Abstract
Although the endocrine disruptor bisphenol A (BPA) is reported to inhibit nerve conduction, the underlying mechanisms are unclear. Therefore, in the present study, we examined the effect of BPA on compound action potentials (CAPs) recorded from the frog sciatic nerve using the air-gap method. Treatment of the sciatic nerve with BPA (0.5 mM) for 20 min reduced the peak amplitude of the CAP by approximately 60% in a partially reversible manner. The reduction in the CAP peak amplitude was concentration-dependent, with a half-maximal inhibitory concentration (IC50) value of 0.31 mM. This effect of BPA was unaffected by an estrogen-receptor antagonist, 4-hydroxytamoxifen, which by itself reduced CAP peak amplitude, with an IC50 value of 0.26 mM (comparable to that of BPA). The natural estrogen 17β-estradiol, at the highest dissolvable concentration (0.05 mM), had an effect similar to that of BPA. The IC50 value of BPA was comparable to those of some local anesthetics in inhibiting frog CAPs. Our findings suggest that BPA inhibits nerve conduction in a manner independent of estrogen receptors. This action of BPA may underlie, at least in part, the neurotoxicity of the compound.
Abbreviations: BPA, bisphenol A; CAP, compound action potential; DMSO, dimethyl sulfoxide; DRG, dorsal root ganglion; IC50, half-maximal inhibitory concentration; ERα, estrogen receptor α; ERβ, estrogen receptor β; ERRγ, estrogen-related receptor γ; nH, Hill coefficient; 4-OHT, 4-hydroxytamoxifen; LA, local anesthetic; TTX, tetrodotoxin
Keywords: Bisphenol A, 17β-Estradiol, Local anesthetic, Nerve conduction inhibition, Sciatic nerve, Frog
Highlights
-
•
Bisphenol A acutely inhibits compound action potentials in nerve fibers.
-
•
The effect of bisphenol A is not mediated by estrogen receptors.
-
•
The effect of bisphenol A is comparable to those of local anesthetics.
1. Introduction
Bisphenol A [BPA; 2,2-bis (4-hydroxyphenyl)propane], an organic synthetic compound, is easily absorbed into the body through food and drink, owing to its lipophilicity (for review see [1], [2], [3]). Because BPA has an estrogenic action, albeit comparatively weak [4], [5], [6], [7], it perturbs normal endocrine functions (for review see [8], [9]). Furthermore, there are numerous studies demonstrating that BPA acts on the nervous system (for review see [3], [9], [10]). For example, BPA has been shown to affect, in a sex-specific manner, the motivation to explore- and anxiety-related behavior in rats [11]. BPA also impacts the behavioral response to pain produced by the subcutaneous injection of formalin in rats [12]. Interestingly, Pandey and Deshpande [13] reported that BPA inhibits fast-conducting compound action potentials (CAPs) in the frog (Rana tigrina) sciatic nerve. This effect of BPA was found to be sensitive to the estrogen-receptor antagonist tamoxifen, as well as Ca2+-free and voltage-gated L-type Ca2+-channel antagonists (i.e., nifedipine and diltiazem), but not T- or P-type Ca2+-channel antagonist (Ni2+). Accordingly, the researchers concluded that BPA modulates voltage-gated L-type Ca2+ channels via estrogen receptor α (ERα). However, Ca2+-channel involvement appears unlikely in the frog (Rana nigromaculata) sciatic nerve, because CAPs in this preparation are completely blocked by a voltage-gated Na+-channel blocker, tetrodotoxin (TTX; [14]). Therefore, the effects of BPA on nerve conduction are currently unclear.
The present study was undertaken to evaluate the effect of BPA on the TTX-sensitive CAPs recorded from the frog sciatic nerve using the air-gap method. We chose this preparation because it has been extensively used to examine the effects of various drugs on nerve activity (for review see [15]). In contrast to the previous study [13], we use the estrogen-receptor antagonist trans-4-hydroxytamoxifen (4-OHT) in the present study because it is about 150-fold more effective than tamoxifen in inhibiting human breast epithelial cell growth induced by a natural estrogen (17β-estradiol) [16]. Additionally, 4-OHT binds to and deactivates estrogen-related receptor γ (ERRγ; [17], [18]), for which BPA has a high binding affinity [19], [20], [21]. We also examine whether BPA activity is mimicked by 17β-estradiol, which is much more effective than BPA in stimulating prolactin secretion [5] and uterotrophic activity [6]. Because BPA has been reported to bind to a local anesthetic (LA) receptor site on human cardiac Na+ channels [22], we further examine the effects of prilocaine and pramoxine, which are amide-type and ester-type LAs, respectively [23], on frog sciatic nerve CAPs. A part of the present study has been reported in abstract form [24].
2. Materials and methods
This study was approved by the Animal Care and Use Committee of Saga University, and was conducted in accordance with the Guiding Principles for the Care and Use of Animals in the Field of Physiological Science of the Physiological Society of Japan. All efforts were made to minimize animal suffering and the number of animals used.
2.1. Preparation of frog sciatic nerves
The method used for obtaining frog sciatic nerve preparations has been described previously [14], [15], [25], [26]. Because there appears to be no difference between females and males in the overall distribution pattern of ERα in the rodent spinal cord or dorsal root ganglion (DRG) [27], [28] (data on the frog sciatic nerve are lacking), we used either sex of the frog Rana nigromaculata. In brief, frogs were decapitated and then pithed. Thereafter, the sciatic nerve (length: 3–4 cm; diameter: 0.4–1 mm) was dissected from the lumbar plexus to the knee in Ringer's solution. The isolated sciatic nerve was carefully desheathed under a binocular microscope and then loosely placed on five platinum wires that were glued to a Lucite plate, and the two ends of the nerve were tied to the wires with threads. The plate was put on a beaker containing Ringer's solution, immersing the sciatic nerve. Throughout the experiments, the Ringer's solution was continuously stirred at a rate of about 350 rpm with a Teflon-covered magnetic stir bar to maintain a uniform and steady solute concentration around the sciatic nerve. The composition of the Ringer's solution used was (mM): NaCl, 115.5; KCl, 2.0; CaCl2, 1.8; Na2HPO4, 1.3; and NaH2PO4, 0.7 (pH=7.0). Before the start of the experiment, the sciatic nerve was preincubated for at least 15 min with Ringer's solution.
