Abstract
The bacterium Burkholderia ubonensis is commonly co-isolated from environmental specimens harbouring the melioidosis pathogen, Burkholderia pseudomallei. B. ubonensis has been reported in northern Australia and Thailand but not North America, suggesting similar geographic distribution to B. pseudomallei. Unlike most other Burkholderia cepacia complex (Bcc) species, B. ubonensis is considered non-pathogenic, although its virulence potential has not been tested. Antibiotic resistance in B. ubonensis, particularly towards drugs used to treat the most severe B. pseudomallei infections, has also been poorly characterised. This study examined the population biology of B. ubonensis, and includes the first reported isolates from the Caribbean. Phylogenomic analysis of 264 B. ubonensis genomes identified distinct clades that corresponded with geographic origin, similar to B. pseudomallei. A small proportion (4%) of strains lacked the 920kb chromosome III replicon, with discordance of presence/absence amongst genetically highly related strains, demonstrating that the third chromosome of B. ubonensis, like other Bcc species, probably encodes for a nonessential pC3 megaplasmid. Multilocus sequence typing using the B. pseudomallei scheme revealed that one-third of strains lack the “housekeeping” narK locus. In comparison, all strains could be genotyped using the Bcc scheme. Several strains possessed high-level meropenem resistance (≥32 μg/mL), a concern due to potential transmission of this phenotype to B. pseudomallei. In silico analysis uncovered a high degree of heterogeneity among the lipopolysaccharide O-antigen cluster loci, with at least 35 different variants identified. Finally, we show that Asian B. ubonensis isolate RF23-BP41 is avirulent in the BALB/c mouse model via a subcutaneous route of infection. Our results provide several new insights into the biology of this understudied species.
Author summary
The pathogenic bacterium Burkholderia pseudomallei causes the disease melioidosis, which occurs in most tropical regions across the globe. The true burden of melioidosis is unknown but has been predicted to affect 165,000 people every year, resulting in 89,000 deaths. B. pseudomallei is easily confused with its close relative B. ubonensis as both species are frequently found in the same environmental niche and can appear phenotypically identical using serotyping and laboratory culture methods. B. ubonensis is a poorly characterised species but has recently gained interest in the research community as a potential biocontrol agent in B. pseudomallei-endemic regions, and for production of unusual and versatile biocompounds that are now being exploited for industrial applications. B. ubonensis is thought to be non-pathogenic, although other members of the B. cepacia complex to which it belongs are known for their ability to cause clinical disease that can be fatal in immunocompromised patients and people with cystic fibrosis. In this study, we investigated the biology of B. ubonensis to better understand its genetics, genomics, global distribution, virulence potential and antibiotic resistance. We show that this organism is highly genetically diverse, is avirulent in the mouse model, and can naturally encode high levels of meropenem resistance. We also identify B. ubonensis in the Caribbean for the first time, with phylogenomic analysis revealing distinct clades corresponding to geographic origin.
Introduction
The Gram-negative soil- and water-dwelling bacterium B. ubonensis is a member of the Burkholderia cepacia complex (Bcc) [1], a genetically related group of metabolically diverse, highly adaptable and widely dispersed environmental species [2]. The Bcc, which comprises at least 20 species, includes some members known for their ability to cause clinical disease, such as severe sepsis in the immunocompromised and progressive pulmonary disease in cystic fibrosis patients [3]. Many Bcc species are also recognised for their unique biotechnological potential, particularly in bioremediation applications and in the production of antibiotic and antifungal compounds [4]. Novel compounds produced by B. ubonensis have been proposed as potential agents in biocontrol against Burkholderia pseudomallei [5] and in biodiesel catalysis [6].
B. pseudomallei, the causative agent of the tropical infectious disease melioidosis, is frequently isolated from the same soil samples as B. ubonensis in regions where both species are endemic [7]. Melioidosis is a diagnostically challenging and often deadly disease that affects humans and many animals, and remains underdiagnosed in many regions across the globe [8]. As B. pseudomallei is not a part of the healthy human flora, the ‘gold standard’ method for melioidosis confirmation is growth of B. pseudomallei from clinical specimens. For maximum isolation of B. pseudomallei from non-sterile sites such as sputum and pus, clinical laboratories require selective culture methods such as Ashdown’s agar containing gentamicin [9] and Ashdown’s broth containing colistin [10]. These media have also been used to successfully isolate B. pseudomallei from microbiologically complex environmental samples such as soil and surface water, which would otherwise yield growth and dominance of many other species [11]. We have previously demonstrated that B. ubonensis is the most commonly co-isolated species when using B. pseudomallei enrichment methods in the melioidosis-endemic “Top End” of the Northern Territory, Australia, in part due to the indistinguishable nature of certain B. ubonensis and B. pseudomallei morphotypes [7]. In addition, it has been reported that the atypical B. pseudomallei O-antigen type B is found in 25% of B. ubonensis strains from Australia [12], further complicating the differentiation between these species due to their immunological cross-reactivity.
Little is currently known about the population biology and genomics of B. ubonensis, although a clearer picture is emerging. The first B. ubonensis isolate (“B. uboniae” EY 3383, isolated from soil in Ubon Ratchathani in 1989) was reported in 2000 [13], and the first B. ubonensis genome (MSMB0022, isolated from soil in Darwin, Australia, in 2001) was sequenced to closure in 2015 [14]. The MSMB0022 genome encodes three circular replicons totalling ~7.2Mbp, which is approximately the same size as the two-chromosome B. pseudomallei genome. In Bcc species, the third replicon, a megaplasmid called pC3 (formerly chromosome III), has been shown to be important for stress resistance, virulence, and antifungal and proteolytic activity in several strains [15, 16]. This replicon is not essential for survival, with ~4% of tested Bcc isolates having spontaneously lost pC3, and additional strains able to be cured of this replicon either by plasmid incompatibility or by removal or toxin-antitoxin systems [15]. Although pC3 loss in B. ubonensis has been achieved in vitro, pC3 loss in wild-type B. ubonensis strains has not yet been identified.
Previous work has shown that Bcc species can encode for innate high-level resistance towards many clinically relevant antibiotics, including the carbapenem antibiotic meropenem [17]. Meropenem is a critical antibiotic for melioidosis therapy, being considered the treatment of choice for those with life-threatening sepsis [18, 19]. To date, the vast majority of B. pseudomallei isolates have been fully susceptible to meropenem [20], although recent evidence has shown that decreased susceptibility towards meropenem can occur after prolonged use of this antibiotic in melioidosis patients with severe sepsis [21]. Certain Bcc species such as B. vietnamiensis, B. cepacia and B. cenocepacia [22], as well as B. pseudomallei [23, 24], exhibit high rates of intra-species recombination. This observation raises the concern that antibiotic resistance genes may spread amongst Burkholderia species in the environment and potentially to the globally important pathogen B. pseudomallei.
The current study describes the first comprehensive analysis of the population biology of B. ubonensis from Australia and Asia. In addition, we identify the first B. ubonensis isolates from the Caribbean. Using large-scale comparative genome analysis, we interrogated 264 B. ubonensis genomes to better understand the geographic distribution and genetic diversity of this species, including potential loss of the pC3 megaplasmid. We also explored rates of meropenem resistance in Asian and Australian B. ubonensis strains, lipopolysaccharide (LPS) O-antigen cluster prevalence and diversity, and the virulence potential of an Asian B. ubonensis strain in the BALB/c mouse model.
Methods
Ethics statement
Procedures and ethics approval for collection of the environmental specimens from which the B. ubonensis isolates were recovered has been previously described [7, 25]. The murine challenge work was conducted according to the specific guidelines provided by the United States Department of Agriculture Animal Welfare Act under approved protocols from the Northern Arizona University IACUC (Protocol 14–011) and the USA Department of Defense Animal Care and Use Review Office (ACURO approval for HDTRA1-12-C-0066_Wagner).
