Abstract
We previously reported that ubiquitin-specific protease (USP) 2 in macrophages down-regulates genes associated with metabolic diseases, suggesting a putative anti-diabetic role for USP2 in macrophages. In this study, we evaluate this role at both cellular and individual levels. Isolated macrophages forcibly expressing Usp2a, a longer splicing variant of USP2, failed to modulate the insulin sensitivity of 3T3-L1 adipocytes. Similarly, macrophage-selective overexpression of Usp2a in mice (Usp2a transgenic mice) had a negligible effect on insulin sensitivity relative to wild type littermates following a three-month high-fat diet. However, Usp2a transgenic mice exhibited fewer M1 macrophages in their mesenteric adipose tissue. Following a six-month high-fat diet, Usp2a transgenic mice exhibited a retarded progression of insulin resistance in their skeletal muscle and liver, and an improvement in insulin sensitivity at an individual level. Although conditioned media from Usp2a-overexpressing macrophages did not directly affect the insulin sensitivity of C2C12 myotubes compared to media from control macrophages, they did increase the insulin sensitivity of C2C12 cells after subsequent conditioning with 3T3-L1 cells. These results indicate that macrophage USP2A hampers obesity-elicited insulin resistance via an adipocyte-dependent mechanism.
Abbreviations: DMEM, Dulbecco's modified Eagle medium; ELISA, enzyme-linked immunosorbent assay; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HFD, high-fat diet; HOMA-IR, homeostatic model assessment as an index of insulin resistance; IL, interleukin; IR, insulin receptor; IRS, insulin receptor substrate; KD, knock down; KO, knockout; NCD, normal chow diet; NEFA, nonesterified fatty acid; pAkt, phosphorylated Akt; PDK, phosphoinositide-dependent kinase; PI3K, phosphatidylinositol 3-phosphate kinase; pIRβ, phosphorylated insulin receptor β chain; SOCS, suppressor of cytokine signaling; Tg, transgenic; T2DM, type 2 diabetes mellitus; USP, ubiquitin-specific protease
Keywords: Diabetes, Obesity, Macrophage, Insulin, USP
Highlights
-
•
USP2A controls macrophage population in mesenteric adipose tissue during obesity.
-
•
Overexpression of USP2A in macrophages retards progression of insulin resistance.
-
•
Overexpression of USP2A in macrophages represses high-fat diet-induced obesity.
-
•
Macrophage USP2A controls insulin sensitivity of muscle dependent on adipocytes.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a disease with globally expanding incidence, and there is therefore much interest in the medical field in the development of novel therapies for this condition. In obese individuals, adipocytes release several soluble factors, which cause insulin resistance in skeletal muscle and liver and inhibit insulin secretion by pancreatic β cells [1], [2]. Thus, control of communication from adipose tissue to other metabolic organs is crucially important for preventing obesity-induced T2DM. Accumulating evidence indicates that chronic inflammation of adipose tissues accelerates the release of diabetic adipocytokines and lipokines from adipocytes [1], [2]. Of the various types of inflammatory cells, macrophages constitute the largest cellular population in adipose tissues and play a pivotal role in the establishment of “meta-inflammation” [3]. They secrete a variety of cytokines that modulate adipocytokine secretion from adipocytes and control carbohydrate metabolism and tissue remodeling [3]. Thus, therapeutic interventions targeting adipose tissue macrophages are considered an effective approach to overcoming T2DM [4].
Molecular mechanisms underlying insulin signaling have been well documented. After insulin binds insulin receptor (IR), IRα and -β subunits dimerize and are auto-phosphorylated by their tyrosine kinase domain. Subsequently, insulin receptor substrate (IRS) 1 is recruited to the receptor complex, followed by sequential phosphorylation of phosphatidylinositol 3-phosphate kinase (PI3K), phosphoinositide-dependent kinase (PDK)-1, and Akt. Consequently, the IR/IRS1/PI3K/PDK-1/Akt cascade controls glucose metabolism, protein synthesis, and lipolysis in myocytes, hepatocytes, and adipocytes [5]. In T2DM patients, Akt phosphorylation is disrupted and thus inhibited [5], [6]. The phosphorylation state of Akt is therefore a useful index for insulin resistance at both the cellular and individual levels.
Ubiquitination and deubiquitination are reversible processes regulating the digestion and functional modulation of target proteins. The ubiquitin-specific proteases (USPs) are the largest family contributing to deubiquitination [7]. Each USP affects different target proteins, implying different cellular roles. USP2, which is expressed in a wide range of cells, performs various cellular functions, such as carcinogenesis, sodium channel regulation, interferon production, and cell death [8], [9], [10], [11]. USP2 has two splicing variants, namely USP2A (USP2-69) and USP2B (USP2-45) [12]. Both variants share a ubiquitin isopeptidase domain at the C-terminus, but the first structure at the N-terminus differs. So far, we have found that USP2A downregulates genes associated with metabolic diseases, such as plasminogen activator inhibitor-1 and adipocyte protein 2 in macrophages, in a relatively selective manner [13]. Moreover, macrophage-selective Usp2a transgenic (Tg) mice showed a lower accumulation of total macrophages in their visceral adipose tissues while on a high-fat diet (HFD), suggesting that USP2A may have an anti-diabetic role in macrophages. However, whether USP2 in macrophages is a key determinant of metabolic state in obese individuals is still unclear. In this study, we assessed the effects of macrophage USP2 on glucose metabolism and insulin sensitivity using gene-engineered cells and mice.
2. Materials and methods
2.1. Cells
Mouse peritoneal macrophages were prepared as described previously [14]. USP2 knockdown (KD) and their control cells were generated based on human myeloid HL-60 cells [13]. One day prior to examination, the cells were treated with phorbol 12-myristate 13-acetate (30 nM; Sigma-Aldrich, St Louis, MO, USA) to induce macrophage-like differentiation. Expression levels of Usp2 variants were downregulated by ~20% relative to control cells [13]. Mouse 3T3-L1 cells were purchased from Takara Bio (Kusatsu, Japan) and were differentiated into mature adipocytes by adding Adipoinducer cocktail for one week (Takara Bio). Mouse C2C12 myoblasts were obtained from RIKEN BioResource Center (Tsukuba, Japan), and were differentiated into myotubes in Dulbecco's modified Eagle medium (DMEM) (Nacalai, Kyoto, Japan) containing 4.5 g/L of glucose and 2% horse serum (Thermo Fischer Scientific, Waltham, MA, USA) for five days.