2.2. Recordings of CAPs from frog sciatic nerve fibers
As described previously [14], [15], [25], [26], the Lucite plate with the platinum wires attached to the sciatic nerve was moved from the beaker containing Ringer's solution (100 mL) to an empty beaker, and CAPs were recorded in air using a preamplifier. Two of the platinum wires were used to record CAPs, and the other two were used to stimulate the sciatic nerve. The stimulation was performed at a frequency of 1 Hz with a stimulator, using rectangular pulses of 0.1-ms duration and of various amplitudes. To prevent the sciatic nerve from drying out in air, this procedure was quickly performed (within about 30 s), with time intervals of 2 min. The data were monitored on a storage oscilloscope while being recorded on a thermal array recorder with a wave form storage module. The data were stored on magnetic tape with a PCM tape recorder for later analyses. In several cases, the data were analyzed with a personal computer using pCLAMP 8.0 software (Molecular Devices, Foster City, CA, USA). Stimulating the sciatic nerve produced a CAP following a stimulus artifact. The peak amplitude of the CAP was measured as the difference between baseline and CAP peak level, as done previously [14], [15], [25], [26]. The peak amplitude of the CAP depended on the strength of the stimulus given to the sciatic nerve, and the CAP peak amplitude increased with increasing stimulus strength, eventually attaining a maximal value (where all fibers contained in the nerve were excited; see below). As done previously [14], [15], [25], [26], we analyzed the peak amplitude of the maximal CAP. When the effect of a drug on CAPs was examined, the sciatic nerve was soaked for 20 min in Ringer's solution containing the drug (sufficient for a near-maximal effect) and then for ≤60 min in drug-free Ringer's solution. Each sciatic nerve was used only once to examine the effect of a drug, unless otherwise mentioned, because the effects of many of the drugs were partially reversible. The conduction velocity was determined using the fifth electrode as an additional stimulation site and by measuring the duration between the stimulus artifact and the peak of the CAP. All experiments were carried out at room temperature (20–28 °C).
2.3. Drugs
The drugs used were BPA (Tokyo Chemical Industries, Co. Ltd., Tokyo, Japan), 17β-estradiol, 4-OHT (≥98% trans isomer), pramoxine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) and prilocaine hydrochloride (Wako Pure Chemical Industries, Ltd., Osaka, Japan). All of the drugs (except for pramoxine and prilocaine, which were directly dissolved in Ringer's solution) were first dissolved in dimethyl sulfoxide (DMSO) to make a stock solution, and then diluted to the desired concentrations in Ringer's solution immediately before use, where the concentration of DMSO was less than 1%. BPA, 17β-estradiol and 4-OHT were tested at concentrations less than their highest dissolvable concentration.
2.4. Data analysis
The concentration-dependence curve for the reduction of the peak amplitude of the CAP in the sciatic nerve soaked with a drug for 20 min was analyzed using the following Hill equation:
CAP amplitude (% of control) =100/(1+([Drug]/IC50)nH),
where [Drug] is drug concentration, IC50 is the concentration of the drug for half-maximal inhibition, and nH is the Hill coefficient. Data were indicated as mean±S.E.M., and statistical significance was set at p<0.05 using paired or unpaired Student's t-test. In all cases, n refers to the number of sciatic nerves studied. An average of the peak amplitudes of five CAPs measured during 10 min before drug application was taken as control.
3. Results
The effects of various drugs on fast-conducting CAPs were examined in a total of 82 sciatic nerves, and the CAPs had an average peak amplitude of 21.8±0.2 mV (n=82). The conduction velocity of the fibers averaged 26±2 m/s (range: 16–49 m/s; n=28). These values were comparable to those reported previously [14], [26], [29], [30], [31], [32], [33], [34], [35]. DMSO at 1% (the maximal concentration used in the present study) did not affect CAPs; the peak amplitude of the CAP was 106±2% of control (taken as 100%; 32.2±3.5 mV; n=4) (p>0.05), 20 min after treatment with DMSO.
3.1. Effect of BPA on frog sciatic nerve CAPs
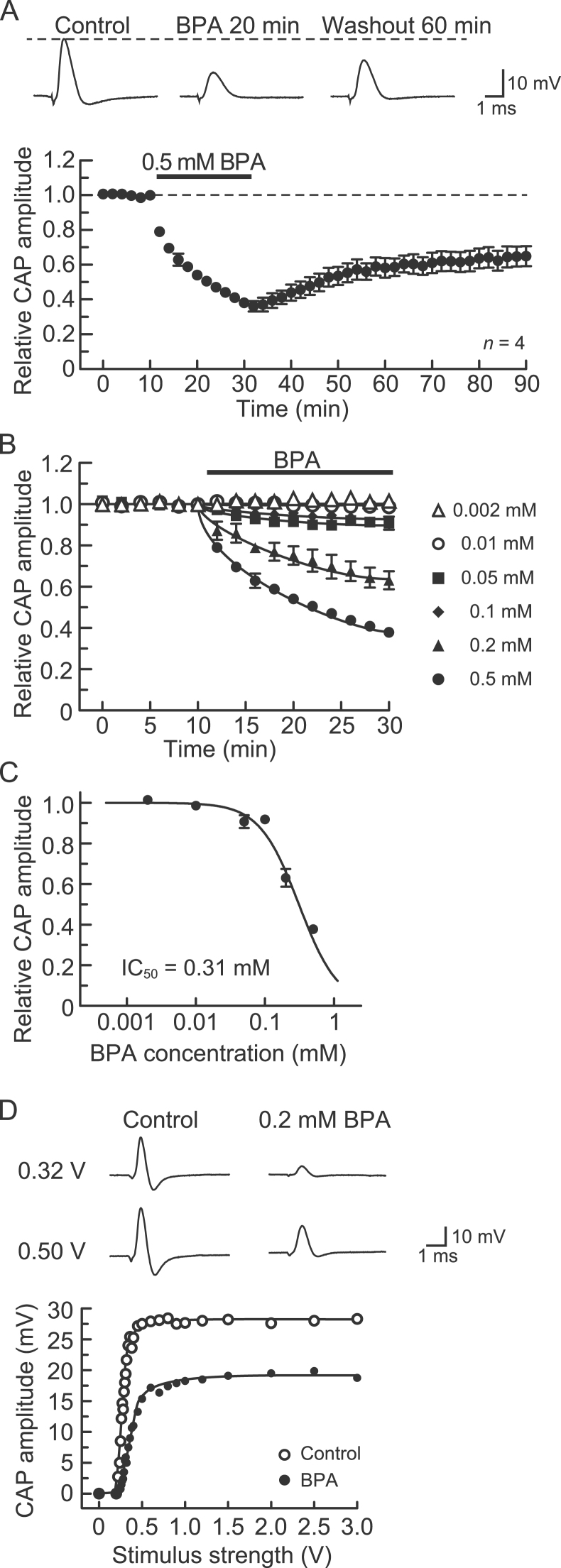
First, we examined the effect of BPA on CAPs recorded from the frog sciatic nerve. Soaking the sciatic nerve into Ringer's solution containing BPA at 0.5 mM (a concentration enough to inhibit frog sciatic nerve CAPs [13] and cardiac Na+ channels [22]) for 20 min reduced the peak amplitude of the CAP (Fig. 1A, top). Fig. 1A, bottom, shows the average time course of the change in CAP peak amplitude after incubation in the BPA (0.5 mM) solution, relative to that before application (control), obtained from four sciatic nerves. The BPA-induced reduction in CAP peak amplitude was near maximal 20 min following exposure, where the peak amplitude of the CAP was 38±2% (p<0.05) of control (22.1±0.8 mV; n=4). In nerves treated with BPA and then returned to drug-free Ringer's solution (washout) for up to 60 min, the CAP amplitude recovered to 65±6% (p<0.05; n=4) of the control level (Fig. 1A). Fig. 1B shows the time course of the changes in CAP peak amplitude after treating the sciatic nerve with BPA of various concentrations ranging from 0.002 mM to 0.5 mM. The rate of the reduction in CAP peak amplitude during 20 min of exposure to BPA increased with increasing concentration of the compound. The CAP amplitude reduction after 20 min of exposure increased in magnitude with increasing BPA concentration. The concentration-response curve for the BPA-induced reduction in CAP amplitude in the nerve trunks is given in Fig. 1C. The IC50 value obtained from analysis based on the Hill equation was 0.31 mM. In the following experiments, the inhibitory action of BPA on CAPs was examined at 0.2 mM, a value similar to the IC50.