Isolates and species determination
The 264 B. ubonensis isolates examined in this study originated from northern Australia (n = 238), Central Australia (n = 4), Ubon Ratchathani, Thailand (n = 15), Papua New Guinea (PNG; n = 1), and Puerto Rico (n = 6), and were obtained from samples of soil (n = 160), water (n = 15), or plant material (n = 2) (S1 Table). DNA was extracted using protocols optimised for B. pseudomallei [26], and quality-checked using a NanoDrop UV spectrophotometer. Prior to WGS, all isolates were verified as B. ubonensis using the Bu550 real-time PCR [7], which targets the conserved iron-containing redox enzyme family protein encoded by BW23_5472 on chromosome II of MSMB0022 (also referred to as MSMB22 [14]).
Genome sequencing, assemblies and annotation
Genomic data were already publicly available for 230 of the 264 isolates [14, 27]. For completeness, we performed paired-end sequencing of the remaining 34 isolates using a HiSeq2000 instrument (Illumina Inc., San Diego, CA) at the Translational Genomics Research Institute (Phoenix and Flagstaff; AZ, USA). Assemblies were performed with the Microbial Genome Assembler Pipeline (MGAP; https://github.com/dsarov/MGAP---Microbial-Genome-Assembler-Pipeline), which incorporates Trimmomatic [28], Velvet [29], VelvetOptimiser (https://github.com/tseemann/VelvetOptimiser), GapFiller [30], PAGIT [31] and SSPACE [32] into its workflow, using the closed B. ubonensis MSMB0022 genome [14] as a reference for aligning, reordering and orientating contigs. All assemblies were quality-assessed by BLAST against phiX, with any contigs corresponding to this bacteriophage removed. Assemblies were annotated using PGAP [33]. Reference accessions for all 264 genomes are listed in S1 Table.
Comparative genome analysis
The default settings of SPANDx v3.0 [34] were used to identify biallelic single-nucleotide polymorphisms (SNPs) from the 264 B. ubonensis genomes for phylogenetic analysis. B. ubonensis MSMB0022 was used as a reference genome for paired-end read alignment. BEDTools [35], which is run by default in SPANDx, was used to determine gene presence/absence relative to MSMB0022 using a 1kb locus ‘window’ size. Loci were considered variable if they had ≤99% read coverage in one or more strains, and conserved otherwise. To confirm the loss of pC3 (previously called chromosome III) in 10 isolates and to rule out alternative replicons being present in these strains, the unmapped reads from SPANDx for each strain were assembled using MGAP.
BEDTools was also used to determine LPS O-antigen type based on mapping quality against both known and novel LPS O-antigen clusters. Known clusters included B. pseudomallei K96243 (Type A LPS; GenBank reference BX571965.1; coordinates 3196645–3215231), B. ubonensis MSMB0057 (Type B LPS; GenBank reference JF745807), B. pseudomallei 576 (Type B LPS; GenBank reference NZ_CP008777.1; coordinates 1383179–1418799), B. ubonensis MSMB0122 (Type B2 LPS; GenBank reference HQ908420.1), B. pseudomallei MSHR0840 (Type B2 LPS; GenBank reference GU574442.1), B. thailandensis 82172 (Type B2 LPS; GenBank reference JQ783347.1) and B. humptydooensis MSMB0043 (novel LPS; GenBank reference CP013380.1; coordinates 971381–996024). B. ubonensis type strains for determining the prevalence of novel LPS O-antigen genotypes were: A21, BDU9, BDU12, BDU14, INT1-BP158, MSMB0022, MSMB0054, MSMB0063, MSMB0083, MSMB0103, MSMB0268a, MSMB0609, MSMB0742, MSMB0782, MSMB0827, MSMB1058, MSMB1137, MSMB1172, MSMB1173, MSMB1178, MSMB1189, MSMB1206, MSMB1304, MSMB1471, MSMB1517, MSMB1586, MSMB1591, MSMB2105, MSMB2123, MSMB2166, MSMB2180, MSMB2207, RF23-BP17, and RF32-BP11. Sequence coordinates for these LPS O-antigen clusters were extracted from MGAP-assembled genomes based on Mega BLAST analysis against the MSMB0057 O-antigen biosynthesis cluster [12].
Multilocus sequence typing (MLST)
In silico MLST was carried out on all isolates using the Bcc scheme (http://pubmlst.org/bcc/), and on 173 of the 264 B. ubonensis isolates based on the B. pseudomallei scheme (http://pubmlst.org/bpseudomallei/). Ninety-one strains could not be genotyped using the B. pseudomallei scheme as they lack the narK housekeeping locus [36]. Sequence types (STs) were determined from assemblies using the BIGSdb tool, which is integrated into these MLST websites [37]. ST assignments for both schemes are listed in S1 Table and are also searchable on the online databases.
Phylogenetic analysis
The maximum parsimony function of PAUP v4.0a153 [38] was used for phylogenetic reconstruction of genome-wide variants. The Ortho_SNP_matrix.nex output automatically generated by SPANDx was used as the PAUP input. Trees were constructed based on a heuristic search and bootstrapped using 100 replicates. FigTree (http://tree.bio.ed.ac.uk/software/figtree/) was used to visualise PAUP outputs.
Laboratory passaging for pC3 megaplasmid loss
To promote pC3 loss in vitro, phylogenetically unrelated B. ubonensis strains MSMB0782 and MSMB1215 were passaged five times on Ashdown’s agar (37°C for 24-48h), and strains INT1-BP274 and RF23-BP41 were passaged 10 times. MSMB0782 and INT1-BP274 were also subjected to five freeze/thaws ranging from -80°C to room temperature, and INT1-BP274 was passaged seven times at 42°C or room temperature. Eighteen colonies of MSMB2036, which is the same ST as the pC3-negative strain MSMB2035, were then examined for pC3 loss by passaging once on Luria-Bertani agar and growing at 37°C for 48h. DNA from all laboratory-passaged strains was extracted using a chelex heat soak procedure [39] and diluted 1:10 prior to PCR. pC3 detection was carried out with primers Bu_pC3_For1 (5’-CGATGAGCTATTCGTTCGATCT) and Bu_ pC3_Rev1 (5’-AACGTGATCCGGTACAGCAC) to generate a 52bp amplicon, using a slowdown PCR for GC-rich templates [40]. MSMB2035 was included as the pC3-negative control. All DNA was verified for quality using the Bu550 assay [7].
Minimum inhibitory concentration (MIC) determination
Etests (bioMérieux, Baulkham Hills, NSW, Australia) were used to determine meropenem MICs in 40 B. ubonensis strains (S1 Table). This subset of strains was chosen to represent geographically and phylogenetically diverse taxa, and to identify potential MIC differences among strains of the same ST. Isolates were grown on Mueller Hinton agar for 24h at 37°C in an oxygenated environment prior to MIC assessment.
B. ubonensis murine challenge
The ability of B. ubonensis to cause disease via the subcutaneous (sc) route of infection was examined in a murine BALB/c model using a Thai environmental isolate, RF23-BP41 (S1 Table), collected by Northern Arizona University in 2007. We compared the results to sc infection with B. thailandensis type strain E264, which is known to cause death in mice at high doses (>106 colony forming units, or CFU) when delivered via the intraperitoneal [41], intranasal [42, 43] or aerosol [44] routes. Virulence testing was performed in a similar manner as previously described [45]. After shipping, mice were acclimatised for five days before the experiment; food and water were provided ad libitum throughout the study. Mice were lightly anaesthetised with vaporised isoflurane and injected via a single 100μL sc injection in the scruff of the neck. All mice in a single cage received the same infectious dose (B. ubonensis: 1.71 x 104, 105 or 106 CFU). Three infection control mice were injected in an identical way, but with 100μL of sterile 1x PBS instead of bacterial culture. Mice were monitored daily for health status and euthanased on day 21 post-injection with CO2 gas followed by exsanguination.
