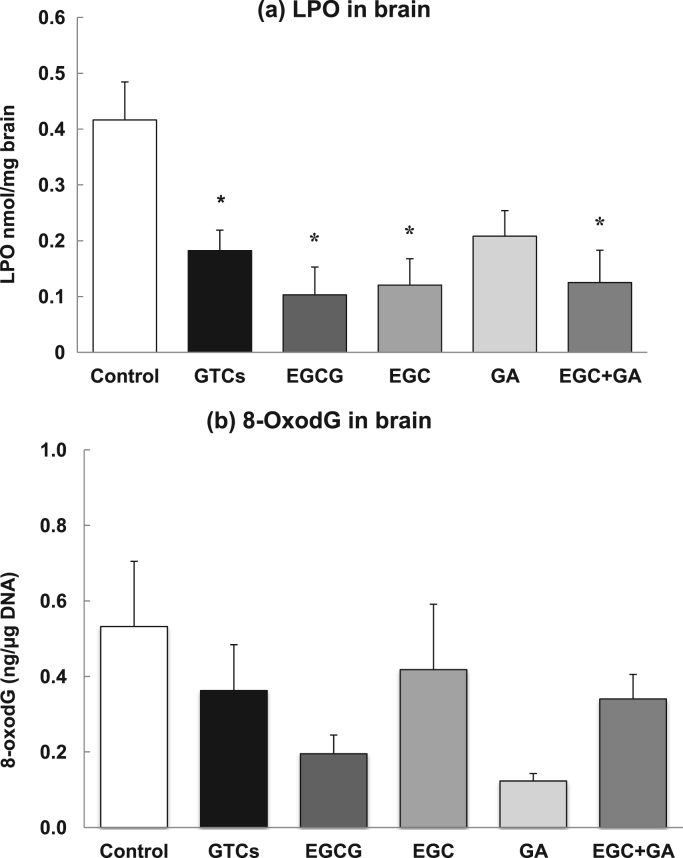
3.4. Levels of oxidative damage in the brain
The levels of LPO in brain were significantly lower in SAMP10 mice that ingested GTCs, EGCG, EGC, and combined ingestion of EGC and GA than in control mice (Fig. 4a). The level of 8-oxodG, a marker of DNA oxidative damage, in the brain tended to be lower, but not significantly different, in mice that ingested EGCG and GA than in control mice (Fig. 4b).
Fig. 4.
Levels of oxidative damage in the brain. LPO (a), and 8-oxodG (b) in the brain. Each value represents the mean±SEM (n=4). Asterisks represent a significant difference (* p<0.05, Bonferroni's t-test).
4. Discussion
4.1. BBB permeability of EGCG, EGC and GA
The distribution ratios of C and EC in the rat brain were 0.0726±0.0376 and 0.1065±0.0531 [15], and the transport efficacy (%, in 1 h) in a BBB rat model were 7.4±0.7 and 15.4±0.6 [16], respectively. In our study, the in vitro BBB permeability of EGCG, EGC and GA (%, in 1 h) was 5.6±0.2, 6.8±0.6 and 13.0±1.2, respectively. A stereoselective process was reported to be involved during the passage of C and EC across the BBB [16]. Since EGC has one more hydroxyl bond than EC, its BBB permeability may be much lower than that of EC. On the other hand, the size effect between EGCG and EGC on BBB permeability may be counteracted by the hydrophobicity of the galloyl bond.
The BBB permeability of EGCG was significantly suppressed by EGC and that of EGC was significantly suppressed by GA. This suggests that the distribution ratio in the brain of mice that ingested EGCG is altered by the coexistence of EGC and GA in plasma.
4.2. Effect of EGCG, EGC and GA on nerve cell growth, differentiation and cognitive function
Adult hippocampal neurogenesis is essential for learning and memory [23]. Dietary interventions such as caloric restriction and diet supplementation with polyphenols and polyunsaturated fatty acids have been shown to alter brain plasticity, including adult hippocampal neurogenesis and synaptogenesis [24]. Recently, EGCG has been reported to modulate brain plasticity and to promote neuronal differentiation of isolated adult hippocampal precursor cells at low concentrations 1 nM to 10 µM) [25]. Neuritogenic ability in PC12 cells was promoted by EGCG (<0.5 µM) [26]. In our study, EGCG significantly increased the proliferation of SH-SY5Y cells at 0.05 µM. Although the dosages were slightly different among these studies, their results suggest that EGCG promotes brain plasticity at nanomolar concentrations. The effect of a low concentration of EGCG has been suggested to produce sublethal levels of H2O2 as a secondary messenger [26]. Since H2O2 has been shown to induce redox signaling in several growth factor-induced signaling cascades [27], EGCG incorporated into brain parenchyma may promote neuronal cell proliferation and differentiation, resulting in the prevention of cognitive dysfunction.