Fig. 1.
Bisphenol A (BPA) inhibits compound action potentials (CAPs) recorded from frog sciatic nerve fibers without changing the threshold for CAP generation. (A) BPA at a concentration of 0.5 mM reduces CAP peak amplitudes in a partially reversible manner. Top of figure: recordings of CAPs in the control, at 20 min after exposure to BPA, and 60 min following BPA washout. Bottom of figure: average time course of changes in CAP peak amplitudes following exposure to BPA for 20 min, relative to that before exposure, obtained from four sciatic nerves. In this and subsequent figures, the stimulus strength used to elicit CAPs was one at which a maximal CAP was produced (see below), unless otherwise mentioned; each point with vertical bars represents the mean and S.E.M.; the S.E.M. of a value without a visible vertical bar is smaller than the size of the data point. (B) Comparison of the average time course of CAP peak amplitude reductions produced by BPA at 0.002–0.5 mM, obtained from 24 sciatic nerves. (C) Plot of the peak amplitudes of CAPs, relative to control, recorded from sciatic nerve fibers treated with BPA of various concentrations for 20 min. Each of the data points was obtained from 3 to 6 sciatic nerves. The concentration-response curve was drawn according to the Hill equation (half-maximal inhibitory concentration, IC50=0.31 mM; Hill coefficient, nH=1.5). (D) Stimulus strength dependence for CAP peak amplitude reduction produced by BPA. The peak amplitudes of CAPs before (open circles) and after exposure to BPA (0.2 mM; closed circles) for 20 min, plotted against the stimulus strength used to elicit the CAP. Insets show recordings of CAPs elicited at 0.32 and 0.50 V in the control (left) and after exposure to BPA for 20 min (right). The solid lines in (B) and (D) were drawn arbitrarily.
Fig. 1D shows the dependency of the peak amplitude of the CAP on stimulus intensity in the control and after 20 min of exposure to BPA (0.2 mM). In the absence and presence of BPA, the CAP amplitude increased with increasing stimulus intensity, eventually reaching a maximal value. The inhibitory effect of BPA was seen for CAPs evoked by the maximal stimulus strength, without affecting the threshold to elicit the CAP. This finding was obtained in four other nerves.
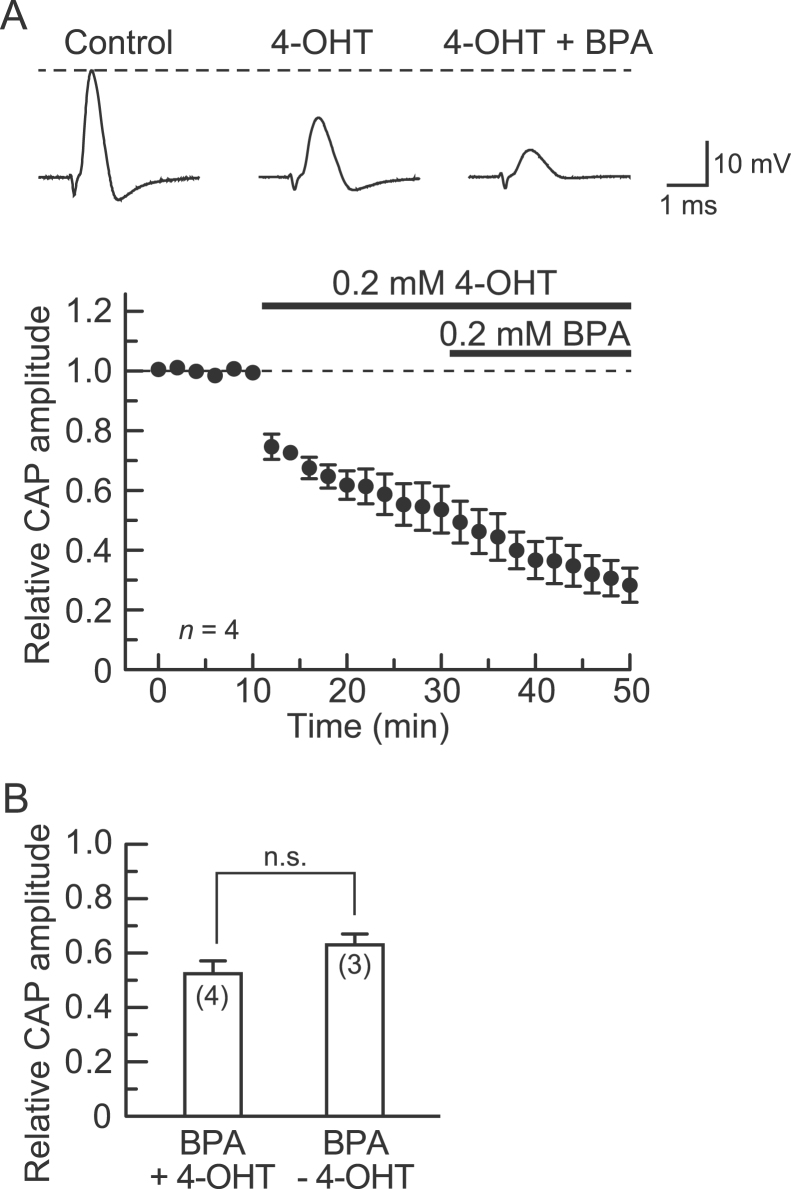
To reveal whether the CAP inhibition produced by BPA is mediated by estrogen receptors, especially ERRγ, we examined the effect of 4-OHT, administered at the same concentration as BPA (0.2 mM). Pretreatment with 4-OHT for 20 min reduced the peak amplitude of the CAP. This reduction was stable within this period (Fig. 2A, top). There was no difference in the relative CAP amplitude between 4-OHT treatments of 18 and 20 min (55±8 and 54±8%, respectively; p>0.05; n=4), indicative of a stable effect of 4-OHT (0.2 mM) itself. Following this pretreatment, adding BPA (0.2 mM) to the 4-OHT-containing Ringer's solution markedly inhibited the CAP (Fig. 2A, top). The average result obtained from four nerves is given in Fig. 2A, bottom. The peak amplitude after treatment with both 4-OHT and BPA for 20 min was reduced to 52±5% (p<0.05; n=4) of that just before the co-treatment. The CAP peak amplitude after 20 min of co-treatment with BPA and 4-OHT was not different from that obtained with BPA alone (p>0.05; Fig. 2B).