Results and discussion
Phylogeographic analysis of B. ubonensis
The true global distribution of B. ubonensis is not known. To date, strains have only been reported from the environment in Wuhan, China [6], Ubon Ratchathani, Thailand [13], northern and Central Australia [7], and PNG [5]. In this study, we identified B. ubonensis in the Caribbean environment for the first time, with six isolates retrieved from soil obtained from the north-central and north-eastern regions of Puerto Rico (Juncos, Ceiba and Barceloneta). A recent study of soil samples in the southern United States to determine the presence of Burkholderia spp., and particularly B. pseudomallei, did not yield a single B. ubonensis or B. pseudomallei isolate, although several other Bcc species were retrieved [40]. It is thus probable that neither B. ubonensis nor B. pseudomallei are naturally found in the environment in North America. It remains to be determined whether B. ubonensis is found in other melioidosis-endemic regions such as Africa, Central America, the Indian Ocean islands, South America or South Asia.
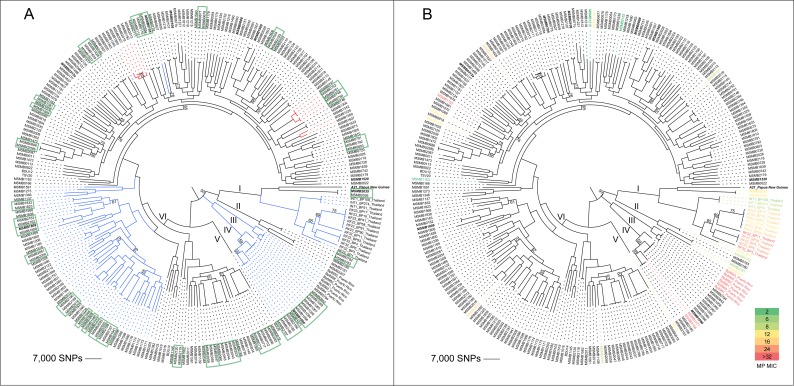
A B. ubonensis phylogeny was reconstructed from 264 genomes derived from Australian, Thai, PNG and Puerto Rican isolates to determine the existence of a continental phylogeographic signal, a phenomenon that has been described in B. pseudomallei [23, 46, 47]. Based on 589,433 biallelic SNPs, six distinct and well-supported clades were identified. Clades II, IV, V and VI solely contained Australian B. ubonensis isolates (n = 240), whereas Clade I contained all isolates from Thailand (n = 15), the PNG isolate A21, and two Australian strains from the tropical “Top End” region of the Northern Territory, and Clade III was comprised of the six Puerto Rican isolates (Fig 1; S1 Fig). Subclades within Clade I showed that the Thai strains clustered most closely with one another (Fig 1), with A21 residing on its own branch and the two Australian strains, MSMB2035 and MSMB2036, sharing a node with the PNG isolate. The Puerto Rican isolates share a node with the Clade IV Australian isolates (Fig 1). Due to limited availability of B. ubonensis from PNG, it could not be determined whether other PNG isolates group with A21, although we hypothesise that PNG B. ubonensis strains will be related based on the relatively narrow genetic diversity observed in PNG B. pseudomallei populations [47, 48]. Within Clade IV, four isolates from the arid region of Central Australia (MSMB2166, MSMB2167, MSMB2185 and MSMB2186), which were obtained from the same soil sample, grouped with other Australian strains, with the most closely related isolates originating from the “Top End” region. Taken together, these results demonstrate that, like B. pseudomallei, B. ubonensis populations exhibit a continental phylogeographic signal, although more samples from Asia and PNG would be needed to improve resolution of subclades within Clade I.
Fig 1. Phylogenomic analysis of 264 Burkholderia ubonensis genomes.
A midpoint-rooted maximum parsimony phylogeny was constructed using 589,433 biallelic core-genome SNPs. A) Strains lacking the B. pseudomallei multilocus sequence typing (MLST) gene narK are labelled with blue branches, and those lacking pC3 (previously known as chromosome III) are in bold italics. Highly related strains retrieved from single environmental samples are outlined by green boxes. Red branches indicate instances where isolates could be differentiated by the B. pseudomallei MLST scheme, but not the Bcc scheme. B) Heatmap of the meropenem minimum inhibitory concentration values for 40 tested B. ubonensis isolates. In both trees, the six distinct clades (I, II, III, IV, V and VI) are labelled. Consistency index = 0.25. Bootstrap values below 80% are labelled on their corresponding branches.
Comparison of phylogenomic structure and MLST
We compared B. ubonensis MLST genotypes obtained using both the B. pseudomallei and Bcc MLST schemes with phylogenomic assignment to determine whether the STs reflected isolate relatedness on the genome level [49], or whether homoplasy was evident among STs as has been observed with certain B. pseudomallei STs [50, 51]. For both MLST schemes, the ST and genomic data showed excellent concordance and no evidence of ST homoplasy, with all identical STs clustering closely on the phylogeny (Fig 1A; green box outlines) and non-identical STs residing on separate branches. Unlike the Bcc scheme, where STs could be assigned from all genomes, STs were not able to be determined for 91 (35%) isolates using the B. pseudomallei MLST scheme due to these strains lacking the “housekeeping” locus narK [36]. We identified five separate clusters within our phylogeny that lacked narK (Fig 1A; blue branches). The first included all the Thai isolates (n = 15), with the remaining four comprising all Puerto Rican (Clade III; n = 6) and Clade IV (n = 14) isolates, plus 57 isolates within Clade VI that were isolated from various “Top End” locales. These results show that certain B. ubonensis strains cannot be fully genotyped with the B. pseudomallei MLST scheme. However, in three instances where strains could be genotyped, the B. pseudomallei scheme was superior at differentiating strains that were related yet distinct on a genomic level (Fig 1, red branches; S1 Table). MSMBs 1225 and 1559 were both ST-1187 using the Bcc scheme but were different STs using the B. pseudomallei scheme; MSMB2013 was assigned ST-1235 by both schemes but the other Bcc ST-1235 strains were found to be ST-1226 according to the B. pseudomallei scheme; and the Bcc ST-1148 strains were separated into ST-1266 and ST-1267 based on the B. pseudomallei alleles. In all cases where additional STs were found, the isolates were obtained from distinct soil samples, indicating greater resolving power of the B. pseudomallei MLST scheme in these cases.
Comparison of phylogenomic structure and meropenem MICs
We mapped meropenem MICs for 40 strains against the genome phylogeny to ascertain whether meropenem-resistant, meropenem-intermediate or meropenem-sensitive strains belonged to a single clade. Eleven strains (Bp8955, Bp8958, Bp8960, Bp8961, Bp8962, Bp8964, MSMB1162, MSMB1471, MSMB2166, RF32-BP11 and RF32-BP3) showed high-level resistance (≥32 μg/mL) towards this antibiotic, including all six Puerto Rican strains. In contrast, two Australian strains (MSMB1215 and MSMB2152) exhibited the lowest MICs at 2–3 μg/mL (Fig 2). Both highly resistant and highly sensitive (2–6 μg/mL) strains were found in the Asian and Australian populations, demonstrating that these phenotypes are not restricted to a certain clade and that B. ubonensis populations from these two geographic regions encode for a range of meropenem MICs (Fig 1B). Although our testing was not comprehensive, we did observe similar MICs for closely related strains. For example, the closely related Thai strains RF23-BP93, RF32-BP4 and RF32-BP6 all exhibited MICs of 24 μg/mL (Fig 1B). The lack of phylogenetic congruence of high-level meropenem-resistant strains supports the hypothesis that the genetic mechanism conferring resistance is laterally transferred among strains. Alternatively, resistance may have arisen multiple times or through multiple mechanisms during the evolution of B. ubonensis due to similar environmental pressures.