2.2. Mice
Macrophage-selective Usp2a Tg mice were described previously [13]. The mice have a Usp2a transgene that is exclusively expressed in macrophages under the control of an fms intronic regulatory element [15].
Mice were fed a 60% kcal high-fat diet (HFD) (D12492; Research Diets, New Brunswick, NJ, USA) or normal chow diet (NCD) (Oriental Yeast, Tokyo, Japan) from age five weeks. All animal experiments were approved by the Animal Ethics Committees of Nagoya City University (Permit Number: H22M-54) and Rakuno Gakuen University (Permit Number: VH15A10).
2.3. Preparation and treatment of macrophage- and adipocyte-conditioned media
Peritoneal macrophages or HL-60 derivatives were cultured in Opti-medium (Thermo Fisher Scientific) for five days, and the media were diluted to half its original concentration with DMEM. These macrophage-conditioned media were applied to 3T3-L1 cells or C2C12 cells for 10 h and three days, respectively. These cells were then stimulated with insulin (200 nM) for 10 min. In some experiments, the macrophage-conditioned media were harvested again 12 h after addition to 3T3-L1, diluted to half its original concentration with DMEM, and then added to C2C12 cells for three days.
2.4. Immunoprecipitation and Western blotting analysis
Cells and tissues were homogenized in a Radio-Immunoprecipitation Assay buffer containing 50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, a complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and phosphatase inhibitor cocktail (Sigma-Aldrich). In some experiments, lysate of insulin-treated 3T3-L1 cells was subjected to immunoprecipitation using an anti-insulin receptor antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Pierce protein-G magnetic beads (Thermo Fisher Scientific, Waltham, MA, USA). The homogenates or immunoprecipitants were then subjected to sodium dodecyl sulfate -polyacrylamide gel electrophoresis and transferred to an Immobilon-P polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). After blocking with Blocking One (Nacalai) or Blocking One-P (Nacalai) solution, the first antibodies dissolved in Hikari enhancer solution (Nacalai) were added to the membrane. Anti-phosphorylated Akt (pAkt) (Ser473) and Akt were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-phosphorylated IRβ chain (pIRβ) (Tyr1162/1163), IRβ, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Santa Cruz Biotechnology. After washing, horseradish peroxidase-conjugated anti-rabbit antibody (Cell Signaling Technology) was added. Immunocomplexes were visualized using Chemilumi One Super reagent (Nacalai), and the images were captured using bioimage analyzers BAS-3000 (Fuji Film, Tokyo, Japan) and EZ-capture (Atto, Tokyo, Japan).
2.5. Blood tests
Blood glucose, triglycerides, nonesterified fatty acids (NEFAs), and total cholesterol were measured using Test Wako kits (Wako, Osaka, Japan). Blood insulin was measured using an enzyme-linked immunosorbent assay (ELISA) kit purchased from Shibayagi (Gunma, Japan). Mouse homeostasis model assessment-insulin resistance (HOMA-IR) was calculated using plasma from 12 h-fasting mice using the following formula: [blood glucose (mg/dL)×plasma insulin (μU/mL)]/405, as described in [17].
2.6. Insulin tolerance test
An insulin tolerance test was performed as previously described [16]. Briefly, after 6 h of starvation, mice were intraperitoneally injected with human insulin (2.0 U/kg; Eli Lilly, Indianapolis, IN, USA). Blood was collected from the tail vein after the insulin application and glucose levels were measured using a Free Style Freedom Light (Abbott Japan, Tokyo, Japan).
2.7. Histological analyses
Tissues were fixed with 10% formaldehyde neutral buffer solution (Nacalai), and were dipped in 30% sucrose solution overnight at 4 °C, embedded in OCT compound (Sakura Finetek, Tokyo, Japan). Frozen sections, approximately 14 µm thick, were mounted on gelatin-coated glass slides. After pretreatment with 0.3% Triton X-100-containing PBS (pH 7.2) for 30 min and preincubation in 10% normal donkey serum, the sections were incubated with a rat anti-F4/80 monoclonal antibody (Biolegend) overnight at room temperature, followed by incubation with horseradish peroxidase-labeled anti-rat IgG antibody (Nichirei, Tokyo, Japan) for 2 h. The sites of the antigen-antibody reaction were detected by 3,3′-diaminobenzidine staining in the presence of hydrogen peroxide. Images were acquired using a DP70 digital camera mounted on a BX51 microscope (Olympus, Tokyo, Japan). The average F4/80-positive area of each individual was determined based on 10 microscope fields of the images using Image J software [18].
2.8. FACS analysis
FACS analysis of the vascular stromal cell fraction of the adipose tissue was performed as described previously [16]. Briefly, the adipose tissue was cut into small pieces and digested with collagenase (Sigma Aldrich). After centrifugation, hemolysis was conducted using a hypotension buffer (150 mM ammonium chloride, 10 mM potassium bicarbonate, 100 μM EDTA, pH 7.2). After filtration and washing with PBS, the cells in the vascular stromal fraction were blocked with TruStain FcX (Biolegend, San Diego, CA, USA) and then labeled with phycoerythrin-conjugated anti-F4/80 and AlexaFluor488-conjugated anti-CD11b, or AlexaFluor488-conjugated anti-CD11c and allophycocyanin-conjugated anti-CD206 (all Biolegend). The labeled cells were monitored using FACS Canto2 (BD Bioscience) and analyzed using FlowJo software (Ashland, OR, USA).
2.9. Statistics
All statistical analysis was based on Student's t-test (for single-time-point comparisons) or two-way analysis of variance (for time-course experiments). For some experiments, the Holm-Bonferroni test was also used.