Fig. 2.
The CAP peak amplitude reduction produced by BPA (0.2 mM) is resistant to 4-hydroxytamoxifen (4-OHT; 0.2 mM). (A) The effect of BPA on CAPs in the presence of 4-OHT. Top of figure: recordings of CAPs in the absence (left) and presence of 4-OHT alone (middle) or together with BPA (right). Bottom of figure: average time course of changes in CAP peak amplitudes following treatment with 4-OHT for 20 min and then with both 4-OHT and BPA for 20 min, relative to those before drug treatment, obtained from four sciatic nerves. Note that 4-OHT alone reduced CAP peak amplitude. (B) Comparison of CAP peak amplitude reductions produced by BPA in the presence (+) and absence (-) of 4-OHT. The reduction in CAP amplitude produced by the combination of BPA and 4-OHT did not differ in magnitude from that produced by BPA alone. Value in parentheses indicates the number of nerve trunks tested. Statistical significance between data is denoted by a horizontal line; n.s.: not significant.
3.2. Effect of 4-OHT on frog sciatic nerve CAPs
Because 4-OHT alone inhibited CAPs, we examined this inhibition in great detail. 4-OHT, at a concentration of 0.1 mM, irreversibly reduced CAP peak amplitude (Fig. 3A, top). Fig. 3A, bottom, shows the average time course of the change in the CAP peak amplitude following exposure to 4-OHT (0.1 mM), relative to control, which was obtained from four sciatic nerves. Like BPA, 4-OHT maximally reduced CAP amplitude within 20 min of immersion in the 4-OHT-containing solution; the peak amplitude was reduced to 76±7% (p<0.05) of control (24.5±0.9 mV; n=4). As seen in Fig. 3B, the CAP peak amplitude reduction produced by 4-OHT increased with increasing concentration. The IC50 obtained from analysis based on the Hill equation was 0.26 mM.
Fig. 3.
Effect of 4-OHT on CAPs recorded from frog sciatic nerve fibers. (A) 4-OHT at a concentration of 0.1 mM irreversibly reduced CAP peak amplitudes. Top of figure: recordings of CAPs in the control, at 20 min after exposure to 4-OHT, and at 30 and 60 min after washout. Bottom of figure: average time course of changes in CAP peak amplitudes following exposure to 4-OHT for 20 min, relative to that before exposure, obtained from four sciatic nerves. (B) Concentration dependency of the CAP peak amplitude reduction produced by 4-OHT. Plot of the peak amplitudes of CAPs, relative to control, recorded from sciatic nerve fibers treated with 4-OHT at various concentrations for 20 min. Each of the data points was obtained from 3 to 4 sciatic nerves. The concentration-response curve was drawn according to the Hill equation (IC50=0.26 mM, nH=1.3).
3.3. Effect of 17β-estradiol on frog sciatic nerve CAPs
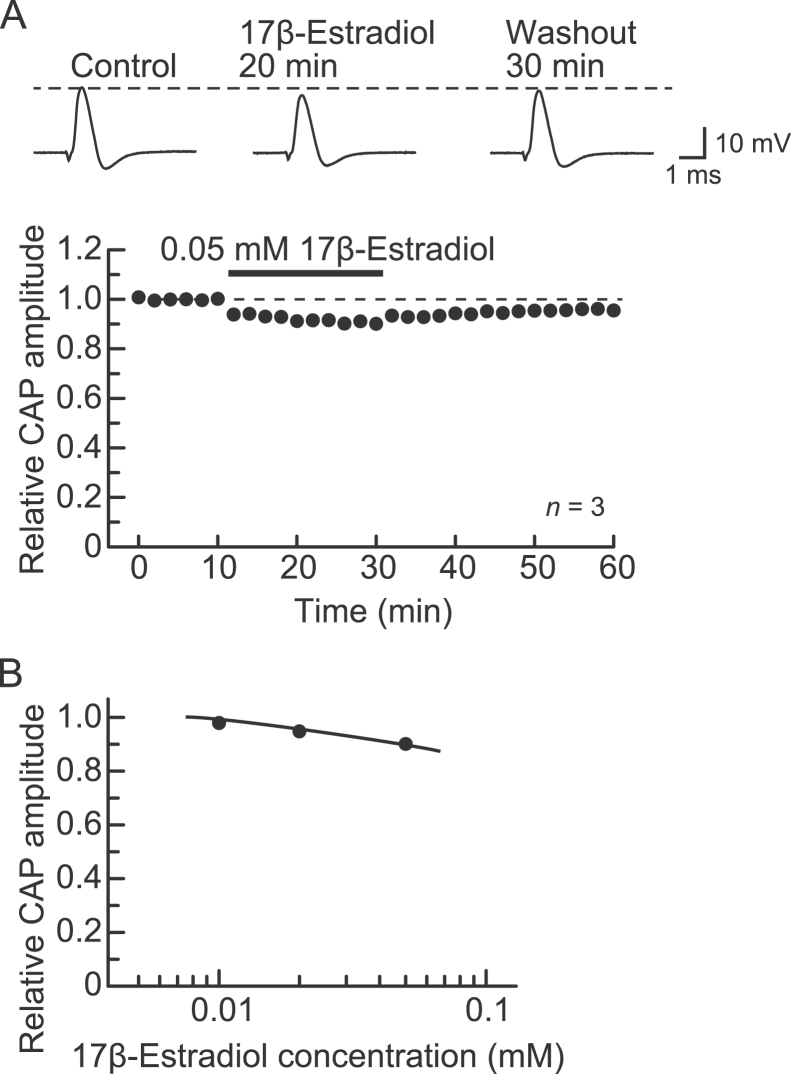
Next, we compared the effect of BPA on CAP inhibition with that of an estrogen-receptor agonist, 17β-estradiol. Similar to BPA, 17β-estradiol at a concentration of 0.05 mM (the maximally dissolvable concentration in Ringer's solution containing 1% DMSO) inhibited CAPs slightly in a partially reversible manner (Fig. 4A, top). The reduction in the CAP peak amplitude produced by 17β-estradiol (0.05 mM) was maximal within 20 min of exposure to the drug; the amplitude was 90±2% (p<0.05) of control (21.7±1.8 mV; n=3; Fig. 4A, bottom). This percentage value was not different from that of 0.05 mM BPA (91±3%; n=5; Fig. 1C) (p>0.05). Fig. 4B shows the effect of 17β-estradiol in a concentration range of 0.01–0.05 mM on CAPs. Although this inhibition by 17β-estradiol weakened with increasing concentration, the relative CAP amplitudes at 0.02 and 0.05 mM were not significantly different (p>0.05). Because the concentration dependency of this effect of 17β-estradiol was very slight, we did not test a wider concentration range, although 17β-estradiol has a biphasic effect on GH cell growth in a manner dependent on the concentration tested [36].