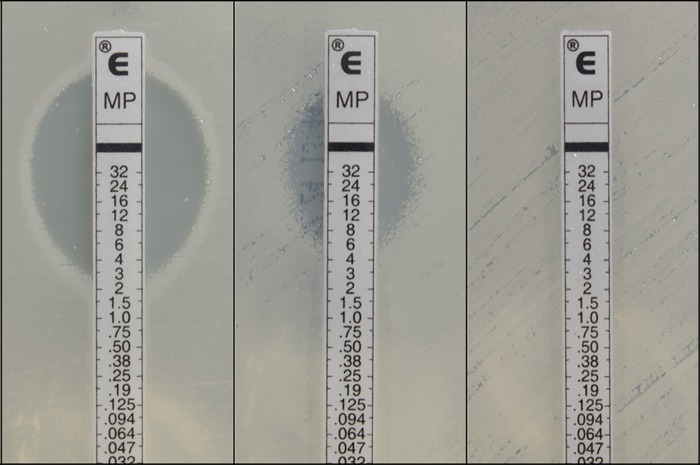
Fig 2. Example Etest results in Burkholderia ubonensis towards meropenem.
Left, sensitive isolate MSMB2152 at a minimum inhibitory concentration (MIC) of 3 μg/mL; centre, intermediate isolate MSMB1183 at an MIC of 6 μg/mL; right, resistant isolate MSMB1162 at an MIC of ≥32 μg/mL.
Many other Bcc species strains can exhibit high-level meropenem resistance [17, 52], indicating that this trait is not specific to B. ubonensis, although the basis for this resistance and its persistence in Bcc populations is not clear. In comparison, the highest meropenem MICs recorded for B. pseudomallei to date are ~4 μg/mL [53, 54], with wild-type strains consistently exhibiting MICs of 0.75–1 μg/mL. Unlike B. pseudomallei, where human-to-human transmission is exceptionally rare and where infections are almost always acquired from the environment [55], Bcc species can transmit between individuals, and indeed this a major clinical issue in the management of cystic fibrosis cohorts [56]. The selective forces acting upon Bcc strains in patients receiving meropenem or other antibiotics may encourage this phenotype to persist in the population, although the lack of human B. ubonensis infections and the identification of high-level meropenem resistance in environmental samples argue against this route of selection in the context of B. ubonensis. B. pseudomallei does not encode a carbapenamase, which likely explains why high-level resistance has not been reported. However, it is conceivable that B. pseudomallei may acquire a carbapenamase whilst residing in the environment, especially from closely related species that share this niche, such as B. ubonensis or other Bcc species. Determining the molecular basis for high-level meropenem resistance in B. ubonensis and in other Bcc species should be a focus of future studies to not only promote a better understanding of resistance mechanisms in these species, but to also provide a basis for proactive monitoring of B. pseudomallei populations in the event of carbapenamase acquisition.
Genetic diversity of B. ubonensis
MLST revealed that B. ubonensis is a highly diverse species. We found 128 STs among the 173 strains that could be genotyped using the B. pseudomallei MLST scheme, and 182 STs among the 264 strains based on the Bcc scheme, although these numbers underestimate diversity due to multiple related isolates being tested from single environmental specimens in our study (S1 Table). Among the 33 Bcc scheme STs represented by two or more B. ubonensis isolates, 27 (82%) of these STs were found within a single sample; such samples are likely to be identical or clonally related due to their physical proximity. We next examined B. ubonensis diversity within our environmental samples. Of the 51 samples where two or more B. ubonensis isolates were retrieved, 26 (51%) exhibited two or more STs, revealing that multiple B. ubonensis genotypes commonly exist within single environmental samples. This result reflects similar observations made in studies examining B. pseudomallei diversity in environmental samples from Thailand [57, 58], B. vietnamiensis in the United States [40], and B. cepacia genomovar III (now known as B. cenocepacia) in the United States, Canada and Australia [59]. Whilst isolation of multiple colonies from a single sample is a laborious endeavour, these studies reinforce the need to collect multiple isolates from individual samples to maximise capture of population diversity.
The megaplasmid pC3 is nonessential to B. ubonensis replication
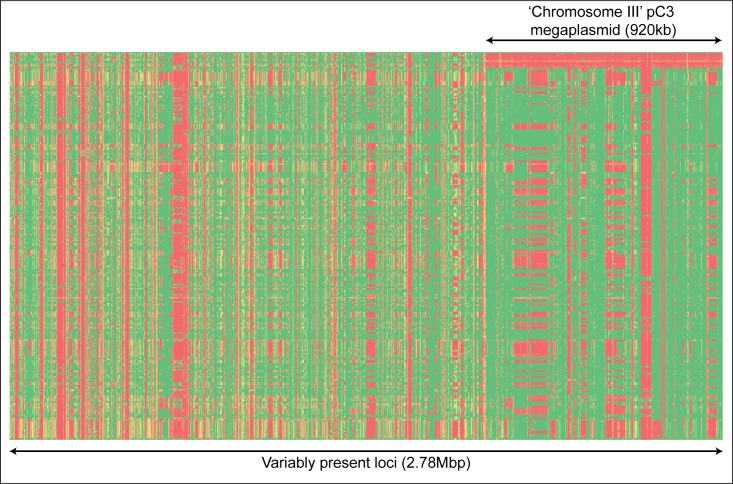
Gene presence/absence analysis of the 264 B. ubonensis genomes against the MSMB0022 reference showed that 2.78Mbp (39%) of the B. ubonensis reference genome was variably present, with the remaining 4.41Mbp conserved across these strains. Ten phylogenetically unrelated strains (A21, MSMB0312a, MSMB0668, MSMB0705, MSMB1080, MSMB1509, MSMB1520, MSMB1809, MSMB2035 and MSMB2108) failed to map reads against the entire sequence for pC3, equating to one-third of the variable regions observed in our dataset (Fig 3). Certain closely related strains did not share this pattern: for example, MSMB2035 and MSMB2036 are clonal according to the two MLST schemes and the WGS phylogeny, yet only MSMB2035 lacked this replicon. Phylogenetic reconstruction using just pC3 as the reference showed no evidence of lateral transfer, with the topology of the tree being highly similar to the phylogenetic tree constructed for chromosomes I and II (Fig 1). This result suggests that pC3 is probably ubiquitous in B. ubonensis strains found in the environment and that it largely follows a vertical path of evolution, but, when propagated under certain conditions, segregation of this replicon can occur spontaneously; in our study, segregation occurred in 4% of strains. Agnoli and coworkers (2014) also observed that four of 110 Bcc isolates tested in their study (4%) had lost pC3, with one of these events having been confirmed to have occurred following laboratory passage [15]. In the type strain MSMB0022, pC3 encodes for 669 genes that are involved in myriad functions (S2 Table). When excluding this replicon, 1.86Mbp (26%) of the B. ubonensis reference genome was variable among the 264 strains.
Fig 3. Heatmap of variably present Burkholderia ubonensis genes across the MSMB0022 genome.
A presence/absence matrix was constructed across 1kb windows of the MSMB0022 reference genome for each of the 264 taxa. Green = 100% mapped reads; red = 0% mapped reads. Taxa are in rows and the 1kb windows are in columns. Only regions containing <80% window coverage for at least one strain are shown, representing 2.78Mbp of the MSMB0022 B. ubonensis genome. The absence of pC3 in 4% of strains demonstrates that this megaplasmid can occasionally segregate, a finding consistent with pC3 in other Bcc species [15].