Indeed, dendrite maturation of immature newborn neurons and hippocampal neurogenesis was observed in young male Balb/C mice that were intraperitoneally administered EGCG for 14 days at 2.5 mg/kg [25]. In our study, cognitive function was higher in SAMP10 mice that ingested EGCG (20 mg/kg) as drinking water than in same-aged control mice. The dose difference in these mice may be explained by the difference between oral and intraperitoneal administrations. Taken together, these data suggest that a low concentration of EGCG promotes adult brain plasticity.
On the other hand, the difference between EGCG and EGC suggests the involvement of a 67-kDa laminin receptor (67LR) that is specific to EGCG [28], [29]. In addition, catechins are excreted by efflux transporters expressed on the cell membrane [30] and the activity of efflux transporters are suppressed by the presence of a gallate moiety [31], [32]. These data suggest a reason for the reduced effects of EGC or GA on SH-SY5Y and on mice.
Since oxidative damage of lipids was fully suppressed in SAMP10 mice that ingested EGC to a similar extent as EGCG, oxidative damage-related perturbations in mitochondrial function and redox-sensitive signaling processes may be suppressed in the brain of these mice [33], [34]. This suggests that the suppression of oxidative damage in lipids and DNA is not an essential reason to prevent cognitive dysfunction.
4.3. The concentration of EGCG in brain parenchyma
Since the concentration of EGCG transferred into brain parenchyma is dependent on plasma concentration and circulating time, we speculated whether the ingestion of GTCs would be able to reach ~0.05 µM EGCG in the brain. The level of EGCG detected in plasma accounts for ~0.1% of the ingested amount [10], [11], [12], [13]. In our study, mice ingested EGCG (20 mg/kg) every day, inferring that the concentration of EGCG reached ~0.6 µM in plasma. Since the in vitro BBB permeability of EGCG in 1 h was 5.6%, ~0.03 µM EGCG was calculated to have reached into the brain in 1 h. If the concentration of EGCG in plasma is maintained at ~1.0 µM for 1 h, the concentration of EGCG in brain parenchyma will be ~0.05 µM based on the permeability of EGCG into the BBB. Furthermore, the additive effect of EGC and GA on nerve cell differentiation in an in vivo study suggests that EGCG sustains a preventive effect after its hydrolysis to EGC and GA.
In humans, drinking two cups of green tea introduced 0.2 µM EGCG into plasma [13], suggesting that 0.01 µM EGCG could be delivered into the brain parenchyma in 1 h. Additional cups of green tea will enhance the concentration in the brain when continuously consumed. However, since no effect of EGCG on nerve cell proliferation was observed at ≥0.5 µM, a high concentration of EGCG is not needed. Consecutive low concentrations of EGCG in the brain are thought to be important for brain plasticity. Epidemiological data shows that drinking green tea daily reduces the risk of cognitive decline is accumulating [35], [36], [37]. Our data suggests a reason for the daily ingestion of green tea namely to prevent age-related cognitive dysfunction.
5. Conclusion
To clarify the preventive effect of GTCs on age-related cognitive function, the permeability of EGCG, EGC and GA into the BBB was examined in vitro. Learning ability was significantly higher in mice ingested EGCG than control mice. In addition, human neuroblastoma cell proliferation was significantly enhanced by ~0.1 µM EGCG. The results suggest that a low concentration of EGCG that was suggested from BBB permeability may prevent cognitive dysfunction by increasing brain plasticity. Furthermore, the additive effect of EGC and GA suggests that EGCG sustains a preventive effect after its hydrolysis to EGC and GA.
Acknowledgements
This research study was supported by a Grant-in-Aid for Scientific Research (KAKENHI 23617014 and 15K00828), Mishima Kaiun Memorial Foundation and a grant for specially promoted research of the University of Shizuoka. The authors thank Dr. Hara M. at the University of Chicago for her valuable discussion.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.12.012.
Appendix A. Transparency document
Transparency document
.