Fig. 4.
Effect of 17β-estradiol on CAPs recorded from frog sciatic nerve fibers. (A) 17β-Estradiol at a concentration of 0.05 mM reduced CAP peak amplitudes in a partially reversible manner. Top of figure: recordings of CAPs in the control, at 20 min after exposure to 17β-estradiol, and 30 min after washout. Bottom of figure: average time course of changes in CAP peak amplitudes following exposure to 17β-estradiol for 20 min, relative to that before exposure, obtained from three sciatic nerves. (B) Plot of the peak amplitudes of CAPs recorded from sciatic nerve fibers treated with 17β-estradiol at various concentrations for 20 min, relative to control. Each of the data points was obtained from 3 sciatic nerves. The solid line was arbitrarily drawn.
3.4. Effects of LAs on frog sciatic nerve CAPs
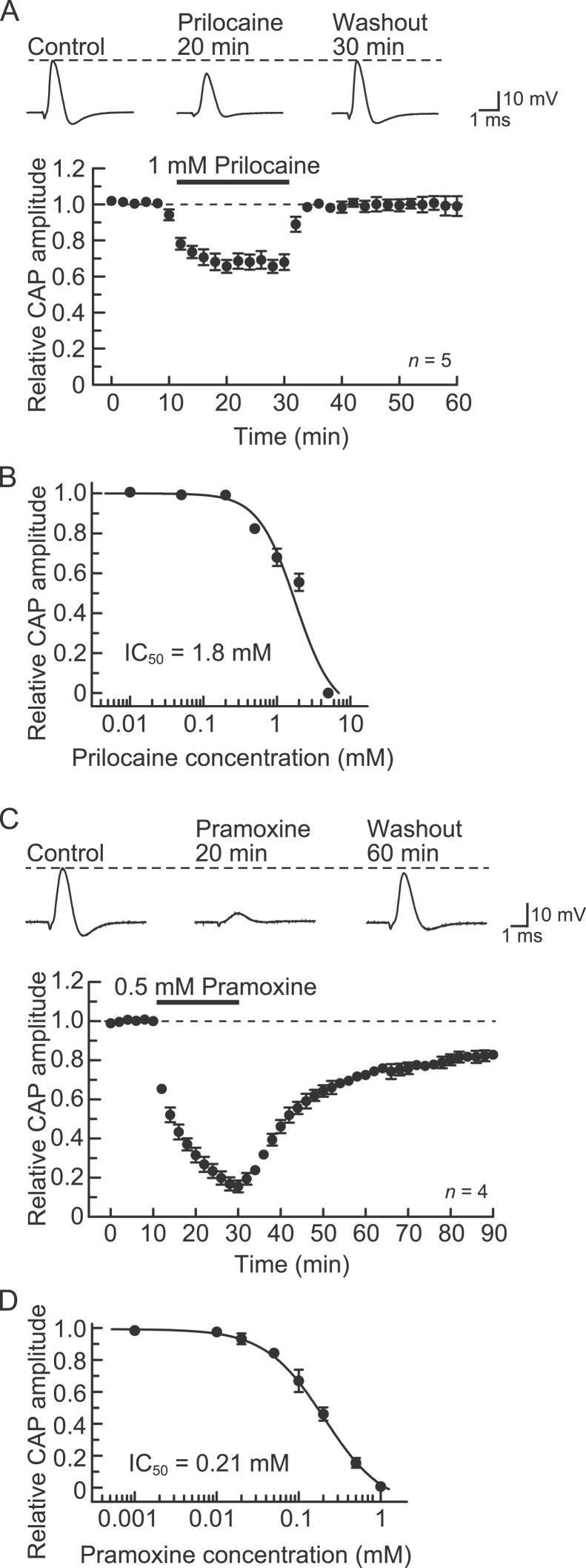
BPA reportedly binds to an LA receptor site on voltage-gated Na+ channels [22]. Therefore, we compared the effect of BPA with those of two different types of LAs (prilocaine and pramoxine). Prilocaine at 1 mM reversibly reduced CAP peak amplitude (Fig. 5A, top). Fig. 5A, bottom, shows the average time course of the change in CAP peak amplitude following exposure to prilocaine (1 mM), relative to control. Prilocaine stably reduced CAP amplitude within 20 min of exposure; the peak amplitude was reduced to 68±4% (n=5; p<0.05) of control (21.9±2.8 mV). The peak amplitude of the CAP 30 min after washout was 101±6% (n=5) of control. This value was not significantly different from 100% (p>0.05). The extent of the reduction in the CAP peak amplitude produced by prilocaine increased with increasing concentration, within a range of 0.01–5 mM (Fig. 5B; IC50=1.8 mM).
Fig. 5.
Effects of prilocaine and pramoxine on CAPs recorded from frog sciatic nerve fibers. (A, C) CAP peak amplitudes are reduced by prilocaine (1 mM; A) and pramoxine (0.5 mM; C); the reductions were reversible and partially reversible, respectively. Top of figure: recordings of CAPs in the control, at 20 min after exposure to prilocaine (A) or pramoxine (C), and after washout of prilocaine (30 min; A) or pramoxine (60 min; C). Bottom of figure: average time course of changes in CAP peak amplitudes following exposure to prilocaine (A) or pramoxine (C) for 20 min, relative to that before exposure, obtained from 5 or 4 sciatic nerves. (B, D) Plot of the peak amplitudes of CAPs, relative to control, recorded from sciatic nerve fibers treated with prilocaine (B) or pramoxine (D) at various concentrations for 20 min. Each of the data points was obtained from 3 to 5 sciatic nerves. The concentration-response curve was drawn according to the Hill equation (B: IC50=1.8 mM, nH=1.7; D: IC50=0.21 mM, nH=1.2).