The conservation of pC3 and its phylogenetic relatedness to chromosomes I and II confirms that pC3 is under strong selection pressure to be maintained in Bcc species, including B. ubonensis. However, certain growth conditions appear to encourage pC3 segregation, raising the possibility that this replicon may be a megaplasmid [60]. Based on the earlier work of Agnoli and colleagues [15, 16], we attempted to cure B. ubonensis strains MSMB0782, MSMB1215, INT1-BP274 and RF23-BP41 of pC3 by performing laboratory passage and growth under varying conditions, including multiple freeze/thaws, growth at 42°C and room temperature, or multiple passages. Despite these attempts, none were successful at segregating pC3. To examine whether an insufficient number of colonies were being tested, we next attempted passage of 18 colonies of MSMB2036, which is closely related to the pC3-lacking strain MSMB2035. Four (22%) colonies lost pC3 after a single passage on Luria-Bertani agar at 37°C for 48h, as observed by a lack of amplification using the Bu_pC3 primers. This finding demonstrates that, as with other Bcc species, the third replicon of B. ubonensis is not necessary for the organism’s survival, at least in a laboratory setting. It remains to be determined whether pC3 replicates independently of the two chromosomes in B. ubonensis. It has been proposed that the second (and where applicable) third ‘chromosomes’ found in approximately 10% of bacterial genomes are in fact ‘chromids’, a term used to define replicons that are not strictly chromosomes or plasmids [61]. To maintain consistency with the work of Agnoli and colleagues [15, 16], we have chosen to refer to this replicon as a pC3 megaplasmid.
At 920kb, the B. ubonensis pC3 megaplasmid is unusually large, although such size is not unprecedented, with B. cenocepacia H111 encoding a curable 1.04Mbp pC3 megaplasmid [16]. Larger megaplasmids have been identified in other soil- and rhizosphere-dwelling organisms including a 1.8Mbp linear megaplasmid identified in the actinomycete Streptomyces clavuligerus [62], and a 1.59Mbp megaplasmid in Azospirillum brasilense [63]. The pC3 replicon of B. ubonensis MSMB0022 failed to be detected as a plasmid using the online PlasmidFinder and VecScreen tools; however, we found that these tools also failed to identify the B. vietnamiensis megaplasmid pBVIE01, possibly because PlasmidFinder has been optimised for plasmid identification in Enterobacteriaceae [64]. BLAST analysis of parA and parB genes from B. vietnamiensis G4 pBVIE01 showed weak evidence of these partitioning system genes in MSMB0022 pC3, although more solid BLAST hits were obtained with chromosome I genes. This result does not rule out the presence of plasmid maintenance loci encoded on this replicon, but rather demonstrates the difficulties in identifying genetic homology across distantly related species. Similarly, the presence of 5S, 16S and 23S ribosomal RNA-encoding genes on pC3 does not necessarily rule out this replicon as being a megaplasmid [16, 60]. Read depth coverage analysis of pC3 showed similar depth to the two chromosomes (e.g. MSMB0011: 108x for pC3 vs 123x for chromosome I and 124x for chromosome II), indicating that this megaplasmid is at a low or single copy number, a finding that is consistent with the generally low copy number of larger plasmids [65].
B. ubonensis exhibits high levels of LPS O-antigen diversity
Earlier work has shown that 25% of Australian B. ubonensis strains possess the unusual B. pseudomallei type B LPS O-antigen [12]. Using our larger dataset, we examined LPS diversity among the 264 strains in silico. Due to insufficient contig coverage across the LPS cluster, 19 strains could not be fully genotyped using this approach; however, these strains did not possess clusters matching to other LPS types. Of the remaining 245 strains that could be genotyped, type B LPS was identified in 20 (8%). In total, 35 different LPS types were found, compared with only four LPS types among 477 global B. pseudomallei strains using the same in silico approach. The most abundant LPS type in the B. ubonensis cohort was MSMB0063 Type Novel, with 28 strains having this genotype; in contrast, eleven LPS types were seen in only a single isolate (S1 Table). LPS genotypes were not restricted to particular STs or geographic regions. For example, the Thai strains RF25-BP1 and RF32-BP3 possessed an LPS cluster that was also found in Australian strains MSMB0782, MSMB0783, MSMB1188, MSMB1562, MSMB1603, and MSMB1635, and among these eight isolates, seven different STs were present. Our findings are consistent with the presence of similar LPS types among Burkholderia species. In addition, we show that B. ubonensis LPS is highly variable and is not associated with the genetic relatedness or geographic origin of an isolate, and would thus be a poor marker for such purposes.
B. ubonensis RF23-BP41 does not cause disease in the immunocompetent BALB/c mouse model
Unlike other Bcc species or B. pseudomallei, B. ubonensis is thought to rarely, if ever, cause disease in humans [66], as evidenced by B. ubonensis being the only Bcc species not yet retrieved from cystic fibrosis sputum [52]. Indeed, there is only a single report of B. ubonensis being isolated from a human infection, a Thai nosocomial case (strain LMG 24263 [1]). Given the absence of other reported B. ubonensis infections to date, the role of B. ubonensis as the aetiologic agent in this Thai case should be treated with scepticism; for instance, testing for the presence of known pathogens in the same clinical specimen was not stated. However, another possibility is that certain B. ubonensis strains are in fact capable of causing disease, with such cases remaining unreported due to insufficient or inaccurate differentiation of B. ubonensis from other Bcc species.
To further examine the virulence potential of B. ubonensis, we inoculated BALB/c mice via sc injection using 1.7x 104, 105, and 106 CFU of the Thai strain RF23-BP41. To our knowledge, B. ubonensis virulence has not yet been tested in the mouse model. RF23-BP41 was chosen for several reasons. First, its Thai origin maximises the probability of genetic relatedness to the putatively pathogenic LMG 24263 strain. Second, RF23-BP41 was isolated from a region where individuals (particularly rice farmers) regularly come into contact with soil bacteria, increasing the likelihood of successful human infection. Third, this strain demonstrated resistance towards meropenem (MIC 16μg/mL), which would potentially confer a selective advantage during antibiotic treatment. Finally, this strain harbours pC3, which has been shown to impart virulence capacity in other Bcc species [15, 16]. Even at the highest dose of 1.7x106 CFU, no mice exhibited weight loss or lethargy during the 21-day challenge experiment, with their health status identical to that of the three control mice. The same result was observed in the BALB/c mice subcutaneously injected with B. thailandensis E264 at a similar dosage range [45]. Certain B. thailandensis strains are capable of infecting immunocompromised humans [67–69], and can be lethal in murine models when administered at high doses via other routes [41–44]. In contrast, in other studies the 10-day LD50 of B. pseudomallei in BALB/c mice was ~1x103 CFU when delivered via the sc route [70], and between 10 and 6x104 CFU when administered via the intraperitoneal route, with virulence reduced but not abolished in highly laboratory-passaged strains [71, 72]. Other mouse model studies have shown that virulence of Bcc species can vary; for example, the epidemic B. cenocepacia strain J2315 caused universal mortality when inoculated at 103 cfu into gp91phox−/− mice via an intratracheal route, whereas other B. cenocepacia strains were less virulent and B. vietnamiensis strain R2 was avirulent [73]. Another study using intranasal inoculation of leukopaenic BALB/c mice with ~104 cfu also showed differential virulence within Bcc species, with some mice clearing their infections [74], indicating that virulence potential varies among strains.
Based on the findings of these earlier studies, pathogenicity may also vary among B. ubonensis strains. Characterising the virulence potential of other B. ubonensis strains may identify unusual pathogenic strains, although we deem this unlikely based on the lack of verified human infections caused by B. ubonensis. In consideration of the IACUC guidelines, we chose not to carry out testing of further strains using the mouse model. We acknowledge that our study only tested B. ubonensis in immunocompetent BALB/c mice via a sc route. The use of immunocompromised or immune-deficient mouse models or infection via different routes may reveal that B. ubonensis can cause disease in such cases. Bcc species carry various virulence factors that are thought to contribute to their pathogenic potential, including extracellular lipases, metalloproteases, serine proteases, flagella, pili, adhesins, toxins, siderophores and lipopolysaccharides [75]. We did not investigate the presence of virulence genes in B. ubonensis compared with other Bcc species but doing so may shed further light on its potential virulence capacity. It may be possible to use such in silico methods rather than further animal experiments to determine whether B. ubonensis is unusual compared with other Bcc species due to a lack of key virulence loci or pathways in its genome.