References
- 1.Unno K., Takabayashi F., Kishido T., Oku N. Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10) Exp Gerontol. 2004;39:1027–1034. doi: 10.1016/j.exger.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 2.Unno K., Takabayashi F., Yoshida H., Choba D., Fukutomi R., Kikunaga N., Kishido T., Oku N., Hoshino M. Daily consumption of green tea catechin delays memory regression in aged mice. Biogerontology. 2007;8:89–95. doi: 10.1007/s10522-006-9036-8. [DOI] [PubMed] [Google Scholar]
- 3.Unno K., Ishikawa Y., Takabayashi F., Sasaki T., Takamori N., Iguchi K., Hoshino M. Daily ingestion of green tea catechins from adulthood suppressed brain dysfunction in aged mice. Biofactors. 2008;34:263–271. doi: 10.3233/BIO-2009-1080. [DOI] [PubMed] [Google Scholar]
- 4.Shimada A., Keino H., Satoh M., Kishikawa M., Seriu N., Hosokawa M. Age-related progressive neuronal DNA damage associated with cerebral degeneration in a mouse model of accelerated senescence. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:B415–B421. doi: 10.1093/gerona/57.12.b415. [DOI] [PubMed] [Google Scholar]
- 5.Shimada A., Keino H., Satoh M., Kishikawa M., Hosokawa M. Age-related loss of synapses in the frontal cortex of SAMP10 mouse: a model of cerebral degeneration. Synapse. 2003;48:198–204. doi: 10.1002/syn.10209. [DOI] [PubMed] [Google Scholar]
- 6.Shimada A., Hasegawa-Ishii S. Senescence-accelerated Mice (SAMs) as a model for brain aging and immunosenescence. Aging Dis. 2011;2:414–435. [PMC free article] [PubMed] [Google Scholar]
- 7.Suganuma M., Okabe S., Oniyama M., Tada Y., Ito H., Fujiki H. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19:1771–1776. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- 8.Kohri T., Matsumoto N., Yamakawa M., Suzuki M., Nanjo F., Hara Y., Oku N. Metabolic fate of (-)-[4-(3)H] epigallocatechin gallate in rats after oral administration. J. Agric. Food Chem. 2001;49:4102. doi: 10.1021/jf001491+. 4012. [DOI] [PubMed] [Google Scholar]
- 9.Kohri T., Suzuki M., Nanjo F. Identification of metabolites of (-)-epicatechin gallate and their metabolic fate in the rat. J. Agric. Food Chem. 2003;51:5561–5566. doi: 10.1021/jf034450x. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa K., Okuda S., Miyazawa T. Dose-dependent incorporation of tea catechins, (-)-epigallocatechin-3-gallate and (-)-epigallocatechin, into human plasma. Biosci. Biotechnol. Biochem. 1997;61:1981–1985. doi: 10.1271/bbb.61.1981. [DOI] [PubMed] [Google Scholar]
- 11.Unno T., Takeo T. Absorption of (-)-epigallocatechin gallate into the circulation system of rats. Biosci. Biotechnol. Biochem. 1995;59:1558–1559. doi: 10.1271/bbb.59.1558. [DOI] [PubMed] [Google Scholar]
- 12.Chen L., Lee M.J., Li H., Yang C.S. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997;25:1045–1050. [PubMed] [Google Scholar]
- 13.Lee M.J., Maliakal P., Chen L., Meng X., Bondoc F.Y., Prabhu S., Lambert G., Mohr S., Yang C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 14.Lin L.C., Wang M.N., Tseng T.Y., Sung J.S., Tsai T.H. Pharmacokinetics of (-)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food Chem. 2007;55:1517–1524. doi: 10.1021/jf062816a. [DOI] [PubMed] [Google Scholar]
- 15.Wu L., Zhang Q.L., Zhang X.Y., Lv C., Li J., Yuan Y., Yin F.X. Pharmacokinetics and blood-brain barrier penetration of (+)-catechin and (-)-epicatechin in rats by microdialysis sampling coupled to high-performance liquid chromatography with chemiluminescence detection. J. Agric. Food Chem. 2012;60:9377–9383. doi: 10.1021/jf301787f. [DOI] [PubMed] [Google Scholar]
- 16.Faria A.1, Pestana D., Teixeira D., Couraud P.O., Romero I., Weksler B., de Freitas V., Mateus N., Calhau C. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011;2:39–44. doi: 10.1039/c0fo00100g. [DOI] [PubMed] [Google Scholar]
- 17.Møller P., Løhr M., Folkmann J.K., Mikkelsen L., Loft S. Aging and oxidatively damaged nuclear DNA in animal organs. Free Radic. Biol. Med. 2010;48:1275–1285. doi: 10.1016/j.freeradbiomed.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki T., Unno K., Tahara S., Shimada A., Chiba Y., Hoshino M., Kaneko T. Age-related increase of superoxide generation in the brains of mammals and birds. Aging Cell. 2008;7:459–469. doi: 10.1111/j.1474-9726.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 19.Kishido T., Unno K., Yoshida H., Choba D., Fukutomi R., Asahina S., Iguchi K., Oku N., Hoshino M. Decline in glutathione peroxidase activity is a reason for brain senescence: consumption of green tea catechin prevents the decline in its activity and protein oxidative damage in ageing mouse brain. Biogerontology. 2007;8:423–430. doi: 10.1007/s10522-007-9085-7. [DOI] [PubMed] [Google Scholar]