Compared with prilocaine, pramoxine at 0.5 mM reduced CAP peak amplitude in a partially reversible manner (Fig. 5C, top). Pramoxine reduced CAP peak amplitude 20 min after exposure to 15±3% (n=4; p<0.05) of control (21.6±3.1 mV). The peak amplitude of the CAP 60 min after washout was 83±3% (n=4; p<0.05; Fig. 5C, bottom) of control. The magnitude of the CAP peak amplitude reduction produced by pramoxine increased with increasing concentration within a range of 0.001–1 mM (Fig. 5D; IC50=0.21 mM).
4. Discussion
We demonstrate that the endocrine disruptor BPA reduces the peak amplitude of fast-conducting and TTX-sensitive CAPs recorded from the frog (Rana nigromaculata) sciatic nerve in a partially reversible manner. This action was concentration-dependent, with an IC50 value of 0.31 mM. This inhibition by BPA was observed for CAPs evoked by a maximal stimulus strength, and BPA did not change the threshold to elicit the CAP. This effect of BPA was mimicked by a natural estrogen, 17β-estradiol; the effect of this estrogen was comparable in magnitude to that of BPA. The CAP inhibition elicited by BPA and 17β-estradiol occurred within 2 min of exposure (see Figs. 1A and 4A). Similar CAP inhibitions were produced by an amide-type LA (prilocaine) and an ester-type LA (pramoxine). Prilocaine reversibly reduced CAP peak amplitude, with an EC50 value of 1.8 mM. In comparison, pramoxine did so with an efficacy (EC50=0.21 mM) higher than that of prilocaine, with a slow recovery to control level.
4.1. No involvement of estrogen receptors or estrogen-related receptors in the CAP inhibition produced by BPA
Because the CAP inhibition is rapid, and our preparation is the dissected sciatic nerve that lacks neuronal cell bodies, it is unlikely that the effect of BPA is mediated by nuclear estrogen receptors that affect gene transcription. Estrogen receptors are located in the plasma membrane as well as the nucleus (for review see [37]). Plasma membrane receptors are involved in many actions of BPA; for example, the modulation of the NMDA receptor (a glutamate receptor)-mediated increase in intracellular Ca2+ concentration in rat hippocampal neurons in culture [38] and the modulation of dopamine transporter function in rat pheochromocytoma cells [39]. ERα and estrogen receptor β (ERβ) are expressed in rat DRG [27] and spinal cord ventral horn neurons [40] whose fibers are contained within the sciatic nerve. The CAP inhibition produced by BPA, therefore, may be mediated by plasma membrane estrogen receptors. However, this possibility is unlikely because this action of BPA is resistant to 4-OHT, which inhibits estrogen receptors and ERRγ, of which the latter is thought to be a target of BPA [19], [20], [21]. 4-OHT itself reduces CAP peak amplitude with almost the same IC50 value (0.26 mM) as that of BPA. This indicates that BPA binds to a site different from that of 4-OHT. Although 17β-estradiol has a much higher affinity (>25,000-fold) for ERα and ERβ than BPA (for review see [41]), both inhibit frog sciatic nerve CAPs to almost the same extent. These results suggest that there is no involvement of estrogen receptors or ERRγ in the action of BPA on CAPs. Our finding differs from that of Pandey and Deshpande [13], who observed that BPA-induced CAP inhibition in the frog (Rana tigrina) sciatic nerve is mediated by ERα. The reason for this discrepancy is unknown.
It is possible that BPA acts on voltage-gated Na+ channels involved in the production of CAPs [14]. Indeed, Wang et al. [42] reported that BPA reduces the peak amplitude of TTX-sensitive voltage-gated Na+-channel currents in mouse DRG neurons. This idea of Na+-channel involvement is supported by the fact that BPA shares a site on human cardiac Na+ channels with LAs ([22]; see below). However, the IC50 value (0.0398 mM) for the Na+-channel inhibition [42] was much lower than that for CAP inhibition obtained in the present study (0.31 mM). Although BPA shifted the voltage-activation curve of the DRG Na+ channel to a more negative potential, the threshold for eliciting the CAPs was not changed by BPA. Because the Na+-channel inhibition produced by BPA in DRG neurons appears to be mediated by protein kinase C/A-dependent signaling pathways [42], this inhibition may be caused by the activation of plasma membrane estrogen receptors, distinct from the mechanism we found in the present study (see above). Although 4-OHT by itself inhibits CAPs, this action also appears to be caused by the inhibition of TTX-sensitive voltage-gated Na+ channels, because tamoxifen reduces TTX-sensitive Na+-channel current amplitudes within 5 min after application in rat cortical astrocytes in culture. Like CAP inhibition by 4-OHT, this reduction was irreversible 10 min after washout [43]. Nonetheless, 4-OHT and BPA appear not to share the same site on voltage-gated Na+ channels, because there is no interaction between 4-OHT and BPA for CAP inhibition (see Fig. 2). A similar CAP inhibition without receptor activation in the frog sciatic nerve has been shown for the actions of a μ-opioid receptor agonist, tramadol, and an α2-adrenoceptor agonist, dexmedetomidine. These agonists inhibit CAPs evoked by a maximal stimulus strength without changing the threshold to elicit them [29], [30].
A direct action of BPA on Na+ channels, as seen in the present study, has been demonstrated for other types of channels as well (see [44] for review). For example, BPA directly modulates GABAA-receptor channels expressed in Xenopus oocytes [45] as well as in acutely-dissociated rat hippocampal CA3 pyramidal neurons [46]. Furthermore, BPA activates large conductance Ca2+/voltage-sensitive K+ channels in coronary smooth muscle cells [47].
4.2. Comparison of BPA with LAs
Because BPA was reported to bind to an LA receptor site on Na+ channels [22], the EC50 value of BPA in inhibiting CAPs was compared with those of LAs (obtained from the present and previous studies) [14], [29], [30], [31], [32]. The efficacy of BPA was similar to those of pramoxine, ropivacaine and levobupivacaine (0.21, 0.34 and 0.23 mM, respectively), higher than those of prilocaine, procaine, lidocaine and cocaine (IC50=1.8, 2.3, 0.74 and 0.80 mM, respectively), and lower than that of tetracaine (0.014 mM). Many plant-derived chemicals have been reported to inhibit frog sciatic nerve CAPs with efficacies similar to those of LAs [26], [31], [33], [34], [35]. Further study is required to clarify the interaction between BPA and LA receptor sites on Na+ channels.
4.3. Actions of BPA on neuronal functions
There is much evidence that BPA acts on the nervous system. For example, BPA increases spontaneous motor activity along with a large reduction in tyrosine hydroxylase activity in the midbrain [48], and it produces memory impairment accompanied by a reduction in acetylcholine production in the hippocampus [49] in rodents. Synaptogenesis induced by 17β-estradiol is inhibited by BPA in the hippocampus of rodents and primates [50], [51]. Additionally, BPA affects exploratory, anxiety- and pain-related behaviors [11], [12]. Many of these effects of BPA might be mediated by estrogen receptors expressed in the central nervous system (for example see [40]). The inhibitory action of BPA on frog sciatic nerve CAPs was seen at concentrations higher than 0.1 mM. Völkel et al. [52] reported that 80 min after the oral administration of BPA (5 mg), the maximal plasma concentration of BPA, albeit in its glucuronide form, attains some 0.8 μM in humans. Even though this concentration is much lower than 0.1 mM, BPA is lipophilic, and therefore may accumulate in fatty tissues, such as the central nervous system, resulting in a high concentration of BPA that inhibits nerve conduction without estrogen-receptor activation.