Conclusions
The metabolic diversity of Bcc species continues to spur interest in this highly adaptable group of bacteria. Our study provides important new insights into the biology of B. ubonensis, a largely neglected member of the Bcc due to its ostensibly avirulent nature. Genomic analysis of 264 B. ubonensis strains from Australia, PNG, Puerto Rico and Thailand revealed that B. ubonensis is a genetically highly diverse organism, with at least 26% of its chromosomal DNA variably present among strains. Like B. pseudomallei, B. ubonensis has a distinct phylogeographic signature that can be distinguished at the genomic level. It remains to be determined whether B. ubonensis is found on other continents. ‘Chromosome III’ encodes a ubiquitous yet apparently dispensable pC3 megaplasmid, similarly to other Bcc species, and can segregate in the laboratory setting. Like other Bcc species, we show that B. ubonensis strains exhibit variable levels of meropenem resistance. Determining the molecular mechanism underpinning high-level meropenem resistance in certain B. ubonensis strains will provide a better understanding of the potential transmission of this phenotype to the melioidosis bacterium B. pseudomallei, which frequently co-resides with B. ubonensis in the environment. Finally, using the immunocompetent BALB/c mouse model, we show that an Asian B. ubonensis strain is not likely to cause disease, providing evidence that at least some members of this species are probably avirulent in immunocompetent individuals. Further studies are needed to confirm the avirulent nature of B. ubonensis across a greater strain set using both immunocompetent and immunocompromised or immunodeficient animal models, or in silico analysis of the B. ubonensis genome to identify intact virulence determinants. The apparent non-pathogenic nature of certain B. ubonensis strains may make them amenable to large-scale biotechnological applications, such as biocontrol and biofuel production.
Supporting information
B. ubonensis Clades I-VI are labelled, and B. ubonensis strains from regions other than Australia are noted. Consistency index = 0.36. The tree was rooted with the B. pseudomallei complex species. In total, 277 taxa were used to reconstruct this phylogeny, of which 264 were B. ubonensis.
(PDF)
(XLSX)
(XLSX)
Acknowledgments
We wish to thank Ian Harrington and Vanessa Theobald (Menzies School of Health Research) for isolate collection and laboratory assistance, Jay E. Gee and Alex R. Hoffmaster (US Centers for Disease Control and Prevention) for helpful discussions in planning and organising the environmental sampling of Burkholderia spp. in Puerto Rico, and Dr Charlotte Peeters (Ghent University) and Keith Jolley (University of Oxford) for B. cepacia complex MLST database assistance.
Data Availability
Raw sequences or assembled genomes for the 264 B. ubonensis genomes are available from the NCBI GenBank and/or SRA databases. Accession numbers are listed in S1 Table.
Funding Statement
This study was made possible by funding from the Australian National Health and Medical Research Council (grant IDs 236216, 383504, 605820, 1046812 and 1098337; https://www.nhmrc.gov.au/), the US Department of Defense Chemical and Biological Defense Program through the Defense Threat Reduction Agency (grant ID HDTRA1-12-C-0066; www.dtra.mil/), the Australian Research Council (grant ID LP110100691; www.arc.gov.au/), the US Centers for Disease Control and Prevention (grant ID NU50CK000480; www.cdc.gov), and the Northern Territory government (grant IDs NTRIB06 and NTRIB09; https://nt.gov.au/). EPP is funded by a USC Research Fellowship (http://usc.edu.au/), DSS is funded by an Advance Queensland Fellowship (AQRF13016-17RD2; http://advance.qld.gov.au/) and MK is supported by a Swiss National Science Foundation fellowship (PBBSB-111156; http://www.snf.ch/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vanlaere E, Lipuma JJ, Baldwin A, Henry D, De Brandt E, Mahenthiralingam E, et al. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int J Syst Evol Microbiol. 2008;58(Pt 7):1580–1590. doi: 10.1099/ijs.0.65634-0 [DOI] [PubMed] [Google Scholar]
- 2.De Smet B, Mayo M, Peeters C, Zlosnik JE, Spilker T, Hird TJ, et al. Burkholderia stagnalis sp. nov. and Burkholderia territorii sp. nov., two novel Burkholderia cepacia complex species from environmental and human sources. Int J Syst Evol Microbiol. 2015;65(7):2265–2271. doi: 10.1099/ijs.0.000251 [DOI] [PubMed] [Google Scholar]
- 3.Peeters C, Depoorter E, Praet J, Vandamme P. Extensive cultivation of soil and water samples yields various pathogens in patients with cystic fibrosis but not Burkholderia multivorans. J Cyst Fibros. 2016;15(6):769–775. doi: 10.1016/j.jcf.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 4.Depoorter E, Bull MJ, Peeters C, Coenye T, Vandamme P, Mahenthiralingam E. Burkholderia: an update on taxonomy and biotechnological potential as antibiotic producers. Appl Microbiol Biotechnol. 2016;100(12):5215–5229. doi: 10.1007/s00253-016-7520-x [DOI] [PubMed] [Google Scholar]
- 5.Marshall K, Shakya S, Greenhill AR, Padill G, Baker A, Warner JM. Antibiosis of Burkholderia ubonensis againist Burkholderia pseudomallei, the causative agent for melioidosis. Southeast Asian J Trop Med Public Health. 2010;41(4):904–912. [PubMed] [Google Scholar]
- 6.Yang W, He Y, Xu L, Zhang H, Yan Y. A new extracellular thermo-solvent-stable lipase from Burkholderia ubonensis SL-4: Identification, characterization and application for biodiesel production. J Mol Catal B Enzym. 2016;126:76–89. [Google Scholar]
- 7.Price EP, Sarovich DS, Webb JR, Ginther JL, Mayo M, Cook JM, et al. Accurate and rapid identification of the Burkholderia pseudomallei near-neighbour, Burkholderia ubonensis, using real-time PCR. PLoS One. 2013;8(8):e71647 doi: 10.1371/journal.pone.0071647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1(1). doi: 10.1038/nmicrobiol.2015.8 [DOI] [PubMed] [Google Scholar]
- 9.Ashdown LR. An improved screening technique for isolation of Pseudomonas pseudomallei from clinical specimens. Pathology. 1979;11(2):293–297. [DOI] [PubMed] [Google Scholar]
- 10.Walsh AL, Wuthiekanun V, Smith MD, Suputtamongkol Y, White NJ. Selective broths for the isolation of Pseudomonas pseudomallei from clinical samples. Trans R Soc Trop Med Hyg. 1995;89(1):124 [DOI] [PubMed] [Google Scholar]
- 11.Ashdown LR, Clarke SG. Evaluation of culture techniques for isolation of Pseudomonas pseudomallei from soil. Appl Environ Microbiol. 1992;58(12):4011–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone JK, Mayo M, Grasso SA, Ginther JL, Warrington SD, Allender CJ, et al. Detection of Burkholderia pseudomallei O-antigen serotypes in near-neighbor species. BMC Microbiol. 2012;12:250 doi: 10.1186/1471-2180-12-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yabuuchi E, Kawamura Y, Ezaki T, Ikedo M, Dejsirilert S, Fujiwara N, et al. Burkholderia uboniae sp. nov., L-arabinose-assimilating but different from Burkholderia thailandensis and Burkholderia vietnamiensis. Microbiol Immunol. 2000;44(4):307–317. [DOI] [PubMed] [Google Scholar]