3. Results
3.1. USP2-knockdowned macrophage-like cells but not Usp2a-overexpressing macrophages modulate the insulin sensitivity of 3T3-L1 cells
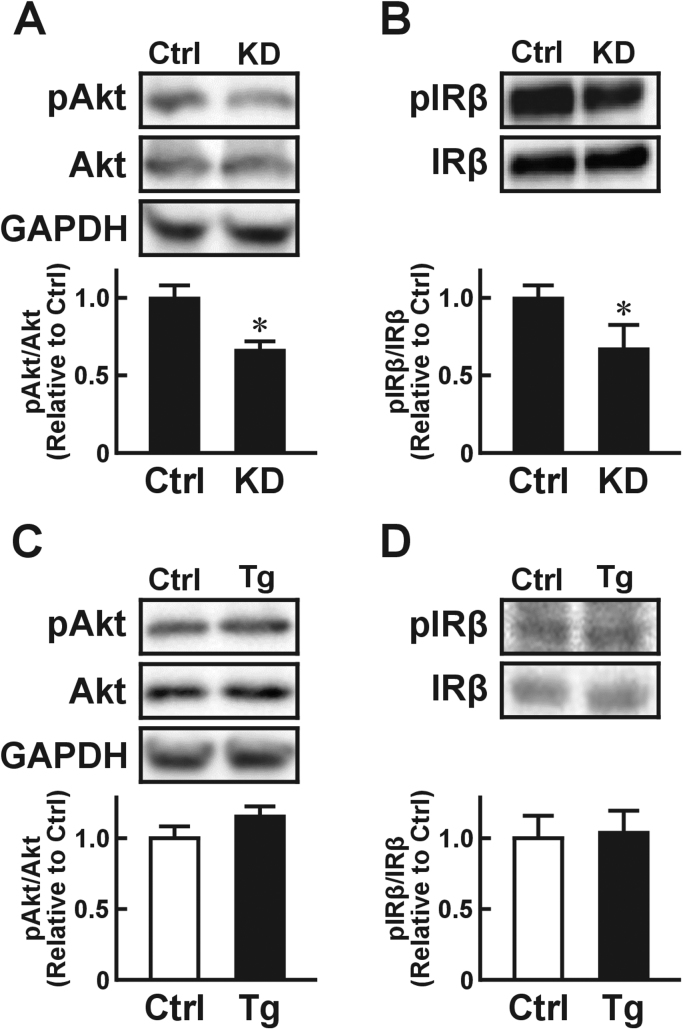
Since USP2 down-regulates meta-inflammation-associated genes in macrophages, we hypothesized that macrophage USP2 might affect the insulin sensitivity of adjacent adipocytes. To explore this idea, we monitored insulin-elicited tyrosine phosphorylation of Akt (Ser473), which is a major index of insulin signaling activation [19], [20], in 3T3-L1 adipocytes after treatment with conditioned media from USP2 KD macrophage-like HL-60 cells. Western blotting showed that supernatant from USP2 KD cells repressed insulin-elicited phosphorylation of Akt in 3T3-L1 cells significantly more than media from control HL-60 cells (Fig. 1A). Similar repression of phosphorylation of IRβ (Tyr1162/1163), another index of insulin sensitivity [21], was observed in 3T3-L1 cells pretreated with the conditioned media from USP2 KD cell culture (Fig. 1B).
Fig. 1.
Effects of USP2 in macrophages on insulin signaling in adipocytes. Changes in insulin-elicited Akt phosphorylation (pAkt; A, C) and insulin receptor β chain (pIRβ; B, D) in 3T3-L1 cells conditioned by USP2 knockdown HL-60 cells (KD; A, B) or Usp2a overexpression isolated mouse macrophages(C, D). 3T3-L1 cells were pretreated with culture media from USP2 KD (A, B), Usp2a overexpression (C, D), or their respective control (Ctrl) cells for 10 h. After insulin (100 nM) treatment, the 3T3-L1 cells were subjected to Western blot (A, C) and immunoprecipitation Western blot (B, D) analyses. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was also detected as reference. Values presented are the mean±SD of three experiments. *P<0.05 vs. control cell-conditioned cells.
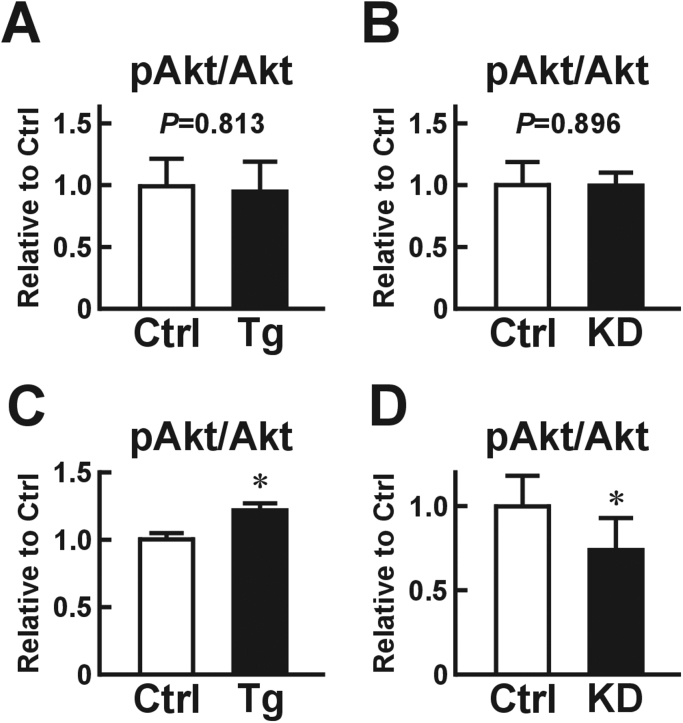
In our previous paper, we demonstrated that a longer splicing variant of USP2, namely USP2A, overcame USP2 KD-elicited aberrant expression of metabolic syndrome-associated genes in macrophages [13]. Thus, overexpression of Usp2a in macrophages might modulate the basal insulin activity of adipocytes. To examine this, we treated 3T3-L1 cells with media conditioned with macrophages isolated from Usp2a Tg mice or control littermates. As shown in Fig. 1C, the Akt phosphorylation state was almost identical in 3T3-L1 cells treated with Usp2a-overexpressing and control macrophage-conditioned media. Similar results were obtained regarding IRβ phosphorylation (Fig. 1D). Therefore, excess USP2A in macrophages does not affect the insulin sensitivity of nascent adipocytes in vitro.