In conclusion, our findings indicate that BPA inhibits nerve conduction independent of estrogen-receptor activation. This action of BPA might contribute to the neurotoxic effects of the compound on the central nervous system.
Acknowledgements
We appreciate Professor Y. Shimohigashi and Dr. A. Matsushima for giving us BPA and 4-OHT. This work was partly supported by Grants-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (KAKENHI: 15K08673).
References
- 1.Kang J.-H., Kondo F., Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226:79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Vandenberg L.N., Hauser R., Marcus M., Olea N., Welshons W.V. Human exposure to bisphenol A (BPA) Reprod. Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Maqbool F., Mostafalou S., Bahadar H., Abdollahi M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016;145:265–273. doi: 10.1016/j.lfs.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan A.V., Stathis P., Permuth S.F., Tokes L., Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 5.Steinmetz R., Brown N.G., Allen D.L., Bigsby R.M., Ben-Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997;138:1780–1786. doi: 10.1210/endo.138.5.5132. [DOI] [PubMed] [Google Scholar]
- 6.Laws S.C., Carey S.A., Ferrell J.M., Bodman G.J., Cooper R.L. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol. Sci. 2000;54:154–167. doi: 10.1093/toxsci/54.1.154. [DOI] [PubMed] [Google Scholar]
- 7.Li W., Seifert M., Xu Y., Hock B. Comparative study of estrogenic potencies of estradiol, tamoxifen, bisphenol-A and resveratrol with two in vitro bioassays. Environ. Int. 2004;30:329–335. doi: 10.1016/S0160-4120(03)00183-1. [DOI] [PubMed] [Google Scholar]
- 8.Sohoni P., Sumpter J.P. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- 9.Wetherill Y.B., Akingbemi B.T., Kanno J., McLachlan J.A., Nadal A., Sonnenschein C., Watson C.S., Zoeller R.T., Belcher S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007;24:178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Ogiue-Ikeda M., Tanabe N., Mukai H., Hojo Y., Murakami G., Tsurugizawa T., Takata N., Kimoto T., Kawato S. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res. Rev. 2008;57:363–375. doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Farabollini F., Porrini S., Dessì-Fulgheri F. Perinatal exposure to the estrogenic pollutant bisphenol A affects behavior in male and female rats. Pharmacol. Biochem. Behav. 1999;64:687–694. doi: 10.1016/s0091-3057(99)00136-7. [DOI] [PubMed] [Google Scholar]
- 12.Aloisi A.M., Della Seta D., Rendo C., Ceccarelli I., Scaramuzzino A., Farabollini F. Exposure to the estrogenic pollutant bisphenol A affects pain behavior induced by subcutaneous formalin injection in male and female rats. Brain Res. 2002;937:1–7. doi: 10.1016/s0006-8993(02)02446-0. [DOI] [PubMed] [Google Scholar]
- 13.Pandey A.K., Deshpande S.B. Bisphenol A depresses compound action potential of frog sciatic nerve in vitro involving Ca2+-dependent mechanisms. Neurosci. Lett. 2012;517:128–132. doi: 10.1016/j.neulet.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 14.Mizuta K., Fujita T., Nakatsuka T., Kumamoto E. Inhibitory effects of opioids on compound action potentials in frog sciatic nerves and their chemical structures. Life Sci. 2008;83:198–207. doi: 10.1016/j.lfs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Kumamoto E., Mizuta K., Fujita T. Peripheral nervous system in the frog as a tool to examine the regulation of the transmission of neuronal information. In: Murray J.L., editor. Frogs: Biology, Ecology and Uses. Nova Science Publishers, Inc; New York, USA: 2012. pp. 89–106. [Google Scholar]
- 16.Malet C., Gompel A., Spritzer P., Bricout N., Yaneva H., Mowszowicz I., Kuttenn F., Mauvais-Jarvis P. Tamoxifen and hydroxytamoxifen isomers versus estradiol effects on normal human breast cells in culture. Cancer Res. 1988;48:7193–7199. [PubMed] [Google Scholar]
- 17.Heard D.J., Norby P.L., Holloway J., Vissing H. Human ERRγ, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol. Endocrinol. 2000;14:382–392. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- 18.Coward P., Lee D., Hull M.V., Lehmann J.M. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor γ. Proc. Natl. Acad. Sci. USA. 2001;98:8880–8884. doi: 10.1073/pnas.151244398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takayanagi S., Tokunaga T., Liu X., Okada H., Matsushima A., Shimohigashi Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicol. Lett. 2006;167:95–105. doi: 10.1016/j.toxlet.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Okada H., Tokunaga T., Liu X., Takayanagi S., Matsushima A., Shimohigashi Y. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-γ. Environ. Health Perspect. 2008;116:32–38. doi: 10.1289/ehp.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X., Matsushima A., Okada H., Shimohigashi Y. Distinction of the binding modes for human nuclear receptor ERRγ between bisphenol A and 4-hydroxytamoxifen. J. Biochem. 2010;148:247–254. doi: 10.1093/jb/mvq056. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly A.O., Eberhardt E., Weidner C., Alzheimer C., Wallace B.A., Lampert A. Bisphenol A binds to the local anesthetic receptor site to block the human cardiac sodium channel. PLoS One. 2012;7:e41667. doi: 10.1371/journal.pone.0041667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catterall W.A., Mackie K. In: Local Anesthetics, Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th ed. Brunton L.L., Chabner B.A., Knollmann B.C., editors. McGraw-Hill, Medical Publishing Division; New York, USA: 2011. pp. 565–582. [Google Scholar]
- 24.Mizuta K., Fujita T., Yamagata H., Lommi M., Yasaka T., Kumamoto E. Bisphenol A inhibits compound action potentials in frog sciatic nerves. J. Physiol. Sci. 2010;60(Suppl. 1):S121. [Google Scholar]
- 25.Kumamoto E., Mizuta K., Fujita T., Kosugi T., Katsuki R. Neurotransmitter receptor agonists having the inhibitory actions of nerve conduction – structure-function relationship. In: Pandalai S.G., editor. Vol. 2. Research Signpost; Kerala, India: 2011. pp. 1–26. (Recent Res. Devel. Pharmacol.). [Google Scholar]