- 14.Johnson SL, Bishop-Lilly KA, Ladner JT, Daligault HE, Davenport KW, Jaissle J, et al. Complete genome sequences for 59 Burkholderia isolates, both pathogenic and near neighbor. Genome Announc. 2015;3(2):e00159–15. doi: 10.1128/genomeA.00159-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agnoli K, Frauenknecht C, Freitag R, Schwager S, Jenul C, Vergunst A, et al. The third replicon of members of the Burkholderia cepacia complex, plasmid pC3, plays a role in stress tolerance. Appl Environ Microbiol. 2014;80(4):1340–1348. doi: 10.1128/AEM.03330-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agnoli K, Schwager S, Uehlinger S, Vergunst A, Viteri DF, Nguyen DT, et al. Exposing the third chromosome of Burkholderia cepacia complex strains as a virulence plasmid. Mol Microbiol. 2012;83(2):362–378. doi: 10.1111/j.1365-2958.2011.07937.x [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Chen Y, Tabibi S, Alba L, Garber E, Saiman L. Antimicrobial susceptibility and synergy studies of Burkholderia cepacia complex isolated from patients with cystic fibrosis. Antimicrob Agents Chemother. 2007;51(3):1085–1088. doi: 10.1128/AAC.00954-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng AC, Fisher DA, Anstey NM, Stephens DP, Jacups SP, Currie BJ. Outcomes of patients with melioidosis treated with meropenem. Antimicrob Agents Chemother. 2004;48(5):1763–5. doi: 10.1128/AAC.48.5.1763-1765.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med. 2015;36(1):111–125. doi: 10.1055/s-0034-1398389 [DOI] [PubMed] [Google Scholar]
- 20.Crowe A, McMahon N, Currie BJ, Baird RW. Current antimicrobial susceptibility of first-episode melioidosis Burkholderia pseudomallei isolates from the Northern Territory, Australia. Int J Antimicrob Agents. 2014;44(2):160–162. doi: 10.1016/j.ijantimicag.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 21.Price EP, Laird Smith M, Paxinos EE, Tallon LJ, Sadzewicz L, Sengamalay N, et al. Whole-genome sequences of Burkholderia pseudomallei isolates exhibiting decreased meropenem susceptibility. Genome Announc. 2017;5(14):e00053–17. doi: 10.1128/genomeA.00053-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldwin A, Mahenthiralingam E, Thickett KM, Honeybourne D, Maiden MC, Govan JR, et al. Multilocus sequence typing scheme that provides both species and strain differentiation for the Burkholderia cepacia complex. J Clin Microbiol. 2005;43(9):4665–4673. doi: 10.1128/JCM.43.9.4665-4673.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson T, Giffard P, Beckstrom-Sternberg S, Auerbach R, Hornstra H, Tuanyok A, et al. Phylogeographic reconstruction of a bacterial species with high levels of lateral gene transfer. BMC Biol. 2009;7:78 doi: 10.1186/1741-7007-7-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nandi T, Holden MT, Didelot X, Mehershahi K, Boddey JA, Beacham I, et al. Burkholderia pseudomallei sequencing identifies genomic clades with distinct recombination, accessory, and epigenetic profiles. Genome Res. 2015;25(4):608 [PMC free article] [PubMed] [Google Scholar]
- 25.Warner JM, Pelowa DB, Gal D, Rai G, Mayo M, Currie BJ, et al. The epidemiology of melioidosis in the Balimo region of Papua New Guinea. Epidemiol Infect. 2008;136(7):965–71. doi: 10.1017/S0950268807009429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Currie BJ, Gal D, Mayo M, Ward L, Godoy D, Spratt BG, et al. Using BOX-PCR to exclude a clonal outbreak of melioidosis. BMC Infect Dis. 2007;7:68 doi: 10.1186/1471-2334-7-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahl JW, Vazquez AJ, Hall CM, Busch JD, Tuanyok A, Mayo M, et al. The effects of signal erosion and core genome reduction on the identification of diagnostic markers. MBio. 2016;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–829. doi: 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boetzer M, Pirovano W. Toward almost closed genomes with GapFiller. Genome Biol. 2012;13(6):R56 doi: 10.1186/gb-2012-13-6-r56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swain MT, Tsai IJ, Assefa SA, Newbold C, Berriman M, Otto TD. A post-assembly genome-improvement toolkit (PAGIT) to obtain annotated genomes from contigs. Nat Protoc. 2012;7(7):1260–1284. doi: 10.1038/nprot.2012.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27(4):578–579. doi: 10.1093/bioinformatics/btq683 [DOI] [PubMed] [Google Scholar]
- 33.Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44(14):6614–6624. doi: 10.1093/nar/gkw569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarovich DS, Price EP. SPANDx: a genomics pipeline for comparative analysis of large haploid whole genome re-sequencing datasets. BMC Res Notes. 2014;7:618 doi: 10.1186/1756-0500-7-618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26(6):841–842. doi: 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price EP, MacHunter B, Spratt BG, Wagner DM, Currie BJ, Sarovich DS. Improved multilocus sequence typing of Burkholderia pseudomallei and closely related species. J Med Microbiol. 2016. [DOI] [PubMed] [Google Scholar]
- 37.Jolley KA, Maiden MC. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595 doi: 10.1186/1471-2105-11-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods) Version 4. Sunderland, Massachusetts: Sinauer Associates; 2002. [Google Scholar]
- 39.de Lamballerie X, Zandotti C, Vignoli C, Bollet C, de Micco P. A one-step microbial DNA extraction method using "Chelex 100" suitable for gene amplification. Res Microbiol. 1992;143(8):785–790. [DOI] [PubMed] [Google Scholar]
- 40.Hall CM, Busch JD, Shippy K, Allender CJ, Kaestli M, Mayo M, et al. Diverse Burkholderia species isolated from soils in the southern United States with no evidence of B. pseudomallei. PLoS One. 2015;10(11):e0143254 doi: 10.1371/journal.pone.0143254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeShazer D. Virulence of clinical and environmental isolates of Burkholderia oklahomensis and Burkholderia thailandensis in hamsters and mice. FEMS Microbiol Lett. 2007;277(1):64–69. doi: 10.1111/j.1574-6968.2007.00946.x [DOI] [PubMed] [Google Scholar]
- 42.Morici LA, Heang J, Tate T, Didier PJ, Roy CJ. Differential susceptibility of inbred mouse strains to Burkholderia thailandensis aerosol infection. Microbial Pathogenesis. 2010;48(1):9–17. doi: 10.1016/j.micpath.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiersinga WJ, de Vos AF, de Beer R, Wieland CW, Roelofs JJ, Woods DE, et al. Inflammation patterns induced by different Burkholderia species in mice. Cell Microbiol. 2008;10(1):81–87. doi: 10.1111/j.1462-5822.2007.01016.x [DOI] [PubMed] [Google Scholar]
- 44.West TE, Frevert CW, Liggitt HD, Skerrett SJ. Inhalation of Burkholderia thailandensis results in lethal necrotizing pneumonia in mice: a surrogate model for pneumonic melioidosis. Trans R Soc Trop Med Hyg. 2008;102 Suppl 1:S119–S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuanyok A, Mayo M, Scholz H, Hall CM, Allender CJ, Kaestli M, et al. Burkholderia humptydooensis sp. nov., a new species related to Burkholderia thailandensis and the fifth member of the Burkholderia pseudomallei complex. Appl Environ Microbiol. 2017;83(5):e02802–16. doi: 10.1128/AEM.02802-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price EP, Sarovich DS, Smith EJ, MacHunter B, Harrington G, Theobald V, et al. Unprecedented melioidosis cases in northern Australia caused by an Asian Burkholderia pseudomallei strain identified by using large-scale comparative genomics. Appl Environ Microbiol. 2016;82(3):954–963. doi: 10.1128/AEM.03013-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarovich DS, Garin B, De Smet B, Kaestli M, Mayo M, Vandamme P, et al. Phylogenomic analysis reveals an Asian origin for African Burkholderia pseudomallei and further supports melioidosis endemicity in Africa. mSphere. 