USP2 is also expressed in a wide variety of cells, including adipocytes. Thus, it is possible that USP2 expressed in adipocytes also has the potential to modulate the insulin sensitivity of cells. To analyze this, we generated three Usp2 knockout (KO) 3T3-L1-derived clones using the CRISPR/Cas9 systems. None of the clones exhibited substantial changes in Akt phosphorylation after insulin stimulation (Fig. 1). Hence, USP2 expressed in adipocytes is not a determinant of the insulin sensitivity of cells.
3.2. Adiposity and glucose metabolism marginally affected in macrophage-selective Usp2a transgenic mice fed a three-month high-fat diet
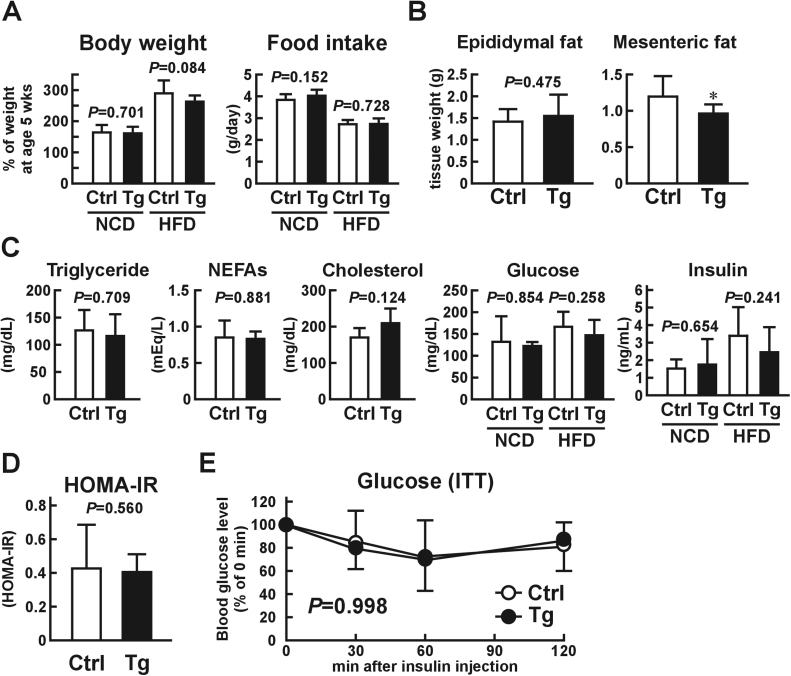
As shown in Fig. 1C and D, excess USP2A in macrophages did not increase the insulin sensitivity of intact adipocytes. However, this result does not exclude the possibility that USP2A expressed in macrophages had beneficial effects with respect to T2DM in obese individuals. We thus investigated whether overexpression of Usp2a in macrophages impeded the progress of T2DM in HFD-induced obese mouse models. After being fed an HFD for three months, there were no obvious differences in food consumption between Usp2a Tg mice and control littermates (Fig. 2A). However, body weight gain was slightly smaller in HFD-fed Usp2a Tg mice (n=9–10; P=0.084, Student's t-test; P=0.025, Holm-Bonferroni test). There was no significant difference in the wet tissue weight of epididymal adipose tissue between the Usp2a Tg and control mice (Fig. 2B). In contrast, the mesenteric adipose tissue mass was slightly, but significantly, lower in Usp2a Tg mice. Blood glucose, triglyceride, NEFAs, total cholesterol, and insulin levels were not modulated in the obese Usp2a Tg mice (Fig. 2C). The HOMA-IR and insulin tolerance test also suggested that overexpression of Usp2a in macrophages had failed to improve insulin sensitivity in an HFD-induced obese model at this point in the trial (Fig. 2D and E). Taken together, these results indicate that overexpression of Usp2a in macrophages has limited effects on glucose metabolism in the early phases of obesity, although it slightly represses weight gain and hypertrophy of mesenteric adipose tissue.
Fig. 2.
Phenotypic changes in macrophage-selective Usp2a transgenic (Tg) mice after being fed a high-fat diet (HFD) or normal chow diet (NCD) for three months. Tg mice and their control littermates were fed an HFD from the age of five weeks. (A) Body weight and food consumption of Usp2a Tg mice and control mice after a three-month HFD or NCD. (B) Wet tissue weight of epididymal and mesenteric adipose tissues. (C) Plasma triglyceride, non-esterified fatty acids (NEFAs), total cholesterol, glucose, and insulin levels under normal feeding conditions in the two diet treatments. Note that these blood indices were not affected by overexpression of Usp2a in macrophages during a once-off 12-h fast. (D) HOMA-IR and (E) insulin tolerance in Usp2a Tg mice and control mice after a three-month HFD. Values presented are the mean±SD of nine to 15 (A, E) or four (B-D) mice. *P<0.05 vs. control mice.
We also investigated the effects of macrophage-selective Usp2a-overexpression on glucose metabolism in lean mice. Relative to control littermates, Usp2a Tg mice did not exhibit changes in body weight gain, blood glucose, lipids, insulin, or insulin sensitivity after being fed an NCD for three months (Fig. 2A and C, and Fig. 2A-C).