- 26.Ohtsubo S., Fujita T., Matsushita A., Kumamoto E. Inhibition of the compound action potentials of frog sciatic nerves by aroma oil compounds having various chemical structures. Pharmacol. Res. Perspect. 2015;3:e00127. doi: 10.1002/prp2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taleghany N., Sarajari S., DonCarlos L.L., Gollapudi L., Oblinger M.M. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J. Neurosci. Res. 1999;57:603–615. [PubMed] [Google Scholar]
- 28.Vanderhorst V.G.J.M., Gustafsson J.-A., Ulfhake B. Estrogen receptor-α and -β immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J. Comp. Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- 29.Katsuki R., Fujita T., Koga A., Liu T., Nakatsuka T., Nakashima M., Kumamoto E. Tramadol, but not its major metabolite (mono-O-demethyl tramadol) depresses compound action potentials in frog sciatic nerves. Br. J. Pharmacol. 2006;149:319–327. doi: 10.1038/sj.bjp.0706868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosugi T., Mizuta K., Fujita T., Nakashima M., Kumamoto E. High concentrations of dexmedetomidine inhibit compound action potentials in frog sciatic nerves without α2 adrenoceptor activation. Br. J. Pharmacol. 2010;160:1662–1676. doi: 10.1111/j.1476-5381.2010.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomohiro D., Mizuta K., Fujita T., Nishikubo Y., Kumamoto E. Inhibition by capsaicin and its related vanilloids of compound action potentials in frog sciatic nerves. Life Sci. 2013;92:368–378. doi: 10.1016/j.lfs.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Uemura Y., Fujita T., Ohtsubo S., Hirakawa N., Sakaguchi Y., Kumamoto E. Effects of various antiepileptics used to alleviate neuropathic pain on compound action potential in frog sciatic nerves: comparison with those of local anesthetics. Biomed. Res. Int. 2014;2014:540238. doi: 10.1155/2014/540238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawasaki H., Mizuta K., Fujita T., Kumamoto E. Inhibition by menthol and its related chemicals of compound action potentials in frog sciatic nerves. Life Sci. 2013;92:359–367. doi: 10.1016/j.lfs.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Matsushita A., Ohtsubo S., Fujita T., Kumamoto E. Inhibition by TRPA1 agonists of compound action potentials in the frog sciatic nerve. Biochem. Biophys. Res. Commun. 2013;434:179–184. doi: 10.1016/j.bbrc.2013.02.127. [DOI] [PubMed] [Google Scholar]
- 35.Matsushita A., Fujita T., Ohtsubo S., Kumamoto E. Traditional Japanese medicines inhibit compound action potentials in the frog sciatic nerve. J. Ethnopharmacol. 2016;178:272–280. doi: 10.1016/j.jep.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Amara J.F., Dannies P.S. 17 β-Estradiol has a biphasic effect on GH cell growth. Endocrinology. 1983;112:1141–1143. doi: 10.1210/endo-112-3-1141. [DOI] [PubMed] [Google Scholar]
- 37.Watson C.S., Gametchu B. Membrane-initiated steroid actions and the proteins that mediate them. Proc. Soc. Exp. Biol. Med. 1999;220:9–19. doi: 10.1046/j.1525-1373.1999.d01-2.x. [DOI] [PubMed] [Google Scholar]
- 38.Tanabe N., Kimoto T., Kawato S. Rapid Ca2+ signaling induced by bisphenol A in cultured rat hippocampal neurons. Neuro. Endocrinol. Lett. 2006;27:97–104. [PubMed] [Google Scholar]
- 39.Alyea R.A., Watson C.S. Differential regulation of dopamine transporter function and location by low concentrations of environmental estrogens and 17β-estradiol. Environ. Health Perspect. 2009;117:778–783. doi: 10.1289/ehp.0800026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shughrue P.J., Lane M.V., Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 41.le Maire A., Bourguet W., Balaguer P. A structural view of nuclear hormone receptor: endocrine disruptor interactions. Cell. Mol. Life Sci. 2010;67:1219–1237. doi: 10.1007/s00018-009-0249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Q., Cao J., Zhu Q., Luan C., Chen X., Yi X., Ding H., Chen J., Cheng J., Xiao H. Inhibition of voltage-gated sodium channels by bisphenol A in mouse dorsal root ganglion neurons. Brain Res. 2011;1378:1–8. doi: 10.1016/j.brainres.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Smitherman K.A., Sontheimer H. Inhibition of glial Na+ and K+ currents by tamoxifen. J. Membr. Biol. 2001;181:125–135. doi: 10.1007/s00232-001-0016-2. [DOI] [PubMed] [Google Scholar]
- 44.Soriano S., Ripoll C., Alonso-Magdalena P., Fuentes E., Quesada I., Nadal A., Martinez-Pinna J. Effects of bisphenol A on ion channels: experimental evidence and molecular mechanisms. Steroids. 2016;111:12–20. doi: 10.1016/j.steroids.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Aoshima H., Hossain S.J., Imamura H., Shingai R. Effects of bisphenol A and its derivatives on the response of GABAA receptors expressed in Xenopus oocytes. Biosci. Biotechnol. Biochem. 2001;65:2070–2077. doi: 10.1271/bbb.65.2070. [DOI] [PubMed] [Google Scholar]
- 46.Choi I.-S., Cho J.-H., Park E.-J., Park J.-W., Kim S.-H., Lee M.-G., Choi B.-J., Jang I.-S. Multiple effects of bisphenol A, an endocrine disrupter, on GABAA receptors in acutely dissociated rat CA3 pyramidal neurons. Neurosci. Res. 2007;59:8–17. doi: 10.1016/j.neures.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Asano S., Tune J.D., Dick G.M. Bisphenol A activates Maxi-K (KCa1.1) channels in coronary smooth muscle. Br. J. Pharmacol. 2010;160:160–170. doi: 10.1111/j.1476-5381.2010.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishido M., Masuo Y., Kunimoto M., Oka S., Morita M. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J. Neurosci. Res. 2004;76:423–433. doi: 10.1002/jnr.20050. [DOI] [PubMed] [Google Scholar]
- 49.Miyagawa K., Narita M., Narita M., Akama H., Suzuki T. Memory impairment associated with a dysfunction of the hippocampal cholinergic system induced by prenatal and neonatal exposures to bisphenol-A. Neurosci. Lett. 2007;418:236–241. doi: 10.1016/j.neulet.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 50.MacLusky N.J., Hajszan T., Leranth C. The environmental estrogen bisphenol A inhibits estradiol-induced hippocampal synaptogenesis. Environ. Health Perspect. 2005;113:675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leranth C., Hajszan T., Szigeti-Buck K., Bober J., MacLusky N.J. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc. Natl. Acad. Sci. USA. 2008;105:14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Völkel W., Colnot T., Csanády G.A., Filser J.G., Dekant W. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem. Res. Toxicol. 2002;15:1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]