2016;1(2):e00089–15. doi: 10.1128/mSphere.00089-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker A, Pearson T, Price EP, Dale J, Keim P, Hornstra H, et al. Molecular phylogeny of Burkholderia pseudomallei from a remote region of Papua New Guinea. PLoS One. 2011;6(3):e18343 doi: 10.1371/journal.pone.0018343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peeters C, Cooper VS, Hatcher PJ, Verheyde B, Carlier A, Vandamme P. Comparative genomics of Burkholderia multivorans, a ubiquitous pathogen with a highly conserved genomic structure. PLoS One. 2017;12(4):e0176191 doi: 10.1371/journal.pone.0176191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Smet B, Sarovich DS, Price EP, Mayo M, Theobald V, Kham C, et al. Whole-genome sequencing confirms that Burkholderia pseudomallei multilocus sequence types common to both Cambodia and Australia are due to homoplasy. J Clin Microbiol. 2015;53(1):323–326. doi: 10.1128/JCM.02574-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aziz A, Sarovich DS, Harris T, Kaestli M, McRobb E, Mayo M, et al. Suspected cases of intracontinental Burkholderia pseudomallei sequence type homoplasy resolved using whole-genome sequencing. Microb Genom. 2017;Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peeters E, Nelis HJ, Coenye T. In vitro activity of ceftazidime, ciprofloxacin, meropenem, minocycline, tobramycin and trimethoprim/sulfamethoxazole against planktonic and sessile Burkholderia cepacia complex bacteria. J Antimicrob Chemother. 2009;64(4):801–809. doi: 10.1093/jac/dkp253 [DOI] [PubMed] [Google Scholar]
- 53.Sarovich DS, Price EP, Von Schulze AT, Cook JM, Mayo M, Watson LM, et al. Characterization of ceftazidime resistance mechanisms in clinical isolates of Burkholderia pseudomallei from Australia. PLoS One. 2012;7(2):e30789 doi: 10.1371/journal.pone.0030789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayden HS, Lim R, Brittnacher MJ, Sims EH, Ramage ER, Fong C, et al. Evolution of Burkholderia pseudomallei in recurrent melioidosis. PLoS One. 2012;7(5):e36507 doi: 10.1371/journal.pone.0036507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dance DAB. Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human-animal hosts. Acta Trop. 2000;74(2–3):159–168. [DOI] [PubMed] [Google Scholar]
- 56.Vinion-Dubiel AD, Spilker T, Dean CR, Monteil H, LiPuma JJ, Goldberg JB. Correlation of wbiI genotype, serotype, and isolate source within species of the Burkholderia cepacia complex. J Clin Microbiol. 2004;42(9):4121–4126. doi: 10.1128/JCM.42.9.4121-4126.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chantratita N, Wuthiekanun V, Limmathurotsakul D, Vesaratchavest M, Thanwisai A, Amornchai P, et al. Genetic diversity and microevolution of Burkholderia pseudomallei in the environment. PLoS Negl Trop Dis. 2008;2(2):e182 doi: 10.1371/journal.pntd.0000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wuthiekanun V, Limmathurotsakul D, Chantratita N, Feil EJ, Day NP, Peacock SJ. Burkholderia pseudomallei is genetically diverse in agricultural land in Northeast Thailand. PLoS Negl Trop Dis. 2009;3(8):e496 doi: 10.1371/journal.pntd.0000496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coenye T, LiPuma JJ. Population structure analysis of Burkholderia cepacia genomovar III: varying degrees of genetic recombination characterize major clonal complexes. Microbiology. 2003;149(Pt 1):77–88. doi: 10.1099/mic.0.25850-0 [DOI] [PubMed] [Google Scholar]
- 60.Bentley SD, Parkhill J. Comparative genomic structure of prokaryotes. Annu Rev Genet. 2004;38:771–792. doi: 10.1146/annurev.genet.38.072902.094318 [DOI] [PubMed] [Google Scholar]
- 61.Harrison PW, Lower RP, Kim NK, Young JP. Introducing the bacterial 'chromid': not a chromosome, not a plasmid. Trends Microbiol. 2010;18(4):141–148. doi: 10.1016/j.tim.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 62.Medema MH, Trefzer A, Kovalchuk A, van den Berg M, Muller U, Heijne W, et al. The sequence of a 1.8-Mb bacterial linear plasmid reveals a rich evolutionary reservoir of secondary metabolic pathways. Genome Biol Evol. 2010;2:212–224. doi: 10.1093/gbe/evq013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Acosta-Cruz E, Wisniewski-Dye F, Rouy Z, Barbe V, Valdes M, Mavingui P. Insights into the 1.59-Mbp largest plasmid of Azospirillum brasilense CBG497. Arch Microbiol. 2012;194(9):725–36. doi: 10.1007/s00203-012-0805-2 [DOI] [PubMed] [Google Scholar]
- 64.Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58(7):3895–3903. doi: 10.1128/AAC.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith MA, Bidochka MJ. Bacterial fitness and plasmid loss: the importance of culture conditions and plasmid size. Can J Microbiol. 1998;44(4):351–355. [PubMed] [Google Scholar]
- 66.Levy A, Merritt AJ, Aravena-Roman M, Hodge MM, Inglis TJ. Expanded range of Burkholderia species in Australia. Am J Trop Med Hyg. 2008;78(4):599–604. [PubMed] [Google Scholar]
- 67.Lertpatanasuwan N, Sermsri K, Petkaseam A, Trakulsomboon S, Thamlikitkul V, Suputtamongkol Y. Arabinose-positive Burkholderia pseudomallei infection in humans: case report. Clin Infect Dis. 1999;28(4):927–928. [DOI] [PubMed] [Google Scholar]
- 68.Dharakul T, Tassaneetrithep B, Trakulsomboon S, Songsivilai S. Phylogenetic analysis of Ara+ and Ara- Burkholderia pseudomallei isolates and development of a multiplex PCR procedure for rapid discrimination between the two biotypes. J Clin Microbiol. 1999;37(6):1906–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glass MB, Gee JE, Steigerwalt AG, Cavuoti D, Barton T, Hardy RD, et al. Pneumonia and septicemia caused by Burkholderia thailandensis in the United States. J Clin Microbiol. 2006;44(12):4601–4604. doi: 10.1128/JCM.01585-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barnes JL, Ketheesan N. Route of infection in melioidosis. Emerg Infect Dis. 2005;11(4):638–639. doi: 10.3201/eid1104.041051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welkos SL, Klimko CP, Kern SJ, Bearss JJ, Bozue JA, Bernhards RC, et al. Characterization of Burkholderia pseudomallei strains using a murine intraperitoneal infection model and in vitro macrophage assays. PLoS One. 2015;10(4):e0124667 doi: 10.1371/journal.pone.0124667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Challacombe JF, Stubben CJ, Klimko CP, Welkos SL, Kern SJ, Bozue JA, et al. Interrogation of the Burkholderia pseudomallei genome to address differential virulence among isolates. PLoS One. 2014;9(12):e115951 doi: 10.1371/journal.pone.0115951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sousa SA, Ulrich M, Bragonzi A, Burke M, Worlitzsch D, Leitao JH, et al. Virulence of Burkholderia cepacia complex strains in gp91phox-/- mice. Cell Microbiol. 2007;9(12):2817–2825. doi: 10.1111/j.1462-5822.2007.00998.x [DOI] [PubMed] [Google Scholar]
- 74.Chu KK, Davidson DJ, Halsey TK, Chung JW, Speert DP. Differential persistence among genomovars of the Burkholderia cepacia complex in a murine model of pulmonary infection. Infect Immun. 2002;70(5):2715–2720. doi: 10.1128/IAI.70.5.2715-2720.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leitão JH, Sousa SA, Ferreira AS, Ramos CG, Silva IN, Moreira LM. Pathogenicity, virulence factors, and strategies to fight against Burkholderia cepacia complex pathogens and related species. Appl Microbiol Biotechnol. 2010;87(1):31–40. doi: 10.1007/s00253-010-2528-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
B. ubonensis Clades I-VI are labelled, and B. ubonensis strains from regions other than Australia are noted. Consistency index = 0.36. The tree was rooted with the B. pseudomallei complex species. In total, 277 taxa were used to reconstruct this phylogeny, of which 264 were B. ubonensis.
(PDF)
(XLSX)
(XLSX)
Data Availability Statement
Raw sequences or assembled genomes for the 264 B. ubonensis genomes are available from the NCBI GenBank and/or SRA databases. Accession numbers are listed in S1 Table.