3.3. Fewer inflammatory macrophages in the mesenteric adipose tissue of Usp2a transgenic mice
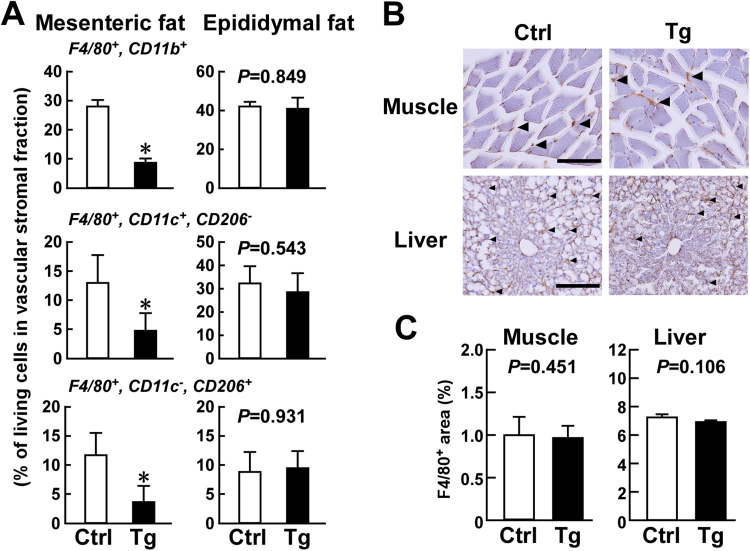
Consumption of an HFD for three months had a significant effect on mesenteric adipose tissue mass in Usp2a Tg mice (Fig. 2B). This agrees with our previous report that overexpression of Usp2a in macrophages affects total cell number and gene expression in mesenteric adipose tissue macrophages after three months of an HFD [13]. Since mesenteric adipose tissue mass is strongly correlated with T2DM progression [22], we further characterized the macrophage subpopulation in this tissue in obese Usp2a Tg mice. In agreement with previous reports, overexpression of Usp2a in macrophages prevented the infiltration of F4/80+, CD11b+ macrophages in mesenteric adipose tissue (Fig. 3A). Further FACS analysis showed that there were fewer F4/80+, CD11c+, CD206− inflammatory M1-like macrophages and F4/80+, CD11c−, CD206+ anti-inflammatory M2-like macrophages in the adipose tissue of Usp2a Tg mice. In contrast to the mesenteric adipose tissue, there was no difference between Usp2a Tg mice and control mice in the abundance of total, M1-like, or M2-like macrophages in the epididymal adipose tissue. Moreover, Usp2a Tg mice fed an HFD did not show obvious changes in the number of F4/80+ macrophages in the gastrocnemius muscle or liver relative to control mice (Fig. 3B and C). These results show that overexpression of Usp2a in macrophages prevents chronic inflammation in the mesenteric adipose tissue.
Fig. 3.
Macrophages in adipose tissues, skeletal muscle, and liver in macrophage-selective Usp2a transgenic (Tg) mice after three months of a high-fat diet (HFD). Usp2a Tg mice and control C57BL/6 littermates were fed a 60% kcal HFD from age five weeks to four months.(A) FACS analysis data for F4/80+, CD11b+ total macrophages, F4/80+, CD11c+, CD206− M1-like macrophages, and F4/80+, CD11c−, CD206+ M2-like macrophages in mesenteric and epididymal adipose tissues. Values presented are the mean±SD of three experiments. *P<0.05 vs. control mice. (B) Immunohistochemical detection of F4/80+ macrophages in gastrocnemius muscle and liver. Representative images of three replicates are shown. Arrowheads indicate macrophages. Scale bar represents 100 µm (muscle) and 200 µm (liver). (C) Average macrophage-positive area. The areas in 10 microscope fields were measured for each sample. Values presented are the mean of the mean±SD for three individuals.
3.4. Macrophage-selective Usp2a transgenic mice show an improvement in metabolic disorders after being fed a high-fat diet for a prolonged period
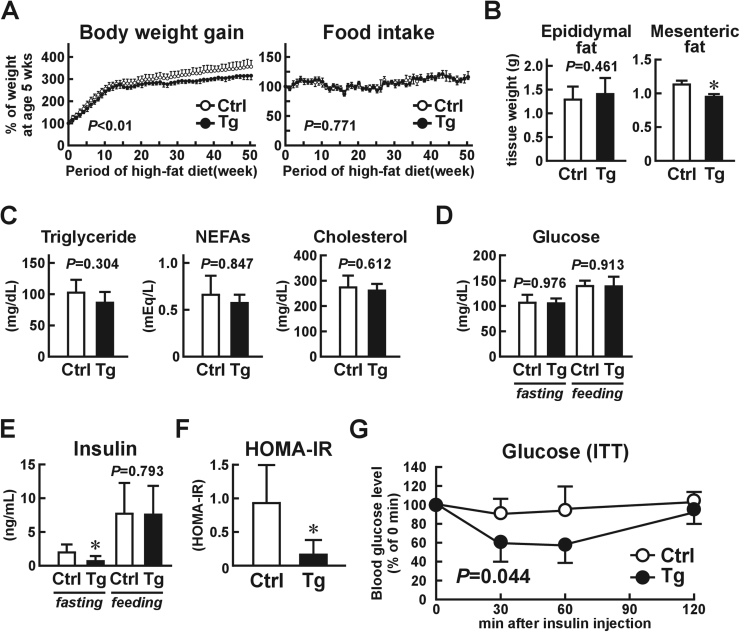
Based on the decrease in mesenteric adipose tissue weight and repression of macrophage infiltration into the tissues of Usp2a Tg mice, we suspected that HFD consumption for a longer period might affect the glucose metabolism of the mice. We thus extended the HFD period to one year and monitored their glucose metabolism. As shown in Fig. 4A, body weight gain attenuated in the Usp2a Tg mice after six months of HFD consumption. After the full year, the body weight of the Tg mice was ~13% lower than that of the control mice, even though there was no difference in food consumption. As with the three-month HFD, the wet tissue weight of the mesenteric adipose tissue, but not the epididymal adipose tissue (~111%), was smaller (~84%) in the Usp2a Tg mice than in the control mice (Fig. 4B). There were no differences between the blood glucose, triglyceride, NEFAs, or total cholesterol levels of the Usp2a Tg mice and the control mice (Fig. 4C and D), although plasma insulin levels were slightly lower during fasting (but not under normal feeding conditions) in the Usp2a Tg mice (Fig. 4E). The HOMA-IR values indicated a significant improvement in insulin resistance in the Usp2a Tg mice (Fig. 4F), and the insulin tolerance test showed that their insulin sensitivity was also greater (Fig. 4G). Taken together, these results show that overexpression of Usp2a in macrophages improves insulin resistance in individuals consuming a high-fat diet for a prolonged period.
Fig. 4.
Phenotypic changes in macrophage-selective Usp2a transgenic (Tg) mice after being fed a high-fat diet (HFD) for one year. The Tg mice and their control C57BL/6 littermates were fed an HFD from the age of five weeks to one year. (A) Changes in body weight and food consumption. (B) Wet tissue weight of epididymal and mesenteric adipose tissues. (C) Plasma triglyceride, non-esterified fatty acids (NEFAs), and total cholesterol levels under normal feeding conditions in high-fat diet treatment. (D, E) Blood glucose (D) and insulin (E) were measured during both feeding and fasting conditions. (F) HOMA-IR. (G) Insulin tolerance test. Values presented are the mean±SD of four mice. *P<0.05 vs. control mice.