- 20.Takagaki A., Nanjo F. Metabolism of (-)-epigallocatechin gallate by rat intestinal flora. J. Agric. Food Chem. 2010;58:1313–1321. doi: 10.1021/jf903375s. [DOI] [PubMed] [Google Scholar]
- 21.Simpson P.B., Bacha J.I., Palfreyman E.L., Woollacott A.J., McKernan R.M., Kerby J. Retinoic acid evoked-differentiation of neuroblastoma cells predominates over growth factor stimulation: an automated image capture and quantitation approach to neuritogenesis. Anal Biochem. 2001;298:163–169. doi: 10.1006/abio.2001.5346. [DOI] [PubMed] [Google Scholar]
- 22.Price R.D., Oe T., Yamaji T., Matsuoka N. A simple, flexible, nonfluorescent system for the automated screening of neurite outgrowth. J. Biomol. Screen. 2006;11:155–164. doi: 10.1177/1087057105283344. [DOI] [PubMed] [Google Scholar]
- 23.Deng W., Aimone J.B., Gage F.H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy T., Dias G.P., Thuret S. Effects of diet on brain plasticity in animal and human studies: mind the gap. Neural Plast. 2014;2014:563160. doi: 10.1155/2014/563160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz-López L., Márquez-Valadez B., Gómez-Sánchez A., Silva-Lucero M.D., Torres-Pérez M., Téllez-Ballesteros R.I., Ichwan M., Meraz-Ríos M.A., Kempermann G., Ramírez-Rodríguez G.B. Green tea compound epigallo-catechin-3-gallate (EGCG) increases neuronal survival in adult hippocampal neurogenesis in vivo and in vitro. Neuroscience. 2016;322:208–220. doi: 10.1016/j.neuroscience.2016.02.040. doi: 10.1016/j.neuroscience. 2016.02.040. [DOI] [PubMed] [Google Scholar]
- 26.Gundimeda U., McNeill T.H., Fan T.K., Deng R., Rayudu D., Chen Z., Cadenas E., Gopalakrishna R. Green tea catechins potentiate the neuritogenic action of brain-derived neurotrophic factor: role of 67-kDa laminin receptor and hydrogen peroxide. Biochem. Biophys. Res. Commun. 2014;445:218–224. doi: 10.1016/j.bbrc.2014.01.166. [DOI] [PubMed] [Google Scholar]
- 27.Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J. Biol. Chem. 2014;289:8735–8741. doi: 10.1074/jbc.R113.544635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tachibana H., Koga K., Fujimura Y., Yamada K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 29.Fujimura Y., Umeda D., Yamada K., Tachibana H. The impact of the 67 kDa laminin receptor on both cell-surface binding and anti-allergic action of tea catechins. Arch. Biochem. Biophys. 2008;476:133–138. doi: 10.1016/j.abb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Kadowaki M., Sugihara N., Tagashira T., Terao K., Furuno K. Presence or absence of a gallate moiety on catechins affects their cellular transport. J. Pharm. Pharmacol. 2008;60:1189–1195. doi: 10.1211/jpp.60.9.0011. [DOI] [PubMed] [Google Scholar]
- 31.Annaba F., Kumar P., Dudeja A.K., Saksena S., Gill R.K., Alrefai W.A. Green tea catechin EGCG inhibits ileal apical sodium bile acid transporter ASBT. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G467–G473. doi: 10.1152/ajpgi.00360.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farabegoli F., Papi A., Bartolini G., Ostan R., Orlandi M. (-)-Epigallocatechin-3-gallate downregulates Pg-P and BCRP in a tamoxifen resistant MCF-7 cell line. Phytomedicine. 2010;17:356–362. doi: 10.1016/j.phymed.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Müller W.E., Eckert A., Kurz C., Eckert G.P., Leuner K. Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer's disease--therapeutic aspects. Mol. Neurobiol. 2010;41:159–171. doi: 10.1007/s12035-010-8141-5. [DOI] [PubMed] [Google Scholar]
- 34.Dröge W., Schipper H.M. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noguchi-Shinohara M., Yuki S., Dohmoto C., Ikeda Y., Samuraki M., Iwasa K., Yokogawa M., Asai K., Komai K., Nakamura H., Yamada M. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9:e96013. doi: 10.1371/journal.pone.0096013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomata Y., Sugiyama K., Kaiho Y., Honkura K., Watanabe T., Zhang S., Sugawara Y., Tsuji I. Green tea consumption and the risk of incident dementia in elderly Japanese: The Ohsaki cohort 2006 study. Am. J. Geriatr. Psychiatry. 2016;24:881–889. doi: 10.1016/j.jagp.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura K., Watanabe Y., Nakamura K., Sanpei K., Wakasugi M., Yokoseki A., Onodera O., Ikeuchi T., Kuwano R., Momotsu T., Narita I., Endo N. Modifiable factors associated with cognitive impairment in 1143 Japanese outpatients: The project in Sado for total health (PROST) Dement. Geriatr. Cogn. Dis. Extra. 2016;6:341–349. doi: 10.1159/000447963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document