We also monitored the metabolic state of Usp2a Tg mice and their control littermates after being fed an NCD for a prolonged period. The Usp2a mice did not exhibit differences in body weight gain, blood glucose, lipids, or insulin sensitivity after NCD consumption for six months (Fig. 2D-G).
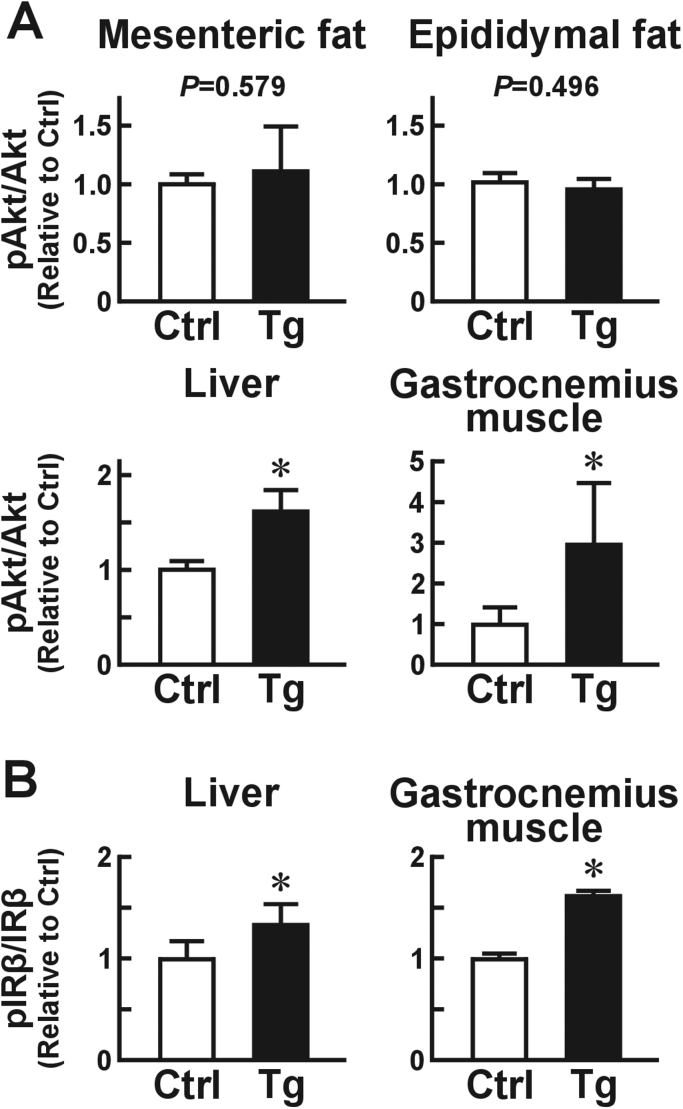
We next examined whether overexpression of Usp2a in macrophages improves insulin sensitivity in the glucose metabolism-competent organs, namely the adipose tissues, skeletal muscle, and liver, in obese individuals fed an HFD for six months. After brief (~8 min) stimulation with insulin, there was no difference in the proportion of pAkt to total Akt in the mesenteric adipose tissue or the epididymal adipose tissue between Usp2a Tg mice and their control littermates (Fig. 5A). The Usp2a Tg mice also did not exhibit changes in the cellular size of adipocytes in the adipose tissues (Fig. 3). In sharp contrast, Akt phosphorylation was strongly promoted in the gastrocnemius muscle (~301%) and liver (~164%) of Usp2a Tg mice relative to control mice. The ratio of pIRβ to total IRβ was also higher in the gastrocnemius muscle and liver of Usp2a Tg mice (Fig. 5B). These results show that macrophage USP2A hampers obesity-elicited insulin resistance in skeletal muscle and liver.
Fig. 5.
Effects of macrophage USP2 on the insulin sensitivity of glucose metabolism-competent organs. Macrophage-selective Usp2a Tg mice and control littermates were fed an HFD for six months. (A) Ratio of phosphorylated Akt (pAkt) to total Akt in mesenteric and epididymal adipose tissues, liver, and gastrocnemius muscle. (B) Ratio of phosphorylated insulin receptor β chain (pIRβ) to total IRβ in gastrocnemius muscle and liver. Values presented are the mean±SD of five mice. *P<0.05 vs. control mice.
3.5. Macrophage USP2 controls insulin sensitivity in myotubes via adipocyte-derived factors
Although Usp2a Tg mice exhibited no obvious changes in the number of macrophages in their skeletal muscle, macrophages forcibly expressing Usp2a might directly potentiate insulin sensitivity in myocytes. To elucidate this, we collected conditioned media from macrophages isolated from Usp2a Tg mice or control mice, and applied these media to C2C12 myotubes. After stimulation with insulin, the ratio of pAkt to total Akt was comparable among the two groups of myotubes (Fig. 6A). Likewise, conditioned media from USP2 KD HL-60 cells failed to modulate Akt phosphorylation in C2C12 cells (Fig. 6B). From this we conclude that macrophages exhibiting aberrant expression of USP2 do not directly regulate the insulin sensitivity of adjacent myocytes.
Fig. 6.
Direct and indirect effects of macrophage USP2 on the insulin-elicited phosphorylation of Akt in C2C12 myotubes. Conditioned media from peritoneal macrophages isolated from Usp2a transgenic (Tg) mice or their control littermates (A, C), or USP2 KD HL-60 cells or control cells (B, D) were collected. (A, B) C2C12 cells were treated with the macrophage-conditioned media for three days. (C, D) The macrophage-conditioned media were added to 3T3-L1 cells. After culturing for 12 h, the media from the 3T3-L1 cells were collected and applied to the C2C12 cells for three days. The C2C12 cells were then stimulated with insulin (200 nM) for 10 min and subjected to Western blot analyses. Values presented are the mean±SD of three experiments. *P<0.05 vs. control cell-conditioned C2C12 cells.
Growing evidence indicates that mild inflammation of the adipose tissue is a triggering event for insulin resistance in major glucose metabolism organs, such as skeletal muscle, via adipocyte-derived factors [1], [2]. Thus, we hypothesized that macrophage USP2 controls the insulin signaling in skeletal muscle that is mediated by the modulation of adipocytes. To check this possibility, we treated C2C12 cells with media collected from 3T3-L1 cells conditioned with Usp2a-overexpressing or control macrophages. As we expected, Akt phosphorylation was significantly higher in the cells treated with media from 3T3-L1 cells with Usp2a-overexpressing macrophages (Fig. 6C). Conversely, C2C12 myocytes treated with conditioned media from 3T3-L1 cells with USP2 KD HL-60 cells had a lower proportion of pAkt than those treated with media from 3T3-L1 cells with control HL-60 cells (Fig. 6D). We also examined the effects of conditioned media from Usp2 KO adipocytes on insulin-elicited Akt phosphorylation in C2C12 cells. As shown in Fig. 4, however, these media did not affect the ratio of pAkt to Akt. These results collectively indicate that macrophage USP2 indirectly controls the insulin sensitivity of myocytes by modulating the release of certain adipocyte-derived soluble factors.
4. Discussion
In this study, overexpression of Usp2a in macrophages resulted in lower blood insulin levels during fasting after a one-year HFD, suggesting improved T2DM. Moreover, the insulin tolerance test and Western blot analysis of pAkt in muscle and liver tissues demonstrated increased insulin sensitivity in the Usp2a Tg mice. Macrophage USP2A thus potentiates insulin sensitivity during an HFD. Previously, hepatic USP2B, a shorter variant of USP2, was shown to contribute to diurnal glucose metabolism [23]. Furthermore, expression of USP2 in epithelial and breast cancer cells, and in the hypothalamus and cerebral cortex, has been shown to be modulated by adiponectin and hypoglycemia, respectively [24], [25]. USP2 can also determine the turnover of low-density lipoprotein receptors in HepG2, A431, and HeLa cells [26]. These observations collectively indicate that USP2 is closely related to carbohydrate and lipid metabolism, and they thus highlight its regulatory roles in energy homeostasis.
Our current cellular experiments suggest that macrophage USP2 inhibits the insulin resistance of myocytes in an adipocyte-dependent manner. However, it is still unclear which adipocyte-derived factor(s) is regulated by macrophage USP2A and in turn modulates the insulin response of distant myocytes. Previously we demonstrated that USP2 KD HL-60 cells increase the expression of interleukin (IL)-6 in 3T3-L1 cells [13]. Since IL-6 induces insulin resistance in muscle and liver tissues through the induction of suppressor of cytokine signaling (SOCS) 3 [27], [28], we considered that Usp2a-overexpressing macrophages repress IL-6 production by adipocytes, resulting in a decrease in the insulin resistance of skeletal muscle and liver. However, our preliminary studies indicated that neither the levels of circulating IL-6 nor SOCS3 expression in skeletal muscle were affected in Usp2a Tg mice after HFD consumption for one year. Further studies are thus required to clarify the adipocyte molecule(s) that is controlled by macrophage USP2 and regulates the insulin sensitivity of myocytes.
In this study, the Usp2a Tg mice exhibited a more gradual increase in body weight than the control mice while being fed an HFD. This difference might be attributable to a decrease in some of the visceral adipose tissues, including the mesenteric adipose tissue. To date, several lines of research indicate that adipose tissue macrophages stimulate adipogenesis [29], [30], [31]. A decrease in macrophage abundance in the mesenteric adipose tissue might repress adipose tissue remodeling and consequently suppress hypertrophy of the tissue. Alternatively, energy consumption might increase in Usp2a Tg mice when they become obese. Macrophages are known to induce increases in the beige cell population in subcutaneous adipose tissue [32]. It is possible that USP2 confers this beige cell-inducing activity to macrophages. The relationship between USP2 in macrophages and the number of beige adipocytes is therefore an issue to address in future studies.
The change in glucose metabolism we observed in the macrophage-selective Usp2a Tg mice was relatively slight, especially when mice were young. Despite this, the differences in insulin sensitivity between the Usp2a Tg and control mice became significant after six months. Growing evidence demonstrates that the production of proinflammatory cytokines changes in an age-and adiposity-dependent manner [33]. These observations indicate that age-and adiposity-dependent factors accelerate the production of proinflammatory cytokines by leukocytes. Macrophage USP2 may therefore selectively prevent age- and/or adiposity-related chronic inflammation and subsequent insulin resistance.
5. Conclusions
USP2A expressed in macrophages slightly but significantly improved insulin sensitivity in obese individuals. Macrophage USP2A therefore has potential as a therapeutic target in T2DM.
Acknowledgements
This work was supported by the Kakenhi Program (24500494 and 15K06805) of the Japan Society for the Promotion of Science, and by the Suhara Memorial Foundation. The authors acknowledge the editorial assistance of Uni-Edit.
Footnotes
Transparency document associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2017.01.009.
Contributor Information
Natsuko Saito, Email: s21361144@stu.rakuno.ac.jp.
Shunsuke Kimura, Email: skimu@hokudai.ac.jp.
Tomomi Miyamoto, Email: tomomi@med.nagoya-cu.ac.jp.
Sanae Fukushima, Email: sana_fukushima@brain.riken.jp.
Misato Amagasa, Email: s21361055@stu.rakuno.ac.jp.
Yoshinori Shimamoto, Email: shimamot@rakuno.ac.jp.
Chieko Nishioka, Email: chieko@brain.riken.jp.
Shiki Okamoto, Email: shiki@nips.ac.jp.
Chitoku Toda, Email: chitoku.toda@yale.edu.
Kohei Washio, Email: s21003068@stu.rakuno.ac.jp.
Atsushi Asano, Email: atasano@vet.kagoshima-u.ac.jp.
Ichiro Miyoshi, Email: ichirom@nagoya-cu.ac.jp.
Eiki Takahashi, Email: etakahashi@brain.riken.jp.
Hiroshi Kitamura, Email: ktmr@rakuno.ac.jp.
Appendix A. Transparency document
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Abranches M.V., Oliveira F.C., Conceição L.L. Obesity and diabetes: the link between adipose tissue dysfunction and glucose homeostasis. Nutr. Res. Rev. 2015;28(2):121–132. doi: 10.1017/S0954422415000098. [DOI] [PubMed] [Google Scholar]
- 2.Klöting N., Blüher M M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014;15(4):277–287. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- 3.McNelis J.C., Olefsky J.M. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Donath M.Y. Multiple benefits of targeting inflammation in the treatment of type2 diabetes. Diabetologia. 2016;59(4):679–682. doi: 10.1007/s00125-016-3873-z. [DOI] [PubMed] [Google Scholar]
- 5.Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse model to disease mechanisms. J. Endocrinol. 2014;220(2):T1–T23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fröjdö S., Vidal H., Pirola L. Alterations of insulin signaling in type 2 diabetes: a review of the current evidence from humans. Biochim. Biophys. Acta. 2009;1792(2):83–92. doi: 10.1016/j.bbadis.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Faesen A.C., Luna-Vargas M.P., Sixma T.K. The role of UBL domains in ubiquitin-specific proteases. Biochem. Soc. Trans. 2012;40(3):539–545. doi: 10.1042/BST20120004. [DOI] [PubMed] [Google Scholar]
- 8.Benassi B., Flavin R., Marchionni L. MYC is activated by USP2a-mediated modulation of microRNAs in prostate cancer. Cancer Discov. 2010;2(3):236–247. doi: 10.1158/2159-8290.CD-11-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pouly D., Debonneville A., Ruffieux-Daidié D. Mice carrying ubiquitin-specific protease 2 (Usp2) gene inactivation maintain normal sodium balance and blood pressure. Am. J. Physiol. 2013;305(1):F21–F30. doi: 10.1152/ajprenal.00012.2013. [DOI] [PubMed] [Google Scholar]
- 10.Mahul-Mellier A.L., Pazarentzos E., Datler C. De-ubiquitinating protease USP2a targets RIP1 and TRAF2 to mediate cell death by TNF. Cell Death Diff. 2012;19(5):891–899. doi: 10.1038/cdd.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L., Zhao X., Zhang M. Ubiquitin-specific protease 2b negatively regulates IFN-β production and antiviral activity by targeting TANK‐binding kinase 1. J. Immunol. 2014;193(5):2230–2237. doi: 10.4049/jimmunol.1302634. [DOI] [PubMed] [Google Scholar]
- 12.Gousseva N., Baker R.T. Gene structure, alternate splicing, tissue distribution, cellular localization, and developmental expression pattern of mouse deubiquitinating enzyme isoforms Usp2-45 and Usp2-69. Gene Expr. 2003;11(3–4):163–179. doi: 10.3727/000000003108749053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitamura H., Kimura S., Shimamoto Y. Ubiquitin-specific protease 2-69 in macrophages potentially modulates metainflammation. FASEB J. 2013;27(12):4940–4953. doi: 10.1096/fj.13-233528. [DOI] [PubMed] [Google Scholar]
- 14.X. Xhang, R. Goncalves, D.M. Mosser. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol., vol. 83 (14.1), pp. 1–14. http://dx.doi.org/10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed]
- 15.Sasmono R.T., Oceandy D., Pollard J.W. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101(3):1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 16.Kitamura H., Naoe Y., Kimura S. Beneficial effects of Brazilian propolis on type 2 diabetes in ob/ob mice: possible involvement of immune cells in mesenteric adipose tissue. Adipocyte. 2013;2(4):227–236. doi: 10.4161/adip.25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berglund E.D., Li C.Y., Poffenberger G. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57(7):1790–1799. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schindelin J., Arganda-Carreras I I., Frise E. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X., Lin J., Zhang Y. MicroRNA-18b improves glucose homeostasis and insulin sensitivity by regulating endothelial function in white adipose tissue. Circ. Res. 2016;118(5):810–821. doi: 10.1161/CIRCRESAHA.115.308166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikolaidis L.A., Sturzu A., Stolarski C. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc. Res. 2004;61(2):297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Furuhashi R. Fucho, Görgün C.Z. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J. Clin. Invest. 2008;118(7):2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wueest S., Item F., Lucchini F.C. Mesenteric fat lipolysis mediates obesity-associated hepatic steatosis and insulin resistance. Diabetes. 2016;65(1):140–148. doi: 10.2337/db15-0941. [DOI] [PubMed] [Google Scholar]
- 23.Moulsky M.M., Li S., Ma D. Ubiquitin-specific protease 2 regulates hepatic gluconeogenesis and diurnal glucose metabolism through 11β-hydroxysteroid dehydrogenase 1. Diabetes. 2012;61(5):1025–1035. doi: 10.2337/db11-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treeck O., Lattrich C., Juhasz-Boess I. Adiponectin differentially affects gene expression in human mammary epithelial and breast cancer cells. Br. J. Cancer. 2008;99(8):1246–1250. doi: 10.1038/sj.bjc.6604692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastaitis J.W., Wurmbach E., Cheng H. Acute induction of gene expression in brain and liver by insulin-induced hypoglycemia. Diabetes. 2005;54(4):952–958. doi: 10.2337/diabetes.54.4.952. [DOI] [PubMed] [Google Scholar]
- 26.Nelson J.K., Sorrentino V., Avagliano Trezza R. Deubiquitylase USP2 regulates the LDLR pathway by counteracting the E3-Ubiquitin ligase IDOL. Circ. Res. 2016;118(3):410–419. doi: 10.1161/CIRCRESAHA.115.307298. [DOI] [PubMed] [Google Scholar]
- 27.Ueki K., Kondo T., Kahn C.R. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol. Cell. Biol. 2004;24(12):5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorgensen S.B., O’Neil H.M., Sylow L. Deletion of skeletal muscle SOCS3 prevents insulin resistance in obesity. Diabetes. 2013;62(1):56–64. doi: 10.2337/db12-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boutens L., Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59(5):879–894. doi: 10.1007/s00125-016-3904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chazenbalk G., Bertolotto C., Heneidi S. Novel pathway of adipogenesis through cross-talk between adipose tissue macrophages, adipose stem cells and adipocytes: evidence of cell plasticity. PLoS One. 2011;6(3):e17834. doi: 10.1371/journal.pone.0017834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaragosi L.E., Wdziekonski B., Villageois P. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes. 2010;59(10):2513–2521. doi: 10.2337/db10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui X., Gu P., Zhang J. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. 2015;22(2):279–290. doi: 10.1016/j.cmet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Mirsoian A., Bouchlaka M.N., Sckisel G.D. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J. Exp. Med. 2014;211(12):2373–2383. doi: 10.1084/jem.20140116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material